Abstract
Background:
Eighty percent of premature mortality from cardiovascular disease occurs in low-and middle-income countries. Hypertension, diabetes, and smoking are the top risk factors causing this disease burden.
Objectives:
The study aimed to test the hypothesis that utilizing community health workers (CHWs) to manage hypertension, diabetes and smoking in an integrated manner would lead to improved control of these conditions.
Methods:
This was a 2-year cluster (n = 12) randomized controlled trial of 3,556 adults (35 to 70 years of age) in a single town in India, who were screened at home for hypertension, diabetes, and smoking. Of these adults, 1,242 (35%) had at least 1 risk factor (hypertension = 650, diabetes = 317, smoking = 500) and were enrolled in the study. The intervention group had behavioral change communication through regular home visits from community health workers. The control group received usual care in the community. The primary outcomes were changes in systolic blood pressure, fasting blood glucose, and average number of cigarettes/bidis smoked daily among individuals with respective risk factors.
Results:
The mean ± SD change in systolic blood pressure at 2 years was −12.2 ± 19.5 mm Hg in the intervention group as compared with −6.4 ± 26.1 mm Hg in the control group, resulting in an adjusted difference of −8.9 mm Hg (95% confidence interval [CI]: −3.5 to −14.4 mm Hg; p = 0.001). The change in fasting blood glucose was −43.0 ± 83.5 mg/dl in the intervention group and −16.3 ± 77.2 mg/dl in the control group, leading to an adjusted difference of −21.3 mg/dl (95% CI: 18.4 to −61 mg/dl; p = 0.29). The change in mean number of cigarettes/bidis smoked was nonsignificant at +0.2 cigarettes/bidis (95% CI: 5.6 to −5.2 cigarettes/bidis; p = 0.93).
Conclusions:
A population-based strategy of integrated risk factor management through community health workers led to improved systolic blood pressure in hypertension, an inconclusive effect on fasting blood glucose in diabetes, and no demonstrable effect on smoking. (Study of a Community-Based Approach to Control Cardiovascular Risk Factors in India [SEHAT]; NCT02115711).
Noncommunicable diseases (NCDs) are the leading cause of premature deaths worldwide, with 80% of premature mortality from NCDs occurring in low-and middle-income countries (LMICs) [1]. In response to the high burden of NCDs worldwide, the World Health Organization (WHO) in May 2012 adopted a global target of 25% reduction in premature mortality from NCDs by 2025 [2]. Subsequently, a set of 9 voluntary targets were developed, aimed at the 4 principal causes of deaths from NCDs: cardiovascular disease (CVD), cancer, chronic lung disease, and diabetes. Eight of these 9 targets pertain to cardiovascular risk factors, with reduction in harmful alcohol intake being the sole exception. These 8 targets are focused on hypertension, diabetes and smoking along with their precursors such as high salt intake, physical inactivity, and obesity [2].
In India, at the rate of deaths in 2014, the probability of a person ≥15 years of age dying before 70 years of age is a remarkable 50% for men and 40% for women, with vascular diseases contributing the largest fraction to this burden [3]. Of the 321 million years of life lost globally to CVD in 2015, 25% (81 million) were lost in South Asia alone [4]. Given that hypertension, diabetes and smoking are the top modifiable risk factors causing this disease burden, successfully targeting them can cause a substantial decrease in premature mortality [5]. In India, control rates of hypertension and diabetes are abysmal, and smoking cessation services are not widely available [6,7]. A major cause for this is a health care system that is designed to provide episodic care for acute disease conditions, and has not been able to adequately adapt to provide longitudinal care for chronic conditions. In this system, the adverse effects of poor health literacy and poverty get magnified, resulting in poor risk factor control. Although there have been recent steps to address NCDs though a national comprehensive primary care program, its effects on outcomes are currently uncertain [8].
Community health workers (CHWs) are lay individuals who undergo brief periods of training, usually aimed at a specific health task or disease. Unlike other nonphysician health workers such as nurses or pharmacists, CHWs typically do not have formal health care degrees and work in the community setting, outside the traditional health care system [9]. Historically, CHWs have played a pivotal role in progress toward achieving the Millennium Development Goals related to health: improvement in maternal health, reduction in infant mortality, and reduction of the burden of human immunodeficiency virus and acquired immunodeficiency syndrome [10]. Given their effectiveness in advancing the Millennium Development Goals, CHWs could play a prominent role in making progress toward the WHO goal of 25% reduction in premature mortality from NCDs by 2025 [11]. Toward that end, CHWs have been shown to successfully execute community-based cardiovascular risk factor screening in LMICs [12]. Over the last decade, they have also been shown to improve control of single cardiovascular risk factors in LMICs, usually by providing longitudinal follow-up and improving individual health behaviors [9,13]. However, few such studies have employed a robust randomized controlled trial design, and all of them have focused on a single cardiovascular risk factor [14,15]. Moreover, some well-designed studies have also found neutral results for blood pressure control using CHWs, possibly as a result of specific program features [16]. Given that cardiovascular risk factors are often coexistent and interrelated, implementing vertical programs for each cardiovascular risk factor is neither feasible nor advisable. It is likely that the full potential of cardiovascular risk factor control will be better realized by targeting multiple risk factors (hypertension, diabetes, and smoking) in an integrated manner. Moreover, although incorporating care for single cardiovascular risk factors into existing CHW programs may be feasible, it is unclear how multiple cardiovascular risk factors can be successfully integrated—either into existing CHW programs or in new CHW programs. To our knowledge, no previous trial has tested this idea, though an ongoing study is evaluating a related approach to integrated cardiovascular risk factor management in India [17].
We therefore designed a trial, Project SEHAT (Study to Enhance Heart Associated Treatments), to test the hypothesis that use of CHWs to manage hypertension, diabetes, and smoking in an integrated manner would result in improved control of these risk factors, compared with a control group. A cluster randomized design was chosen to minimize contamination of the control group by the behavioral interventions. The main outcomes were at the individual level.
METHODS
The study protocol received approval from the Institutional Review Board at University Hospitals/Case Western Reserve University and Society for the Promotion of Ethical Clinical Trials, an independent review committee in India. All study participants provided written, informed consent. For illiterate participants, verbal consent was obtained in the presence of a witness. The study is registered on ClinicalTrials.gov (NCT02115711).
