Abstract
Background
The duration of ventricular repolarization (VR) and its spatial and temporal heterogeneity are central elements in arrhythmogenesis. We studied the adaptation of VR duration and dispersion and their relationship in healthy human subjects during atrial pacing.
Methods
Patients 20‐50 years of age who were scheduled for ablation of supraventricular tachycardia without preexcitation but otherwise healthy were eligible. Vectorcardiography recordings with Frank leads were used for data collection. Incremental atrial pacing from a coronary sinus electrode was performed by decrements of 10ms/cycle from just above sinus rate, and then kept at a fixed heart rate (HR) just below the Wenckebach rate for ≥5min and then stopped. VR duration was measured as QT and VR dispersion as T area, T amplitude and ventricular gradient. The primary measure (T90 End) was the time to reach 90% change from baseline to the steady state value during and after pacing.
Results
A complete study protocol was accomplished in 9 individuals (6 women). VR duration displayed a monophasic adaptation during HR acceleration lasting on average 20s. The median (Q1‐Q3) T90 End for QT was 85s (51‐104), a delay by a factor >4. All dispersion measures displayed a tri‐phasic response pattern during HR acceleration and T90 End was 3‐5 times shorter than for VR duration.
Conclusions
Even during close to “physiological” conditions, complex and differing response patterns in VR duration and dispersion measures followed changes in HR. Extended knowledge about these responses in disease conditions might assist in risk evaluation and finding therapeutic alternatives.
Keywords: hysteresis, QT dispersion, restitution, vectorcardiography, ventricular repolarization
1. INTRODUCTION
The duration of ventricular repolarization (VR) and its spatial and temporal heterogeneity (from here dispersion) are central elements in arrhythmogenesis (Eisner, Dibb, & Trafford, 2009; Han & Moe, 1964; Kuo, Munakata, Reddy, & Surawicz, 1983). Furthermore, many arrhythmias occur in situations with changes in heart rate (HR), usually related to changes in autonomous nervous system (ANS) activity (Bergfeldt, Lundahl, Bergqvist, Vahedi, & Gransberg, 2017; Bueno‐Orovio, Hanson, Gill, Taggart, & Rodriguez, 2014; Verrier & Tan, 2009; Zaza, Ronchi, & Malfatto, 2018). The interplay between VR duration and dispersion on one hand and changes in HR and ANS activity on the other hand is therefore of both theoretical and clinical interest.
The physiology of VR duration adaptation to an increase in HR is well described and this regulatory mechanism displays hysteresis (Guzman, Jing, & Patwardhan, 2010; Nolasco & Dahlen, 1968; Ozgen & Rosen, 2009; Pueyo et al., 2010; Rosen & Bergfeldt, 2015; Seethala, Shusterman, Saba, Mularski, & Nemec, 2011). That is, the shortening of the QT interval (approximately equal to mechanical systole) starts immediately after a change in cycle length but subsequently adapts slowly to the new HR. The purpose of hysteresis during changes in HR is to optimize the time for ventricular filling and ejection, to optimize coronary blood flow and to increase electrical stability (Eisner et al., 2009; Ozgen & Rosen, 2009; Rosen & Bergfeldt, 2015; Taggart & Lab, 2008; Vahedi, Haney, Jensen, Näslund, & Bergfeldt, 2011). Apart from VR duration, VR dispersion is of well‐known importance for the propensity for arrhythmias and different dispersion measures are markers for increased arrhythmia risk in patients with cardiovascular disease (Bueno‐Orovio, Hanson, Gill, Taggart, & Rodriguez, 2012; Lingman et al., 2016). The knowledge about VR adaptation to HR changes rests mainly on experimental studies on cell preparations and in animals. There are only a few studies on humans describing VR responses to increased HR (from chronotropic drugs or pacing) (Bergfeldt et al., 2017; Bueno‐Orovio et al., 2012; Seethala et al., 2011). The relation between VR duration and dispersion adaptation during different phases of atrial pacing and normal conduction (simulating physiologic conditions but without ANS activation) has not been thoroughly investigated.
The aim of this study was therefore to explore the pattern of adaptation of VR duration and dispersion and their temporal relation following increasing and fixed‐rate atrial pacing and after sudden return to sinus rhythm.
