Key Points
Question
Does an association exist between dietary protein choice, particularly from various food sources, and long-term overall mortality or cause-specific mortality in the US population?
Findings
In this cohort of 237 036 men and 179 068 women with 16 years of observation and nearly 78 000 deaths, greater intake of plant protein was significantly associated with lower overall mortality and cardiovascular disease mortality independent of several other risk factors.
Meaning
This study provides evidence for public health recommendations regarding dietary modifications in choice of protein sources that may promote health and longevity.
Abstract
Importance
Although emphasis has recently been placed on the importance of high-protein diets to overall health, a comprehensive analysis of long-term cause-specific mortality in association with the intake of plant protein and animal protein has not been reported.
Objective
To examine the associations between overall mortality and cause-specific mortality and plant protein intake.
Design, Setting, and Participants
This prospective cohort study analyzed data from 416 104 men and women in the US National Institutes of Health–AARP Diet and Health Study from 1995 to 2011. Data were analyzed from October 2018 through April 2020.
Exposures
Validated baseline food frequency questionnaire dietary information, including intake of plant protein and animal protein.
Main Outcomes and Measures
Hazard ratios and 16-year absolute risk differences for overall mortality and cause-specific mortality.
Results
The final analytic cohort included 237 036 men (57%) and 179 068 women. Their overall median (SD) ages were 62.2 (5.4) years for men and 62.0 (5.4) years for women. Based on 6 009 748 person-years of observation, 77 614 deaths (18.7%; 49 297 men and 28 317 women) were analyzed. Adjusting for several important clinical and other risk factors, greater dietary plant protein intake was associated with reduced overall mortality in both sexes (hazard ratio per 1 SD was 0.95 [95% CI, 0.94-0.97] for men and 0.95 [95% CI, 0.93-0.96] for women; adjusted absolute risk difference per 1 SD was −0.36% [95% CI, −0.48% to −0.25%] for men and −0.33% [95% CI, −0.48% to −0.21%] for women; hazard ratio per 10 g/1000 kcal was 0.88 [95% CI, 0.84-0.91] for men and 0.86 [95% CI, 0.82-0.90] for women; adjusted absolute risk difference per 10 g/1000 kcal was −0.95% [95% CI, −1.3% to −0.68%] for men and −0.86% [95% CI, −1.3% to −0.55%] for women; all P < .001). The association between plant protein intake and overall mortality was similar across the subgroups of smoking status, diabetes, fruit consumption, vitamin supplement use, and self-reported health status. Replacement of 3% energy from animal protein with plant protein was inversely associated with overall mortality (risk decreased 10% in both men and women) and cardiovascular disease mortality (11% lower risk in men and 12% lower risk in women). In particular, the lower overall mortality was attributable primarily to substitution of plant protein for egg protein (24% lower risk in men and 21% lower risk in women) and red meat protein (13% lower risk in men and 15% lower risk in women).
Conclusions and Relevance
In this large prospective cohort, higher plant protein intake was associated with small reductions in risk of overall and cardiovascular disease mortality. Our findings provide evidence that dietary modification in choice of protein sources may influence health and longevity.
To provide evidence for public health recommendations regarding dietary modifications in choice of protein sources that may promote long-term health outcomes, this cohort study assesses overall mortality and cause-specific mortality associated with consumption of plant protein and animal protein in a large US population with 16 years of follow-up.
Introduction
Great emphasis has been placed on the human health effects of a high-quality protein diet owing to its proposed benefits regarding weight management, metabolism, and healthy aging.1 Previous studies, including meta-analyses of short-term clinical trials, have shown that high-protein diets lead to weight loss and fat mass loss, possibly from the negative energy balance that results from sustained satiety and energy expenditure.2,3,4,5 In addition, cardiovascular metrics, including blood pressure, circulating lipid and lipoprotein profiles, and glycemic modulation, have shown improvement with protein substitution for carbohydrate.4,5,6,7,8 Notwithstanding such evidence and interest in the beneficial health effects of dietary protein, recommendations regarding food sources of protein have yet to be made. A thorough analysis of disparate long-term health outcomes resulting from choices of dietary protein sources (ie, plant protein or animal protein) remains to be elucidated given the different dimensions of nutritional profiles, including macronutrients, micronutrients, polyphenols, and amino acid combinations from varied protein sources.9,10
The association between dietary protein sources and mortality has been examined in only a few population-based studies.11,12,13 The Iowa Women’s Health Study showed that plant protein substitution for animal protein is associated with reduced coronary heart disease mortality but not with overall or cancer mortality.11 The Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS) cohorts with 131 342 participants found lower overall and cardiovascular disease (CVD) mortality with greater plant protein intake,12 whereas reduced risk of mortality overall and mortality related to cancer or CVD was associated with a 3% energy substitution of plant protein for red meat protein in the Japan Public Health Center-Based Prospective Cohort Study of 70 696 participants.13 Additional evidence is needed regarding the association between the intake of plant protein and animal protein and mortality in a large cohort that examines specific plant and animal protein sources in detail as well as in population subgroups based on risk factors.
