Abstract
Introduction
The Brugada syndrome is associated with arrhythmic events, which may even lead to sudden cardiac death (SCD) as it causes arrhythmic events. A typical Brugada syndrome ECG type I can be triggered at fever situations. The aim of this pooled meta‐analysis is to further explore the baseline characteristics and the association of fever to BrS‐related arrhythmic events.
Methods
We compiled data from a search of databases (PubMed, Web of Science, Cochrane Library, and Google Scholar). We included 17 studies including 14 case reports and a total of 53 patients.
Results
Our population including 53 patients showed a male predominance of 92% with a mean age of 40.6 ± 17.7 years. 58% of patients had a family history of SCD or BrS. Genetic screening was performed in 14 patients (26%) and revealed a SCN5A mutation in 21% of the patients. ICD implantation was initiated in six patients. 75% (n = 39) of patients did not have symptoms before the fever event. Symptoms at fever included life‐threatening arrhythmia such as ventricular fibrillation (VF) or ventricular tachycardia (VT; 17%), syncope (13%), and cardiac arrest or aborted SCD (13%). One patient developed electrical storm which led to not aborted SCD.
Conclusion
Fever is a great risk factor for arrhythmia events in BrS patients. Patients with known fever triggered Brugada syndrome should be surveilled closely during fever and be started on antipyretic therapy as soon as possible.
Keywords: arrhythmic events, Brugada, fever
1. INTRODUCTION
The type I Brugada syndrome (BrS) is electrographically characterized by a coved type ST segment elevation in precordial leads (V1‐V3) and right bundle branch block (RBBB). The global prevalence varies between 5‐ 20/10.000 inhabitants with a higher incidence in endemic areas such as in Asian countries. In many cases, an SCN5A mutation can be identified. Moreover, it is considered as one of the leading causes of death in men under 40‐year‐old men excluding accidents.
Fever not only unmasks a Brugada‐type electrocardiogram (ECG) but also increases the risk of ventricular tachyarrhythmias such as ventricular fibrillation (VF) or sudden cardiac death (SCD; Morita, Nagase, Kusano, & Ohe, 2002; Saura et al., 2002). The underlying mechanism of this finding might involve a decrease of the sodium current during a rise in temperature which has been recently described by our group using human cardiomyocytes from induced pluripotent stem cells (El‐Battrawy et al., 2016).
The aim of this study is to describe the baseline characteristics and outcomes of BrS patients unmasked by fever.
2. METHODS
In this analysis, we included all the patients that presented with a type I Brugada syndrome induced by fever. We recruited a total of 53 BrS patients that were described in 17 articles including 14 case reports, Figure 1. (Adler et al., 2013; Aramaki, Okumura, & Shimizu, 2005; Baranchuk & Simpson, 2011; Barra, Providencia, & Nascimento, 2013; Chockalingam, Rammeloo, & Postema, 2011; Daimi et al., 2015; Dinckal et al., 2003; Erdogan & Hunuk, 2013; Gotz, Beckmann, Kaab, & Hinterseer, 2009; Grogan, Cube, & Edwards, 2011; Junttila, Gonzalez, Lizotte, & Benito, 2008; Keller et al., 2006; McIntyre et al., 2012; Mendes, Elvas, Ramos, & Pego, 2017; Ortega‐Carnicer, Benezet, & Ceres, 2003; Picetti, Zoerle, Cattani, & Caspani, 2014; Probst et al., 2007; Rattanawong et al., 2016; Rebollo et al., 2000; Samani et al., 2009; Skinner et al., 2007; Sovari, Prasun, Kocheril, & Brugada, 2006; Tsarouhas et al., 2010).
Figure 1.