Study design and setting
This was a parallel, 1:1 cluster randomized controlled trial conducted at a single site in Dalkhola, India, from May 2014 to February 2017. The trial ended after completing the prespecified 2 years of assigned interventions. Dalkhola is a semiurban town in the state of West Bengal, with an approximate population of 20,000 individuals and an agriculture-based economy. The town is located in the district of Uttar Dinajpur, which in the 2011 census had a literacy rate of 60%, well below the national average of 74% [18]. The town has a single government primary health center, with no secondary or tertiary health care facility. In addition to the single primary health center, health care is provided by private practitioners. The study coordinating center was at University Hospitals, Case Western Reserve University, in Cleveland, Ohio. Study data were entered into REDCap in Dalkhola, India, and was analyzed in Cleveland, Ohio [19].
Participants
Eligibility criteria for screening included age between 35 and 70 years and permanent residence in the area allotted to the given CHW. Participants were screened in their homes by CHWs and field workers using automated blood pressure monitors (Omron HEM 8711 [Omron Automation Pvt. Ltd., Gurgaon, India]) and glucometers (Accuchek Performa Nano [Roche Diabetes Care India Pvt. Ltd, Mumbai India]). Of the screened individuals, those with ≥ 1 cardiovascular risk factor (either one of hypertension [blood pressure ≥ 140/90 mm Hg on 2 separate days or on antihypertensive medication], diabetes [fasting blood glucose (FBG) ≥ 126 mg/dl on 2 separate days or on antihyperglycemic medication], or current daily smoker [self-reported]) were enrolled in the study.
Exclusion criteria included individuals who were bed bound; were deemed unable to participate in the intervention due to significant disabilities such as deafness, blindness, or intellectual disability; and had stayed <6 months in the study area before the screening date. Baseline charac-teristics of the study participants is shown in Table 1.
TABLE 1.
Baseline characteristics of the study population
| Intervention (n = 736) | Control (n = 506) | p Value | |
|---|---|---|---|
| Risk factors | |||
| 1 | 598 (81.3) | 436 (86.2) | 0.31 |
| 2 | 125 (17.0) | 66 (13.0) | |
| 3 | 13 (1.8) | 4 (0.8) | |
| Language | |||
| Bengali | 653 (88.8) | 435 (86.1) | 0.72 |
| Hindi | 82 (11.2) | 70 (13.9) | |
| Male | 440 (59.8) | 321 (63.6) | 0.46 |
| Age, yrs | 52.1 ± 9.6 | 51.7 ± 9.8 | 0.6 |
| Education | |||
| None | 239 (32.5) | 321 (63.4) | 0.35 |
| 1–5 yrs | 132 (17.9) | 58 (11.5) | |
| 6–8 yrs | 123 (16.7) | 46 (9.1) | |
| 9–10 yrs | 139 (18.9) | 57 (11.3) | |
| More than 10 yrs | 103 (8.3) | 24 (4.7) | |
| Community | |||
| Bengali Hindu | 577 (78.4) | 128 (25.3) | <0.001 |
| Bengali Muslim | 46 (6.3) | 224 (44.3) | |
| Marwari | 27 (3.7) | 3 (0.59) | |
| Other | 84 (11.4) | 150 (29.6) | |
| Refused | 2 (0.27) | 1 (0.2) | |
| Marital status | |||
| Married | 636 (86.4) | 437 (86.4) | 0.99 |
| Not married | 100 (13.6) | 69 (13.6) | |
| Work status | |||
| Works | 388 (52.7) | 266 (52.7) | 0.99 |
| Income* | |||
| ≤25,000 INR | 136 (18.5) | 78 (15.5) | 0.52 |
| 26,000–50,000 INR | 187 (25.4) | 175 (34.7) | |
| >50,000–100,000 INR | 212 (28.8) | 175 (34.7) | |
| >100,000–200,000 INR | 131 (17.8) | 32 (6.3) | |
| >200,000 INR | 59 (8.0) | 6 (1.2) | |
| Refused | 11 (1.5) | 39 (7.7) | |
| For people with hypertension | |||
| Systolic blood pressure, mm Hg | 155.0 ± 18.6 | 160.0 ± 21.0 | 0.11 |
| Diastolic blood pressure, mm Hg | 92 | 92 | 0.35 |
| Control rate of hypertension, % | 18.8 | 14.4 | 0.40 |
| For people with diabetes | |||
| Fasting blood glucose, mg/dl | 192.0 ± 7.3 | 181.0 ± 71.0 | 0.56 |
| Fasting blood glucose, mg/dl Control rate of diabetes, % |
6.2 | 14.5 | 0.21 |
| For people using tobacco | |||
| Cigarettes/bidis smoked | 11.0 ± 7.4 | 10.6 ± 7.5 | |
| Weight, kg | 56.5 ± 11.6 | 57.3 ± 11.8 | 0.38 |
| Waist, cm | 84.8 ± 12.8 | 83.1 ± 12.7 | 0.44 |
Values are n (%) or mean ± SD, unless otherwise indicated.
USD$1 = Rs. 65. INR = Indian rupees.
Outcomes
The primary outcomes were change in SBP from visit 1 to post-intervention among people with hypertension, change in FBG from visit 1 to post-intervention among people with diabetes, and change in self-reported mean number of daily cigarettes/bidis smoked from visit 1 to post-intervention among individuals who smoked cigarettes/bidis. Bidis are a form of hand-rolled cigarettes that are commonly used in India.
Secondary outcomes included mean reduction in diastolic blood pressure among participants with hypertension, control rates of hypertension and diabetes, proportion of participants with diabetes who were on a statin or aspirin, proportion of participants with hyper-tension and tobacco use on a statin, proportion of tobacco users who quit smoking, and mean reduction in weight and waist circumference for those who were overweight or had increased waist size at baseline, respectively. Control of hypertension was defined as blood pressure <140/90 mm Hg whereas control of diabetes was defined as FBG <126 mg/dl. All outcomes were measured 2 years after the start of the intervention.
Sample size
The sample size calculations accounted for lack of independence in observations due to the clustered study design, by multiplying the standard sample size calculation by the design effect, {1 + [(average cluster size−1) × intracluster correlation coefficient [ICC]}. Based on previous studies, we used various estimates of ICC as detailed in Table 2. We decided to use more conservative estimates of ICC for FBG and number of cigarettes/bidis smoked, as there was a paucity of reliable data on ICC for our study context.
TABLE 2.
