Key Points
Question
Did disparities between rural vs urban residents following an acute stroke or transient ischemic attack in care and patient outcomes change from 2008 to 2017?
Findings
In this cohort study, rural vs urban disparities in use of certified stroke centers and neurologist consultations narrowed over the past decade. In contrast, disparities in outcomes, such as mortality or morbidity, have not changed or have worsened over the same period.
Meaning
More focus is needed on reducing long-standing rural-urban disparities in high-quality stroke care and patient outcomes.
This cohort study describes trends among rural and urban patients with acute ischemic stroke or transient ischemic attack in the type of health care centers to which patients were admitted, what care was provided, and patient outcomes.
Abstract
Importance
Over the last decade or so, there have been substantial investments in the development of stroke systems of care to improve access and quality of care in rural communities. Whether these have narrowed rural-urban disparities in care is unclear.
Objective
To describe trends among rural and urban patients with acute ischemic stroke or transient ischemic attack in the type of health care centers to which patients were admitted, what care was provided, and the outcomes patients experienced.
Design, Setting, and Participants
This descriptive observational study included 100% claims for beneficiaries of traditional fee-for-service Medicare from 2008 through 2017. All rural and urban areas in the US were included, defined by whether a beneficiary’s residential zip code was in a metropolitan or nonmetropolitan area. All admissions in the US among patients with traditional Medicare who had a transient ischemic attack or acute stroke (N = 4.01 million) were eligible to be included in this study. Admissions for beneficiaries with end-stage kidney disease (n = 85 927 [2.14%]), beneficiaries with unidentified Rural-Urban Commuting Area codes (n = 12 797 [0.32%]), and beneficiaries not continuously enrolled in traditional Medicare in the 12 months before and 3 months after their admission (n = 442 963 [11.0%]) were excluded.
Exposures
Residence in an urban or rural area; admission to a hospital with a transient ischemic attack or acute stroke.
Main Outcomes and Measures
Discharge from a certified stroke center, receiving a neurology consultation during admission, treatment with alteplase, days institutionalized, and 90-day mortality.
Results
The final sample included 3.47 million admissions from 2008 through 2017. In this sample, 2.01 million patients (58.0%) were female, and the mean (SD) age was 78.6 (10.5) years. In 2008, 24 681 patients (25.2%) and 161 217 patients (60.6%) in rural and urban areas, respectively, were cared for at a certified stroke center (disparity, −35.4%). By 2017, this disparity was −26.6%, having narrowed by 8.7 percentage points (95% CI, 6.6-10.8 percentage points). There was also narrowing in the rural-urban disparity in neurologist evaluation during admission (6.3% [95% CI, 4.2%-8.4%]). However, the rural-urban disparity widened or was similar with regard to receiving alteplase (0.5% [95% CI, 0.1%-0.8%]), mean days in an institution from admission (0.5 [95% CI, 0.2-0.8] days), and mortality at 90 days (0.3% [95% CI, −0.02% to 0.6%]), respectively.
Conclusions and Relevance
In the last decade, care for rural residents with acute ischemic stroke and transient ischemic attack has shifted to certified stroke centers and now more likely includes neurologist input. However, disparities in access to treatments, such as alteplase, and outcomes persist, highlighting that work still is needed to extend improvements in stroke care to all US residents.
Introduction
Rural US residents have historically had greater stroke mortality than their urban peers.1,2,3,4,5 One potential driver of this disparity is that rural residents are less likely to receive timely evaluation and treatment by a stroke expert,6,7,8,9 the need for which has become even more important, given the time-sensitive benefits of alteplase10 and thrombectomy.11
To address this disparity, there have been substantial investments in improving access to stroke expertise through the development of regional systems of care for stroke.12 Transfers and protocols that preferentially route patients directly to stroke centers have become more common,13 as have the number of certified stroke centers.14 Hundreds of hospitals now use telestroke to provide timely access to stroke specialists via telemedicine.15 It is unclear whether these care delivery changes have changed where rural residents go for care and whether they have attenuated rural-urban disparities in stroke treatment and patient outcomes.16
Prior studies have shown that patients with stroke who are admitted to rural hospitals receive lower-quality care17,18,19 and have higher mortality rates.20,21 However, prior studies have 2 substantial limitations: they generally use the address of the hospital (rather than patient residence) to determine rural and urban locations, and the analyses are several years old and therefore do not account for recent changes in stroke care patterns. Describing such trends is also complicated by better imaging methods that have led more patients to be diagnosed with an acute ischemic stroke vs a transient ischemic attack (TIA),22 as well as changes in reimbursement that have led to greater use of observation stays instead of inpatient admissions.23
To advance understanding of current rural-urban disparities in stroke care, we compared trends in 2008 through 2017 among rural and urban Medicare beneficiaries with acute ischemic stroke or TIA in where they received care, what care they were given at those locations, and their subsequent outcomes. We focused on the Medicare population, given that most cerebrovascular disease occurs in those older than 65 years, and examined ischemic stroke and TIA together.24 In addition, we focused on differences between rural vs urban communities using the patient’s residence (instead of the hospital’s location) and included all hospital stays, including observation stays, in our analysis.
Methods
Setting and Study Population
We used a 100% sample of Medicare Inpatient and Outpatient Standard Analytic Files to identify all stroke and TIA admissions in the population receiving fee-for-service Medicare from calendar year 2008 through calendar year 2017. Stroke and TIA were identified using the primary diagnosis code. Stroke diagnoses included International Classification of Diseases, Ninth Revision (ICD-9) codes 433.X (except for 433.10, which is how most elective carotid stenosis admissions are classified), 434.X, and 43625 and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes I63 through I66 (except I65.2) and I67.89. Diagnoses of TIA were identified with ICD-9 codes 435.X and ICD-10 codes G45.X. The study was approved by the Harvard Medical School Committee on Human Studies, which granted a waiver of informed consent because the study involved no more than minimal risk and analyzed already collected deidentified data.
We included as admissions all contiguous inpatient hospitalizations, outpatient observation stays, or emergency department visits in short-term acute care or critical access hospitals for which the primary diagnosis was TIA or acute stroke. The term contiguous was defined as no intervening days spent at home or in rehabilitation. If the patient was seen at an emergency department and transferred to an admitting institution or transferred after hospital admission while under a primary diagnosis of TIA or acute stroke, all care was combined into 1 admission record. Subsequent hospital use under other primary diagnoses was not included as part of the admission. We excluded patients not continuously enrolled in Medicare parts A and B in the 12 months prior to or the 3 months after their admission, as well as those currently enrolled with end-stage kidney disease (eFigure 1 in the Supplement for a diagram of our study sample).