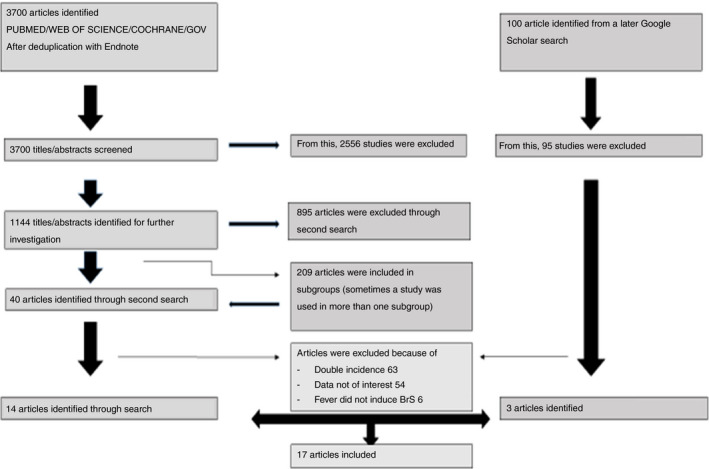
Flow chart of the search strategy
To diagnose BrS, the electrodes are placed in the second, third, or fourth intercostal space. A type I ECG is defined by a descending ST segment with a negative T wave in at least one right precordial lead (V1‐V3) and a terminal r‘ wave with a J‐point elevation of ≥0.2 mV.
2.1. Data collection of different studies
We evaluated demographic data such as age at fever event, gender and clinical data such as family history of SCD or BrS, symptoms before fever and at fever event, results of drug tests as well as results of electrophysiological studies and genetic testing (Figure 1). If follow‐up was performed, we collected the data with special consideration of arrhythmic events that recurred at the reappearance of fever. Since not every study especially case reports documented follow‐up, only those patients with follow‐up were included in the statistics of recurrent events.
2.2. Pooled literature review
A thorough search of leading data banks such as PubMed, Web of Science, Google scholar, and Cochrane Library was performed including publications until 2018, English or German language and human subjects. Other criteria were a type I Brugada pattern and fever. Later we limited the latter through focussing on those patients whose Brugada patterns were induced by fever. Case reports and studies that did not perform follow‐up were also included. At first, we conducted a wide search only using the term Brugada and Syndrome, please deleted the following words BrugadaANDsyndrome, later as we wanted to search more specifically we used the term Brugada AND syndrome AND fever OR hyperthermia.
2.3. Statistics
For continous variables with normal distribution, data are presented as mean ± SD. Data without normal distribution are presented as median ± interquartile range. For categorical variables, frequency (%) was used. To compare categorical variables, the chi‐squared test came into use. The Student's t test and Mann–Whitney U test were to compare continuous non‐normal distributions. For assessing normal distribution, the Kolmogorov–Smirnov test was applied.
3. RESULTS
3.1. Demographics
Our analysis of 53 patients (Table 1 and Table S1) with a mean age of 40.6 ± 17.7 revealed a male predominance of 92%. 58% of patients presented with a family history of SCD or BrS. The BrS pattern was unmasked in eight of 10 patients (80%) by a drug test after fever had subsided. Drug tests were performed to further secure diagnosis of BrS Sovari et al. (2006) or for risk stratification (Barra et al., 2013). Symptoms before fever included syncope in two patients (4%) and VT/VF in one (2%).
Table 1.