Sample size calculations
| Variable | Assumed SD | ICC | MDD | Sample Size | Adjusted Sample Size | Prevalence in Population (%) | Screening Sample Size |
|---|---|---|---|---|---|---|---|
| SBP | 16 | 0.004 | 5 | 228 | 326 | 20 | 3,258 |
| 0.052 | 9 | 126 | 180 | 1,800 | |||
| FBG | 26 | 0.02 | 12 | 162 | 232 | 13 | 3,570 |
| 0.07 | 18 | 96 | 138 | 2,124 | |||
| Cigarettes/bidis per day | 10 | 0.01 | 4 | 150 | 215 | 15 | 2,858 |
| 0.05 | 6 | 96 | 138 | 1,828 |
FBG = fasting blood glucose; ICC = intracluster correlation coefficient; MDD = minimum detectable difference; SBP = systolic blood pressure.
The minimum detectable difference chosen was 7 ± 2 mm Hg for SBP, 15 ± 3 mg/dl for FBG, and 5 ± 1 for number of cigarette/bidis smoked per day. Based on these ranges of ICCs and MDD, we calculated the range of sample sizes needed for a study with a power of 80%, alpha of 0.05, attrition of 30%, and number of clusters per group as 6. We fixed the number of cluster size for each group at 6 based on our financial and technical capabilities. Based on these assumptions, the sample sizes were calculated and are displayed in Table 2. We used conservative estimates of prevalence of diabetes, hyper-tension, and smoking to determine the population required to be screened. We then verified that 6 was an adequate number of clusters based on the following formula: k > Ni ● ρ, where k is the minimum number of clusters, Ni is the number of individuals required under individual randomization, and ρ is the ICC [20]. Thus, we decided to use a screening sample size of 3,570, the largest required sample size for screening among all the possible combinations.
Randomization
Dalkhola was divided into 36 geographic clusters to ensure approximately 300 screening eligible individuals in each cluster. Using a simple random sampling technique, authors A.K. and R.P. selected 12 of 36 clusters equally divided between the intervention and control groups. After randomization, one control cluster had to be changed, as it was adjacent to an intervention cluster, which was not allowed per protocol to minimize contamination. After clusters were finalized, one CHW was recruited in each of the intervention clusters, resulting in a total of 6 CHWs. CHWs and field workers enrolled participants and collected baseline data in the intervention and control groups, respectively. The final data collection was done by the same CHWs (in intervention clusters) and field workers (in control clusters). The role of the field workers was limited to study procedures in the control area, and they were trained separately from the CHWs.
Intervention and CHWs
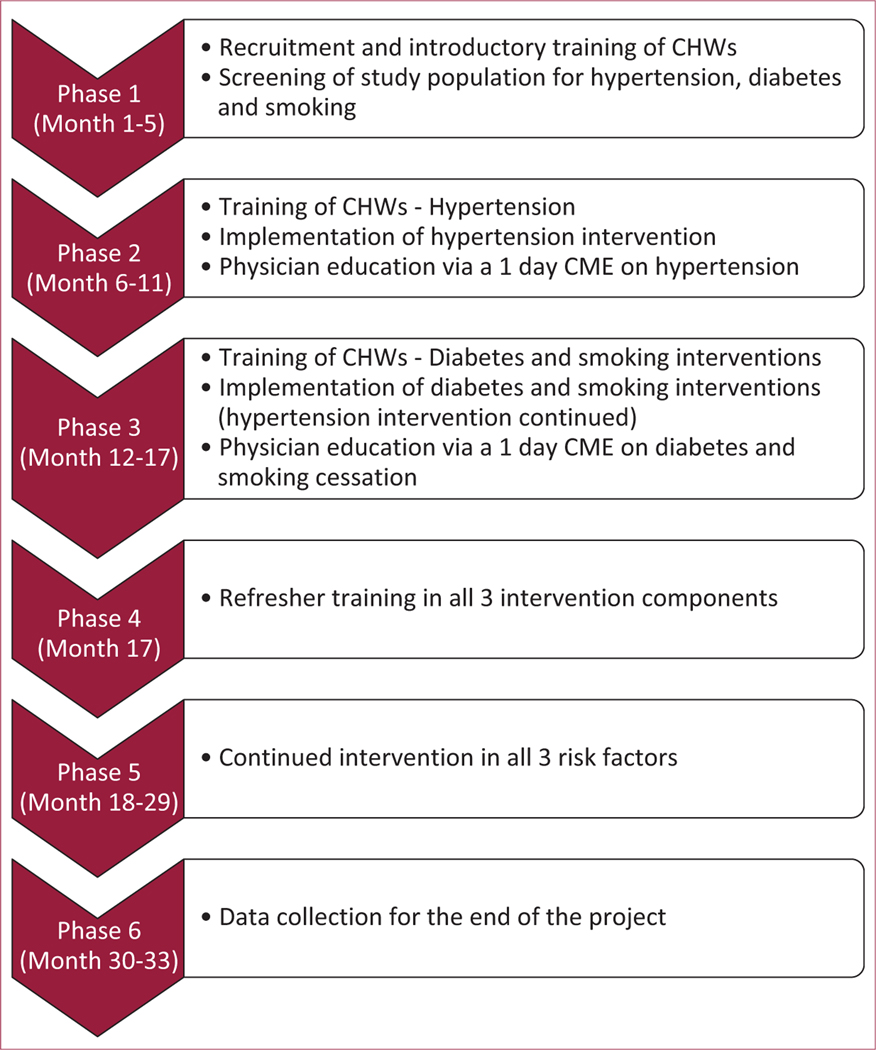
The intervention timeline is described in Figure 1. Details of recruitment, training, compensation, and supervision of study CHWs have been published previously and are described here in brief [21]. All CHWs, field workers and supervisors were recruited for the purpose of the study, and were not previously a part of the health system. Interventions for the 3 risk factors were staggered over time. This was to ensure CHWs had sufficient time to become comfortable with the knowledge and work required for each risk factor, before moving on to incorporate the next risk factor. Correspondingly, the training of the CHWs was also staggered, with initial training and work focused on hypertension, followed by diabetes and then smoking. Training for each risk factor was delivered over 1 to 2 weeks (3 h/day). All CHWs were retained from the start to the end of the intervention, with zero attrition. Once patients were enrolled at the end of the second screening visit, CHWs provided home based counseling to people with hypertension. This usually lasted for around an hour, and consisted of a behavior change strategy focused on modifying the individual’s lifestyle (diet and physical activity), improving health care-seeking behavior, and addressing barriers to medication adherence. Importantly, in addition to lifestyle modifications, CHWs specifically focused on encouraging physician visits, medication purchase, and medication adherence. The communication was conducted in the native language of the participant. CHWs had a flipbook aid that summarized these strategies and provided a template for discussion (see Online Video 1). As many of our patients were illiterate, the patient facing side of the flipbook only had pictures, with all textual information conveyed verbally. Involvement of family members was encouraged. CHWs visited patients with hypertension every 2 months till the end of the study. At follow-up visits, CHWs had a template to use for their encounter, which was focused on reinforcing previous recommendations, understanding barriers to behavior change, behavior change communication, and problem solving. Each CHW had a paper diary in which they recorded details of their patient encounters, in a predefined format.