For each admission, we identified patient characteristics from the Master Beneficiary Summary Files, including birth and death dates, sex, race/ethnicity, original Medicare entitlement reason, Medicaid enrollment in the prior 12 months, residential zip code, and 27 indicators for chronic diagnoses that were identified prior to admission. We classified patients as rural or urban based on their home zip code’s Rural-Urban Commuting Area (RUCA) code. Patients living in metropolitan areas or RUCA codes 1 through 3 were designated urban and patients living in nonmetropolitan areas or RUCA codes 4 through 10 were designated rural. Patients were excluded (n = 12 797 [0.32%]) if we could not identify a RUCA for their residence.
Study Outcomes
Admission Type and Transfers
We identified ambulance use and mileage on the first day of patient admissions. Ambulance information was identified in the carrier (part B) claim file using Healthcare Common Procedure Coding System modifier codes RH and SH for residence-to-hospital and scene-to-hospital rides, respectively. We created an indicator for whether an ambulance was used on the first day of an admission and a mileage variable that captured the number of miles billed for by the ambulance service. Because of data availability, this was limited to a random 20% sample of the study population.
Using 100% outpatient and inpatient revenue files, we identified whether the patient was under observation (codes 0760 and 0762) during their admission. We identified whether the patient was admitted as an inpatient during their admission and created an indicator for admissions during which the patient was outpatient only. We identified whether a patient was transferred by observing that more than 1 hospital submitted claims during an admission.
Site of Care, Stroke Evaluation, and Interventions
We focused on both the first hospital we observed where care was received (the presenting hospital) and the discharge hospital from that stay. Hospitals were identified using the US Centers for Medicare & Medicaid Services certification number. We created an indicator for whether a hospital was accredited by the Joint Commission as a comprehensive stroke center or primary stroke center in 2016 and 2017 (the last years of our analysis). We did not focus on accreditation in 2016 or 2017 vs the year that care was received because we were concerned that, in prior years, many hospitals had sufficient stroke expertise but had not yet gone through the accreditation process.26
To assess whether a neurologist was consulted (either in person or via telemedicine) during an admission, we identified all professional claims with the clinician specialty code 13 over the period of the admission. We also examined whether neurology consultations occurred on the first day that the patient was admitted. As was the case for ambulance services, evaluation of professional services was limited to a 20% random sample of the study population.
Using 100% inpatient and outpatient claim records, alteplase use was identified from both the procedure code for whether the hospital administered alteplase and the diagnosis code for whether alteplase was administered prior to arriving at the hospital (also known as drip and ship) (eFigure 2 in the Supplement for the share of alteplase use identified through a drip-and-ship diagnosis code). Alteplase procedure codes included ICD-9 code 99.10 and ICD-10 codes 3E03317 through 3E08317. Drip-and-ship diagnoses codes included ICD-9 code V45.88 and ICD-10 code Z9282. Thrombectomy procedures were identified using procedure ICD-9 code 39.74 and ICD-10 codes 03CG3ZZ through 03CV3ZZ. We created an indicator termed any intervention for whether a patient received either alteplase or a thrombectomy during their admission.
Patient Outcomes
We measured patient mortality at 30, 90, and 180 days from admission using beneficiary death dates and 30-day all-cause returns to hospital as the occurrence of any inpatient admission, outpatient emergency department visit, or observation stay in any short-term acute care or critical access hospital 30 days from discharge (among those discharged alive). Building on prior work,27,28 we assessed functional outcomes using the proxy of time spent within an institution during the 90 days after admission. To do so, we counted the number of days from hospital admission spent in a hospital, inpatient rehabilitation facility, or skilled nursing facility, and we identified whether patients were still in an institution on day 90.
Finally, we measured all part A and B spending during the first 90 days of each admission. Spending included the amount paid by Medicare, the beneficiary, and the primary payer and was extracted from the inpatient, outpatient, skilled nursing facility, hospice, home health agency, durable medical equipment, and (for a 20% sample only) professional claim files. Total spending was the summation of each component (again, for a 20% sample only).
Statistical Analysis
Unadjusted mean values of patient characteristics were compared between urban and rural residents over time. To capture changes in the overall risk of the population, we created an expected mortality measure. Using 2008 data only, we modeled 180-day mortality from admission in 2008 based on patient demographics, enrollment in Medicaid in the prior 12 months, original entitlement reason, inpatient and postacute care facility use in the prior 12 months, and chronic disease burden (using 27 disease indicators) prior to admission. Using the parameters from this model (eTable 1 in the Supplement), we calculated the expected mortality risk for each admission in our study sample through 2017 and the fraction of admissions in the top quartile of risk.
We also compiled unadjusted means for all study outcomes for rural and urban residents in each year of the study period, 2008 through 2017. We calculated unadjusted differences over the full study period for each group by subtracting the urban mean value for each characteristic from the rural mean value in the initial study year (2008) and the final year (2017).
We use the term disparities when describing aspects of care where higher or lower rates likely represent better outcomes and care quality (eg, mortality, use of alteplase, institutional time, receipt of care at a stroke center). However, we use the term difference when it is unclear if higher or lower rates represent better or worse outcomes for patients (eg, patient sex, hospital transfer).
To assess whether disparities in stroke care and patient outcomes changed for rural beneficiaries, we estimated the differential change in each of our study outcomes using linear models of our initial and final years of data only, controlling for patient age, sex, race/ethnicity, Medicaid enrollment in prior 12 months, original Medicare entitlement reason (whether for age, disability, or end-stage kidney disease), and 27 indicators for chronic conditions prior to admission (eTable 2 in the Supplement). We clustered the standard errors in our models at the state level and considered P values less than .05 significant. All analyses were conducted using Stata version 15 (StataCorp LLC) from October 2018 to December 2019.
Results
Study Population
There were 4.01 million admissions in the US among patients with traditional Medicare who had a transient ischemic attack or acute stroke in the study period. The final sample included 3.47 million admissions from 2008 through 2017. In this sample, 2 013 131 patients (58.0%) were female, and the mean (SD) age was 78.6 (10.5) years.