Demographics of fever‐induced Brugada syndrome patients
| Number of patients | 53 |
| Age mean ± SD | 40.6 ± 17.7 |
| Male, n (%) | 33 (92) |
| Family history of SCD or BrS, n (%) | 7 (58) |
| Asymptomatic before fever | 39 (75) |
| Events | |
| Life‐threatening arrhythmia (VF and/or VT) | 9 (17) |
| Syncope | 7 (13) |
| Cardiac arrest/aSCD/SCD | 7 (13) |
| Atrial arrhythmias | 1 (2) |
| ECG data, n (%) | |
| Drug‐induced BrS | 8 (80) |
| Spontaneous/persistent BrS type I | 0 (0) |
| Fever‐induced BrS type I | 53 (100) |
| ICD implantation before fever, n (%) | 0 (0) |
| ICD implantation after fever, n (%) | 6 (11) |
| Recurrence of arrhythmic events with recurrence of fever | |
| Yes, n (%) | 2 (40) |
| Adequate shocks, n (%) | 0 (0) |
| EP study | 3 (6) |
| Induction of VF or VT | 1 (33) |
| Genetic screening, n (%) | |
| SCN5a | 4 (28.6) |
| Mortality during fever, n (%) | 1 (2) |
| Electrical storm regarding fever, n (%) | 1 (2) |
| Inappropriate ICD discharge regarding fever, n (%) | 0 (0) |
| Follow‐up, months | 6.4 ± 7.2 |
An electrophysiological study was performed in three patients (6%) of which 33% were inducible of VF or ventricular tachycardia (VT). The study was performed due to syncopes in two cases (67%) and due to monomorphic VT in one case (33%).Genetic screening was performed in 14 patients (26%) and revealed a SCN5A mutation in 29% of the patients. The SCN5A mutation was not further categorized in two patients, in one patient, the mutation was a missense mutation (c.931 + 1G > A), and another patient presented with a mutation that was located on exon 24. Other than mutations in the SCN5A gene, no further mutations were identified in our study population.
An implanted cardioverter defibrillator (ICD) was implanted in six patients (11%) after fever event. Neither appropriate nor inappropriate shocks were documented in the literature. The reasons for implantation varied. In three patients (50%), the reason was syncope, in two patients (33%), the ICD was implanted due to VF, and in one patient (17%) because of VF followed by cardiac arrest.
3.2. Reasons for fever
In 14 patients, the reason for fever was presented in the publication. In 36% of cases, fever was caused by pneumonia, in 36% by an upper respiratory tract infection, in 7% by cholecystitis, in 7% by gastroenteritis, in 7% by urinary tract infection, and in 7% by appendicitis.
3.3. Symptoms at fever
The exact temperature at fever was not known in two case reports, other than that fever was defined as a temperature of over 38 Celsius (100.4° Fahrenheit).
During fever, 38% of the patients had an arrhythmic event. Four patients presented with more than one symptom at fever. Nine patients (17%) presented with life‐threatening arrhythmia such as VF or VT, seven patients (13%) suffered from syncope during fever event, and 7 (13%) had either cardiac arrest, aborted SCD or SCD. One patient developed incessant monomorphic tachyarrhythmia, which leads to electrical storm that was eventually fatal, and the patient died. Only one patient (2%) developed atrial arrhythmia. Statistical analysis did not show a correlation between the seriousness or frequency of antiarrhythmic events with higher temperature.
In five patients with previous symptoms at fever, a follow‐up was conducted. Of these five BrS subjects, life‐threatening arrhythmia such as VF, VT, cardiac arrest, or aborted SCD reoccurred with a later feverish episode in two patients (40%).
4. DISCUSSION
In our pooled meta‐analysis, we described a total of 53 patients that presented with BrS type I pattern induced by fever. From our findings, we deduced the following conclusions:
The incidence of arrhythmic events in patients with BrS I during fever was very high (38%).
Often the diagnostic BrS pattern is only unmasked by fever and will subside when the temperature goes down which can make it difficult for physicians to establish a connection between BrS and arrhythmic event.
In some cases, the life‐threatening arrhythmia will recur with another episode of fever (40%).
If someone presents with arrhythmic events or syncope during fever, the Brugada syndrome should be considered as a possible diagnosis and an ECG should be written. Particular patients such as patients presenting with a coinciding positive family history should be kept under close surveillance. An EP study might be helpful in symptomatic patients (Sroubek et al., 2016) in the presence of spontaneous BrS ECG or drug‐induced ECG. There are limited data regarding the impact of EP study in BrS patients triggered by fever.
Up to this point, it is well known that a loss of function in the peak sodium channel current is an underlying cause of the arrhythmogenesis in Brugada syndrome; furthermore, mutations in other channels such as mutations in the calcium channel or mutations in potassium channel currents have been described (Dinckal et al., 2003; Junttila et al., 2008).