FIGURE 1. Intervention timeline.
CHWs = community health workers; CME = continuing medical education.
Six months after the start of the hypertension intervention, CHWs underwent training for diabetes and started visiting patients with diabetes. These visits followed a similar format and frequency as hypertension, and a separate flipbook was provided for diabetes counseling. Two months after the start of the diabetes intervention, CHWs received training for the smoking intervention and started visiting patients who smoked, aided by a flipbook. However, the frequency and nature of visits for smoking were customized depending on whether the participant was contemplative or pre-contemplative about quitting smoking. More intensive support was provided to participants who were contemplative about quitting smoking. Once all the 3 interventions were underway, CHWs continued to follow all participants under their care until the end of the study. For a patient with multiple risk factors (e.g., hypertension and diabetes), CHW visits at the start of the study focused on hypertension, and after undergoing diabetes training at the 6-month mark, CHWs also began to counsel the patient on diabetes (while continuing the hypertension intervention).
The 6 intervention CHWs were recruited through a written test and an interview. The CHWs were recruited as volunteers, and were free to pursue other vocations in addition to their work as CHWs. On an average, they worked 40 to 60 h/month, and after screening, cared for an average of 120 participants. They were paid a honorarium of Rs. 2,000/month (around US$350 annually; median per capita annual income in India in 2013 was $616) [22]. They were also provided a phone credit of Rs. 100 (<$2)/ month, which allowed around 200 min of outgoing calls per month. This was directly transferred to their phone. We purposefully avoided performance based cash incentives, the current method for paying CHWs (called accredited social health activist [ASHA] workers) in India. Outcome-based remuneration can diminish the value of nontangible benefits (e.g., respect from society, opportunity to learn and serve), and in the case of India’s ASHA program, has been shown to become an institutional limitation by itself [23,24].
To provide supervision and support to CHWs, 1 supervisor was appointed for every 3 CHWs. The supervisors randomly verified 10% of the work done by CHWs every 2 months, following a standard protocol, which varied according to patient and trial progress. For instance, in hypertension, the automated blood pressure measurement was verified, knowledge level of the patient regarding hypertension assessed, and open ended feedback sought. The supervisors also provided support to CHWs, helping them identify areas of improvement, discussing challenging situations, and accompanying them on visits as the need for help arose. Both CHWs and supervisors maintained close contact with 1 study investigator (a physician), and were free to approach the investigator directly for any help.
All physicians in the study area were invited for 1-day continuing medical education (CME) on hypertension at the start of the study, and a 1-day CME on diabetes and smoking at the end of 6 months. The CME was provided at no cost to the physicians. This component of the intervention was not randomized as being a small town, we expected intervention and control participants to seek care from the same set of physicians. During CHW visits, participants were recommended to visit a MBBS physician and made aware of options; however, the choice of physician (including whether public or private) was left to the participant.
Control group
The control group received a handout at the end of the screening process, that explained to them their respective risk factor(s) (hypertension, diabetes, or smoking). They also received brief verbal advice regarding the same from the field worker. After screening, participants in the control group had 1 visit at the end of the first year of the intervention and a final visit at the end of the study. There was no other contact between control group participants and the study.
Statistical methods
Outcomes were examined for normality and outliers at baseline and post-intervention. Variables were normally distributed except for cigarette/bidi count which was therefore log transformed. Outliers (n = 6) for cigarette/ bidi at post-intervention were replaced with baseline values if mean counts were >30/day. Complete case analyses (i.e., using only those participants who were available post-intervention) were performed for each variable. Descriptive statistics, including mean ± SD or percentage, were applied at baseline and post-intervention.
Given that there were 3 co-primary outcomes, the level of statistical significance was revised by Bonferroni correction to 0.05/3. The p value threshold to meet statistical significance was therefore fixed at <0.016, to account for multiplicity of statistical testing.
Two way analysis of covariance analysis was done to determine the interaction of clustering effect, age, gender, marital status, work status, level of education, and income with the intervention (SBP, FBG, and cigarettes/bidis smoked). Age was taken as a continuous variable while cluster number and income were taken as categorical variables. Marital status (married or single), work status (working or not working), gender (male/female) and education (education up to 10 years/education more than 10 years) were taken in the model as binary values. Regression accounting for the clustering effect was done in a stepwise approach.
For SBP, except income, all other variables showed an interaction with the intervention in reducing SBP (p < 0.05). Therefore, in multiple linear regression analysis, all independent variables were included except for income. After adjusting for the clustering effect and other variables as mentioned above, the intervention variable remained in the model as a significant predictor contributing 8.9 mm of Hg (p < 0.017) of SBP reduction.
For FBG, except for cluster effect, no other baseline variable showed a significant interaction with intervention in reducing FBG level (p < 0.05). Therefore, in multiple linear regression analysis, only cluster variable and intervention were included. The final model showed that after adjusting for the clustering effect, the intervention variable (FBG) was not significant. The intervention showed reduction in fasting blood sugar values by 21 mg/dl which was not statistically significant.
For cigarettes/bidis, cluster effect and gender showed a significant interaction with intervention in reducing number of cigarettes/bidis smoked (p < 0.05). Therefore, in multiple linear regression analysis, cluster variable, gender and intervention were included. In unadjusted analysis as well as the final adjusted model, there were was no significant effect of the intervention variable on reducing number of cigarettes/bidis smoked.
To assess the effect of missing values, demographic factors between those who completed the study and those who were lost to follow-up was assessed. There were no significant differences in any of the demographic factors. In addition, there were no differences in SBP (p = 0.39) or FBG (p = 0.76) at baseline between those who completed the study and those who were lost to follow-up. Similarly, there were no differences in cigarette/bidi use (p = 0.53) at baseline between those who completed the study and those who were lost to follow-up. In addition, there were no differences between study groups in SBP (p = 0.48) or FBG (p = 0.37) at baseline in those who were lost to follow-up. Cigarettes/bidi use at baseline differed between groups in those who were lost to follow-up (intervention 11.8 ± 7.9 cigarettes/bidis, control 7.8 ± 6.4 cigarettes/bidis; p = 0.04).