Incidence of acute ischemic stroke and TIA per capita were higher among residents of rural areas throughout the period (total admissions per 1000 beneficiaries: 2008, 13.1 in rural areas vs 11.7 in urban areas; 2017, 11.4 in rural areas vs 10.1 in urban areas) (Table 1). Among both residents of rural and urban areas, rates of acute ischemic stroke and TIA declined over the decade (in rural areas, from 13.1 to 11.4 admissions per 1000 beneficiaries; in urban areas, from 11.7 to 10.1 admissions per 1000 beneficiaries). Among all patients with either acute ischemic stroke or TIA, the fraction of patients diagnosed with an acute ischemic stroke increased from 59.5% (216 628 of 364 246 admissions) in 2008 to 66.5% (218 812 of 329 063 admissions) in 2017.
Table 1. Characteristics of Rural and Urban Medicare Residents Diagnosed With Acute Stroke or Transient Ischemic Attack in 2008 and 2017a.
| Characteristic | Admissions, No. (%) | |||||
|---|---|---|---|---|---|---|
| 2008 | 2017 | |||||
| Rural | Urban | Difference | Rural | Urban | Difference | |
| Admissions, No. | 98 008 | 266 238 | 168 230 | 86 750 | 242 313 | 155 563 |
| No. per 1000 beneficiaries | 13.1 | 11.7 | 1.4 | 11.4 | 10.1 | 1.3 |
| Primary diagnosis | ||||||
| Acute ischemic stroke | 56 037 (57.2) | 160 590 (60.3) | −3.1 (−3.5 to −2.8) | 55 780 (64.3) | 163 077 (67.3) | −2.9 (−3.3 to −2.6) |
| Transient ischemic attack | 40 452 (41.3) | 104 788 (39.4) | 1.9 (1.6-2.3) | 29 322 (33.8) | 78 267 (32.3) | 1.4 (1.1-1.8) |
| Age, mean (SD), y | 78.3 (10.2) | 79.0 (10.1) | −0.6 (−0.7 to −0.5) | 77.6 (10.5) | 78.6 (10.5) | −1.0 (−1.1 to −0.9) |
| Female | 57 189 (58.4) | 159 210 (59.8) | −1.4 (−1.8 to −1.1) | 47 799 (55.1) | 136 665 (56.4) | −1.3 (−1.7 to −0.9) |
| Race/ethnicity | ||||||
| White | 88 753 (90.6) | 222 722 (83.7) | 6.9 (6.6-7.2) | 77 985 (89.9) | 199 891 (82.5) | 7.4 (7.1-7.7) |
| Black | 6987 (7.1) | 31 666 (11.9) | −4.8 (−5.0 to −4.5) | 5986 (6.9) | 28 835 (11.9) | −5.0 (−5.2 to −4.7) |
| Asian | 202 (0.2) | 3499 (1.3) | −1.1 (−1.2 to −1.0) | 174 (0.2) | 4119 (1.7) | −1.4 (−1.5 to −1.4) |
| Other | 1384 (1.4) | 1.2 (3 316) | 0.2 (0.1-0.3) | 1909 (2.2) | 5573 (2.3) | 0.0 (−0.1 to 0.1) |
| Hispanic | 682 (0.7) | 5033 (1.9) | −1.2 (−1.3 to −1.1) | 607 (0.7) | 4119 (1.7) | −0.9 (−1.0 to −0.8) |
| Medicaid enrollment in prior 12 mo | 26 301 (26.8) | 57 847 (21.7) | 5.1 (4.8-5.4) | 20 560 (23.7) | 47 251 (19.5) | 4.3 (3.9-4.6) |
| Original entitlement reason | ||||||
| Age >65 y | 78 720 (80.3) | 223 103 (83.8) | −3.5 (−3.8 to −3.2) | 66 624 (76.8) | 197 970 (81.7) | −5.0 (−5.3 to −4.6) |
| Disabled | 19 107 (19.5) | 42 627 (16.0) | 3.5 (3.2-3.8) | 20 039 (23.1) | 43 859 (18.1) | 5.0 (4.7-5.3) |
| End-stage kidney disease | 181 (0.2) | 506 (0.2) | 0.0 (0.0-0.0) | 174 (0.2) | 485 (0.2) | 0.0 (0.0-0.0) |
| Inpatient stay in prior 12 mo | 40 461 (41.3) | 109 424 (41.1) | 0.2 (−0.2 to 0.5) | 29 322 (33.8) | 86 021 (35.5) | −1.7 (−2.1 to −1.3) |
| Postacute care stay in prior 12 mo | 14 747 (15.1) | 40 903 (15.4) | −0.3 (−0.6 to −0.1) | 11 625 (13.4) | 35 862 (14.8) | −1.4 (−1.7 to −1.1) |
| Chronic conditions, mean (SD), No. | 7.8 (3.6) | 8.2 (3.7) | −0.4 (−0.4 to −0.3) | 8.6 (3.9) | 9.0 (4.0) | −0.4 (−0.5 to −0.4) |
| Expected 180-d mortality risk, mean (SD) | 17.7 (11.7) | 17.9 (11.8) | −0.2 (−0.3 to −0.1) | 16.4 (11.3) | 17.2 (11.8) | −0.8 (−0.9 to −0.7) |
| In top quartile of mortality risk | 23 982 (24.5) | 67 079 (25.2) | −0.7 (−1.0 to −0.4) | 18 391 (21.2) | 58 155 (24.0) | −2.8 (−3.1 to −2.5) |
The Table presents a comparison of the characteristic differences between rural and urban Medicare beneficiaries in 2008 and 2017. Differences are unadjusted and are the urban value in each year subtracted from the rural value. Negative values indicate that a characteristic was less common among rural beneficiaries in a given year, while positive values indicate that the characteristic was more common.
Patient demographics of rural and urban resident samples changed differentially over the decade. Among patients with acute stroke and TIA, the top quartile of expected mortality risk fell 2.2 percentage points more among residents of rural areas (from 24.5% in 2008 to 21.2% in 2017) than among residents of urban areas (from 25.2% in 2008 to 24.0% in 2017; an 8.7% relative change from 2008; P < .001), indicating that compared with residents of urban areas, residents of rural areas were differentially becoming healthier on observed patient characteristics, in particular age groups, over the study period (Table 1).
Care Prior to Hospital Presentation and Classification of Hospital Stay
In 2008, residents of rural areas were 5.3% less likely to use an ambulance than residents of urban areas (25.6% vs 30.9%, respectively), while in 2017, they were 6.6% less likely (4904 [28.2%] vs 16 943 [34.8%]) (Table 2). While ambulance miles changed only slightly for urban residents over the study period, mean ambulance ride distance increased by 1.5 miles (from 11.3 to 12.8 miles) in rural areas from 2008 to 2017.