2. METHODS
2.1. Study subjects
During 4 years (2014–2018), referral notes and records for patients scheduled for ablation of paroxysmal supraventricular tachycardia without preexcitation were screened for participation in the study. Patients were eligible based on the following criteria: 18–50 years of age, no acute or chronic illness besides supraventricular tachycardia, no family history of sudden cardiac death, no ECG or echocardiographic abnormalities and no medication known to affect VR (except antiarrhythmic drugs withdrawn prior to the procedure). In total, 35 patients were eligible for participation in the study and 27 agreed to participate. The study was performed following the principles of the Declaration of Helsinki and was approved by the regional ethics committee, and written informed consent was obtained from all participants.
2.2. Procedure and protocol
The study was performed in the electrophysiology (EP) laboratory at Sahlgrenska University Hospital. Antiarrhythmic drugs were withdrawn 3 days prior to study participation. Patients were connected to the vectorcardiography (VCG) equipment upon arrival to the EP laboratory. They were otherwise prepared according to the routine protocol for invasive catheterization including electrodes for 12 lead ECG‐monitoring and patches for radiofrequency catheters and a three‐dimensional catheter navigation system.
Local anesthetic was applied in the groin prior to venous punctures, but no other medications were administrated. A decapolar 6–7 F catheter was inserted to the coronary sinus via an introducer from the right femoral vein. No other catheters were placed in the heart prior to study completion in order to avoid extra beats and tachycardias.
The first step was to establish the Wenckebach cycle length (WbCL). Then the patients rested for 5 min and thereafter the VCG recording during sinus rhythm started. After 5 min, atrial pacing started from a CL slightly shorter than that of the baseline sinus rhythm. The pacing CL was decreased in decrements of 10 ms/cycle until a CL 20‐30 ms longer than the WbCL was reached where after fixed‐rate pacing followed. The per protocol shortest paced CL was chosen at 430 ms (140 bpm) in any patient. Pacing continued at the fixed rate for at least 5 (and up to 8) minutes and then stopped abruptly. After that 5 more minutes of sinus rhythm was recorded, after which the study was completed. Thereafter the EP study and ablation continued according to the clinical routine.
2.3. Data acquisition and analysis
2.3.1. Recording procedure
A CoroNet II system (Ortivus AB) was connected to 8 electrodes positioned according to Frank. Signals were sampled at 500 Hz with an amplifier bandwidth of 0.03–170 Hz. VCG recording and analysis were performed as described in detail in recent publications from our group (Bergfeldt et al., 2017; Vahedi et al., 2011). In this study, we used the same measures and terminology. The system automatically annotates onset and offset points for P, QRS, and T waves (T end by the tangent method) on a global QRST complex (magnitude complex) derived from the XYZ leads. The data were then transferred to a database and analyzed offline using customized software for beat‐to‐beat analysis.
2.3.2. Adaptation of VR duration and dispersion
Ventricular repolarization duration and dispersion were evaluated in relation to changes in RR intervals. All consecutive RR intervals during the registration were analyzed. We measured and compared the T90 End for RR intervals and VR duration and dispersion measures. T90 End is the time from a change in heart rate (e.g., beginning of atrial pace) to the time‐point of 90% change in either measure. T90 End for RR was defined from the exact duration of incremental pacing. In order to identify T90 End for QT (and the dispersion measures) a curve fit method was applied using nonlinear optimization solution in Excel's Problem Solver, using single or double exponential functions. (Figure 1). The concept of T90 End follows examples from in vivo experiments on action potential duration (APD90 at 90% repolarization) as well as system control in engineering (Bergfeldt et al., 2017). The difficulties in identifying the exact time‐point where, for example, QT has reached steady state are also overcome by the use of T90 End. The steady state value is easy to identify and from this follows that the time‐point where 90% of the change has occurred can be identified on the optimized curve fit with high precision.
Figure 1.

A mono‐exponential function curve fits to the adaptation of the QT interval to increasing heart rate. Gray line is raw data and black line the exponential curve fit
We studied three dispersion measures: T‐wave area (T area; global ventricular dispersion), T‐wave amplitude (T amplitude), and ventricular gradient (VG; dispersion of action potential morphology). VG (also QRST area) and T area are created by the moving heart vector and calculated from the QRST complex in the 3 orthogonal directions (X, Y, and Z). The ventricular gradient (VG) is the sum of the QRS‐area and the T‐area vectors. T amplitude is the maximal amplitude of the T‐wave vector inscribed in the T loop. These measures are all global measures of VR dispersion and provide information beyond what can be obtained from routine 12‐lead ECG measures (Draisma, Schalij, Wall, & Swenne, 2006; Geselowitz, 1983; Hurst, 2005; Man, Maan, Schalij, & Swenne, 2015; Vahedi et al., 2013; Van Huysduynen et al., 2005; Wecke et al., 2013; Wecke, Rubulis, Lundahl, Rosen, & Bergfeldt, 2007). In the analysis, we primarily focused on changes in these parameters during acceleration of HR. While local and regional differences cannot be discerned, they will be inscribed in the T loop and the T‐loop vector and contribute to the measures of global dispersion at rest and during VR adaptation to the HR changes.