To this end, we examined whether plant protein intake and animal protein intake from various sources are associated with overall and cause-specific mortality in the National Institutes of Health (NIH)-AARP Diet and Health Study, a large prospective cohort of more than 400 000 participants with more than 77 000 deaths.
Methods
Study Population
Details of the NIH-AARP Diet and Health Study have been described.14 In brief, 617 119 participants 50 to 71 years of age who returned a baseline questionnaire were enrolled from 6 states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and 2 metropolitan areas (Atlanta and Detroit) in 1995 and 1996. The study was approved by the Special Studies institutional review board of the US National Cancer Institute. Completion of the self-administered questionnaire was considered to imply informed consent to participate in the study and consistent with the Common Rule requirements. No one received compensation or was offered any incentive for participating in the study.
Exposure Assessment
At baseline, in addition to completing a questionnaire of demographic and lifestyle characteristics, participants completed the National Cancer Institute Diet History Questionnaire (DHQ) of 124 dietary items.14 The DHQ included 124 food items and portion sizes, and it was linked with the US Department of Agriculture 1994-1996 Continuing Survey of Food Intakes by Individuals food composition nutrient database to provide an accurate representation relative to the dietary habits and nutrition of the general US population. Validity and reliability of the DHQ has been confirmed and reported.15 Total dietary protein intake included plant protein and animal protein. Primary dietary sources for plant protein (mean percentages of total plant protein intake) included bread, cereal, and pasta (45.8%), nuts (4.5%), beans and legumes (8.0%), and other plant protein (41.7%). Primary dietary sources of animal protein (mean percentages of total animal protein intake) were red meat (eg, fresh and processed red meat) (30.6%), white meat (eg, poultry, fish, and processed white meat) (31.3%), and other animal protein (eg, dairy products [31.6%] and eggs [4.0%]). Dietary intake of protein, fiber, fruits, and vegetables were adjusted for total energy intake (per 1000 kcal intake) according to the nutrient-density approach.
Mortality Assessment
Vital status of participants was ascertained via linkage with the Social Security Administration Death Master File, and specific causes of death were identified through linkage with the National Death Index Plus, updated by the National Center for Health Statistics (for details, see eAppendix 1 in the Supplement).
Statistical Analysis
Applying the nutrient density method to adjust for caloric intake,16,17 we divided all nutritional variables by a standardized calorie intake (grams per 1000 kcal) in addition to including energy as a continuous covariate in the model because it can be independently associated with mortality.18 Person-time of observation was calculated for cohort participants from study entry in 1995 to 1996 until date of death or the end of follow-up (December 31, 2011), whichever occurred first. Cox proportional hazards regression models using person-time as the underlying time metric were used to estimate hazard ratios (HRs) and 95% CIs for the association between plant protein intake (per 1 SD or 10 g/1000 kcal increment) and mortality risk, including overall and cause-specific mortality. We used the nutrient-density model (ie, grams of protein per 1000 kcal dietary intake) and reported risk estimates for men and women separately. In the models of cause-specific mortality, mortality from causes other than the cause of interest was censored at the time of death. To test for proportional hazards, we included an interaction of intervals of follow-up time as categories with plant protein intake in the regression model, and P values for the significance of the interaction were determined from likelihood ratio tests. However, we did find several quantitative interactions with follow-up time (ie, significant risk estimates in the same direction, with only magnitudes differing by the mortality outcome and protein source), but not qualitative interactions (ie, risk estimates in opposite directions). Because interactions were only quantitative in nature, we report main analyses without stratifying by follow-up time intervals and separately present models stratified by follow-up time. For each HR from the primary analysis, the adjusted absolute risk difference (ARD) was estimated for each 1 SD or 10 g/1000 kcal increment of plant protein intake per day at the end of the observation follow-up of 16 years. The corresponding 95% CIs for ARD were calculated based on 100 bootstrap samples. To elucidate residual confounding status, propensity scores reflecting the association between plant protein intake and potential confounding covariables were generated and included in the models.19 To evaluate the possibility that the inverse mortality associations may be explained by other confounding factors, we further adjusted for consumption of sugar-sweetened beverages (ie, regular soft drinks and regular fruit drinks) and total carbohydrates, as well as median household income (as a census variable in group levels). To minimize the influence of potential bias from reverse causality, we also performed sensitivity analyses that excluded the first 5 years of follow-up.
