Abstract
All stereotactic neurosurgical procedures utilize coordinate systems to allow navigation through the brain to a target. During the surgical planning, indirect and direct targeting determines the planned target point and trajectory. This targeting allows a surgeon to precisely reach points along the trajectory while minimizing risks to critical structures. Oftentimes, once a target point and a trajectory are determined, a frame-based coordinate system is used for the actual procedure. Considerations include the use of various coordinate spaces such as the anatomical ( ), the frame (
), the frame ( ), the head-stage (
), the head-stage (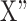 ), and an atlas. Therefore, the relationships between these coordinate systems are integral to the planning and implementation of the neurosurgical procedure. Although coordinate transformations are handled in planning via stereotactic software, critical understanding of the mathematics is required as it has implications during surgery. Further, intraoperative applications of these coordinate conversions, such as for surgical navigation from the head-stage, are not readily available in real-time. Herein, we discuss how to navigate these coordinate systems and provide implementations of the techniques with samples.
), and an atlas. Therefore, the relationships between these coordinate systems are integral to the planning and implementation of the neurosurgical procedure. Although coordinate transformations are handled in planning via stereotactic software, critical understanding of the mathematics is required as it has implications during surgery. Further, intraoperative applications of these coordinate conversions, such as for surgical navigation from the head-stage, are not readily available in real-time. Herein, we discuss how to navigate these coordinate systems and provide implementations of the techniques with samples.
Keywords: cartesian coordinate system, euclidean space, stereotactic and functional, coordinate transformation, stereotactic frame
Introduction
In 1908, Sir Victor Horsley (1857-1916), a neurosurgeon, and Robert Clarke (1850-1926), a physiologist, ignited the field of stereotactic neurosurgery by introducing a frame to navigate the structures of the Macacus rhesus cerebellum methodically using "electrolytic" lesions [1, 2]. By 1947, Ernest Spiegel (1895-1985), a neurologist, and Henry Wycis (1911-1972), a neurosurgeon, applied these frame techniques for human use in the treatment of pain, epilepsy, mental disorders, movement disorders, and tumors [3-5]. While at that time the devices were primitive, the idea was revolutionary as it was built on mathematics applied to an apparatus designed to navigate regions of the brain. In 1978, the next big leap forward was an invention by Russell Brown that precisely mapped computed tomography (CT) imaging with a stereotactic frame using an N-localizer [6-8]. This creation, which later merged with image fusion and magnetic resonance imaging (MRI), allowed precise stereotactic targeting in neurosurgery.
Historically, coordinate systems are a key element in the practice of stereotactic neurosurgery. These systems are utilized during the surgical procedures; therefore, a robust understanding is critical for those using them. For this reason, we hope to re-introduce some important concepts previously published and expand upon them further as a complete solution for the surgical technique.
Technical report
Background transformations
Various Cartesian coordinate systems in Euclidean space are utilized in stereotactic neurosurgery. The affine conversion of one coordinate system to another is often computed from one coordinate set 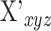 to the other coordinate set
to the other coordinate set 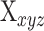 using matrices that specifies information on rotation
using matrices that specifies information on rotation 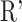 , scaling
, scaling 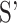 , and translation
, and translation 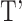 (Equation 1). These conversion matrices can be solved using three or more points using various methods [9-11]. The components of
(Equation 1). These conversion matrices can be solved using three or more points using various methods [9-11]. The components of 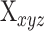 and
and 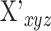 consists of
consists of 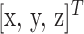 and
and 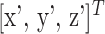 , respectively. The rotational matrix consists of nine components
, respectively. The rotational matrix consists of nine components 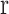 , while the scaling
, while the scaling 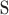 and translation
and translation 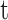 components have three (Equations 2). It should also be noted that the matrix components can also be treated as individual functions in a non-linear fashion. For example, scaling factors can be functions (
components have three (Equations 2). It should also be noted that the matrix components can also be treated as individual functions in a non-linear fashion. For example, scaling factors can be functions (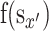 ,
, 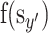 or
or 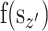 ) used to compensate for distortions of the brain relative to an atlas.
) used to compensate for distortions of the brain relative to an atlas.
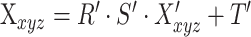 |
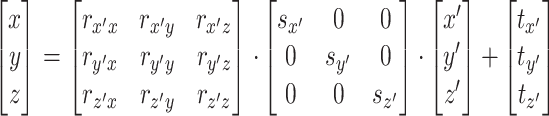 |
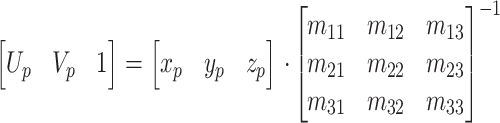
The constitution of the component matrices (Equation 2), which allow conversion from one coordinate to another, depends on the space for which the mathematical operation is being performed. Some of these coordinate spaces include an anatomical space  , a frame-based space
, a frame-based space  , and a head-stage space
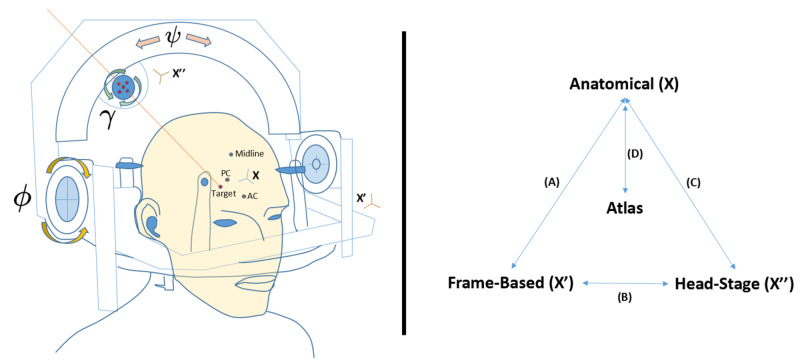
, and a head-stage space 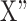 (Figure 1). Frequently, an atlas is used in reference to the anatomical space. The general scheme of Equation 1 can be applied in the transformations of these coordinate spaces for which each operation is discussed in detail (Figure 1, Right Pane, A-D) in subsequent sections. Of note, the x-axis is generally considered the left-right (LAT) direction, the y-axis is the back-front (AP) direction, and finally the z-axis the down-up (VERT) direction, which is right-anterior-superior (RAS) convention; however, some conventions flip the x-y axes making the x-axis AP and y-axis LAT. Lastly, anatomical space is built off of reference points in the brain, such as the anterior commissure (AC), posterior commissure (PC), and a midline point (Midline), whereas frame-based space is generated using an N-localizer [6-8]. We also discuss head-stage to anatomical transformation in frameless stereotaxy in addition to computation of coordinates along a fixed trajectory, both of which are generally in anatomical space.
(Figure 1). Frequently, an atlas is used in reference to the anatomical space. The general scheme of Equation 1 can be applied in the transformations of these coordinate spaces for which each operation is discussed in detail (Figure 1, Right Pane, A-D) in subsequent sections. Of note, the x-axis is generally considered the left-right (LAT) direction, the y-axis is the back-front (AP) direction, and finally the z-axis the down-up (VERT) direction, which is right-anterior-superior (RAS) convention; however, some conventions flip the x-y axes making the x-axis AP and y-axis LAT. Lastly, anatomical space is built off of reference points in the brain, such as the anterior commissure (AC), posterior commissure (PC), and a midline point (Midline), whereas frame-based space is generated using an N-localizer [6-8]. We also discuss head-stage to anatomical transformation in frameless stereotaxy in addition to computation of coordinates along a fixed trajectory, both of which are generally in anatomical space.