2.3.3. Statistics
The median (Q1; Q3) was used for descriptive statistics. Wilcoxon signed rank test was used for testing differences and Spearman's regression coefficient for correlation analysis between changes in RR and VR duration and dispersion measures. Differences in median with confidence intervals were calculated by Hodges‐Lehmann Median Difference Test. IBM Statistical Package for the Social Sciences (SPSS, version 24) was used for statistical calculations.
3. RESULTS
In nine patients (six women) of the 27 enrolled, the study protocol was successfully completed. All were healthy apart from the supraventricular tachycardia and for all but one AV node reentrant tachycardia (AVNRT) proved to be the clinical arrhythmia. Table 1 shows patient characteristics. The high rate of failures depended mainly on difficulties with AV node conduction, either Wenckebach block at a lower HR than during the initial testing or irregularities due to variation in conduction over fast and slow pathways. In a few cases, technical or clinical circumstances precluded completion of the study protocol.
Table 1.
Patient characteristics and RR interval (ms) at baseline and during steady state atrial pacing (AP)
| Study subject | Sex/Age | Arrhythmia | RR Baseline |
RR AP |
Difference |
|---|---|---|---|---|---|
| 1 | F/23 | AVNRT | 689 | 430 | 259 |
| 2 | F/25 | AVNRT | 796 | 490 | 306 |
| 3 | F/24 | AVNRT | 818 | 430 | 388 |
| 4 | F/23 | AVNRT | 1,033 | 600 | 433 |
| 5 | F/39 | AVNRT | 969 | 560 | 409 |
| 6 | F/39 | AVNRT | 660 | 470 | 190 |
| 7 | M/37 | AVRT | 865 | 450 | 415 |
| 8 | M/21 | AVNRT | 604 | 430 | 174 |
| 9 | M/18 | AVNRT | 849 | 550 | 299 |
| Mean | 809 | 490 | 319 | ||
| SD | 141 | 65 | 98 |
The data from four patients required manual editing due to T‐P‐merging. In a few patients, a jump from fast to slow pathway in the AVN conduction occurred during incremental pacing, which was accepted. One patient developed AVNRT at an appropriate CL during incremental stimulation, which we recorded during 8 min instead of atrial stimulation. It was stopped by brief atrial overdrive pacing. We considered this deviation from the study protocol to be insignificantly different from fixed‐rate pacing (and an advantage because of no T‐P merging).
3.1. VR duration and dispersion responses
RR decreased on average 39% during on average 20 s. This corresponds to an average increase in HR from 74 to 122 beats per minute. Table 2 displays T90 End for RR and VR duration and dispersion measures during incremental pacing and the number of subjects for each measure. There was clear evidence of hysteresis in QT adaptation to RR decrease and increase, and the response pattern was mono‐exponential with an initial short and rapid phase followed by a slower and more prolonged (Figure 2, panel a, Table 2).
Table 2.
T90 End for RR interval and ventricular repolarization duration and dispersion measures during acceleration of heart rate. Dispersion measures are T area, T amplitude and ventricular gradient (VG). Correlations between T90 End for RR and duration and dispersion measures. Below are the differences between T90 End for QT and dispersion measures
| Measure | n | Median [s] (Q1‐Q3) | RR correlation |
|---|---|---|---|
| RR | 9 | 18 (12–25) | – |
| QT | 9 | 85 (51–104) | 0.59 (NS) |
| T area | 6 | 23 (19–36) | 0.88 (p < .05) |
| T amplitude | 7 | 17 (15–29) | 0.79 (p < .05) |
| VG | 5 | 27 (20–59) | 0.50 (NS) |
| Difference versus QT [s] | ||
|---|---|---|
| Measure | Median (95% CI) | p |
| T area | 53 (34–74) | p < .05 |
| T amplitude | 64 (42–82) | p < .05 |
| VG | 44 (24–76) | p < .05 |
Figure 2.