Sensitivity analyses were also performed stratified by age at entry (<60, 60 to <65, and ≥65 years), cigarette-smoking status (never, former, or current), diabetes (no or yes), body mass index (BMI [calculated as weight in kilograms divided by height in meters squared], <18.5, 18.5 to <25, 25 to <30, 30 to <35, and ≥35), alcohol consumption (0 to <1, 1 to <3, and ≥3 drinks per day), consumption of fruits and vegetables (median split low/high), supplemental vitamin use (no or yes), self-reported health status (poor to fair, good, and very good to excellent), postmenopausal hormone therapy use (for women; no or yes), and mortality follow-up period (0 to <5, 5 to <10, and ≥10 years). We used likelihood-ratio tests to evaluate P values for interactions by comparing Cox proportional hazards regression models with or without the cross-product terms for the preselected factors and dietary plant protein intake (per 1 SD).
We examined mortality associations of substituting 3% of energy from plant protein with an equal decrement in animal protein from various sources, including red meat, white meat, dairy, and eggs. Plant, animal, and total protein were modeled as percentages of energy, using energy from the specific protein divided by total energy intake. The regression coefficient for plant protein in the model was the log hazard ratio for a 3% increment in energy from plant protein with an equal decrement in animal protein while total energy from protein was held constant. Missing values for any single covariable were less than 5%; we included a missing value indicator for each covariable in the models.
All analyses were conducted from October 2018 through April 2020 using SAS software, version 9.4 (SAS Institute Inc). All P values are 2-sided at a type I error rate of 0.05. The Bonferroni correction threshold was used to account for multiple comparisons and define statistical significance (.05/18 = .0028 for primary and secondary analyses [9 tests each for men and for women], and .05/21 = .0024 for the subgroup interaction tests). Details of covariate adjustment and sensitivity analyses are described in eAppendix 1 in the Supplement.
Results
Participants
After checking dietary questionnaire responses for data quality, 566 398 participants constituted the baseline cohort. After excluding those with proxy questionnaires (n = 15 760), previous diagnoses of cancer except nonmelanoma skin cancer (n = 51 334), self-reported end-stage kidney disease (n = 997), or heart disease or stroke (n = 73 549), and those with only a death record for cancer (n = 4253), extreme daily caloric consumption (n = 4372), or zero years of follow-up (n = 29), the final analytic cohort included 416 104 individuals comprising 237 036 men (57%) and 179 068 women. Their overall median (SD) ages were 62.2 (5.4) years for men and 62.0 (5.4) years for women.
Dietary Plant and Animal Protein Intake, Diet, and Lifestyle Factors
In our study, the median percentage of daily energy intake from total protein was 15.3%, and the median percentage of daily dietary protein intake was 40% from plant sources and 60% from animal protein sources (including 19% from dairy foods). Table 1 provides cohort baseline characteristics according to categories (quintiles) of plant protein intake. The median plant protein intake was 26.9 g/d (14.4 g/1000 kcal per day) for men and 21.6 g/d (14.9 g/1000 kcal per day) for women. Participants with higher plant protein intake were more likely to have diabetes, higher educational level, lower BMI and total energy intake, and higher fiber, fruit, and vegetable intake; be more physically active and use vitamin supplements; and be less likely to be current smokers or report their health as poor or fair (Table 1). Cohort characteristics by quintiles of animal protein intake are presented in eAppendix 2 and eTable 1 in the Supplement.