Secondary outcomes were compared using percentages and means, as appropriate. Owing to multiple secondary outcomes, they were treated as exploratory analyses only, and no formal hierarchical testing or adjustment for multiple testing was done. Therefore, p values for secondary outcomes are listed as nonsignificant.
All analyses were performed using STATA 11.2 (StataCorp, College Station, Texas) and SPSS 16.0 (SPSS Inc., Chicago, Illinois).
Further details of the rationale and design of the study have been published previously [25].
RESULTS
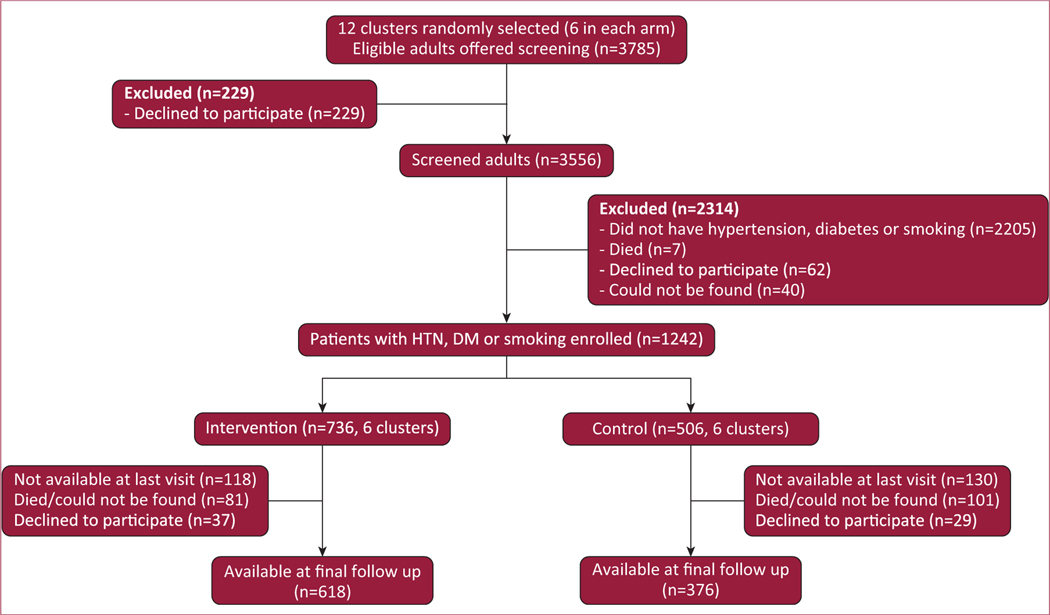
The participant flow is summarized in Figure 2. A total of 3,556 participants were screened and 1,242 individuals were enrolled in 12 clusters. Recruitment was done from May 2014 to September 2014, and the intervention took place from October 2014 to October 2016. Final data collection was completed from November 2016 to February 2017.
FIGURE 2. Study flow diagram.
Twelve clusters (6 in the intervention arm, 6 in the control arm) were randomly selected from a total of 36 clusters. Screening commenced after randomization. DM = diabetes; HTN = hypertension.
Baseline characteristics
Baseline characteristics of intervention and control group participants are shown in Table 1. The majority of enrolled participants (>80% in both groups) had a single risk factor, with <2% in both groups diagnosed with 3 risk factors. The groups were similar to each other in terms of gender, age, work status, education, income levels, weight, and waist circumference (all p > 0.05). However, the intervention group had more people of the Hindu religion, while the control group had more people of the Muslim faith (p < 0.05).
Loss to follow-up
At the end of the study, 248 (20%) participants were not available for follow-up. This included 118 (16%) participants in the intervention group and 130 (26%) participants in the control group. Overall, 55 (4.4%) participants were confirmed to have died and 66 (5.3%) participants refused participation. A total of 127 (10.2%) participants could not be found for the last follow-up.
Blood pressure control
The primary outcome of change in SBP is shown in Table 3. In unadjusted analysis, the decrease in SBP was significantly greater (difference of −5.8 mm Hg; 95% confidence interval [CI]: −1.9 to −9.8 mm Hg; p = 0.004) in the intervention group (−12.2 19.5 mm Hg) than in the control group (−6.4 ± 26.1 mm Hg). After adjusting for the confounders while taking into account the study design, the decrease remained statistically significant (difference of −8.9 mm Hg; 95% CI: −3.5 to −14.4 mm Hg; p = 0.001).
TABLE 3.
Primary outcomes
| Intervention | Control | Intervention vs. Control (Adjusted) | Intervention vs. Control (Unadjusted) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Baseline vs. Post-Intervention | Mean ± SD | n | Baseline vs. Post-Intervention | Mean ± SD | Difference | 2-Tailed p Value | 95% Cl | Difference | 2-Tailed p Value | 95% Cl |
| SBP | 341 | 154.9 vs. 142.8 | −12.2 ± 19.5 | 186 | 159.5 vs. 153.2 | −6.4 ± 26.1 | −8.9 | 0.001 | −14.4 to −3.5 | −5.8 | 0.004 | −9.8 to −1.9 |
| FBG | 177 | 192.4 vs. 149.4 | −43.0 ± 53.5 | 61 | 181.2 vs. 164.9 | −16.3 ± 77.2 | −21.3 | 0.29 | −61.0 to 18.3 | −26.6 | 0.029 | −50.7 to −2.7 |
| Cigarettes/bidis | 219 | 11.0 vs. 7.9 | −3.1 ± 8.6 | 171 | 10.6 vs. 7.3 | −3.3 ± 9.2 | 0.2 | 0.62 | −5.2 to 5.6 | 0.2 | 0.93 | −5.5 to 5.8 |
SBP model adjusted for cluster, education, marital status, cluster, age, gender, and marital status. FBG model adjusted for cluster. Cigarettes/bid is model adjusted for cluster and gender ICC data: SBP = 0.014; FBG = 0.017; for cigarettes/bid is = 0.09.
Cl = confidence interval; other abbreviations as in Table 2.
Blood glucose control
The primary outcome of change in FBG is shown in Table 3. In unadjusted analysis, the decrease in FBG was greater (difference of −26.6 mg/dl; 95% CI: −2.7 to −50.7 mg/dl) in the intervention group (−43.0 ± 83.5 mg/dl) than in the control group (−16.3 ± 77.2 mg/dl). However, this difference was statistically not significant (p = 0.029). After adjusting for the study design, the difference remained nonsignificant (difference of −21.3 mg/dl; 95% CI: 18.3 to −61 mg/dl; p = 0.29).