Table 2. Admission Types of Rural and Urban Medicare Residents Diagnosed With Acute Stroke or Transient Ischemic Attack in 2008 and 2017a.
| Type | Admissions, No. (%) | |||||
|---|---|---|---|---|---|---|
| 2008 | 2017 | |||||
| Rural | Urban | Difference | Rural | Urban | Difference | |
| Ambulance used | 5000 (25.6) | 16 699 (30.9) | −5.3 (−6.0 to −4.5) | 4904 (28.2) | 16 943 (34.8) | −6.6 (−7.4 to −5.8) |
| Distance traveled, mean (SD), mi | 11.3 (12.7) | 7.6 (7.4) | 3.6 (3.4-3.9) | 12.8 (13.8) | 7.9 (7.9) | 4.9 (4.6-5.2) |
| Observation stay used | 11 423 (11.7) | 24 582 (9.2) | 2.4 (2.2-2.6) | 22 411 (25.8) | 66 549 (27.5) | −1.6 (−2.0 to −1.3) |
| Outpatient admission only | 27 851 (28.4) | 47 149 (17.7) | 10.7 (10.4-11.0) | 31 325 (36.1) | 61 437 (25.4) | 10.8 (10.4-11.1) |
| Transfer during admission | 5283 (5.4) | 4553 (1.7) | 3.7 (3.6-3.8) | 10 083 (11.6) | 9921 (4.1) | 7.5 (7.3-7.7) |
The Table presents a comparison of the admission type differences between rural and urban Medicare beneficiaries in 2008 and 2017. Differences are unadjusted and are the urban value in each year subtracted from the rural value. Negative values indicate that the admission type was less common among rural beneficiaries in a given year, while positive values indicate that the admission type was more common.
The use of observation stays increased substantially between 2008 and 2017 for residents of both rural and urban areas. For rural residents, they increased from 11.7% to 25.8% (from 11 423 to 24 582 patients) and for urban residents, from 9.2% to 27.5% (from 22 411 to 66 549 patients). In 2008, residents of rural areas were 3.7 percentage points more likely to be transferred during their hospital stay compared with residents of urban areas (5.4% vs 1.7%). By 2017, that difference doubled to 7.5 percentage points (11.6% vs 4.1%).
Site of Care, Stroke Evaluation, and Interventions
Between 2008 and 2017, the care locations of rural residents changed considerably. In 2008, 25.2% of patients in rural areas (24 681 patients) and 60.6% of patients in urban areas (161 217 patients) received care at stroke centers (an unadjusted disparity of 35.4 percentage points) (Figure). By 2017, the adjusted disparity fell an absolute 8.7 (95% CI, 6.7-10.6) percentage points, a 25% relative reduction from 2008. The attenuation in the disparity was achieved primarily through more residents of rural areas initially presenting at certified hospitals vs increased transfers to certified stroke centers; differences presenting at a stroke center fell 5.2 (95% CI, 3.1-7.4) percentage points (Table 3). More details on changes over time in the presenting hospital for rural vs urban residents are presented in eTable 3 in the Supplement.
Figure. Trends in Acute Stroke and Transient Ischemic Attack Care for Rural and Urban Residents From 2008 Through 2017.
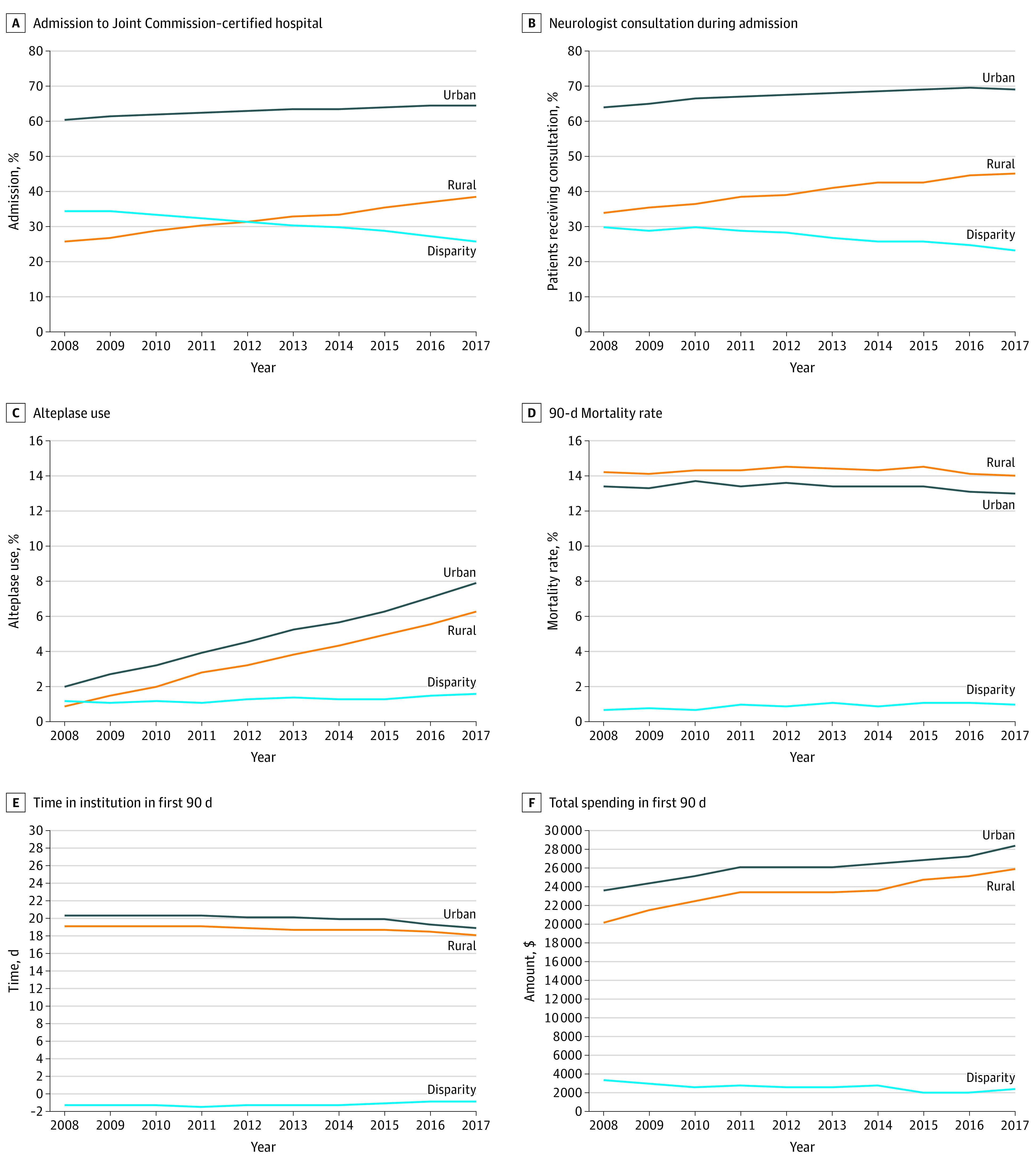
Adjusted rural and urban trends are shown for each outcome over the period 2008 through 2017. Trends were adjusted for patient age, sex, race/ethnicity, Medicaid enrollment in prior 12 months, original Medicare entitlement reason (whether for age, disability, or end-stage kidney disease), and 27 indicators for chronic conditions prior to admission. The disparity line for each outcome is the absolute differences between the adjusted trend lines. Downward sloping lines indicate a dissipating disparity, while upward sloping lines indicate a growing disparity. Flat lines indicate relatively little change in the level of a disparity over time.