Figure 1. Frame-based stereotaxis involves several coordinate spaces, which include the anatomical space 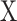 , the frame space
, the frame space 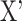 , and the head-stage space
, and the head-stage space 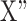 . These spaces are defined in surgery once a frame is attached to a patient and has undergone stereotactic localization (Left). Then, one can perform coordinate transformations between all three systems and the anatomical space to an atlas for which the mathematical computations are referenced in later sections of the article (Right, A-D). Conversion D can also be utilized with computed tomography (CT) or magnetic resonance imaging (MRI) rather than an atlas. Important reference points include the anterior commissure (AC), posterior commissure (PC), and the midline. In frames that operate around an isocenter, which are known as target-centered frames, the head-stage rotates around the frame-space. Three angles can be applied to the head-stage, which are in frame space the ring angle
. These spaces are defined in surgery once a frame is attached to a patient and has undergone stereotactic localization (Left). Then, one can perform coordinate transformations between all three systems and the anatomical space to an atlas for which the mathematical computations are referenced in later sections of the article (Right, A-D). Conversion D can also be utilized with computed tomography (CT) or magnetic resonance imaging (MRI) rather than an atlas. Important reference points include the anterior commissure (AC), posterior commissure (PC), and the midline. In frames that operate around an isocenter, which are known as target-centered frames, the head-stage rotates around the frame-space. Three angles can be applied to the head-stage, which are in frame space the ring angle 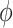 , the arc angle
, the arc angle 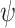 , and the axial angle
, and the axial angle 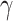 representing rotations about the x-axis, the y-axis, and the z-axis, respectively. In anatomical space,
representing rotations about the x-axis, the y-axis, and the z-axis, respectively. In anatomical space, 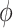 represents the sagittal angle, whereas
represents the sagittal angle, whereas 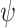 represents the coronal angle. The
represents the coronal angle. The 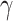 angle can also be used for electrode rotation. In addition, there are movements in the head-stage along the AP, LAT, and Vertical (depth) axes.
angle can also be used for electrode rotation. In addition, there are movements in the head-stage along the AP, LAT, and Vertical (depth) axes.
AP = Antero-Posterior; LAT = Lateral; VERT = Vertical; X = Anatomical space; X' = Frame-based space; X'' = Head-stage space; AC = Anterior Commissure; PC = Posterior Commissure; Midline = Midline structure
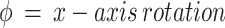
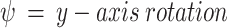
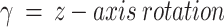
Left Image Pane. Depiction of the Cosman-Roberts-Wells (CRW) Stereotactic Apparatus (Radionics CRW Stereotactic System, Integra LifeSciences Corporation, Plainsboro, New Jersey)
Anatomy-Frame Transformation (A)
A method of rigid coordinate transformation in stereotactic neurosurgery without the need for scaling because the systems are all in units of millimeters (mm), using three points has been previously published, but is worth reviewing [12-15]. In this 3-point transformation (3PT), one can think of the problem in matrix form where 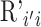 represents an unknown rotational matrix from frame-to-anatomic systems,
represents an unknown rotational matrix from frame-to-anatomic systems, 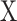 represents the anatomic coordinate space,
represents the anatomic coordinate space, 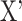 is the frame coordinate space, and
is the frame coordinate space, and 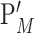 represents a translation (Equation 3). The unknowns are
represents a translation (Equation 3). The unknowns are 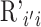 , which represent the unknown elements of the rotational matrix, and
, which represent the unknown elements of the rotational matrix, and 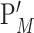 , which represents a translation (Equation 4). Likewise, the reverse coordinate transformation is possible (Equations 5, 6), where (-1) represents the matrix inverse.
, which represents a translation (Equation 4). Likewise, the reverse coordinate transformation is possible (Equations 5, 6), where (-1) represents the matrix inverse.
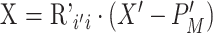 |
 |
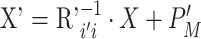 |
 |
Now we identify the components in more detail. First, we consider the stereotactic space relative to the middle of the anterior commissure (AC, 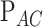 ) and posterior commissure (PC,
) and posterior commissure (PC, 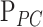 ), which we refer to as
), which we refer to as 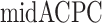 but is alternatively termed as the mid-commissural coordinate system. At this point, the
but is alternatively termed as the mid-commissural coordinate system. At this point, the 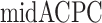 space
space 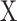 is 0 in the antero-posterior (AP), 0 in the lateral (LAT), and 0 in the vertical (VERT) (
is 0 in the antero-posterior (AP), 0 in the lateral (LAT), and 0 in the vertical (VERT) (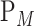 = {0,0,0}). This same point, however, exists in stereotactic frame-space
= {0,0,0}). This same point, however, exists in stereotactic frame-space 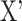 . The
. The 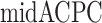 point in frame-space
point in frame-space 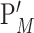 will generally never be {0,0,0}, but rather some other values, by virtue of frame-based coordinates design or misalignment of the two systems during frame placement.
will generally never be {0,0,0}, but rather some other values, by virtue of frame-based coordinates design or misalignment of the two systems during frame placement.
To facilitate subsequent transformation, it is now useful to obtain three points of reference in frame-based space. Generally, these points consist of the AC ( 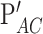 ) , PC (
) , PC ( 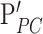 ), and a midpoint (
), and a midpoint ( 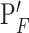 ) above or below a line connecting AC to PC, but not along that line; for instance, the falx cerebri or a midline ventricular structure are good locations to obtain
) above or below a line connecting AC to PC, but not along that line; for instance, the falx cerebri or a midline ventricular structure are good locations to obtain 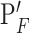 . Using
. Using 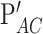 and
and 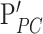 , we can now compute the
, we can now compute the 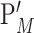 by taking their simple average (Equation 7).
by taking their simple average (Equation 7).
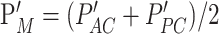 |
To complete the conversion, we must now determine the components of the rotational matrix 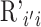 . In
. In 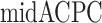 space, the basis unit vector is defined as (1,0,0), (0,1,0), and (0,0,1), which are
space, the basis unit vector is defined as (1,0,0), (0,1,0), and (0,0,1), which are 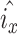 ,
, 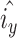 ,
,  respectively. In frame-based space, two vectors,
respectively. In frame-based space, two vectors, 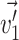 and
and 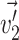 , are created by using the three points,
, are created by using the three points, 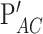 ,
, 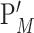 , and
, and 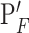 , which will allow us to compute the unit vectors
, which will allow us to compute the unit vectors 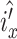 ,
, 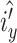 ,
, 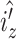 (Equations 8, 9).
(Equations 8, 9).
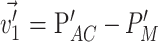 |
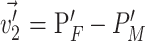 |
Using the two vectors 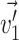 and
and 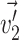 , we can next convert these into unit vectors
, we can next convert these into unit vectors 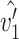 and
and 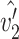 , by dividing them by their own magnitude (Equations 10, 11).
, by dividing them by their own magnitude (Equations 10, 11).
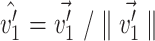 |
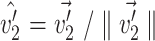 |
The unit vector 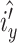 is equivalent to
is equivalent to 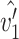 . The result of the cross-product of
. The result of the cross-product of 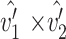 gives
gives 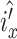 , when divided by the resulting magnitude. Finally, the result of the cross-product
, when divided by the resulting magnitude. Finally, the result of the cross-product 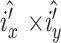 gives
gives 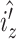 , when divided by the resulting magnitude. These cross-products follow the right-hand rule convention and if the midpoint (
, when divided by the resulting magnitude. These cross-products follow the right-hand rule convention and if the midpoint ( 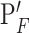 ) is below the AC-PC line, then the cross-product for
) is below the AC-PC line, then the cross-product for 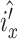 would be
would be 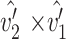 . Next, the dot-products of the unit vectors between the coordinate systems give us the components of the matrix
. Next, the dot-products of the unit vectors between the coordinate systems give us the components of the matrix 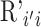 (Equation 12).
(Equation 12).