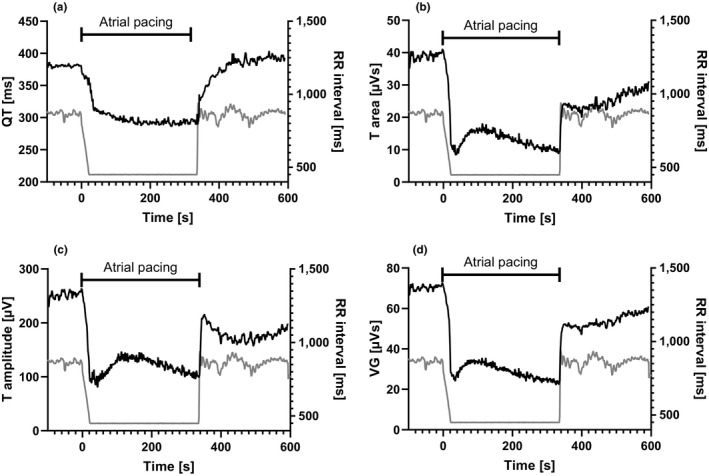
Ventricular repolarization duration and dispersion measures for one of the study patients during changes in RR intervals (right Y‐axis in all panels). QT describes a long adaptation time to sudden changes in RR, but the reactions in the three dispersion measures are more rapid and complex including an overshoot (downward dip in the curves). The dispersion reactions following increasing versus decreasing RR intervals are also different; the tri‐phasic reaction following decreasing RR is less pronounced on increasing RR intervals. For T amplitude, the same tendency to overshoot is, however, present. Gray lines display RR intervals, and black lines are VR measures. (a) QT, (b) T area, (c) T amplitude, (d) ventricular gradient (VG)
In contrast, the response pattern of the VR dispersion measures T area, T amplitude, and VG following decrease in RR intervals was more complex (Figure 2, panels b‐d). First, the median T90 End during incremental pacing was much shorter for these measures than for QT by a factor 3–5 (Figure 3). T area and T amplitude showed the fastest and almost immediate reaction to the HR increase. Secondly, all VR dispersion measures showed a tri‐phasic response pattern but to varying extent. The first part was a very rapid decrease with an overshoot followed by a return toward baseline and then a final phase of slow decrease until pacing stopped. Upon cessation of pacing, there was a sudden return toward the baseline with larger dispersion, for example, T area increased immediately to a level at approximately half of the baseline value. During this rapid initial response, T amplitude displayed a tendency to “overshoot” similar to what was seen following decreasing RR intervals. After this, a slower return toward the baseline level followed for all three dispersion measures.
Figure 3.

T90 End for RR interval and ventricular duration and dispersion measures described in box plots. Median, Q1, and Q3 are displayed in boxes and whiskers display the whole range. The adaptation time for the dispersion measures is more rapid than the adaptation time for QT. Left: increasing heart rate during incremental pacing. Right: decreasing heart rate after abrupt termination of fixed‐rate pacing. VG: ventricular gradient
4. DISCUSSION
The purpose of our study was to investigate adaptation of global VR duration and dispersion measures and their relation during changes in HR using atrial pacing in otherwise healthy patients who subsequently received ablation therapy for supraventricular tachycardia. The main findings were as follows: (a) clear evidence of hysteresis in the adaptation of VR duration to HR change which showed a mono‐exponential response pattern; and (b) VR dispersion measures (T area, T amplitude, VG) showed a “tri‐phasic” response pattern during HR acceleration where the first phase was very rapid—closer to the change in RR than QT—followed by an overshoot. At the abrupt return to sinus rhythm, there was a sudden increase in VR dispersion, followed by a slow increase during the remaining recording time. The response on return to sinus rhythm was very similar for T area and VG, but T amplitude also featured an overshoot quite similar to that seen in response to increasing HR. At HR changes during normal ventricular activation, there was thus a complex relation between the responses of VR duration and dispersion measures.
4.1. VR duration response
We observed hysteresis in the VR duration response to changes in HR in this human model applying atrial pacing, in line with previous studies (Berger, 2004; Bueno‐Orovio et al., 2012; Eisner et al., 2009; Guzman et al., 2010; Nolasco & Dahlen, 1968; Pueyo et al., 2010; Rosen & Bergfeldt, 2015). A delay in QT response followed both incremental pacing and cessation of atrial stimulation (Figure 1). The time from pace stop to steady state was not significantly longer than the adaptation following rapid pacing in line with observations by Seethala et al. (2011), but the median values of the response times were slightly longer when sinus rhythm resumed. The reaction to cessation of pacing was, however, not as uniform among the study subjects as the response to incremental pacing. More prolonged sinus node recovery times after pace stop in some patients might be one reason.