Smoking
The primary outcome of average number of cigarettes/bidis smoked is shown in Table 3. Although both groups had a significant decrease (p = 0.02) in daily smoking (intervention = −3.1 ± 8.6 cigarettes/bidis; control −3.3 ± 9.2 cigarettes/bidis), there was no difference between groups in the change between baseline and post-intervention (difference of +0.2 cigarettes/bidis; 95% CI: 5.6 to −5.2 mg/dl; p = 0.62).
Secondary outcomes
The secondary outcomes are listed in Table 4. The diastolic blood pressure decreased more in the intervention group, as compared with the control group, though this difference was not statistically significant. The dichotomized control rates for both hypertension and diabetes improved more in the intervention group, as compared with the control group, though this difference was again statistically nonsignificant (Table 4). The use of statin and aspirin, for any subgroup of patients, was negligible in both study groups. The number of people who quit smoking was similar in both groups. Although mean reduction in weight and waist circumference were prespecified as secondary outcomes, we could not collect adequate data on these measurements during the final follow-up.
TABLE 4.
Secondary outcomes
| Intervention |
Control |
||||||
|---|---|---|---|---|---|---|---|
| Baseline | Intervention Follow-Up | Change | Baseline | Control Follow-Up | Change | Difference in Change | |
| Mean reduction in diastolic blood pressure, mm Hg* | 92 | 87 | −5.1 ± 13.5 | 92 | 89 | −3.0 ± 14.7 | −2.1 mm Hg (95% CI: −4.5 to 0.3; p = 0.09) |
| Control rates of hypertension, %† | 18.80 | 36.40 | 17.6 | 14.0 | 22.6 | 8.6 | 9 (p = 0.23) |
| Control rates of diabetes, %‡ | 6.20 | 34.30 | 28.1 | 14.5 | 29.0 | 14.5 | 13.6 (p = 0.66) |
| Statin use in participants with diabetes | 3 (1.5) | 7 (4.0) | 2.5 | 0 | 1 (1.6) | 1.6 | 0.9 (p = NS) |
| Statin use in participants with hypertension, who also smoke tobacco | 1 (0.5) | 0 | −0.5 | 0 | 0 | 0 | −0.5 (p = NS) |
| Aspirin use in participants with diabetes |
1 (0.6) | 4 (2.3) | 1.7 | 0 | 1 (1.6) | 1.6 | 0.1 (p = NS) |
| Participants who quit smoking at final follow-up | 11.8 (26/220) | 11.0 (19/173) | 0.8 (p = 0.90) | ||||
Values are mean ± SD, n (%), or % (n/n), unless otherwise indicated.
Among participants with hypertension at baseline: intervention = 341, control = 186.
Defined as blood pressure <140/90 mm Hg: intervention = 341, control = 186.
Defined as fasting blood sugar <126 mg/dl: intervention = 177, control = 61.
CI = confidence interval.
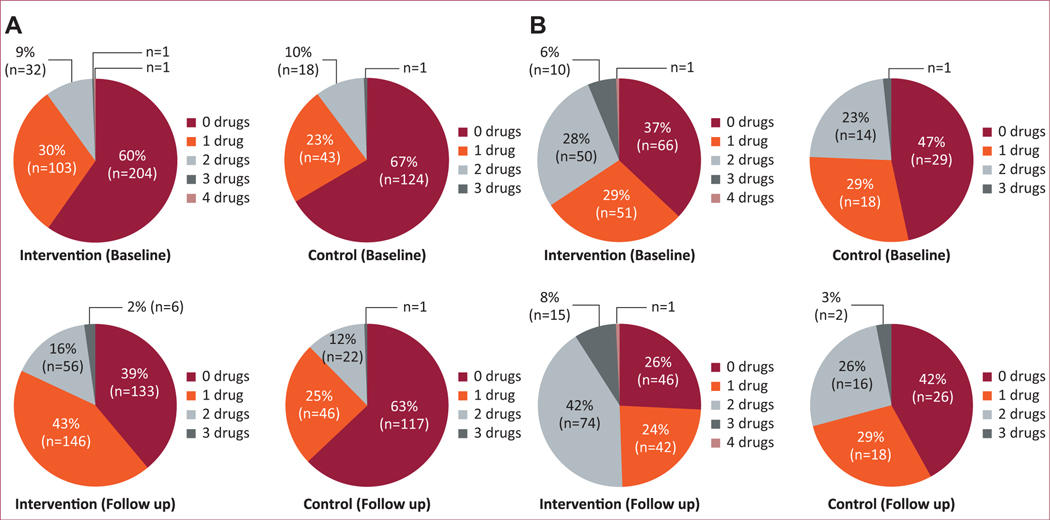
The mean number of medications used for hypertension and diabetes increased within the intervention group as well as the control group, with a greater increase in the intervention group for both conditions (Figures 3A and 3B), though this was not statistically significant.
FIGURE 3. (A) Number of antihypertensive medications used by study group.
Only individuals available for follow-up at the end of 2 years were analyzed (intervention group = 341 participants, control group = 186 participants). The percentage of participants on 0 drugs decreased from 60% at baseline to 39% at the end of 2 years in the intervention group (difference of −21%), while in the control group it decreased from 67% to 63% (difference of −4%). Correspondingly, the percentage of participants on ≥ 2 drugs increased from 10% to 18% (difference of 8%) in the intervention group, while in the control group it increased from 10% to 12% (difference of 2%). (B) Number of antihyperglycemic medications used by study group. Only individuals available for follow-up at the end of 2 years were analyzed (intervention group 178 participants, control group 62 participants). The percentage of participants on 0 drugs decreased from 37% at baseline to 26% at the end of 2 years in the intervention group (difference of −11%) while in the control group it decreased from 47% to 42% (difference of −5%). Correspondingly, the percentage of participants on ≥2 drugs increased from 34% to 51% (difference of 17%) in the intervention group, while in the control group it increased from 24% to 29% (difference of 5%).
There were no significant harms or unintended effects noted during the course of the study from study-related interventions.
DISCUSSION
In SEHAT, we show that an integrated approach to improving control of hypertension, diabetes, and smoking is feasible through CHWs, leading to improved SBP in hypertension, an inconclusive effect on FBG in diabetes, and no demonstrable impact on smoking.