Table 1. Baseline Characteristics of Study Participants Stratified by Quintiles of Daily Dietary Plant Protein Intakea.
| Characteristicb | Quintiles of plant protein intake for men | Quintiles of plant protein intake for women | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (N = 47 407) | 2 (N = 47 407) | 3 (N = 47 408) | 4 (N = 47 407) | 5 (N = 47 407) | 1 (N = 35 813) | 2 (N = 35 814) | 3 (N = 35 814) | 4 (N = 35 814) | 5 (N = 35 813) | |
| Plant protein intake, mean (SD), g/1000 kcal | 9.8 (1.6) | 12.6 (0.6) | 14.4 (0.5) | 16.3 (0.6) | 19.9 (2.4) | 10.4 (1.6) | 13.2 (0.5) | 15.0 (0.5) | 16.8 (0.6) | 20.6 (2.6) |
| Age, mean (SD), y | 61.4 (5.4) | 61.7 (5.4) | 61.8 (5.4) | 61.9 (5.4) | 61.9 (5.3) | 61.3 (5.5) | 61.7 (5.4) | 61.7 (5.4) | 61.8 (5.4) | 61.7 (5.4) |
| BMI, mean (SD) | 27.5 (4.4) | 27.4 (4.3) | 27.3 (4.2) | 27.1 (4.2) | 26.6 (4.2) | 27.1 (6.3) | 26.9 (5.9) | 26.8 (5.9) | 26.6 (5.7) | 26.0 (5.7) |
| Non-Hispanic white, % | 91.5 | 92.6 | 93.1 | 92.6 | 91.4 | 86.9 | 89.7 | 90.6 | 90.7 | 89.5 |
| Vigorous physical activity, ≥5/wk, % | 17.0 | 18.4 | 20.3 | 21.9 | 27.0 | 12.9 | 13.9 | 15.4 | 17.7 | 21.9 |
| Educational level, college or postgraduate, % | 37.2 | 43.0 | 46.6 | 49.4 | 52.3 | 24.6 | 28.6 | 30.8 | 32.3 | 36.7 |
| Married, % | 81.1 | 85.3 | 86.5 | 86.9 | 84.8 | 41.9 | 45.5 | 46.2 | 46.4 | 44.0 |
| Family history of cancer, % | 46.1 | 47.2 | 48.0 | 48.0 | 46.5 | 49.9 | 51.1 | 52.2 | 51.5 | 51.1 |
| Diabetes, % | 6.3 | 7.0 | 7.9 | 9.3 | 10.4 | 5.2 | 5.8 | 6.1 | 6.5 | 6.6 |
| Current smoker, % | 20.0 | 12.6 | 9.6 | 7.6 | 6.0 | 23.4 | 16.0 | 13.0 | 11.4 | 9.5 |
| Alcoholic drinks, >3/d, % | 29.5 | 13.0 | 7.3 | 4.0 | 2.0 | 9.3 | 2.4 | 1.0 | 0.6 | 0.2 |
| Energy intake, mean (SD), kcal | 2323 (1016) | 2088 (837) | 1991 (786) | 1909 (746) | 1863 (757) | 1712 (747) | 1607 (654) | 1556 (623) | 1502 (598) | 1468 (610) |
| Fiber intake, mean (SD), g/1000 kcal | 15.9 (8.3) | 18.3 (8.1) | 20.0 (8.6) | 21.7 (9.3) | 26.4 (12.5) | 13.2 (6.7) | 15.9 (7.2) | 17.5 (7.8) | 19.1 (8.5) | 23.3 (11.6) |
| Foods, servings, mean (SD) | ||||||||||
| Fruits | 2.4 (2.4) | 2.8 (2.4) | 3.0 (2.4) | 3.1 (2.4) | 3.3 (2.6) | 2.6 (2.5) | 2.9 (2.4) | 3.0 (2.3) | 3.1 (2.3) | 3.2 (2.4) |
| Vegetables | 3.0 (1.8) | 3.6 (2.0) | 3.9 (2.1) | 4.2 (2.3) | 5.2 (3.3) | 2.8 (1.7) | 3.4 (1.9) | 3.7 (2.1) | 4.1 (2.3) | 5.2 (3.4) |
| Vitamin supplement use, % | 47.4 | 49.9 | 52.4 | 54.2 | 56.4 | 56.9 | 59.8 | 61.1 | 63.1 | 62.9 |
| Prior or current postmenopausal hormone therapy, % | NA | NA | NA | NA | NA | 49.8 | 53.0 | 55.0 | 55.5 | 55.7 |
| Self-reported health, poor or fair, % | 9.3 | 7.5 | 6.9 | 6.2 | 5.9 | 12.3 | 10.0 | 9.1 | 8.9 | 7.8 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared), NA, not applicable.