The response to accelerating HR confirms previous knowledge from experimental models as well a study on human subjects. Seethala et al. (2011) applied sudden onset atrial pacing at different cycle lengths for 300 s each (before and during dobutamine infusion) and identified two phases of the QT adaptation: one rapid phase followed by a slower decrease to a new steady state; a mono‐exponential pattern like in our study. In a previous study from our group, atropine was used for HR acceleration, and T90 End for QT was 130 s and the relative delay from the adaptation of the RR interval was 5.7 compared to 4.7 in the present study; the RR acceleration time was similar to that in this study (Bergfeldt et al., 2017).
4.2. VR dispersion response
The initial overshoot and reversion corresponds to what we observed in a previous study on healthy subjects in whom a rapid HR increase followed an i.v. bolus dose of atropine 0.04 mg/kg (Bergfeldt et al., 2017). In the atropine study, however, after the initial rapid response with overshoot, the dispersion response curve flattened into a steady state as the HR remained at the same high level. In the present study, we noticed a tri‐phasic response with a continuously slow change in dispersion until pacing was stopped. This tri‐phasic response pattern of VR dispersion during atrial pacing is similar to the observations during sudden onset ventricular pacing at cycle lengths around 500 ms described previously by Bueno‐Orovio et al. (2012). They paced the right ventricle in otherwise healthy patients prior to ablation of atrial fibrillation and measured the differences in rate adaptation of action potential duration between one site each in the right and left ventricles. The response pattern of dispersion measures was thus similar during incremental atrial pacing and sudden onset ventricular pacing without any drugs active on the autonomic nervous system but different from the response after pharmacologic parasympathetic blockade. One possible explanation to the differences in response pattern might be variations in the autonomic nervous system tone.
4.3. Differences between duration and dispersion: timing and pattern
Bueno‐Orovio et al. (2012) demonstrated interventricular heterogeneity during the slow phase of APD adaptation during right ventricular pacing, which is consistent with our findings using the global QT and global VR dispersion measures. Both studies show that during the QT adaptation the repolarization heterogeneity is unstable and fluctuates considerably. Furthermore, both the present study and our previous atropine study show that during the rapid phase of QT adaptation there is an even more rapid decrease in VR dispersion followed by a rebound during the remaining phase of QT adaptation (Figure 1). The relationship between VR duration and dispersion responses during HR changes in disease states and their role in arrhythmogenesis remains to be elucidated. For different cardiac diseases, there is, however, a propensity for malignant arrhythmias following changes in HR. We know from ambulatory recordings of sudden cardiac death victims that monomorphic VT is often preceded by increasing HR and extra beats (and thereby associated with increased sympathetic activity), while Torsades de Pointes (TdP) ventricular tachycardia is more likely to occur during relative bradycardia and variability in coupling intervals (Bayes de Luna, Coumel, & Leclercq, 1989).
Following abrupt cessation of pacing, there was a rapid increase in VR dispersion followed by a slower return to the initial level during sinus rhythm. Sudden changes in HR (increasing as well as decreasing) seem therefore to precipitate an immediate response in VR dispersion followed by a slower adaptation in QT. This might be one mechanistic factor behind the propensity for Torsades de Pointes ventricular tachycardia in high‐degree AV‐block and the bradycardia‐dependent acquired long QT syndromes (Cho et al., 2015; El‐Sherif, Turitto, & Boutjdir, 2019). For congenital long QT syndrome (LQTS) the arrhythmic events are often related to exercise, stress or sudden stimuli—depending on the LQTS genetic subtype (type 1 and 2). There is evidence to support that the arrhythmia (TdP) in congenital LQTS is initiated by early after depolarizations (EAD) in the Purkinje system and sustained by VR dispersion by means of unstable reentry circuits around lines of functional block (Chinushi et al., 2001; El‐Sherif, Chinushi, Caref, & Restivo, 1997; El‐Sherif, Turitto, & Boutjdir, 2017). In our study, all three dispersion measures displayed rapid reaction times and a tri‐phasic response during the time of QT adaptation. This could point to a vulnerable state in global VR dispersion adaptation that might be more pronounced in heart diseases associated with increased arrhythmia risk. The difference in adaptation between QT and the dispersion measures is suggestive of regional heterogeneity in rate response, which probably can be increased in the case of cardiac disease.
The dispersion of repolarization is of importance for arrhythmogenesis in inherited as well as acquired cardiac syndromes. Exaggerated heterogeneity of the action potential duration (APD) is a common finding in the diseased heart, for example in heart failure where the prolongation of the AP initially is an adaptive feature aiming to increase influx of calcium to promote contraction (Osadchii, 2017). If the regional differences are accentuated from structural disease (hypertrophy, fibrosis) or by inherited ion channel disease, this will probably manifest as an increase in global measures of dispersion during VR adaptation to HR variations. Future studies on, for example LQTS, would therefore be of great interest to identify differences from healthy individuals in global VR duration and dispersion adaptation to HR changes.