Our study has several strengths. First, systematically targeting hypertension and diabetes through a community based approach, rather than opportunistic screening through a health care facility, allows targeting of the substantial burden of undiagnosed hypertension and diabetes in the community. In our study, only 45% of participants with hypertension were previously aware of their diagnosis. For diabetes, only 60% of participants were previously aware of their diabetes [26]. Given that home-based screening was carried out in both the intervention and control groups, the trial would not be expected to convey the benefits of uncovering this substantial burden of previously undiagnosed risk factors. Second, our intervention is in the real world, and is simple and replicable with limited resources. Third, our recruitment and staggered training of CHWs is standardized, results in high knowledge retention, and is associated with high CHW satisfaction. We have described the training and overall role development of the CHWs in this study in a previous publication [21]. Ensuring holistic role development of CHWs is likely essential to the success of a CHW program, and interested readers are encouraged to refer to the prior publication, which we have not discussed in this manuscript due to space constraints.
The effect size for reduction in SBP in our study is similar to a previous RCT from Pakistan (COBRA [Control of Blood Pressure and Risk Attenuation] trial) in which the intervention involved physician education regarding hypertension and home health education through CHWs [14]. This shows that the efficacy of CHWs in improving SBP in hypertension does not attenuate with the addition of care for more risk factors. Moreover, the finding of similar efficacy for SBP reduction across 2 different randomized controlled trials makes it more generalizable.
The mechanism of improvement in SBP in SEHAT is likely at least in part due to increased use of medicines. Though it did not reach statistical significance, there was a trend toward increased use of medicines for both hypertension and diabetes in the intervention group, as compared with the control group. The rest of the improvement could possibly be from lifestyle changes, though we did not track this specifically.
Although the diabetes intervention showed a significant reduction in FBG in unadjusted analysis, a null effect could not be excluded after adjusting for the study design. A potential explanation is that while we planned our sample size assuming a 13% prevalence of diabetes in the screening population, the actual prevalence of diabetes was only 9%. This may have led to the study being underpowered to detect an effect of the intervention on FBG. Future studies with larger sample sizes could provide more definitive evidence for the effect of CHWs in reducing FBG in people with diabetes.
In our intervention, patients had to pay out of pocket for physician visits and medicines. 47% of our patients had an annual household income Rs. <50,000 (~US$800) [26]. Reducing out-of-pocket expenses for physician visits and medicines could potentially further improve the efficacy of our intervention. However, given that 45% of our patients had no formal education, it is unclear if merely reducing these out of pocket expenses, without concomitant education and support through CHWs, could achieve similar outcomes. Similarly, it is unclear if merely providing screening for diabetes and hypertension through CHWs, without providing education and follow-up would result in any benefit.
CHW-based studies in hypertension have previously proposed that hypertension care should be integrated with existing CHW programs. However, many existing CHW programs focused on infectious diseases or maternal and child health are already overburdened, and are qualitatively different from cardiovascular risk factor control [27,28]. Moreover, going beyond hypertension and including diabetes in existing CHW programs will only add to potential CHW burden. We, therefore, advocate the creation and support of a new cadre of CHWs that can use a multifactorial approach to cardiovascular risk factor reduction. As opposed to other nonphysician health workers such as nurses, pharmacists, or social workers that require formal degree-level training, and hence limit the number of personnel available for a national program, previous experience from India’s CHW program (ASHA) focusing on maternal and child care shows that such a CHW pro-gram can be created in a few years [29]; this can help achieve the WHO goal of 25% reduction in mortality from NCDs by 2025, provided government and private investment is ramped up in deploying these CHW cadres in LMICs [2]. The recently launched Ayushman Bharat initiative in India aims to establish 150,000 health and wellness centers in the country by 2022 [8]. These centers will aim to provide high-quality, equitable, and comprehensive primary care. Task sharing among CHWs (ASHA workers), physicians, and a new cadre of midlevel health providers forms the basis for these centers. However, no large-scale recruitment of new ASHA workers has taken place until now, to provide human resources for additional services in NCDs. We propose that recruiting and training an additional ASHA worker, who can focus on NCDs through home-based care (screening and follow-up visits), may better serve the aim of high quality and comprehensive primary care.
Our smoking intervention did not result in any appreciable difference in the intervention group, as compared with the control group. A previous study from Pakistan that provided behavioral support through DOT (Directly Observed Therapy) facilitators to patients suspected of tuberculosis had proven to be effective in achieving smoking cessation [30]. A single-visit smoking intervention, delivered through CHWs, has also been shown to have a small but significant effect on smoking cessation [15]. The lack of an urgent health issue in our patients, enrolling patients who were pre-contemplative as well as contemplative and the use of CHWs (as opposed to traditional health care professionals) are all plausible explanations for the lack of a significant impact on smoking. Future studies using CHWs could target a more selective population that is contemplative about quitting smoking as well as explore other means of more frequent interactions (e.g., mobile instant messaging) to enhance the effectiveness of a CHW-led smoking intervention.
Study Limitations
First, our data collection was not blinded. Owing to the small size of the town, home-based intervention, and cluster randomization, blinded assessment was deemed technically infeasible. However, key outcomes of SBP and FBG were measured using automated devices, which minimized the risk of biased outcome assessment. Second, 248 (20%) participants were not available for final follow-up. A total of 55 (4.4%) of these participants had died during the course of the study. Although loss to follow-up has a potential to introduce bias, our sample size calculations accounted for a 30% rate of loss to follow-up. In addition, the loss to follow-up must be considered in the context of our broad eligibility criteria, as 94% of individuals offered screening agreed to participate in the study. We did not exclude people at risk for poor adherence to the intervention, to enhance the generalizability of the study. Moreover, our rate of loss to follow-up is similar to previous such community-based studies [14]. However, the differential loss to follow-up in the intervention (16%) and control groups (26%) has the potential to bias study results. Third, the trial design may underestimate the overall benefits of a CHW-based intervention as the control group also received systematic screening (hypertension and diabetes) and brief education. The reduction in SBP and FBG in the control group may be partly explained by the effects of screening, though secular trends and regression to the mean also likely contributed. Fourth, it was a single-site study in a semiurban Indian town, so it may not be generalizable to other settings. However, many developing countries share a similar health care environment as our study setting, making our results broadly relevant. Adapted versions of our intervention can be tested in other settings, especially in health systems that have a successful history of using CHWs for improvement of maternal and child health.
CONCLUSIONS
SEHAT demonstrates that an integrated intervention through CHWs can lead to improved control of hypertension in a developing country, with an inconclusive effect on controlling diabetes. Given the simplicity of the intervention and robust results, these findings have potential implications for all developing countries that face a similar burden of NCDs.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge the support of Jayant Raikhelkar, MD; Stephen Ganocy, PhD; Pradeep Dixit, MD; and Keith Armitage, MD, the inspiration of Andrew Drexler, MD, as well as thank the people of Dalkhola for their participation.