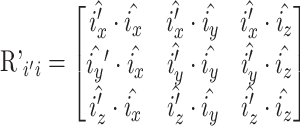 |
Head-Stage-Frame Transformation (B)
Whereas the target is defined by a point, the trajectory through the brain to a target is defined by a direction and a probe-insertion depth. In frame-based stereotaxis, the surgical space has a coordinate basis related to the surgical head-stage  for which the trajectory angles and the electrode/probe depth need to be calculated. This important coordinate system is directly utilized by the surgeon in real-time after the anatomic and frame-based systems are set. We consider here isocentric frame-based systems, which allow rotations around a target (target-centered), but maintain the same radial distance to target. In most frame systems, there is an arc angle
for which the trajectory angles and the electrode/probe depth need to be calculated. This important coordinate system is directly utilized by the surgeon in real-time after the anatomic and frame-based systems are set. We consider here isocentric frame-based systems, which allow rotations around a target (target-centered), but maintain the same radial distance to target. In most frame systems, there is an arc angle 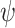 , a ring angle
, a ring angle 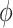 , an "XY Stage", and a depth. The target and frame angles are usually predetermined in planning software, but may be slightly adjusted in real-time to accommodate brain structures, the burr hole, and physiology while the depth is determined manually using fine vernier scales or digitally using microdrives. Generally, the transformation matrix
, an "XY Stage", and a depth. The target and frame angles are usually predetermined in planning software, but may be slightly adjusted in real-time to accommodate brain structures, the burr hole, and physiology while the depth is determined manually using fine vernier scales or digitally using microdrives. Generally, the transformation matrix 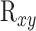 comprises two angles, where
comprises two angles, where 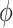 is an angle about the x-axis
is an angle about the x-axis 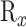 (Equation 13) and
(Equation 13) and 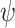 is the angle about the y-axis
is the angle about the y-axis 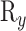 (Equation 14). In some systems, it may be possible to have a rotational matrix
(Equation 14). In some systems, it may be possible to have a rotational matrix 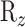 about the z-axis
about the z-axis 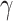 , which can also be used for electrode rotation (Equation 15).
, which can also be used for electrode rotation (Equation 15). 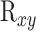 is therefore a combined rotational matrix corresponding to a rotation of
is therefore a combined rotational matrix corresponding to a rotation of 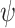 about the AP axis, and a rotation of
about the AP axis, and a rotation of 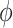 about the LAT axis, and no rotations about the VERT axis (Equation 16). A combination of all three rotational angles into a single matrix
about the LAT axis, and no rotations about the VERT axis (Equation 16). A combination of all three rotational angles into a single matrix 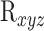 may also be utilized in some systems (Equation 17). Using this transformation, a movement in the direction of AP, LAT, or VERT on the head-stage
may also be utilized in some systems (Equation 17). Using this transformation, a movement in the direction of AP, LAT, or VERT on the head-stage 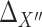 is then converted to a new target frame-based point
is then converted to a new target frame-based point 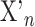 using the current frame-based point
using the current frame-based point 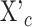 and
and 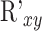 , which has combined rotation angles in the frame-based system (Equation 18). The conversion from
, which has combined rotation angles in the frame-based system (Equation 18). The conversion from 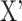 to
to 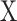 is the same as previously mentioned. Finally, to obtain
is the same as previously mentioned. Finally, to obtain 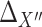 , one can utilize the current frame-based point
, one can utilize the current frame-based point 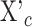 , a desired new target frame-based point
, a desired new target frame-based point 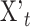 ,
, 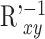 , and rearrange the terms of Equation 18 (Equation 19). Because
, and rearrange the terms of Equation 18 (Equation 19). Because 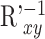 is a combined Euler rotational matrix, it is equivalent to the transpose of
is a combined Euler rotational matrix, it is equivalent to the transpose of 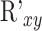 .
.
$$ R_{x} = \begin{bmatrix} 1 & 0 & 0 \\ 0 & cos(\phi) & sin(\phi) \\ 0 & -sin(\phi) & cos(\phi) \end{bmatrix} \tag{13}$$
$$ R_{y} = \begin{bmatrix} cos(\psi) & 0 & sin(\psi) \\ 0 & 1 & 0 \\ -sin(\psi) & 0 & cos(\psi) \end{bmatrix} \tag{14}$$
$$ R_{z} = \begin{bmatrix} cos(\gamma) & sin(\gamma) & 0 \\ -sin(\gamma) & cos(\gamma)& 0 \\ 0 & 0 & 1 \end{bmatrix} \tag{15}$$
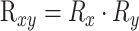 |
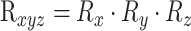 |
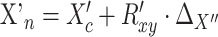 |
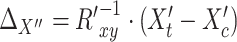 |
It should be noted here that different frame systems have different displacement and angular components. For example, in CRW (Radionics CRW Stereotactic System, Integra LifeSciences Corporation, Plainsboro, New Jersey), LAT to the right is (+), AP towards anterior is (+), and VERT upwards is (+). In Leksell G (Elekta, Stockholm, Sweden), however, LAT to the right is (-), AP anterior is (+), and VERT upwards is (-) (Table 1). It is notable that angular data can also be used in anatomic space and one can also convert to a spherical coordinate system. Further, many of the angles in the systems may be offset from 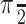 , such as the ring angle in CRW. Also, in CRW, when the
, such as the ring angle in CRW. Also, in CRW, when the 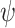 is to the right, the angle is positive, but when it is to the left, it is negative; when
is to the right, the angle is positive, but when it is to the left, it is negative; when 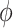 is anterior, the angle is positive, but when it is posterior, it is negative. Another issue is the apparatus assembly, which would flip these angles. Therefore, numerical data for angles in each system require a modification prior to use. Lastly, some frame systems are not target-centered ("isocentric") and other considerations are required.
is anterior, the angle is positive, but when it is posterior, it is negative. Another issue is the apparatus assembly, which would flip these angles. Therefore, numerical data for angles in each system require a modification prior to use. Lastly, some frame systems are not target-centered ("isocentric") and other considerations are required.
Table 1. Direction of displacements changes by target-centered frame-based coordinate system. MidACPC coordinate space axes are similar to CRW, Micromar, FiME, Macom, Zeppelin. However, Leksell G has flipped signs of displacement on the LAT and VERT directions, where the origin is right-posterior-superior. The CRW and Leksell G base rings are fixed inferiorly, whereas the others can be fixed additionally in a more superior position. Lastly, N-localizer dimensions are compared.
*Larger N-localizer for radiosurgery
N-localizer - see references [6-8]
mm = millimeters; AP = Antero-Posterior; LAT = Lateral; VERT = Vertical; H x W = Height and Width; MidACPC = Middle of anterior commissure and posterior commissure
CRW = Cosman-Roberts-Wells stereotactic frame, Radionics CRW Stereotactic System, Integra LifeSciences Corporation, Plainsboro, NJ, USA
Leksell G = Leksell Stereotactic Frame, Elekta, Stockholm, Sweden
Micromar Ind. Com. Ltda, Diadema, Sao Paulo, Brazil
FiMe Medica SRL, Cordoba, Argentina
Macom Instrumental Cirurgico, Sao Paulo, Brazil
Zeppelin Adeor Medical AG, Martinshof 5, D-83626 Valley, Germany
| Offset | |||||||
| Origin-center | Origin-center | Origin-base | N-Localizer | ||||
| Stereotactic Frame | LAT (mm) | AP (mm) | VERT (mm) | Right | Anterior | Superior | [H x W] (mm) |
| CRW | 0 | 0 | -86 | + | + | + | 189 x 140 |
| Leksell G | 100 | 100 | 160 | - | + | - | 120 x 120 |
| Micromar | 0 | 0 | 0 | + | + | + | 140 x 140 & 175 x 140* |
| FiMe | 0 | 0 | 0 | + | + | + | 160 x 160 & 200 x 160* |
| Macom | 0 | 0 | 0 | + | + | + | 140 x 140 |
| Zeppelin | 0 | 0 | 0 | + | + | + | 140 x 140 |
Head-Stage-Anatomy Transformation in Frame-Based Stereotaxy (C)
Multiplying the matrix 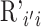 by the matrix
by the matrix 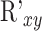 produces the matrix
produces the matrix 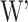 that transforms coordinates from the head-stage to
that transforms coordinates from the head-stage to 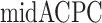 (Equation 20). The complete transformation from a movement on the head-stage
(Equation 20). The complete transformation from a movement on the head-stage 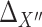 to a new anatomic point
to a new anatomic point 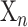 utilizes the current
utilizes the current 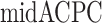 point
point 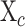 , the
, the  , and
, and 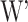 (Equation 21). Finally, to move to a desired
(Equation 21). Finally, to move to a desired 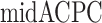 target point
target point 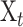 , the head-stage movements can be computed (Equation 22).