Table 3. Changes in Rural-Urban Disparities in the Use of Specialized Stroke Centers, Neurologists, and Stroke Procedures, 2008 vs 2017a.
| Characteristic | Admissions, No. (%) | Adjusted change in disparity from 2008 to 2017, estimate (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| 2008 | 2017 | ||||||
| Rural | Urban | Unadjusted disparity | Rural | Urban | Unadjusted disparity | ||
| Stroke center use during admission | 24 681 (25.2) | 161 217 (60.6) | −35.4 (−35.7 to −35.0) | 33 220 (38.3) | 157 161 (64.9) | −26.6 (−26.9 to −26.2) | −8.7 (−10.8 to −6.6) |
| Presenting hospital | 21 020 (21.4) | 159 096 (59.8) | −38.3 (−38.7 to −38.0) | 25 976 (29.9) | 152 539 (63.0) | −33.0 (−33.4 to −32.6) | −5.2 (−7.4 to −3.1) |
| Discharging hospital | 24 616 (25.1) | 160 942 (60.5) | −35.3 (−35.7 to −35.0) | 33 113 (38.2) | 156 645 (64.6) | −26.5 (−26.8 to −26.1) | −8.8 (−10.9 to −6.7) |
| Neurologist evaluation during admission | 6489 (33.2) | 34 556 (63.9) | −30.7 (−31.5 to −29.9) | 7858 (45.2) | 33 798 (69.4) | −24.2 (−25.0 to −23.4) | −6.3 (−8.4 to −4.2) |
| On first day of admission | 3035 (15.5) | 16 206 (30.0) | −14.4 (−15.1 to −13.7) | 3529 (20.3) | 14 724 (30.2) | −9.9 (−10.7 to −9.2) | −4.4 (−5.9 to −2.9) |
| Treated with alteplase or thrombectomy | 1072 (1.1) | 5908 (2.2) | −1.1 (−1.2 to −1.0) | 6080 (7.0) | 21 659 (8.9) | −1.9 (−2.1 to −1.7) | 0.8 (0.5-1.2) |
| Alteplase use | 1014 (1.0) | 5718 (2.1) | −1.1 (−1.2 to −1.0) | 5466 (6.3) | 19 041 (7.9) | −1.6 (−1.8 to −1.4) | 0.5 (0.1-0.8) |
| Thrombectomy use | 115 (0.1) | 487 (0.2) | −0.1 (−0.1 to −0.0) | 1192 (1.4) | 5015 (2.1) | −0.7 (−0.8 to −0.6) | 0.6 (0.5-0.8) |
Unadjusted disparities are the rural mean in each year less the urban mean. The adjusted disparities shown in the final column are the estimated differences between rural and urban changes in each outcome between 2008 and 2017 after adjusting for patient age, sex, race/ethnicity, Medicaid enrollment in prior 12 months, original Medicare entitlement reason (whether for age, disability, or end-stage kidney disease), and 27 indicators for chronic conditions prior to admission. Standard errors were clustered by beneficiary state and 95% CIs are presented along with our estimates. Negative adjusted changes indicate disparities that have become smaller during the study period. Positive adjusted changes indicate disparities that have become larger during the study period.
In 2008, only 33.2% of residents of rural areas (6489 patients) vs 63.9% of residents of urban areas (34 556 patients) with stroke and TIA (an unadjusted disparity of 30.7 percentage points) were seen by a neurologist during their admission (Table 3). By 2017, the unadjusted disparity fell to 24.1 percentage points (Figure), a narrowing of 6.3 percentage points after adjustment (95% CI, 5.2-9.6 percentage points).
Compared with urban residents, rural residents were less likely to receive alteplase in 2008 (1.0% [1014 patients] vs 2.1% [5718 patients]). In both groups, use of alteplase steadily increased over the study period (Figure) in parallel. By 2017, the disparity between residents of rural and urban areas with respect to alteplase rates had slightly widened (change in adjusted disparity, 0.5% (95% CI, 0.1%-0.8%). We observed very few thrombectomies in 2008 and steady but small growth until 2015, after which the rate accelerated (eFigure 3 in the Supplement for more details). We found that the disparity in thrombectomy use between residents of rural and urban areas widened from 0.1% in 2008 (0.1% [115 patients] in rural areas vs 0.2% [487 patients] in urban areas) to 0.7% in 2017 (1.4% [1192 patients] in rural areas vs 2.1% [5015 patients] in urban areas), a change in the adjusted disparity of 0.6% (95% CI, 0.5%-0.8%). Combined, the disparity between rural and urban patients receiving either procedure widened from 1.1 percentage points in 2008 to 1.9 percentage points in 2017 (an adjusted change in the disparity of 0.8% [95% CI, 0.5%-1.2%]).
Patient Outcomes
In 2008, rural residents had a higher 90-day mortality rate (14.3% [13 981 patients]) than urban residents (13.7% [36 421 patients]) (Table 4 and eAppendix in the Supplement). While the mortality rate declined over this period among both rural and urban residents (Figure), after adjusting for patient characteristics, the rural-urban disparity in 90-day mortality increased slightly by 0.3 percentage points (95% CI, −0.02 to 0.6 percentage points). Thirty-day all-cause returns to hospital were higher among residents of rural areas (24 639 [25.1%]) vs residents of urban areas (57 274 [21.5%]) in 2008, and an unadjusted disparity of 3.6 percentage points that grew larger by an adjusted 0.9 percentage points (95% CI, 0.4-1.5 percentage points) by 2017.