Through a diminishing of the natrium inward current or a reinforcement of the potassium outward current, the balance of the otherwise elaborate process of the action potential is disturbed as positive ions are increasingly lost to the outer cellular membrane (El‐Battrawy et al., 2019; Schmidt et al., 2018). This and a possible loss of the plateau phase physiologically maintained by the calcium channels that is either caused by a loss of function mutation or the fact that the aggravated repolarization through the potassium and sodium channels prevent the activation of the calcium channels lead to a shortening of the action potential, which makes the myocard susceptible to the development of a reentry and with that, that of an extrasystole which might be the start of a arrhythmogenic event.
Some studies implicate a linkage between a rise in temperature and the malfunction of the sodium channel because of a mutation in the SCN5A gene. Cells derived from a patient with a Brugada ECG exceptionally during fever who showed a SCN5A mutation were analyzed in their sodium channel function. The result of the assessment at elevated temperatures up to 40.5°C showed a positive shift of half‐activation voltage and a changing of slope factor during activation compared to wild‐type which the authors assumed to be the underlying cause of a reduction of sodium current amplitude which could possibly unmask a Brugada phenotype (Keller et al., 2006).
Another study has shown that a missense mutation in the SCN5A gene can lead to a slower inactivation of Natrium inward currents if the temperature is increased by 14°C (Amin, Meregalli, Bardai, Wilde, & Tan, 2008).
There is evidence that temperature has a deciding effect on conduction qualities of the sodium channel (El‐Battrawy et al., 2016). In addition, recently published studies presenting an interaction of temperature modulation and antiarrhythmic drugs pose an interesting issue. As illustrated in a study, the inhibitory effects of lidocaine and flecainide on the peak inward sodium channel are increased by an increase of temperature, whereas on the other hand, the inhibitory effects of ajmaline on the peak sodium current are diminished by a rise of temperature (El‐Battrawy et al., 2016). This is an area, which should be investigated further since it has a great impact on how we should treat and diagnose those patients presenting with Brugada syndrome and fever. These data suggest that when it comes to flecainide the shortening of the action potential duration may be more effective in physiological body temperatures than during fever and therefore it should be applied with caution in BrS patients even more so if they have an elevated body temperature (El‐Battrawy et al., 2016). Although various cases of arrhythmia during fever coinciding with Brugada syndrome have been described, there are few larger studies to deal with the topic. The literature is limited to case reports and analysis with few patients as this one. The only multicenter studies dealing with larger cohorts are that of Mizusawa et al. (2016) (88 patients) and that of Michowitz et al. (2018) (2018, 35 of 588 patients presented with AE during fever). Further analysis has to be executed in order to find a common way as how to treat patients that present with fever‐induced BrS as well as to establish the risk of arrhythmic events that might threaten those patients with next fever episode. Nevertheless, studies have shown a higher prevalence of BrS in febrile patients compared to nonfebrile ones (Adler et al., 2013; Rattanawong et al., 2016) showing a prevalence of up to 4% (Rattanawong et al. 2016) with triggered arrhythmic events and therefore highlighting the importance of the topic itself as well as of its further evaluation.
5. CONCLUSION
Fever is a great risk factor in patients with Brugada syndrome which corroborates findings of previous studies and current guidelines. Even if BrS is not diagnosed before fever event, fever can unmask the typical type I pattern as well as trigger arrhythmic events. Patients with Brugada syndrome unmasked by fever should be surveilled closely during an elevated body temperature and be started on antipyretic therapy as soon as possible. This procedure can be recommended for all patients with BrS that develop fever even if BrS is not exclusively fever‐induced since the underlying biochemical mechanisms that make this combination of BrS and fever such a dangerous one are still present in other BrS patients.