All exposures are associated with plant protein intake, with P < .001 for trend across all groups.
All dietary data are per day.
Dietary Plant Protein Intake and Overall Mortality
During 16 years of follow-up (median, 15.5 years; interquartile range, 15.5-15.8) with 6 009 748 accumulated person-years, there were 77 614 deaths (18.7%; 49 297 men and 28 317 women). These deaths included 28 099 from cancer (17 816 men and 10 283 women), 22 228 from CVD (14 647 men and 7581 women), of whom 17 901 were from heart disease and 3748 were from stroke; 5606 from respiratory disease (3135 men and 2471 women); 2923 from infectious disease (1796 men and/1127 women); 2614 from injuries and accidents (1878 men and 736 women); and 16 144 from all other causes combined (10 025 men and 6119 women).
Plant protein intake was significantly inversely associated with age-adjusted mortality from all causes in both men (Table 2) and women (Table 3). After multivariable adjustment, inverse associations remained significant for overall mortality in both sexes. In men, the HRs were 0.95 (95% CI, 0.94-0.97) per 1 SD and 0.88 (95% CI, 0.84-0.91) per 10 g/1000 kcal increment, with adjusted ARDs (at 16 years of follow-up) of −0.36% (95% CI, −0.48% to −0.25%) per 1 SD and −0.95% (95% CI, −1.3% to −0.68%) per 10 g/1000 kcal increment (P < .001) (Table 2). Corresponding adjusted HRs in women were 0.95 (95% CI, 0.93-0.96) per 1 SD and 0.86 (95% CI, 0.82-0.90) per 10 g/1000 kcal increment, with adjusted ARDs of −0.33% (95% CI, −0.48% to −0.21%) per 1 SD and −0.86% (95% CI, −1.3% to −0.55%) per 10 g/1000 kcal increment (P < .001) (Table 3). The propensity score–adjusted risk estimates remained essentially unchanged compared with those from the multivariable-adjusted analyses for both men and women (for overall mortality, propensity score–adjusted HRs were 0.95 [95% CI, 0.94-0.97] per 1 SD for men and 0.92 [95% CI, 0.90-0.93] per 1 SD for women; both P < .001). Additional adjustment for consumption of sugar-sweetened beverages and total carbohydrates as well as for median household income did not materially change our association estimates (eTable 2 and eTable 3 in the Supplement).
Table 2. Risk of Overall and Cause-Specific Mortality Associated With Daily Dietary Plant Protein Intake Among 237 036 Men.
| Cause of death | Per 1-SD increment | Per 10 g/1000 kcal increment | ||||
|---|---|---|---|---|---|---|
| ARD, % (95% CI)a | HR (95% CI) | P value | ARD, % (95% CI)a | HR (95% CI) | P value | |
| Overall | ||||||
| Age adjustedb | −1.2 (−1.3 to −1.1) | 0.86 (0.85 to 0.87) | <.001 | −3.0 (−3.2 to −2.8) | 0.66 (0.64 to 0.67) | <.001 |
| Multivariablec | −0.36 (−0.48 to −0.25) | 0.95 (0.94 to 0.97) | <.001d | −0.95 (−1.3 to −0.68) | 0.88 (0.84 to 0.91) | <.001d |
| Cancer | ||||||
| Age adjustedb | −0.54 (−0.62 to −0.50) | 0.85 (0.84 to 0.87) | <.001 | −1.3 (−1.5 to −1.2) | 0.65 (0.62 to 0.68) | <.001 |
| Multivariablec | −0.073 (−0.17 to 0.0018) | 0.98 (0.96 to 1.00) | .09 | −0.20 (−0.44 to 0.0024) | 0.94 (0.88 to 1.01) | .09 |
| CVD | ||||||
| Age adjustedb | −0.36 (−0.43 to −0.31) | 0.88 (0.86 to 0.90) | <.001 | −0.90 (−1.1 to −0.79) | 0.70 (0.67 to 0.74) | <.001 |
| Multivariablec | −0.13 (−0.22 to −0.050) | 0.95 (0.93 to 0.98) | <.001d | −0.35 (−0.59 to −0.14) | 0.88 (0.81 to 0.95) | <.001d |