Table 4. Changes in Rural-Urban Resident Disparities in Mortality, Hospital Returns, Postadmission Institution Time, and Spending, 2008 vs 2017a.
| Outcome | Patients, No. (%) | Adjusted change in disparity from 2008 to 2017, estimate (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| 2008 | 2017 | ||||||
| Rural | Urban | Unadjusted disparity | Rural | Urban | Unadjusted disparity | ||
| Mortality from admission (%), d | |||||||
| 30 | 9501 (9.7) | 24 353 (9.1) | 0.5 (0.3-0.8) | 8044 (9.3) | 21 212 (8.8) | 0.5 (0.3-0.7) | 0.28 (−0.04 to 0.60) |
| 90 | 13 981 (14.3) | 36 421 (13.7) | 0.6 (0.3-0.8) | 11 610 (13.4) | 31 364 (12.9) | 0.4 (0.2-0.7) | 0.28 (−0.02 to 0.59) |
| 180 | 17 857 (18.2) | 46 991 (17.7) | 0.6 (0.3-0.8) | 14 783 (17.0) | 40 540 (16.7) | 0.3 (0.0-0.6) | 0.28 (−0.11 to 0.66) |
| All-cause returns to hospital 30 d from discharge (%) | 24 639 (25.1) | 57 274 (21.5) | 3.6 (3.3-3.9) | 23 209 (26.8) | 53 790 (22.2) | 4.6 (4.2-4.9) | 0.91 (0.35-1.48) |
| Institution at 90 d (%) | 9856 (10.1) | 28 630 (10.8) | −0.7 (−0.9 to −0.5) | 7393 (8.5) | 21 730 (9.0) | −0.4 (−0.7 to −0.2) | 0.40 (−0.02 to 0.83) |
| Institution time within 90 d, mean (SD), d | 19.0 (26.0) | 20.6 (26.6) | −1.6 (−1.8 to −1.4) | 17.7 (24.7) | 19.0 (24.9) | −1.3 (−1.5 to −1.1) | 0.52 (0.20-0.83) |
| Total spending, mean (SD), $ | 19 858 (20 564) | 23 646 (24 218) | −3788 (−4169 to −3407) | 25 697 (26 979) | 28 701 (29 275) | −3004 (−3500 to −2508) | −849 (−1606 to −92) |
| Inpatient | 7976 (10 091) | 9829 (13 986) | −1852 (−1948 to −1757) | 9632 (12 550) | 11 484 (14 872) | −1852 (−1963 to −1741) | −16 (−235 to 203) |
| Skilled nursing facility | 4429 (10 215) | 4692 (10 434) | −263 (−339 to −187) | 4994 (12 156) | 4975 (11 617) | 19 (−72 to 110) | −369 (−625 to −113) |
| Professional fees | 2615 (2750) | 3346 (3071) | −731 (−780 to −682) | 3622 (3685) | 4110 (3617) | −489 (−552 to −426) | −241 (−324 to −158) |
| Outpatient hospital | 1440 (2205) | 868 (1779) | 572 (558-586) | 2788 (3938) | 1772 (3280) | 1016 (989-1043) | −434 (−558 to −308) |
Unadjusted disparities are the rural mean in each year less the urban mean. The adjusted disparities shown in the final column are the estimated differences between rural and urban changes in each outcome between 2008 and 2017 after adjusting for patient age, sex, race/ethnicity, Medicaid enrollment in prior 12 months, original Medicare entitlement reason (whether for age, disability or end-stage kidney disease), and 27 indicators for chronic conditions prior to admission. Standard errors were clustered by beneficiary state, and 95% CIs are presented along with our estimates. Negative adjusted changes indicate disparities that have become smaller during the study period. Positive adjusted changes indicate disparities that have become larger during the study period. See the eAppendix in the Supplement for a discussion of how our mortality measures differ from the Centers for Medicare & Medicaid Services’ Hospital Compare mortality measure for stroke.
In 2008, the mean (SD) number of days the patient spends in an institution (for example, hospital, skilled nursing facility) after admission was 19.0 (26.0) days among residents of rural areas and 20.6 (26.6) days in residents of urban areas. The number of days fell among both populations over time. After adjusting for patient characteristics, the disparity in the number of institution days increased among rural residents by 0.5 (95% CI, 0.2-0.8) days.
Mean (SD) total spending in the 90 days from admission onward was 19% lower among rural residents in 2008 ($19 858 [$20 564]) than in urban residents ($23 646 [$24 218]). Spending increased by more among rural residents between 2008 and 2017, narrowing the spending gap by an adjusted $849 (95% CI, $92-$1606). The attenuation in the spending difference was driven in part via increased spending on care in skilled nursing facilities, outpatient hospital facilities, and professional services (Table 4).
Discussion
Over the last decade, there have been large investments in developing stroke systems of care throughout the US. During the period of 2008 to 2017, we observed sizeable shifts in the places rural patients with acute ischemic stroke and TIA went for care and the care they received. Rural patients are now more likely to be cared for at certified stroke centers and see a neurologist than they were previously. Broadly, the rural-urban disparity in accessing stroke expertise has narrowed. Despite this substantial shift in care, disparities in revascularization as well as outcomes, such as mortality, have not improved, and in some cases, these are slightly larger than they were a decade ago.
One factor that might explain why outcome disparities have not narrowed is that disparities in the use of alteplase and thrombectomy have not diminished. After accounting for drip-and-ship codes, alteplase use rates among residents of rural areas have increased rapidly but not at the same rate as alteplase use among residents of urban areas, and the disparity has slightly widened. With respect to thrombectomy, we also observe growing use among both patients in rural and urban areas, but the rate of growth is faster in urban areas, and therefore the disparity is growing.
One potential intervention to increase use of revascularization procedures for rural patients is telestroke. Emergency physicians have been reluctant to administer alteplase in the past,29 and neurology input is believed to be associated with greater use of appropriate treatments.30 Telestroke is believed to increase the use of procedures such as alteplase for acute stroke, presumably by facilitating early neurology input.31 While availability of telestroke consultation is becoming more common,32 it is still only available in a minority of hospitals nationally, and availability appears to be similar in rural and urban hospitals,33 which suggests that its use has not differentially benefited patients in rural areas.