5.1. Study limitations
This study has included a number of case reports, which poses the problem of clinical evaluation of patients that may vary and create differences in clinical assessment, clinical tests such as genetic screening or electrophysiological study, follow‐up as well as in treatment methods. In addition, the study population is very small and might be susceptible for random errors. Studies with a larger amount of patients were not included, and among others, we did not include Mizusawa et al. (2016) or Michowitz et al. (2018). Mizusawa et al. included the following authors: Mizusawa Y, Morita H, Adler A, Havakuk O, Thollet A, Maury P, Wang DW, Hong K, Gandjbakhch E, Sacher F, Hu D, Amin AS, Lahrouchi N, Tan HL, Antzelevitch C, Probst V, Viskin S, and Wilde AA. As we were also using Adler et al. in our meta‐analysis, we excluded Mizusawa et al. to avoid overlapping. We did not include Michowitz et al. as we were only including those patients, whose diagnostic Brugada pattern aroused and subsided with the coming and going of fever. Michowitz et al. also included patients who had a type I Brugada pattern also without the appearance of fever which was not the collective we chose to focus on. Moreover, we have not conducted and evaluated a treatment assessment.
CONFLICT OF INTEREST
None.
Supporting information
Roterberg G, El‐Battrawy I, Veith M, et al. Arrhythmic events in Brugada syndrome patients induced by fever. Ann Noninvasive Electrocardiol. 2020;25:e12723 10.1111/anec.12723
Roterberg and El‐Battrawy contributed equally to this work.
REFERENCES
- Adler, A. , Topaz, G. , Heller, K. , Zeltser, D. , Ohayon, T. , Rozovski, U. , … Viskin, S. (2013). Fever‐induced Brugada pattern: How common is it and what does it mean? Heart Rhythm, 10(9), 1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin, A. S. , Meregalli, P. G. , Bardai, A. , Wilde, A. A. M. , & Tan, H. L. (2008). Fever increases the risk for cardiac arrest in the Brugada syndrome. Annals of Internal Medicine, 149(3), 216–218. 10.7326/0003-4819-149-3-200808050-00020 [DOI] [PubMed] [Google Scholar]
- Aramaki, K. , Okumura, H. , & Shimizu, M. (2005). Chest pain and ST elevation associated with fever in patients with asymptomatic Brugada syndrome fever and chest pain in Brugada syndrome. International Journal of Cardiology, 103(3), 338–339. 10.1016/j.ijcard.2004.06.025 [DOI] [PubMed] [Google Scholar]
- Baranchuk, A. , & Simpson, C. S. (2011). Brugada syndrome coinciding with fever and pandemic (H1N1) influenza. Canadian Medical Association Journal, 183(5), 582 10.1503/cmaj.100016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barra, S. , Providencia, R. , & Nascimento, J. (2013). Fever outperforms flecainide test in the unmasking of type 1 Brugada syndrome electrocardiogram. Europace, 15(3), 394 10.1093/europace/eus175 [DOI] [PubMed] [Google Scholar]
- Chockalingam, P. , Rammeloo, L. , & Postema, P. (2011). Fever‐induced life‐threatening arrhythmias in children harboring an SCN5A mutation. Pediatrics. 127(1), e239–e244. 10.1542/peds.2010-1688 [DOI] [PubMed] [Google Scholar]
- Daimi, H. , Khelil, A. H. , Ben Hamda, K. , Aranega, A. , Chibani, J. B. E. , & Franco, D. (2015). Absence of family history and phenotype‐genotype correlation in pediatric Brugada syndrome: More burden to bear in clinical and genetic diagnosis. Pediatric Cardiology, 36(5), 1090–1096. [DOI] [PubMed] [Google Scholar]