| Heart disease | ||||||
| Age adjustedb | −0.30 (−0.36 to −0.25) | 0.88 (0.87 to 0.90) | <.001 | −0.75 (−0.90 to −0.64) | 0.71 (0.68 to 0.75) | <.001 |
| Multivariablec | −0.087 (−0.17 to −0.0088) | 0.97 (0.94 to 0.99) | .02 | −0.23 (−0.44 to −0.028) | 0.91 (0.84 to 0.98) | .02 |
| Stroke | ||||||
| Age adjustedb | −0.074 (−0.10 to −0.049) | 0.86 (0.82 to 0.90) | <.001 | −0.18 (−0.24 to −0.13) | 0.66 (0.58 to 0.75) | <.001 |
| Multivariablec | −0.066 (−0.11 to −0.030) | 0.88 (0.82 to 0.94) | <.001d | −0.17 (−0.26 to −0.08) | 0.70 (0.58 to 0.85) | <.001d |
| Respiratory disease | ||||||
| Age adjustedb | −0.25 (−0.29 to −0.22) | 0.69 (0.66 to 0.72) | <.001 | −0.54 (−0.61 to −0.50) | 0.36 (0.33 to 0.40) | <.001 |
| Multivariablec | −0.018 (−0.066 to 0.027) | 0.98 (0.92 to 1.04) | .42 | −0.049 (−0.17 to 0.071) | 0.93 (0.79 to 1.10) | .42 |
| Infection | ||||||
| Age adjustedb | −0.066 (−0.085 to −0.046) | 0.85 (0.81 to0.90) | <.001 | −0.16 (−0.20 to −0.12) | 0.65 (0.56 to 0.74) | <.001 |
| Multivariablec | −0.0079 (−0.037 to 0.023) | 0.98 (0.91 to 1.06) | .65 | −0.021 (−0.099 to 0.061) | 0.95 (0.77 to 1.18) | .65 |
| Injury and accident | ||||||
| Age adjustedb | −0.052 (−0.077 to −0.030) | 0.89 (0.84 to 0.93) | <.001 | −0.13 (−0.19 to −0.079) | 0.71 (0.62 to 0.82) | <.001 |
| Multivariablec | −0.044 (−0.079 to −0.012) | 0.90 (0.84 to 0.97) | .009 | −0.11 (−0.19 to −0.037) | 0.76 (0.61 to 0.93) | .009 |
| Other causes combined | ||||||
| Age adjustedb | −0.23 (−0.28 to −0.20) | 0.89 (0.87 to 0.91) | <.001 | −0.57 (−0.70 to −0.50) | 0.72 (0.68 to 0.77) | <.001 |
| Multivariablec | −0.18 (−0.25 to −0.13) | 0.91 (0.88 to 0.94) | <.001d | −0.46 (−0.63 to −0.33) | 0.78 (0.71 to 0.85) | <.001d |
Abbreviations: ARD, absolute risk difference; CVD, cardiovascular disease; HR, hazard ratio.
Calculated as a difference of 1-SD or 10 g/1000 kcal increment per day in plant protein intake during the follow-up of 16 years. The 95% CIs were estimated from 100 bootstrap samples.
For age at entry, total energy intake, and animal protein intake.
Adjusted for age at entry (continuous), body mass index (<18.5, 18.5 to <25, 25 to <30, 30 to <35, and ≥35), alcohol consumption (none to ≤1, >1 to 3, and >3 drinks per day), smoking status (never, former with ≤20 cigarettes per day, former with >20 cigarettes per day, current with ≤20 cigarettes per day, current with >20 cigarettes per day, or missing), physical activity (never/rarely, 1-3 times per month, 1-2 times per week, 3-4 times per week, ≥5 times/week, or missing), race or ethnic group (non-Hispanic white, non-Hispanic black, or other race/ethnicity), educational level (<high school, high school graduate, post–high school training or some college,≥college graduate, or missing), marital status (yes vs no), diabetes (yes vs no), health status (poor to fair, good, or very good to excellent, or unknown), vitamin supplement use (yes vs no), and daily dietary total energy, animal protein, saturated fat, polyunsaturated fat, monounsaturated fat, trans fat, fiber, vegetables, and fruits (all continuous). For the end point of cancer mortality, the model was further adjusted for history of cancer in a first-degree relative (yes vs no).