Moreover, while we did observe increases in the use of neurology input among patients in rural areas, most rural residents (55%) still do not receive neurology input during their admission. The need for specialized neurology input may become more important over time as stroke incidence falls nationally34 and emergency medicine physicians in lower-volume hospitals experience fewer stroke cases in the future.
Differences in social determinants of health, including income and education, undoubtedly also contribute to the persistent disparities in outcomes. Hypertension and diabetes are less likely to be treated in rural areas,35 there are fewer primary care physicians per capita in rural areas,36 and there may be disparities in early stroke recognition. Also, inherent differences in rural communities in paramedic availability and transport times may limit the fraction of patients in these areas eligible for alteplase or thrombectomy.
Over the past decade, there was a substantial increase in the use of observation stays, which now account for roughly one-quarter of all hospital stays for TIA and stroke (eFigure 4 in the Supplement shows growth among strokes only). Over time, there has been a steady increase in hospital transfers and fewer patients have been diagnosed with a TIA. Together, these trends affect research on acute cerebrovascular disease and emphasize that it is insufficient to only examine inpatient hospital admissions or TIA and acute stroke separately if one wants to fully capture trends in care.
Limitations
Our study has several key limitations. It is limited to the Medicare population and does not capture enrollees in Medicare Advantage nor those with commercial insurance or Medicaid. While we can control for patient demographics and comorbidities and study a large sample size, we do not have data on stroke severity. In the eTable 3 in the Supplement, we show the changes in rural-urban disparities are affected by shifts in patient demographics and comorbidities in rural and urban communities. Although we do not have functional outcome (eg, modified Rankin) scores to capture patients’ longer-term functional outcomes, previous work has found 90-day time in institution vs home to be a reasonable proxy.37 Also, some of the differences in patient comorbidities over time might be driven by changes in clinician documentation and coding rather than change in patient risk profiles. Our data are limited to administrative claims records and therefore drip-and-ship use may be undercounted because they do not factor into payment to the receiving hospital, and alteplase use may not be recorded in claims if higher reimbursement procedures, such as mechanical thrombectomy, are performed. We are likely underestimating the decrease in the disparity in neurologist visits because many rural patients receive neurologist input via telestroke consultations that are not billed to Medicare. Finally, we do not capture ambulance use from emergency medical services providers that do not bill fees for service, although prior work has found this is uncommon in both urban and rural communities.38
Conclusions
To improve the quality of stroke care, the US has spent considerable effort developing stroke systems of care. This work has led to increased access to acute reperfusion therapies and reduced overall mortality. In an analysis of trends in rural-urban disparities of care, we found that between 2008 and 2017, residents of rural areas are receiving care at certified stroke centers and more likely to receive neurologist input and intervention. However, disparities in the use of alteplase or thrombectomy have persisted or even grown. Disparities in outcomes also remain, highlighting that more work is needed to ensure that the benefits of advanced stroke care translate to better outcomes for all US residents.
eFigure 1. Study sample diagram
eTable 1. Patient mortality risk model
eFigure 2. Share of alteplase identified by a “drip and ship” diagnosis code
eTable 2. Estimates of key outcomes before and after controlling for patient factors
eTable 3. Additional characteristics of the presenting hospital
eFigure 3. Rural and urban trends in thrombectomy use.
eFigure 4. Observation stay use and outpatient only admissions for acute stroke.
eAppendix. Comparison to Medicare’s Mortality Measure for Acute Stroke.
References
- 1.Eberhardt MS, Pamuk ER. The importance of place of residence: examining health in rural and nonrural areas. Am J Public Health. 2004;94(10):1682-1686. doi: 10.2105/AJPH.94.10.1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sergeev AV. Racial and rural-urban disparities in stroke mortality outside the Stroke Belt. Ethn Dis. 2011;21(3):307-313. [PubMed] [Google Scholar]
- 3.Howard G. Ancel Keys lecture: adventures (and misadventures) in understanding (and reducing) disparities in stroke mortality. Stroke. 2013;44(11):3254-3259. doi: 10.1161/STROKEAHA.113.002113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh GK, Siahpush M. Widening rural-urban disparities in all-cause mortality and mortality from major causes of death in the USA, 1969-2009. J Urban Health. 2014;91(2):272-292. doi: 10.1007/s11524-013-9847-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingram DD, Montresor-Lopez JA Differences in stroke mortality among adults aged 45 and over: United States, 2010–2013; NCHS data brief, no 207, July 2015. Published July 2015. Accessed March 20, 2020. https://www.cdc.gov/nchs/products/databriefs/db207.htm [PubMed]
- 6.Barnett HJ, Buchan AM. The imperative to develop dedicated stroke centers. JAMA. 2000;283(23):3125-3126. doi: 10.1001/jama.283.23.3125 [DOI] [PubMed] [Google Scholar]
- 7.Alberts MJ, Hademenos G, Latchaw RE, et al. Recommendations for the establishment of primary stroke centers: Brain Attack Coalition. JAMA. 2000;283(23):3102-3109. doi: 10.1001/jama.283.23.3102 [DOI] [PubMed] [Google Scholar]
- 8.Leira EC, Hess DC, Torner JC, Adams HP Jr. Rural-urban differences in acute stroke management practices: a modifiable disparity. Arch Neurol. 2008;65(7):887-891. doi: 10.1001/archneur.65.7.887 [DOI] [PubMed] [Google Scholar]
- 9.Shultis W, Graff R, Chamie C, et al. Striking rural-urban disparities observed in acute stroke care capacity and services in the Pacific Northwest: implications and recommendations. Stroke. 2010;41(10):2278-2282. doi: 10.1161/STROKEAHA.110.594374 [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480-2488. doi: 10.1001/jama.2013.6959 [DOI] [PubMed] [Google Scholar]