- Dinckal, M. H. , Davutoglu, V. , Akdemir, I. , Soydinc, S. , Kirilmaz, A. , & Aksoy, M. (2003). Incessant monomorphic ventricular tachycardia during febrile illness in a patient with Brugada syndrome: Fatal electrical storm. Europace, 5(3), 257–261. [DOI] [PubMed] [Google Scholar]
- El-Battrawy, I. , Albers, S. , Cyganek, L. , Zhao, Z. , Lan, H. , Li, X. , … Akin, I. (2019). A cellular model of Brugada syndrome with SCN10A variants using human-induced pluripotent stem cell-derived cardiomyocytes. Europace, 21(9), 1410–1421. 10.1093/europace/euz122 [DOI] [PubMed] [Google Scholar]
- El‐Battrawy, I. , Lang, S. , Zhao, Z. , Akin, I. , Yucel, G. , Meister, S. , … Zhou, X. B. (2016). Hyperthermia influences the effects of sodium channel blocking drugs in human‐induced pluripotent stem cell‐derived cardiomyocytes. PLoS ONE, 11(11), e0166143 10.1371/journal.pone.0166143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdogan, O. , & Hunuk, B. (2013). Frequency of Brugada type ECG pattern in male subjects with fever. International Journal of Cardiology, 165(3), 562–563. 10.1016/j.ijcard.2012.09.022 [DOI] [PubMed] [Google Scholar]
- Gotz, O. , Beckmann, B. , Kaab, S. , & Hinterseer, M. (2009). Brugada‐syndrome ‐ diagnosis via 12‐lead‐ECG in a patient with fever and syncope. Notfall & Rettungsmedizin, 12(1), 37–39. [Google Scholar]
- Grogan, S. P. , Cube, R. P. , & Edwards, J. A. (2011). Brugada syndrome unmasked by fever. Military Medicine, 176(8), 946–949. 10.7205/MILMED-D-10-00458 [DOI] [PubMed] [Google Scholar]
- Junttila, M. , Gonzalez, M. , Lizotte, E. , & Benito, B. (2008). Induced Brugada‐type electrocardiogram, a sign for imminent malignant arrhythmias. Circulation. 117(14), 1890–1893. 10.1161/CIRCULATIONAHA.107.746495 [DOI] [PubMed] [Google Scholar]
- Keller, D. I. , Huang, H. , Zhao, J. , Frank, R. , Suarez, V. , Delacretaz, E. , … Chahine, M. (2006). A novel SCN5A mutation, F1344S, identified in a patient with Brugada syndrome and fever‐induced ventricular fibrillation. Cardiovascular Research, 70(3), 521–529. 10.1016/j.cardiores.2006.02.030 [DOI] [PubMed] [Google Scholar]
- McIntyre, W. F. , Femenia, F. , Arce, M. , Trucco, E. , Palazzolo, J. , Perez‐Riera, A. R. , & Baranchuk, A. (2012). Ventricular flutter triggered by fever in a patient with Brugada syndrome. Journal of Electrocardiology, 45(3), 199–202. 10.1016/j.jelectrocard.2011.12.009 [DOI] [PubMed] [Google Scholar]
- Mendes, S. L. , Elvas, L. , Ramos, D. , & Pego, M. (2017). Fever in an elderly patient unmasks Brugada syndrome. Revista Portuguesa De Cardiologia, 36(4), 317–318. 10.1016/j.repce.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Michowitz, Y. , Milman, A. , Sarquella‐Brugada, G. , Andorin, A. , Champagne, J. , Postema, P. G. , … Belhassen, B. (2018). Fever‐related arrhythmic events in the multicenter survey on arrhythmic events in Brugada syndrome. Heart Rhythm, 15(9), 1394–1401. 10.1016/j.hrthm.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Mizusawa, Y. , Morita, H. , Adler, A. , Havakuk, O. , Thollet, A. , Maury, P. , … Wilde, A. A. (2016). Prognostic significance of fever‐induced Brugada syndrome. Heart Rhythm, 13(7), 1515–1520. 10.1016/j.hrthm.2016.03.044 [DOI] [PubMed] [Google Scholar]
- Morita, H. , Nagase, S. , Kusano, K. , & Ohe, T. (2002). Spontaneous T wave alternans and premature ventricular contractions during febrile illness in a patient with Brugada syndrome. Journal of Cardiovascular Electrophysiology, 13(8), 816–818. 10.1046/j.1540-8167.2002.00816.x [DOI] [PubMed] [Google Scholar]