Achieved Bonferroni-corrected threshold (.05/18 = .0028).
Table 3. Risk of Overall and Cause-Specific Mortality Associated With Daily Dietary Plant Protein Intake Among 179 068 Women.
| Cause of death | Per 1 SD | Per 10 g/1000 kcal increment | ||||
|---|---|---|---|---|---|---|
| ARD, % (95% CI)a | HR (95% CI) | P value | ARD, % (95% CI)a | HR (95% CI) | P value | |
| Overall | ||||||
| Age adjustedb | −1.0 (−1.1 to −0.95) | 0.85 (0.83 to 0.86) | <.001 | −2.5 (−2.8 to −2.4) | 0.63 (0.61 to 0.66) | <.001 |
| Multivariablec | −0.33 (−0.48 to −0.21) | 0.95 (0.93 to 0.96) | <.001d | −0.86 (−1.3 to −0.55) | 0.86 (0.82 to 0.90) | <.001d |
| Cancer | ||||||
| Age adjustedb | −0.38 (−0.46 to −0.33) | 0.87 (0.85 to 0.89) | <.001 | −0.96 (−1.1 to −0.84) | 0.68 (0.65 to 0.73) | <.001 |
| Multivariablec | −0.092 (−0.19 to −0.012) | 0.97 (0.94 to 1.00) | .04 | −0.24 (−0.49 to −0.035) | 0.91 (0.84 to 0.99) | .04 |
| CVD | ||||||
| Age adjustedb | −0.32 (−0.38 to −0.27) | 0.85 (0.83 to 0.87) | <.001 | −0.78 (−0.93 to −0.68) | 0.65 (0.60 to 0.69) | <.001 |
| Multivariablec | −0.13 (−0.22 to −0.053) | 0.93 (0.90 to 0.97) | <.001d | −0.35 (−0.57 to −0.15) | 0.83 (0.75 to 0.92) | <.001 |
| Heart disease | ||||||
| Age adjustedb | −0.24 (−0.29 to −0.19) | 0.86 (0.83 to 0.88) | <.001 | −0.59 (−0.72 to −0.49) | 0.66 (0.61 to 0.71) | <.001 |
| Multivariablec | −0.070 (−0.14 to 0.0028) | 0.96 (0.92 to 1.00) | .04 | −0.18 (−0.37 to 0.0032) | 0.89 (0.79 to 0.99) | .04 |
| Stroke | ||||||
| Age adjustedb | −0.079 (−0.11 to −0.049) | 0.85 (0.80 to 0.89) | <.001 | −0.19 (−0.26 to −0.13) | 0.64 (0.55 to 0.74) | <.001 |
| Multivariablec | −0.060 (−0.10 to −0.019) | 0.88 (0.81 to 0.96) | .003d | −0.15 (−0.25 to −0.052) | 0.71 (0.57 to 0.89) | .003d |
| Respiratory disease | ||||||
| Age adjustedb | −0.28 (−0.32 to −0.24) | 0.68 (0.65 to 0.71) | <.001 | −0.60 (−0.69 to −0.55) | 0.35 (0.31 to 0.39) | <.001 |
| Multivariablec | −0.037 (−0.097 to 0.017) | 0.95 (0.89 to 1.01) | .12 | −0.097 (−0.25 to 0.043) | 0.87 (0.73 to 1.04) | .12 |
| Infection | ||||||
| Age adjustedb | −0.072 (−0.098 to −0.049) | 0.81 (0.76 to 0.86) | <.001 | −0.17 (−0.23 to −0.12) | 0.56 (0.47 to 0.67) | <.001 |
| Multivariablec | −0.042 (−0.081 to −0.0035) | 0.89 (0.81 to 0.98) | .02 | −0.10 (−0.20 to −0.013) | 0.73 (0.56 to 0.94) | .02 |
| Injury and accident | ||||||
| Age adjustedb | −0.016 (−0.036 to 0.0051) | 0.93 (0.86 to 1.00) | .06 | −0.042 (−0.090 to 0.012) | 0.82 (0.66 to 1.01) | .06 |
| Multivariablec | −0.013 (−0.042 to 0.018) | 0.94 (0.84 to 1.06) | .32 | −0.035 (−0.11 to 0.045) | 0.85 (0.62 to 1.17) | .32 |
| Other causes | ||||||
| Age adjustedb | −0.22 (−0.28 to −0.18) | 0.87 (0.85 to 0.90) | <.001 | −0.55 (−0.69 to −0.45) | 0.69 (0.64 to 0.74) | <.001 |
| Multivariablec | −0.11 (−0.21 to −0.039) | 0.93 (0.90 to 0.97) | <.001d | −0.29 (−0.53 to −0.11) | 0.83 (0.74 to 0.93) | <.001d |
Abbreviations: ARD, absolute risk difference; CVD, cardiovascular disease; HR, hazard ratio.