- 11.Albers GW, Marks MP, Kemp S, et al. ; DEFUSE 3 Investigators . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708-718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelick PB. Primary and comprehensive stroke centers: history, value and certification criteria. J Stroke. 2013;15(2):78-89. doi: 10.5853/jos.2013.15.2.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song S, Saver J. Growth of regional acute stroke systems of care in the United States in the first decade of the 21st century. Stroke. 2012;43(7):1975-1978. doi: 10.1161/STROKEAHA.112.657809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Joint Commission. Primary stroke center certification. Accessed March 20, 2020. https://www.jointcommission.org/certification/primary_stroke_centers.aspx
- 15.Silva GS, Farrell S, Shandra E, Viswanathan A, Schwamm LH. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke. 2012;43(8):2078-2085. doi: 10.1161/STROKEAHA.111.645861 [DOI] [PubMed] [Google Scholar]
- 16.Dwyer M, Rehman S, Ottavi T, et al. Urban-rural differences in the care and outcomes of acute stroke patients: systematic review. J Neurol Sci. 2019;397:63-74. [DOI] [PubMed] [Google Scholar]
- 17.Messé SR, Khatri P, Reeves MJ, et al. Why are acute ischemic stroke patients not receiving IV tPA? Results from a national registry. Neurology. 2016;87(15):1565-1574. doi: 10.1212/WNL.0000000000003198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales S, Mullen MT, Skolarus L, Thibault DP, Udoeyo U, Willis AW. Progressive rural-urban disparity in acute stroke care. Neurology. 2017;88(5):441-448. doi: 10.1212/WNL.0000000000003562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seabury S, Bognar K, Xu Y, Huber C, Commerford SR, Tayama D. Regional disparities in the quality of stroke care. Am J Emerg Med. 2017;35(9):1234-1239. doi: 10.1016/j.ajem.2017.03.046 [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AI, Suri MF, Nasar A, et al. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke. 2007;38(7):2180-2184. doi: 10.1161/STROKEAHA.106.467506 [DOI] [PubMed] [Google Scholar]
- 21.Lichtman JH, Leifheit-Limson EC, Jones SB, Wang Y, Goldstein LB. 30-Day risk-standardized mortality and readmission rates after ischemic stroke in critical access hospitals. Stroke. 2012;43(10):2741-2747. doi: 10.1161/STROKEAHA.112.665646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Easton JD, Saver JL, Albers GW, et al. ; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease . Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276-2293. doi: 10.1161/STROKEAHA.108.192218 [DOI] [PubMed] [Google Scholar]
- 23.Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff (Millwood). 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention Hospitalization for stroke in U.S. hospitals, 1989–2009: NCHS data brief No. 95, May 2012. Published May 2012. Accessed March 20, 2020. https://www.cdc.gov/nchs/products/databriefs/db95.htm
- 25.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: a systematic review. PLoS One. 2015;10(8):e0135834. doi: 10.1371/journal.pone.0135834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtman JH, Allen NB, Wang Y, Watanabe E, Jones SB, Goldstein LB. Stroke patient outcomes in US hospitals before the start of the Joint Commission Primary Stroke Center certification program. Stroke. 2009;40(11):3574-3579. doi: 10.1161/STROKEAHA.109.561472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Brien EC, Xian Y, Xu H, et al. Hospital variation in home-time after acute ischemic stroke: insights from the PROSPER study (Patient-Centered Research into Outcomes Stroke Patients Prefer and Effectiveness Research). Stroke. 2016;47(10):2627-2633. doi: 10.1161/STROKEAHA.116.013563 [DOI] [PubMed] [Google Scholar]
- 28.Xian Y, Xu H, O’Brien EC, et al. Clinical effectiveness of direct oral anticoagulants vs warfarin in older patients with atrial fibrillation and ischemic stroke: findings from the Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research (PROSPER) study. JAMA Neurol. 2019;76(10):1192-1202. doi: 10.1001/jamaneurol.2019.2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown DL, Barsan WG, Lisabeth LD, Gallery ME, Morgenstern LB. Survey of emergency physicians about recombinant tissue plasminogen activator for acute ischemic stroke. Ann Emerg Med. 2005;46(1):56-60. doi: 10.1016/j.annemergmed.2004.12.025 [DOI] [PubMed] [Google Scholar]
- 30.Jauch EC, Saver JL, Adams HP Jr, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi: 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 31.Schwamm LH, Holloway RG, Amarenco P, et al. ; American Heart Association Stroke Council; Interdisciplinary Council on Peripheral Vascular Disease . A review of the evidence for the use of telemedicine within stroke systems of care: a scientific statement from the American Heart Association/American Stroke Association. Stroke. 2009;40(7):2616-2634. doi: 10.1161/STROKEAHA.109.192360 [DOI] [PubMed] [Google Scholar]
- 32.Akbik F, Hirsch JA, Chandra RV, et al. Telestroke—the promise and the challenge, part one: growth and current practice. J Neurointerv Surg. 2017;9(4):357-360. doi: 10.1136/neurintsurg-2016-012291 [DOI] [PubMed] [Google Scholar]
- 33.Zachrison KS, Boggs KM, M Hayden E, Espinola JA, Camargo CA. A national survey of telemedicine use by US emergency departments. J Telemed Telecare. 2018;X18816112. doi: 10.1177/1357633X18816112 [DOI] [PubMed] [Google Scholar]
- 34.Ramirez L, Kim-Tenser MA, Sanossian N, et al. Trends in acute ischemic stroke hospitalizations in the United States. J Am Heart Assoc. 2016;5(5):e003233. doi: 10.1161/JAHA.116.003233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.US Centers for Medicare & Medicaid Services Rural-urban disparities in health care in Medicare. Published November 2018. Accessed March 20, 2020. https://www.cms.gov/files/document/rural-urban-disparities-health-care-medicare-national-report
- 36.Rosenblatt RA, Hart LG. Physicians and rural America. West J Med. 2000;173(5):348-351. doi: 10.1136/ewjm.173.5.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonarow GC, Liang L, Thomas L, et al. Assessment of home-time after acute ischemic stroke in Medicare beneficiaries. Stroke. 2016;47(3):836-842. doi: 10.1161/STROKEAHA.115.011599 [DOI] [PubMed] [Google Scholar]
- 38.MacKenzie EJ, Carlini AR National Highway Traffic Safety Administration: characterizing local EMS systems (Report No. DOT HS 811 824). Published August 2013. Accessed March 20, 2020. https://www.nhtsa.gov/sites/nhtsa.dot.gov/files/811824.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study sample diagram
eTable 1. Patient mortality risk model
eFigure 2. Share of alteplase identified by a “drip and ship” diagnosis code
eTable 2. Estimates of key outcomes before and after controlling for patient factors
eTable 3. Additional characteristics of the presenting hospital
eFigure 3. Rural and urban trends in thrombectomy use.
eFigure 4. Observation stay use and outpatient only admissions for acute stroke.
eAppendix. Comparison to Medicare’s Mortality Measure for Acute Stroke.