- Ortega‐Carnicer, J. , Benezet, J. , & Ceres, F. (2003). Fever‐induced ST‐segment elevation and T‐wave alternans in a patient with Brugada syndrome. Resuscitation, 57(3), 315–317. 10.1016/S0300-9572(03)00057-1 [DOI] [PubMed] [Google Scholar]
- Picetti, E. , Zoerle, T. , Cattani, L. , & Caspani, M. L. (2014). Fever and Brugada syndrome: A dangerous combination. Minerva Anestesiologica, 80(4), 512–513. [PubMed] [Google Scholar]
- Probst, V. , Denjoy, I. , Meregalli, P. G. , Amirault, J. C. , Sacher, F. , Mansourati, J. , … Wilde, A. A. (2007). Clinical aspects and prognosis of Brugada syndrome in children. Circulation, 115(15), 2042–2048. 10.1161/CIRCULATIONAHA.106.664219 [DOI] [PubMed] [Google Scholar]
- Rattanawong, P. , Vutthikraivit, W. , Charoensri, A. , Jongraksak, T. , Prombandankul, A. , Kanjanahattakij, N. , … Wisaratapong, T. (2016). Fever‐induced Brugada syndrome is more common than previously suspected: A cross‐sectional study from an endemic area. Annals of Noninvasive Electrocardiology. 21(2):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebollo, J. M. G. , Madrid, A. H. , Garcia, A. , de Castro, A. G. , Mejias, A. , & Moro, C. (2000). Recurrent ventricular fibrillation during a febrile illness in a patient with the Brugada syndrome. Revista Espanola De Cardiologia, 53(5), 755–757. [DOI] [PubMed] [Google Scholar]
- Samani, K. , Wu, G. , Ai, T. , Shuraih, M. , Mathuria, N. S. , Li, Z. H. , … Vatta, M. (2009). A novel SCN5A mutation V1340I in Brugada syndrome augmenting arrhythmias during febrile illness. Heart Rhythm, 6(9), 1318–1326. 10.1016/j.hrthm.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura, D. , Garcia‐Alberola, A. , Carrillo, P. , Pascual, D. , Martinez‐Sanchez, J. , & Valdes, M. (2002). Brugada‐like electrocardiographic pattern induced by fever. Pacing and Clinical Electrophysiology, 25(5), 856–859. [DOI] [PubMed] [Google Scholar]
- Schmidt, C. , Wiedmann, F. , El-Battrawy, I. , Fritz, M. , Ratte, A. , Beller, C. J. , … Thomas, D. (2018). Reduced Na+ current in native cardiomyocytes of a brugada syndrome patient associated with β-2-syntrophin mutation. Circulation-Genomic and Precision Medicine., 11(11), e002263 10.1161/CIRCGEN.118.002263 [DOI] [PubMed] [Google Scholar]
- Skinner, J. R. , Chung, S. K. , Nel, C. A. , Shelling, A. N. , Crawford, J. R. , McKenzie, N. , … Rees, M. I. (2007). Brugada syndrome masquerading as febrile seizures. Pediatrics, 119(5), E1206–E1211. 10.1542/peds.2006-2628 [DOI] [PubMed] [Google Scholar]
- Sovari, A. A. , Prasun, M. A. , Kocheril, A. G. , & Brugada, R. (2006). Brugada syndrome unmasked by pneumonia. Texas Heart Institute Journal, 33(4), 501–504. [PMC free article] [PubMed] [Google Scholar]
- Sroubek, J. , Probst, V. , Mazzanti, A. , Delise, P. , Hevia, J. C. , Ohkubo, K. , … Lubitz, S. A. (2016). Programmed ventricular stimulation for risk stratification in the Brugada syndrome a pooled analysis. Circulation, 133(7), 622–630. 10.1161/CIRCULATIONAHA.115.017885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarouhas, K. , Papalexis, P. , Kafantaris, I. , Tsitsimpikou, C. , Vavetsi, S. , & Rentoukas, E. (2010). Electrocardiographic findings compatible with Brugada syndrome in a patient with febrile respiratory infection. Hippokratia, 14(3), 221–223. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