, the head-stage movements can be computed (Equation 22).
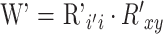 |
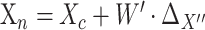 |
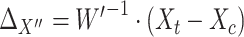 |
Head-Stage-Anatomy Transformation in Frameless Stereotaxy
In some systems, frameless stereotaxy is utilized. In these instances, there is a single anatomical coordinate system, 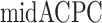 . However, the head-stages may have calibrated displacements
. However, the head-stages may have calibrated displacements 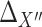 in addition to the optical tracking systems. As some of the systems use all three angles, a combined anatomical rotational matrix
in addition to the optical tracking systems. As some of the systems use all three angles, a combined anatomical rotational matrix 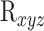 can be utilized, which incorporates a coronal angle
can be utilized, which incorporates a coronal angle 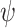 , a sagittal angle
, a sagittal angle 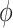 , and an axial angle
, and an axial angle 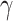 , which are different than the frame-based angles previously mentioned (Equation 17). The complete transformation from a movement on the head-stage
, which are different than the frame-based angles previously mentioned (Equation 17). The complete transformation from a movement on the head-stage 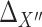 to a new anatomic point
to a new anatomic point 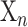 , utilizes the current
, utilizes the current 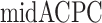 point
point 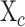 , the
, the 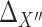 , and
, and 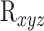 (Equation 23). Finally, to move to a desired
(Equation 23). Finally, to move to a desired 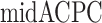 target point
target point 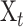 , the head-stage movements can be computed (Equation 24).
, the head-stage movements can be computed (Equation 24).
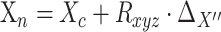 |
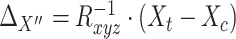 |
Calculation of Points Along a Single Fixed Trajectory
While the above sections provide insight into full computations from head-stage to anatomy, it may be useful to compute points along a single fixed trajectory. For this method, two points are required, an entry 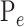 and a target
and a target 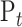 . Also required, the third element is the displacement along the trajectory, which can be treated as a fraction
. Also required, the third element is the displacement along the trajectory, which can be treated as a fraction 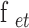 of the vector
of the vector 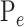 to
to 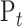 . This fraction is easily derived in stereotactic neurosurgery from the millimeter distances on vernier scales or computerized microdrives from entry to target as the current scalar distance
. This fraction is easily derived in stereotactic neurosurgery from the millimeter distances on vernier scales or computerized microdrives from entry to target as the current scalar distance 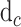 for the numerator, while the denominator is the Euclidean distance
for the numerator, while the denominator is the Euclidean distance 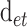 from
from 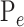 to
to 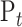 (Equation 25). Finally, a new position
(Equation 25). Finally, a new position 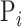 can be computed (Equation 26).
can be computed (Equation 26).
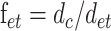 |
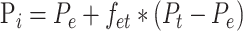 |
Implementation
The following implementation of the discussed mathematics were computed in a Stereotactic Calculator (STACC) and other tools using custom software available at https://app.box.com/s/4zxysoc3bltxvvt4cwcplpbkntoi9cll. A complete plan was utilized from iPlan Stereotaxy 3.0 (Brainlab, Inc., Feldkirchen, Germany) with all the essential coordinates from 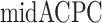 and CRW (Table 2). In this computation, several points are gathered in CRW and
and CRW (Table 2). In this computation, several points are gathered in CRW and 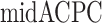 space in order to perform the conversions. A comparison between the coordinate conversion using 3PT versus using 5-points was made. The 5-point conversion utilized a least-squares computation between five pairs of coordinates [9]. Comparing the result with the iPlan target values demonstrates a very slight improvement in the Euclidean error rates when using the 5-point technique as compared to the 3PT. This is likely due to a decimal point round-off error as provided by the planning station.
space in order to perform the conversions. A comparison between the coordinate conversion using 3PT versus using 5-points was made. The 5-point conversion utilized a least-squares computation between five pairs of coordinates [9]. Comparing the result with the iPlan target values demonstrates a very slight improvement in the Euclidean error rates when using the 5-point technique as compared to the 3PT. This is likely due to a decimal point round-off error as provided by the planning station.
Table 2. CRW and MidACPC coordinates obtained from iPlan stereotactic planning software. The Euclidean error in computation of the target point as compared to the point provided by iPlan using 3-points are 0.13 mm and 0.06 mm on the right and left, respectively. The Euclidean error using a 5-point least-squares computation for targets as compared to iPlan are 0.042 mm on both sides. The Euclidean distance from AC to PC was 24.45 mm and half of the value was 12.23 mm, which matched the value provided by the planning system in MidACPC space. Of note, the CRW ring angle is offset by 90 degrees.
iPlan Stereotaxy 3.0 (Brainlab, Inc., Feldkirchen, Germany)
MidACPC = Middle of Anterior Commissure and Posterior Commissure; CRW = Cosman-Roberts-Wells; AP = Antero-Posterior; LAT = Lateral; VERT = Vertical; STN = Subthalamic Nucleus; AC = Anterior Commissure; PC = Posterior Commissure.
| CRW | AP | LAT | VERT | |
| AC CRW | 14.2 | 2.9 | 2.4 | |
| PC CRW | -10.1 | 2.1 | 5 | |
| Mid-Falx CRW | 9.3 | 5.9 | 54.2 | |
| Right CRW Target from Planning Station | -1.8 | 13.6 | 0.3 | |
| Left CRW Target from Planning System | -0.8 | -9.3 | 1.7 | |
| Right CRW Target from Planning Station | -1.8 | 13.6 | 0.3 | |
| Left CRW Target from Planning System | -0.8 | -9.3 | 1.7 | |
| MidACPC | AP | LAT | VERT | |
| AC MidACPC | 12.23 | 0 | 0 | |
| PC MidACPC | -12.23 | 0 | 0 | |
| Mid-Falx MidACPC | 1.98 | 0 | 51.09 | |
| Right STN Target MidACPC | -3 | 11.5 | -3 | |
| Left STN Target | -3 | -11.5 | -3 | |
| Computed Data - 3 Points | AP | LAT | VERT | |
| Right CRW Computed Target | -1.69673 | 13.67524 | 0.334593 | |
| Left CRW Computed Target | -0.79024 | -9.26333 | 1.748831 | |
| Computed Data - 5 Points | AP | LAT | VERT | |
| Right CRW Computed Target | -1.77279 | 13.62566 | 0.318858 | |
| Left CRW Computed Target | -0.77279 | -9.27434 | 1.718858 | |
| CRW Angles | Ring Angle | Arc Angle | Coronal/Lat Angle | Sagittal/AP Angle |
| Right | 51.8 | 20.5 | 20.5 | 38.2 |
| Left | 51.6 | 10.1 | -10.1 | 38.4 |
| MidACPC Angles | Coronal/LAT Angle | Sagittal/AP Angle | ||
| Right | 19.0642 | 32.02104 | ||
| Left | -16.7686 | 32.44535 |
Next, we analyzed the matrix used to compute the conversion between 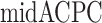 and CRW. The resultant matrices in the forward and reverse match between 3PT and 5-point methods (Table 3). Further, we analyzed the orthonormality of each 1 x 3 vector component, with the expectation that the quantity would be 1. Orthogonality compares the vector components between the first and second rows, the second and third rows, and the first and third rows. In an orthogonal system, one would expect the result to be 0. In Table 1, we can see that we have good normality and orthogonality with all the matrices.
and CRW. The resultant matrices in the forward and reverse match between 3PT and 5-point methods (Table 3). Further, we analyzed the orthonormality of each 1 x 3 vector component, with the expectation that the quantity would be 1. Orthogonality compares the vector components between the first and second rows, the second and third rows, and the first and third rows. In an orthogonal system, one would expect the result to be 0. In Table 1, we can see that we have good normality and orthogonality with all the matrices.