4.4. Limitations
The most obvious limitation is the low number of successfully completed study protocols. The difficulties in achieving a long continuous period of high‐rate atrial pace with 1:1 conduction to the ventricles lie to a great extent in the AV node. In many healthy subjects, the parasympathetic influence on the heart limits AV conduction at rest. The issue with a long AV node refractory period was in the present study underscored by the fact that all but one successfully performed study protocols were achieved in patients with AVNRT where one functional pathway had sufficiently short refractory period to allow atrial pacing at a relatively high rate without drugs such as atropine or isoprenaline. A slightly lower maximum HR might have led to a higher number of successfully performed study protocols. We aimed to pace at a HR slightly below the Wenckebach point, though this is not a fixed point but subject to considerable intrinsic variation due to changes in the ANS activity also during this relatively short study protocol. Our patients were accustomed to clinical tachycardias with mostly much higher HR than the maximum pacing rate and consequently rather relaxed with seemingly parasympathetic dominance during the study.
The VCG‐derived measures of VR dispersion used in this human pacing model reflect global heterogeneity and cannot be used to assess local and regional dispersion. However, Bueno‐Orovio et al. (2012) demonstrated a similar pattern of adaptation in interventricular VR dispersion from differences in local left and right ventricular APD. This suggests that in the healthy heart VR dispersion shows the same pattern of adaptation in both global and regional measurements.
The small number of male participants reduces the possibility to identify any sex‐related differences in the response pattern. There are well‐recognized sex‐related differences in cardiac repolarization (Vink, Clur, Wilde, & Blom, 2018). In our previous study on healthy subjects receiving bolus dose of atropine, women displayed a stronger correlation between changes in RR and changes in VR measures than men (Bergfeldt et al., 2017).
5. CONCLUSIONS
There are complex and differing response patterns in VR duration and dispersion following HR changes even during close to physiological conditions in healthy subjects. Extended knowledge about these responses in disease conditions might assist in risk evaluation and finding therapeutic alternatives.
CONFLICTS OF INTEREST
None.
ACKNOWLEDGMENTS
The Scientific Reader platform used in the customized software development is the property of Ortivus AB, Danderyd, Sweden.
Axelsson K‐J, Brännlund A, Gransberg L, Lundahl G, Vahedi F, Bergfeldt L. Adaptation of ventricular repolarization duration and dispersion during changes in heart rate induced by atrial stimulation. Ann Noninvasive Electrocardiol. 2020;25:e12713 10.1111/anec.12713
REFERENCES
- Bayes de Luna, A. , Coumel, P. , & Leclercq, J. F. (1989). Ambulatory sudden cardiac death: Mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. American Heart Journal, 117(1), 151–159. 10.1016/0002-8703(89)90670-4 [DOI] [PubMed] [Google Scholar]
- Berger, R. D. (2004). Electrical restitution hysteresis: Good memory or delayed response? Circulation Research, 94(5), 567–569. 10.1161/01.RES.0000124605.03595.E4 [DOI] [PubMed] [Google Scholar]
- Bergfeldt, L. , Lundahl, G. , Bergqvist, G. , Vahedi, F. , & Gransberg, L. (2017). Ventricular repolarization duration and dispersion adaptation after atropine induced rapid heart rate increase in healthy adults. Journal of Electrocardiology, 50(4), 424–432. 10.1016/j.jelectrocard.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Bueno‐Orovio, A. , Hanson, B. M. , Gill, J. S. , Taggart, P. , & Rodriguez, B. (2012). In vivo human left‐to‐right ventricular differences in rate adaptation transiently increase pro‐arrhythmic risk following rate acceleration. PLoS ONE, 7(12), e52234 10.1371/journal.pone.0052234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno‐Orovio, A. , Hanson, B. M. , Gill, J. S. , Taggart, P. , & Rodriguez, B. (2014). Slow adaptation of ventricular repolarization as a cause of arrhythmia? Methods of Information in Medicine, 53(4), 320–323. 10.3414/ME13-02-0039 [DOI] [PubMed] [Google Scholar]