The trial was funded by Marwari Yuva Manch, which is a nongovernmental organization based in Dalkhola, West Bengal, India. Dr. Bajaj was supported by a American College of Cardiology Presidential Career Development Award, a Walter B. Frommeyer investigative fellowship, and the National Center for Advancing Translational Research of the National Institutes of Health under award number UL1TR001417.
The funding source had no role to play in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American College of Cardiology or National Institutes of Health.
Footnotes
The authors report no relationships that could be construed as a conflict of interest.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.gheart.2019.08.003.
REFERENCES
- 1.World Health Organization. Noncommunicable diseases. Available at: http://www.who.int/mediacentre/factsheets/fs355/en/. Accessed January 24, 2019.
- 2.World Health Organization. WHO Global NCD Action Plan 2013–2020. Geneva: 2013. Available at: http://www.who.int/nmh/events/ncd_action_plan/en/. Accessed January 24, 2019. [Google Scholar]
- 3.Ram U, Jha P, Gerland P, et al. Age-specific and sex-specific adult mortality risk in India in 2014: analysis of 0–27 million nationally surveyed deaths and demographic estimates from 597 districts. Lancet Glob Health 2015;3:e767–75. [DOI] [PubMed] [Google Scholar]
- 4.Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandona L, Dandona R, Kumar GA, et al. Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the Global Burden of Disease Study. The Lancet 2017. December 2;390(10111):2437–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anchala R, Kannuri NK, Pant H, et al. Hypertension in India: a systematic review and meta-analysis of prevalence, awareness, and control of hypertension. J Hypertens 2014;32:1170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy P, Saddichha S. Tobacco cessation services in India: recent developments and the need for expansion. Indian J Cancer 2010; 47(Suppl 1):69–74. [DOI] [PubMed] [Google Scholar]
- 8.Ved RR, Gupta G, Singh S. India’s health and wellness centres: realizing universal health coverage through comprehensive primary health care. WHO South-East Asia J Public Health 2019;8:18–20. [DOI] [PubMed] [Google Scholar]
- 9.Khetan AK, Purushothaman R, Chami T, et al. The Effectiveness of Community Health Workers for CVD Prevention in LMIC. Glob Heart 2017. September;12:233–243.e6. [DOI] [PubMed] [Google Scholar]
- 10.Lewin S, Munabi-Babigumira S, Glenton C, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev 2010;3:CD004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neupane D, Kallestrup P, McLachlan CS, Perry H. Community health workers for non-communicable diseases. Lancet Glob Health 2014; 10:e567. [DOI] [PubMed] [Google Scholar]
- 12.Gaziano TA, Abrahams-Gessel S, Denman CA, et al. An assessment of community health workers’ ability to screen for cardiovascular disease risk with a simple, non-invasive risk assessment instrument in Bangladesh, Guatemala, Mexico, and South Africa: an observational study. Lancet Glob Health 2015;3:e556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand TN, Joseph LM, Geetha AV, Prabhakaran D, Jeemon P. Task sharing with non-physician health-care workers for management of blood pressure in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Glob Health 2019;7:e761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jafar TH, Hatcher J, Poulter N, et al. Community-based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Ann Intern Med 2009;151:593–601. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar BK, West R, Arora M, Ahluwalia JS, Reddy KS, Shahab L. Effectiveness of a brief community outreach tobacco cessation intervention in India: a cluster-randomised controlled trial (the BABEX Trial). Thorax 2017;72:167–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiris D, Praveen D, Mogulluru K, et al. SMARThealth India: A stepped-wedge, cluster randomised controlled trial of a community health worker managed mobile health intervention for people assessed at high cardiovascular disease risk in rural India. PLoS One 2019;14:e0213708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeemon P, Narayanan G, Kondal D, et al. Task shifting of frontline community health workers for cardiovascular risk reduction: design and rationale of a cluster randomised controlled trial (DISHA study) in India. BMC Public Health 2016;16:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Government of India. Ranking of districts by literacy rate in 2001 and 2011. Available at: http://censusindia.gov.in/2011-prov-results/prov_data_products_wb.html. Accessed August 5, 2019.
- 19.REDCap. Available at: https://projectredcap.org/. Accessed August 5, 2019.
- 20.Hemming K, Girling AJ, Sitch AJ, Marsh J, Lilford RJ. Sample size calculations for cluster randomised controlled trials with a fixed number of clusters. BMC Med Res Methodol 2011;11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khetan A, Patel T, Hejjaji V, et al. Role development of community health workers for cardiovascular disease prevention in India. Eval Program Plann 2018;67:177–83. [DOI] [PubMed] [Google Scholar]
- 22.Gallup. Worldwide, median household income about $10,000. Available at: http://www.gallup.com/poll/166211/worldwide-median-household-income-000.aspx. Accessed August 5, 2019.
- 23.Marshall M, Harrison S. It’s about more than money: financial incentives and internal motivation. Qual Saf Health Care 2005;14:4–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott K, Shanker S. Tying their hands? Institutional obstacles to the success of the ASHA community health worker programme in rural north India. AIDS Care 2010;22(Suppl 2):1606–12. [DOI] [PubMed] [Google Scholar]
- 25.Khetan A, Purushothaman R, Zullo M, et al. Rationale and design of a cluster-randomized controlled trial to evaluate the effects of a community health worker-based program for cardiovascular risk factor control in India. Am Heart J 2017;185:161–72. [DOI] [PubMed] [Google Scholar]
- 26.Khetan A, Zullo M, Hejjaji V, et al. Prevalence and pattern of cardiovascular risk factors in a population in India. Heart Asia 2017;9: e010931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrahams-Gessel S, Denman CA, Montano CM, et al. Training and supervision of community health workers conducting population-based, noninvasive screening for CVD in LMIC: implications for scaling up. Glob Heart 2015;10:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zulu JM, Kinsman J, Michelo C, Hurtig A-K. Integrating national community-based health worker programmes into health systems: a systematic review identifying lessons learned from low-and middle-income countries. BMC Public Health 2014;14:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu A, Sullivan S, Khan M, Sachs S, Singh P. Community health workers in global health: scale and scalability. Mt Sinai J Med N Y 2011;78: 419–35. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqi K, Khan A, Ahmad M, et al. Action to stop smoking in suspected tuberculosis (ASSIST) in Pakistan: a cluster randomized, controlled trial. Ann Intern Med 2013;158:667–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.