Table 3. Comparison of resultant matrices computed from 3-point change of basis technique and 5-point linear least squares method. The matrices are presented from CRW-to-MidACPC and the reverse as 3 x 4, where the 3 x 3 components are the rotational vectors and the last column is the translation. One can observe that the matrices are very similar and a simple delta, subtraction, between each is presented. Analysis of the 3 x 3 vector component of each matrix by orthonormality and orthogonality are close to 1 and 0, respectively, which would be expected in this affine system.
CRW = Cosman-Roberts-Wells; Mid-ACPC = Middle of Anterior Commissure (AC) and Posterior Commissure (PC); AP = Antero-Posterior; LAT = Lateral; VERT = Vertical.
| CRW- | MidACPC- | ||||||||
| MidACPC | Matrix 1: | CRW | Matrix 2: | ||||||
| Change of Basis | 0.993792 | -0.03941 | 0.104036 | 2.05 | 0.993792 | 0.032717 | -0.106 | -1.726 | |
| 3-points | 0.032717 | 0.997329 | 0.065298 | 2.5 | -0.03941 | 0.997329 | -0.061 | -2.185 | |
| -0.10633 | -0.06149 | 0.992428 | 3.7 | 0.104036 | 0.065298 | 0.9924 | -4.049 | ||
| Matrix 3: | Matrix 4: | ||||||||
| Linear Least Squares | 0.993983 | -0.04348 | 0.104011 | 2.021192 | 0.993471 | 0.036872 | -0.107 | -1.707 | |
| 5-points | 0.033201 | 0.995652 | 0.065853 | 2.472828 | -0.03998 | 0.998827 | -0.062 | -2.161 | |
| -0.10593 | -0.06087 | 0.992991 | 3.680035 | 0.103533 | 0.065161 | 0.9919 | -4.021 | ||
| Delta | -0.00019 | 0.004065 | 2.51E-05 | 0.028808 | 0.000322 | -0.00415 | 0.0002 | -0.018 | |
| -0.00048 | 0.001677 | -0.00056 | 0.027172 | 0.000563 | -0.0015 | 0.0006 | -0.024 | ||
| -0.0004 | -0.00062 | -0.00056 | 0.019965 | 0.000503 | 0.000137 | 0.0005 | -0.028 | ||
| Orthonormality | Orthonormality | Orthonormality | Orthogonality | Orthogonality | Orthogonality | ||||
| AP | LAT | VERT | AP-LAT | LAT-VERT | AP-VERT | ||||
| Matrix 1 | 1 | 1 | 1 | -1.39E-15 | 1.19E-15 | 3.25E-15 | |||
| Matrix 2 | 1 | 1 | 1 | 1.26E-15 | -1.33E-15 | -2.73E-15 | |||
| Matrix 3 | 1.000711 | 0.996762 | 1.000959 | -0.00344 | 0.00127 | 0.000633 | |||
| Matrix 4 | 0.999687 | 1.003105 | 0.998815 | 0.003722 | -0.0006 | -0.00038 |
The added value of all of these computations is the ability to move forward and backwards between all the coordinate systems. One of the most valuable conversions during surgery may be the desire to move from a current point to a planned point. These adjustments on the surgical head-stage need to be computed. In our sample we presume the target point (AP, LAT, VERT) coordinates to be (-3, -11.5, -3), but the current position based on intraoperative imaging is (-4.4, -10.3, -4.2). In order to move the electrode point to the target, one would need to move (0.53, -0.71, 2.02) on the head-stage (Table 4). This information is informative as one may also realize that the radial error is 0.89 mm and the Euclidean error is 2.2 mm. Therefore, the largest displacement error is along the trajectory depth.
Table 4. Evaluation of a planned target coordinate versus a current target coordinate. Using the information, one can evaluate the data in MidACPC space and also compute the added movement required on head-stage if a readjustment to target is desired. To go from the current position to the planned target, a movement 0.52 mm AP, LAT to left 0.71 mm, and VERT upwards 2.02 mm needs to be performed. Notably, the radial error is 0.89 mm, and the Euclidean error is 2.2 mm, which leads to the conclusion that the largest error is in the VERT dimension, but the current position is along the planned trajectory.
AP = Antero-Posterior; LAT = Lateral; VERT = Vertical; MidACPC = Middle of anterior commissure and posterior commissure; CRW = Cosman-Roberts-Wells Stereotactic Frame.
| AP | LAT | VERT | |
| CRW Planned Position | -0.8 | -9.3 | 1.7 |
| MidACPC Planned Position | -3 | -11.5 | -3 |
| CRW Current Position | -2.36385 | -8.20936 | 0.615163 |
| MidACPC Current Position | -4.40882 | -10.3171 | -4.21997 |
| To Get From Current to Planned Target | 0.529056 | -0.71043 | 2.021812 |
| Radial Error from Current to Planned Target | 0.88578 | ||
| Euclidean Error from Current to Planned Target | 2.207336 |
It may also be important to analyze how 2 mm adjustments on the head-stage effect the 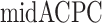 coordinates. Here, we take the target position and make various changes on the head-stage. Each new position is seen as well as their changes to the original position in each dimension. Oftentimes, 2 mm adjustments are made by the surgeon on the head-stage, which are presented in the AP, LAT, and VERT dimension (Table 5). Finally, a new target coordinate is selected and the necessary head-stage operation to reach that coordinate from the original target is computed.
coordinates. Here, we take the target position and make various changes on the head-stage. Each new position is seen as well as their changes to the original position in each dimension. Oftentimes, 2 mm adjustments are made by the surgeon on the head-stage, which are presented in the AP, LAT, and VERT dimension (Table 5). Finally, a new target coordinate is selected and the necessary head-stage operation to reach that coordinate from the original target is computed.
Table 5. AP, LAT, and VERT changes on head-stage coordinates have an effect on the MidACPC coordinates. A head-stage movement is computed by moving +2 mm AP, +2 mm LAT, +2 mm VERT on the left and right trajectories and the resulting MidACPC position is computed. The change in MidACPC from the original target is computed as delta. Finally, a new MidACPC target is chosen on the right and left side and the necessary head-stage adjustments to reach those targets are computed along with the radial error.