- Chinushi, M. , Caref, E. B. , Restivo, M. , Noll, G. , Aizawa, Y. , & El‐Sherif, N. (2001). Cycle length‐associated modulation of the regional dispersion of ventricular repolarization in a canine model of long QT syndrome. Pacing and Clinical Electrophysiology, 24(8 Pt 1), 1247–1257. 10.1046/j.1460-9592.2001.01247.x [DOI] [PubMed] [Google Scholar]
- Cho, M. S. , Nam, G. B. , Kim, Y. G. , Hwang, K. W. , Kim, Y. R. , Choi, H. , … Kim, Y. H. (2015). Electrocardiographic predictors of bradycardia‐induced torsades de pointes in patients with acquired atrioventricular block. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 12(3), 498–505. 10.1016/j.hrthm.2014.11.018 [DOI] [PubMed] [Google Scholar]
- Draisma, H. H. , Schalij, M. J. , van der Wall, E. E. , & Swenne, C. A. (2006). Elucidation of the spatial ventricular gradient and its link with dispersion of repolarization. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 3(9), 1092–1099. 10.1016/j.hrthm.2006.05.025 [DOI] [PubMed] [Google Scholar]
- Eisner, D. A. , Dibb, K. M. , & Trafford, A. W. (2009). The mechanism and significance of the slow changes of ventricular action potential duration following a change of heart rate. Experimental Physiology, 94(5), 520–528. 10.1113/expphysiol.2008.044008 [DOI] [PubMed] [Google Scholar]
- El‐Sherif, N. , Chinushi, M. , Caref, E. B. , & Restivo, M. (1997). Electrophysiological mechanism of the characteristic electrocardiographic morphology of torsade de pointes tachyarrhythmias in the long‐QT syndrome: Detailed analysis of ventricular tridimensional activation patterns. Circulation, 96(12), 4392–4399. 10.1161/01.CIR.96.12.4392 [DOI] [PubMed] [Google Scholar]
- El‐Sherif, N. , Turitto, G. , & Boutjdir, M. (2017). Congenital long QT syndrome and torsade de pointes. Annals of Noninvasive Electrocardiology, 22(6), e12481– 10.1111/anec.12481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Sherif, N. , Turitto, G. , & Boutjdir, M. (2019). Acquired long QT syndrome and electrophysiology of torsade de pointes. Arrhythmia & Electrophysiology Review, 8(2), 122–130. 10.15420/aer.2019.8.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geselowitz, D. B. (1983). The ventricular gradient revisited: Relation to the area under the action potential. IEEE Transactions on Biomedical Engineering, 30(1), 76–77. 10.1109/TBME.1983.325172 [DOI] [PubMed] [Google Scholar]
- Guzman, K. M. , Jing, L. , & Patwardhan, A. (2010). Effects of changes in the L‐type calcium current on hysteresis in restitution of action potential duration. Pacing and Clinical Electrophysiology, 33(4), 451–459. 10.1111/j.1540-8159.2009.02637.x [DOI] [PubMed] [Google Scholar]
- Han, J. , & Moe, G. K. (1964). Nonuniform recovery of excitability in ventricular muscle. Circulation Research, 14, 44–60. 10.1161/01.RES.14.1.44 [DOI] [PubMed] [Google Scholar]
- Hurst, J. W. (2005). Thoughts about the ventricular gradient and its current clinical use (Part I of II). Clinical Cardiology, 28(4), 175–180. 10.1002/clc.4960280404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, C. S. , Munakata, K. , Reddy, C. P. , & Surawicz, B. (1983). Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation, 67(6), 1356–1367. 10.1161/01.CIR.67.6.1356 [DOI] [PubMed] [Google Scholar]
- Lingman, M. , Hartford, M. , Karlsson, T. , Herlitz, J. , Rubulis, A. , Caidahl, K. , & Bergfeldt, L. (2016). Value of the QRS‐T area angle in improving the prediction of sudden cardiac death after acute coronary syndromes. International Journal of Cardiology, 218, 1–11. 10.1016/j.ijcard.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Man, S. , Maan, A. C. , Schalij, M. J. , & Swenne, C. A. (2015). Vectorcardiographic diagnostic & prognostic information derived from the 12‐lead electrocardiogram: Historical review and clinical perspective. Journal of Electrocardiology, 48(4), 463–475. [DOI] [PubMed] [Google Scholar]