AP = Antero-Posterior; LAT = Lateral; VERT = Vertical; MidACPC = Middle of Anterior Commissure and Posterior Commissure; mm = millimeters.
| Head-Stage | AP | LAT | VERT | Delta | ||
| Right Target | -3 | 11.5 | -3 | AP | LAT | VERT |
| +2 mm AP | -1.31 | 11.51 | -4.06 | 1.69 | 0.01 | -1.06 |
| +2 mm LAT | -3.31 | 13.42 | -3.47 | -0.31 | 1.92 | -0.47 |
| +2 mm VERT | -1.98 | 12.06 | -1.37 | 1.02 | 0.56 | 1.63 |
| New Target | -1 | 11.5 | -3 | 1.69347 | -0.3106348 | 1.017677 |
| Radial Error | 1.721724 | |||||
| AP | LAT | VERT | Delta | |||
| Left Target | -3 | -11.5 | -3 | AP | LAT | VERT |
| +2 mm AP | -1.31 | -11.49 | -4.07 | 1.69 | 0.01 | -1.07 |
| +2 mm LAT | -2.75 | -9.56 | -2.58 | 0.25 | 1.94 | 0.42 |
| +2 mm VERT | -1.96 | -11.99 | -1.36 | 1.04 | -0.49 | 1.64 |
| New Target | -3 | -13.5 | -3 | -0.01461 | -1.9382602 | 0.492883 |
| Radial Error | 1.938315 |
Anatomy-Atlas Navigation (D)
An important aid is obtained by mapping a point to an atlas. While simple navigation of a single slice on an atlas is straightforward, we discuss the more general task of navigating all the atlas slices relative to a point. Treating the electrode position as a point ( 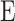 ) and the plane as a slice from an atlas, one can then calculate the closest plane using a Point-to-Plane computation. This is done by picking three points in the plane,
) and the plane as a slice from an atlas, one can then calculate the closest plane using a Point-to-Plane computation. This is done by picking three points in the plane, 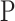 ,
, 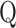 , and
, and 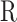 . Two vectors are then created
. Two vectors are then created 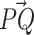 and
and 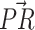 . The normal
. The normal 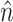 of the plane is then
of the plane is then 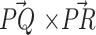 , where
, where 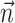 has three components,
has three components, 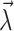 ,
, 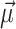 ,
, 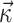 and
and 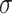 can then be solved for each plane equation of the atlas sections (Equation 27). The distance
can then be solved for each plane equation of the atlas sections (Equation 27). The distance 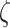 from point to the plane is computed, which gives the closest atlas slice (Equation 28). Then the closest point in the plane
from point to the plane is computed, which gives the closest atlas slice (Equation 28). Then the closest point in the plane 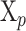 to
to 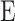 can be computed by first solving for the unknown parameter
can be computed by first solving for the unknown parameter 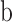 using a known point in the plane, such as
using a known point in the plane, such as 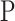 (Equation 29). Then using
(Equation 29). Then using 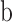 , we can then solve for the final values
, we can then solve for the final values 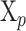 (Equations 30-32). The next task is mapping
(Equations 30-32). The next task is mapping 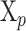 to the screen coordinates. This can be accomplished using 3 pairs of coordinates of the atlas in 3D-2D (three dimensional - two dimensional) using similar mathematics previously published by Brown, where a conversion matrix
to the screen coordinates. This can be accomplished using 3 pairs of coordinates of the atlas in 3D-2D (three dimensional - two dimensional) using similar mathematics previously published by Brown, where a conversion matrix 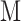 is solved and then
is solved and then 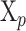 is applied to the inverse matrix to identify the screen coordinates
is applied to the inverse matrix to identify the screen coordinates 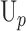 and
and 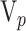 (Equations 33, 34) [16]. In some instances, the determinant of
(Equations 33, 34) [16]. In some instances, the determinant of 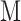 can be zero when using an atlas section with zeros in a dimension, making the matrix not invertible for Equation 34; in this instance, one can alternatively utilize a 2D or alternative approach. The screen coordinates are then placed on the Morel atlas (Figure 2) [17]. Finally, one can use the matrix to compute any point
can be zero when using an atlas section with zeros in a dimension, making the matrix not invertible for Equation 34; in this instance, one can alternatively utilize a 2D or alternative approach. The screen coordinates are then placed on the Morel atlas (Figure 2) [17]. Finally, one can use the matrix to compute any point 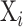 from any screen coordinates
from any screen coordinates 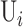 and
and 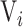 (Equation 35). Notably, this same technique can be utilized with CT or MRI slices.
(Equation 35). Notably, this same technique can be utilized with CT or MRI slices.
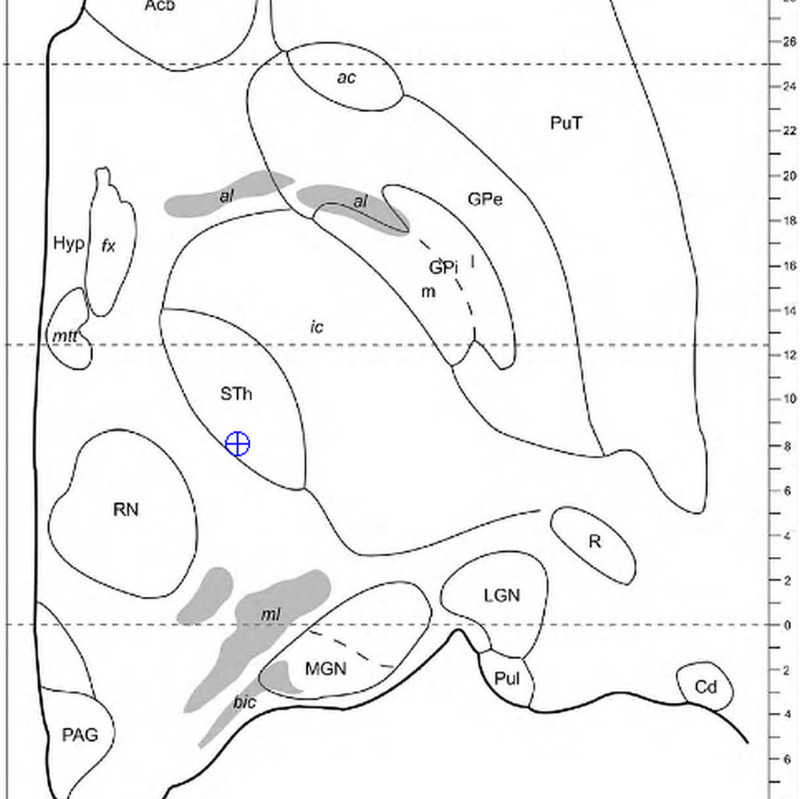
Figure 2. Mapping a 3D intraoperative coordinate to a point on the closest atlas section from a series. Here, we use the current point illustrated in Table 4, {-4.4, -10.3, -4.2}. Using the method, the closest axial slice of the Morel Atlas maps to {-4.4, -10.3, -4.5}. The position of the electrode tip is observed within the margins of the subthalamic nucleus (STN).
All Coordinates are given as {AP, LAT, VERT}
3D = Three Dimensional
Morel Atlas: please refer to reference [17].
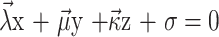 |
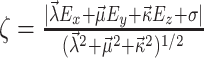 |
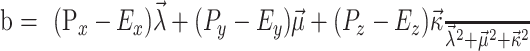 |
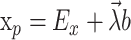 |
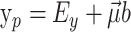 |
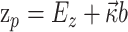 |
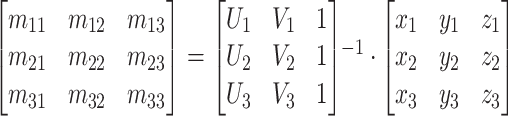 |
 |
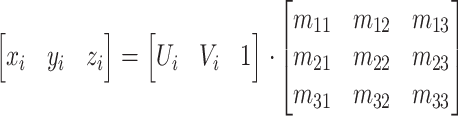 |
If a trajectory is known one can alternatively utilize a line-plane intersection to identify the point in a given atlas section. First, we consider the atlas section of interest, which represents a plane (Equation 27). Then the trajectory can be represented as parametric line equations (Equations 37-39). The point in the plane can then be computed with a line-plane intersection solution (Equations 40-42). Finally, the point in the atlas plane  can then be mapped to the screen coordinates using Equations 33 and 34.
can then be mapped to the screen coordinates using Equations 33 and 34.
 |
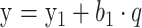 |
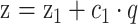 |
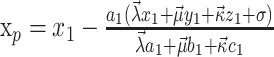 |
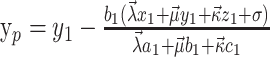 |
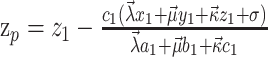 |
Because we can now map points along a trajectory, it may be useful to compute 3D (three-dimensional) kinesthetic points, which are points acquired by along a depth during intraoperative neurophysiological mapping. Then, using stereotactic intraoperative localization (STiL), these kinesthetic points can be mapped directly to an X-ray image, which can be valuable for deep brain stimulation (DBS) programming (Figures 3, 4) [18]. These same kinesthetics can also be mapped to the atlas. Here, we have compiled 2137 individual kinesthetic points matched to the closest point on the atlas slices for the ventral intermediate nucleus of the thalamus (VIM), the globus pallidus interna (GPi), and the subthalamic nucleus (STN) (Figure 5). In VIM, one can observe that arm and leg kinesthetics appear to separate, but in GPi and STN they are largely overlapping.
Figure 3. AP X-ray image showing kinesthetics acquired in 3D space and mapped to the 2D image on the left side. Note the slight disparity between MER tract, with which the kinesthetic points were acquired, and the final DBS electrode position.
AP = Antero-Posterior; AC = Anterior Commissure; PC = Posterior Commissure; STN = Subthalamic Nucleus; DBS = Deep Brain Stimulation; MER = Microelectrode recording; 3D = Three Dimensional; 2D = Two Dimensional.
Figure 4. LAT X-ray image showing kinesthetics acquired in 3D space and mapped to the 2D image on the left side. Note the slight disparity between the left MER tract, with which the kinesthetic points were acquired, and the final electrode position.
AP = Antero-Posterior; AC = Anterior Commissure; PC = Posterior Commissure; STN = Subthalamic Nucleus; DBS = Deep Brain Stimulation; MER = Microelectrode recording; 3D = Three Dimensional; 2D = Two Dimensional.
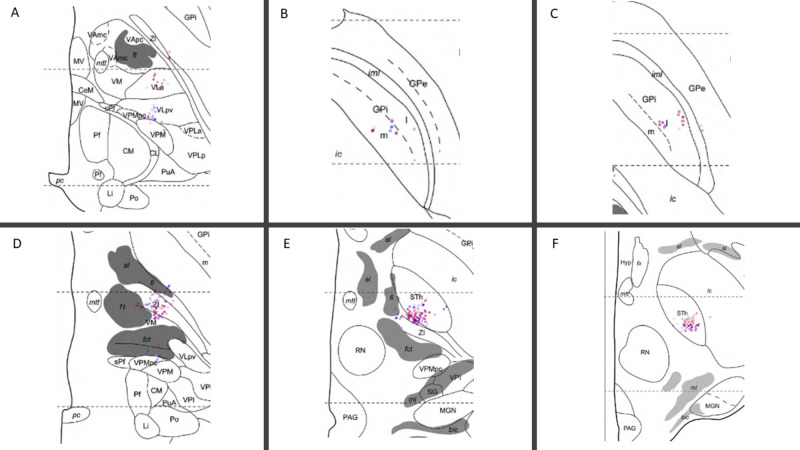
Figure 5. Intraoperative kinesthetic responses were computed in three-dimensional (3D) coordinates along trajectories and then mapped to points on the closest axial Morel Atlas section. These responses were computed during deep brain stimulation (DBS) targeting for ventral intermediate nucleus of the thalamus (VIM), the globus pallidus interna (GPi), and the subthalamic nucleus (STN) regions. Six of twenty-six atlas sections are displayed with the highest density of kinesthetic points acquired during VIM (A), GPi (B, C), and STN (D, E, F) targeting. Lower extremity responses are red and upper extremity responses are blue. In total, these compute to 1336 upper extremity kinesthetics, and 990 lower extremity kinesthetics.
VIM = Ventral Intermediate Nucleus of the Thalamus (A)
GPi = Globus Pallidus Interna (B, C)
STN = Subthalamic Nucleus (D, E, F)
3D = Three Dimensional
Morel Atlas: please refer to reference [17]
Section A: dorsal 0.9 millimeters
Section B: ventral 3.6 millimeters
Section C: ventral 0.9 millimeters
Section D: ventral 0.9 millimeters
Section E: ventral 2.7 millimeters
Section F: ventral 4.5 millimeters
Another approach may normalize an atlas coordinates to anatomical coordinates, or vice versa. Using the above 3PT method, information that can be used is the distance from AC to PC, which is often known in a stereotactic atlas as well. Note that for the 453-patient sample in our series, the average AC-PC distance was 25.016 mm +/- 1.42 mm. While this normalization is only in the AP axis, further sampling of comparable points on the patient MRI and the atlas may theoretically render an improved conversion matrix. While many complex atlas-to-brain normalizations exist, this method is simpler but yet effective for basal ganglia targeting. Normalization in this single AP axis provides significant improvement in registration of the Montreal Neurological Institute (MNI) atlas without any added complexity (Figure 6) [19]. Kinesthetic points can then be placed directly into these images (Figure 7).
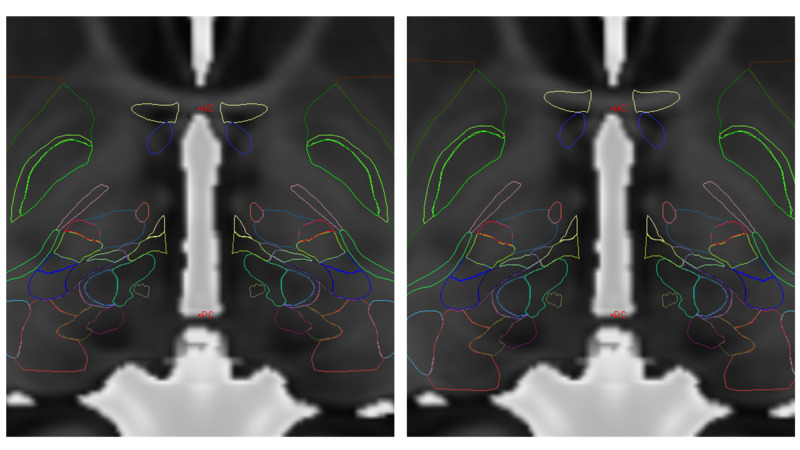
Figure 6. Simple scaling of AP dimension using AC-PC distance in MRI and atlas. The left image has no scaling, but is anchored on MidACPC. The right image has the AP scaling correction on this MNI Atlas over the MRI. This simple method is effective for basal ganglia targeting.
MNI = Montreal Neurological Institute; AC = Anterior Commissure; PC = Posterior Commissure; MRI = Magnetic Resonance Imaging; AP = Antero-Posterior; MidACPC = Middle of Anterior Commissure and Posterior Commissure
MNI Atlas = Montreal Neurological Institute Atlas, please refer to reference [19].
Figure provided by Mevis Stereotactic Planning System (MNPS)
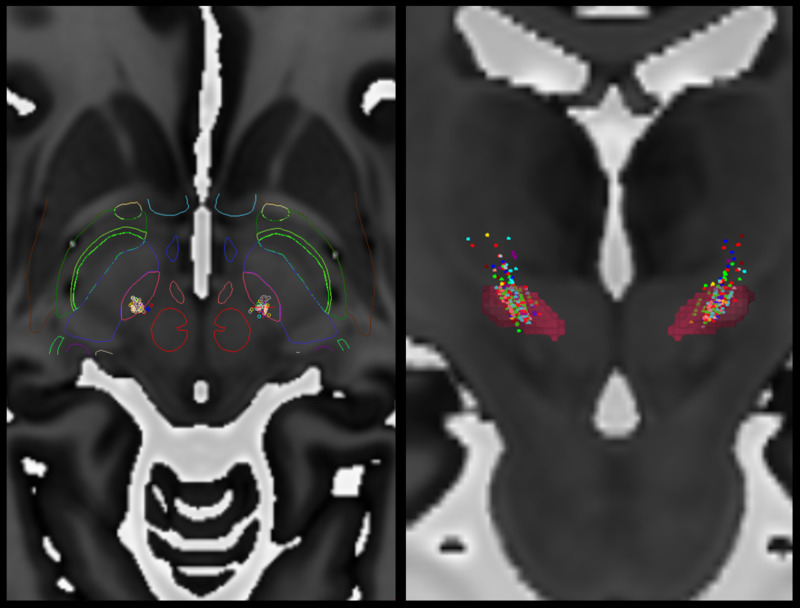
Figure 7. Image of 920 kinesthetic points mapped to MNI Atlas. These kinesthetic points are computed during neurophysiological testing. Axial image with superimposed outlines of subcortical structures (Left). Coronal image shows kinesthetic points along with subthalamic nucleus (red) volume rendering (Right). Note the high density of kinesthetic points in the region of the subthalamic nucleus. In this instance, color coding is arbitrary.