- Nolasco, J. B. , & Dahlen, R. W. (1968). A graphic method for the study of alternation in cardiac action potentials. Journal of Applied Physiology, 25(2), 191–196. 10.1152/jappl.1968.25.2.191 [DOI] [PubMed] [Google Scholar]
- Osadchii, O. E. (2017). Role of abnormal repolarization in the mechanism of cardiac arrhythmia. Acta Physiologica, 220(Suppl 712), 1–71. 10.1111/apha.12902 [DOI] [PubMed] [Google Scholar]
- Ozgen, N. , & Rosen, M. R. (2009). Cardiac memory: A work in progress. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 6(4), 564–570. 10.1016/j.hrthm.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Pueyo, E. , Husti, Z. , Hornyik, T. , Baczkó, I. , Laguna, P. , Varró, A. , & Rodríguez, B. (2010). Mechanisms of ventricular rate adaptation as a predictor of arrhythmic risk. American Journal of Physiology‐Heart and Circulatory Physiology, 298(5), H1577–H1587. 10.1152/ajpheart.00936.2009 [DOI] [PubMed] [Google Scholar]
- Rosen, M. R. , & Bergfeldt, L. (2015). Cardiac memory: The slippery slope twixt normalcy and pathology. Trends in Cardiovascular Medicine, 25(8), 687–696. 10.1016/j.tcm.2015.02.011 [DOI] [PubMed] [Google Scholar]
- Seethala, S. , Shusterman, V. , Saba, S. , Mularski, S. , & Nemec, J. (2011). Effect of beta‐adrenergic stimulation on QT interval accommodation. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 8(2), 263–270. [DOI] [PubMed] [Google Scholar]
- Taggart, P. , & Lab, M. (2008). Cardiac mechano‐electric feedback and electrical restitution in humans. Progress in Biophysics and Molecular Biology, 97(2–3), 452–460. 10.1016/j.pbiomolbio.2008.02.021 [DOI] [PubMed] [Google Scholar]
- Vahedi, F. , Diamant, U.‐B. , Lundahl, G. , Bergqvist, G. , Gransberg, L. , Jensen, S. M. , & Bergfeldt, L. (2013). Instability of repolarization in LQTS mutation carriers compared to healthy control subjects assessed by vectorcardiography. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 10(8), 1169–1175. 10.1016/j.hrthm.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Vahedi, F. , Haney, M. F. , Jensen, S. M. , Näslund, U. , & Bergfeldt, L. (2011). Effect of heart rate on ventricular repolarization in healthy individuals applying vectorcardiographic T vector and T vector loop analysis. Annals of Noninvasive Electrocardiology, 16(3), 287–294. 10.1111/j.1542-474X.2011.00444.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Huysduynen, B. H. , Swenne, C. A. , Draisma, H. H. , Antoni, M. L. , Van De Vooren, H. , Van Der Wall, E. E. , & Schalij, M. J. (2005). Validation of ECG indices of ventricular repolarization heterogeneity: A computer simulation study. Journal of Cardiovascular Electrophysiology, 16(10), 1097–1103. 10.1111/j.1540-8167.2005.40758.x [DOI] [PubMed] [Google Scholar]
- Verrier, R. L. , & Tan, A. (2009). Heart rate, autonomic markers, and cardiac mortality. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 6(11 Suppl), S68–75. 10.1016/j.hrthm.2009.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink, A. S. , Clur, S.‐A.‐B. , Wilde, A. A. M. , & Blom, N. A. (2018). Effect of age and gender on the QTc‐interval in healthy individuals and patients with long‐QT syndrome. Trends in Cardiovascular Medicine, 28(1), 64–75. 10.1016/j.tcm.2017.07.012 [DOI] [PubMed] [Google Scholar]
- Wecke, L. , Poçi, D. , Schwieler, J. , Johansson, B. , Edvardsson, N. , Lundahl, G. , & Bergfeldt, L. (2013). Vectorcardiography shows cardiac memory and repolarization heterogeneity after ablation of accessory pathways not apparent on ECG. International Journal of Cardiology, 166(1), 152–157. 10.1016/j.ijcard.2011.10.106 [DOI] [PubMed] [Google Scholar]
- Wecke, L. , Rubulis, A. , Lundahl, G. , Rosen, M. R. , & Bergfeldt, L. (2007). Right ventricular pacing–induced electrophysiological remodeling in the human heart and its relationship to cardiac memory. Heart Rhythm: The Official Journal of the Heart Rhythm Society, 4(12), 1477–1486. 10.1016/j.hrthm.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Zaza, A. , Ronchi, C. , & Malfatto, G. (2018). Arrhythmias and heart rate: Mechanisms and significance of a relationship. Arrhythmia & Electrophysiology Review, 7(4), 232–237. 10.15420/aer.2018.12.3 [DOI] [PMC free article] [PubMed] [Google Scholar]