MNI Atlas = Montreal Neurological Institute Atlas, please refer to reference [19]
Figure provided by Mevis Stereotactic Planning System (MNPS)
Discussion
Stereotactic operations in neurosurgery have become commonplace in many institutions for the treatment of movement disorders, epilepsy, and psychiatric disease. Essential to these operations is the mathematics related to Cartesian coordinate systems in Euclidean space. Stereotactic planning software generally computes target information for the surgeon, but is often insufficient for guiding decisions during the procedure. Therefore, keen understanding of coordinate systems is critical to enable the neurosurgeon to guide an electrode to the desired location. Clearly, precise targeting can affect patient outcomes [20]. For this reason, we have consolidated a complete, usable, and effective framework that navigates the various coordinate systems with examples. These methods can be implemented during surgical procedures or basic science laboratories.
Herein, we have described coordinate transformations utilized for anatomical space, frame-based space, surgical head-stage space, and atlas space. Importantly, surgical head-stage movement as it relates to the target is required by the surgeon. Further, we have demonstrated previously the ability of using simple biplanar X-ray to achieve real-time sub-millimeter localization of the position of an electrode [18]. Combining these techniques can allow a surgeon to make real-time decisions on electrode position, compute the location where important neurophysiological data are obtained, and assimilate this information into longitudinal data. These techniques may be especially important in some centers in view of the increased occurrence of asleep DBS, the reduction of intraoperative neurophysiological assessments, and the attendant omission of stimulation mapping. Since these asleep surgeries are guided purely by anatomical planning, the assurance of positioning, the comparison to prior data, and the ability to microposition (<2 mm) electrodes at the level of the head-stage may optimize the outcome. Moreover, in standard awake DBS, these techniques can also be utilized and guided by intraoperative mapping. Implementation of these principles to stereotactic neurosurgery may enhance our understanding of the normal and the pathological brain as well as the treatment of various diseases.
Conclusions
Stereotactic neurosurgery frequently involves numerous coordinate systems. An understanding of these coordinate systems may be important for real-time surgical decisions and precise targeting. In frame-based stereotaxis, converting coordinates between different systems is important but beyond the initial stereotactic plan, these techniques are often not provided by the stereotactic planning software. Therefore, we provide a common framework with which these important techniques can be understood and implemented.
Acknowledgments
Maureen Sedrak, MHA; Nathanael Sedrak; Isabelle Sedrak; Gary Heit, PhD, MD; Eric Sabelman, PhD; Bruce Hill, PhD; Patrick Pezeshkian, MD; Ivan Bernstein, PA-C; Diana Bruce, PA-C, DMS
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared financial relationships, which are detailed in the next section.
Armando Bouza declare(s) Owner of company from MNPS. 50% owner of MNPS Stereotactic Software
Human Ethics
Consent was obtained by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Early history of the stereotactic apparatus in neurosurgery. Rahman M, Murad G, Mocco J. Neurosurg Focus. 2009;27:12. doi: 10.3171/2009.7.FOCUS09118. [DOI] [PubMed] [Google Scholar]
- 2.The structure and functions of the cerebellum examined by a new method. By Sir Victor Horsley, FRS, FRCS and R.H. Clarke, MA, MB. Brain 1908: 31; 45-124. Compston A. Brain. 2007;130:1449–1452. [Google Scholar]
- 3.Stereotaxic apparatus for operations on the human brain. Spiegel E, Wycis H, Marks M, Lee AJ. Science. 1947;106:349–350. doi: 10.1126/science.106.2754.349. [DOI] [PubMed] [Google Scholar]
- 4.The origins of human functional stereotaxis: a reappraisal. Rzesnitzek L, Hariz M, Krauss J. Stereotact Funct Neurosurg. 2019;97:49–54. doi: 10.1159/000496157. [DOI] [PubMed] [Google Scholar]
- 5.The history of stereotactic radiosurgery and radiotherapy. Lasak J, Gorecki J. Otolaryngol Clin North Am. 2009;42:593–599. doi: 10.1016/j.otc.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 6.A computerized tomography-computer graphics approach to stereotaxic localization. Brown R. J Neurosurg. 1979;50:715–720. doi: 10.3171/jns.1979.50.6.0715. [DOI] [PubMed] [Google Scholar]
- 7.A stereotactic head frame for use with CT body scanners. Brown R. Invest Radiol. 1979;14:300–304. doi: 10.1097/00004424-197907000-00006. [DOI] [PubMed] [Google Scholar]
- 8.The invention and early history of the N-localizer for stereotactic neurosurgery. Brown R, Nelson J. Cureus. 2016;8:642. doi: 10.7759/cureus.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Automatic calculation of a transformation matrix between two frames. Cashbaugh J, Kitts C. IEEE Access. 2018;6:9614–9622. [Google Scholar]
- 10.Golub G, Van Loan C. Baltimore and London: Johns Hopkins University Press; 1996. Matrix Computations, Third Edition. [Google Scholar]
- 11.Closed-form solution of absolute orientation using unit quaternions. Horn B. J Opt Soc Am. 1987;4:629–642. [Google Scholar]
- 12.An algorithm for the empirical determination of intracranial stereotactic targets. Ott K. Stereotact Funct Neurosurg. 1998;71:29–35. doi: 10.1159/000029644. [DOI] [PubMed] [Google Scholar]
- 13.Mathematical theory of stereotactic coordinate transformation: elimination of rotational targeting error by addition of a third reference point. Taub E. J Neurosurg. 2000;92:884–888. doi: 10.3171/jns.2000.92.5.0884. [DOI] [PubMed] [Google Scholar]
- 14.Trigonometric method of computing the coordinates of invisible targets in functional neurosurgery. Ammannati F, Bordi L, Mennonna P, Gronchi P. Stereotact Funct Neurosurg. 1999;72:70–72. doi: 10.1159/000029673. [DOI] [PubMed] [Google Scholar]
- 15.Alignment correction algorithm for transformation of stereotactic anterior commissure/posterior commissure-based coordinates into frame coordinates for image-guided functional neurosurgery. Krauss J, King D, Grossman R. Neurosurgery. 1998;42:806–811. doi: 10.1097/00006123-199804000-00070. [DOI] [PubMed] [Google Scholar]
- 16.The mathematics of four or more N-localizers for stereotactic neurosurgery. Brown R. Cureus. 2015;7:349. doi: 10.7759/cureus.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morel A. Boca Raton: CRC Press; 2007. Stereotactic Atlas of the Human Thalamus and Basal Ganglia. [Google Scholar]
- 18.Biplanar X-ray methods for stereotactic intraoperative localization in deep brain stimulation surgery. Sedrak M, Sabelman E, Pezeshkian P, et al. Operative Neurosurg. 2019;[Published online ahead of print]:0. doi: 10.1093/ons/opz397. [DOI] [PubMed] [Google Scholar]
- 19.Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. Fonov V, Evans A, McKinstry R, Almli CR, Collins DL. NeuroImage. 2009;47:102. [Google Scholar]
- 20.Review on factors affecting targeting accuracy of deep brain stimulation electrode implantation between 2001 and 2015. Li Z, Zhang J, Ye Y, et al. Stereotact Funct Neurosurg. 2016;94:351–362. doi: 10.1159/000449206. [DOI] [PubMed] [Google Scholar]