Calculated as a difference of 1-SD or 10 g/1000 kcal increment per day in plant protein intake during the follow-up of 16 years. The 95% CIs were estimated from 100 bootstrap samples.
For age at entry, total energy intake, and animal protein intake.
Adjusted as indicated in note c of Table 2, and further adjusted for hormone replacement therapy (yes vs no).
Achieved Bonferroni-corrected threshold (.05/18 = .0028).
By contrast, animal protein intake was not associated with multivariable-adjusted mortality in men (HR, 0.99; 95% CI, 0.98-1.00; P = .18) or women (HR, 0.98; 95% CI, 0.97-1.00; P = .02) (eTable 4 in the Supplement).
Dietary Plant Protein Intake and Cause-Specific Mortality
Plant protein intake was inversely associated with mortality from all CVD, stroke, and “other causes combined,” representing risk reductions of 5% to 12% per 1-SD intake increment and 12% to 30% per 10 g/1000 kcal intake increment in men, and 7% to 12% per 1 SD and 17% to 29% per 10 g/1000 kcal in women (all P < .003). The adjusted ARDs at 16 years of follow-up for CVD mortality were −0.13% (95% CI, −0.22% to −0.05%) per 1-SD intake increment and −0.35% per (95% CI, −0.59% to −0.14%) per 10 g/1000 kcal increment in men, and −0.13% (95% CI, −0.22% to −0.053%) per 1 SD and −0.35% (95% CI, −0.57% to −0.15%) per 10 g/1000 kcal in women. The corresponding adjusted ARDs for stroke mortality were −0.07% (95% CI, −0.11% to −0.03%) per 1-SD intake increment and −0.17% (95% CI, −0.26% to −0.08%) per 10 g/1000 kcal increment in men, and −0.06% (95% CI, −0.1% to −0.019%) per 1-SD intake increment and −0.15% (95% CI, −0.25% to −0.052%) per 10 g/1000 kcal increment in women (Table 2 and Table 3). By contrast, plant protein intake was not significantly associated with mortality from cancer, heart disease, respiratory disease, infections or injuries/accidents in either men or women (all P > .003) (Table 2 and Table 3).
Dietary Plant Protein Intake and Overall Mortality in Cohort Subgroups
The Figure provides key findings from stratified multivariable adjusted analyses of cohort subgroups. As illustrated there, HRs of overall mortality are for 1-SD increment of plant protein intake. Multivariable analyses were adjusted for age at entry (continuous), BMI, alcohol consumption, smoking status (never, former with ≤20 cigarettes a day, former with >20 cigarettes a day, current with ≤20 cigarettes a day, current with >20 cigarettes a day, or missing), physical activity (never or rarely, 1-3 times per month, 1-2 times per week, 3-4 times per week, ≥5 times per week, or missing), race or ethnic group (non-Hispanic white, non-Hispanic black, other race/ethnicity), educational level (<high school, high school graduate, post–high school training or some college, college graduate or higher, or missing), marital status (yes vs no), diabetes, health status, vitamin supplement use, and daily dietary total energy, animal protein, saturated fat, polyunsaturated fat, monounsaturated fat, trans fat, fiber, vegetables, and fruits (all continuous). For women, risk estimates were additionally adjusted for postmenopausal hormone replacement therapy. For the end point of cancer mortality, the model was further adjusted for history of cancer in a first-degree relative (yes vs no). The value of P for interaction was assessed by the likelihood ratio test, entering the cross-product term of plant protein (continuous variable) and the stratification variables (ordinal variable) into the Cox proportional hazards regression model.
Figure. Risk of Overall Mortality Associated With Intake of Plant Protein According to Subgroups Among 237 036 Men and 179 068 Women.
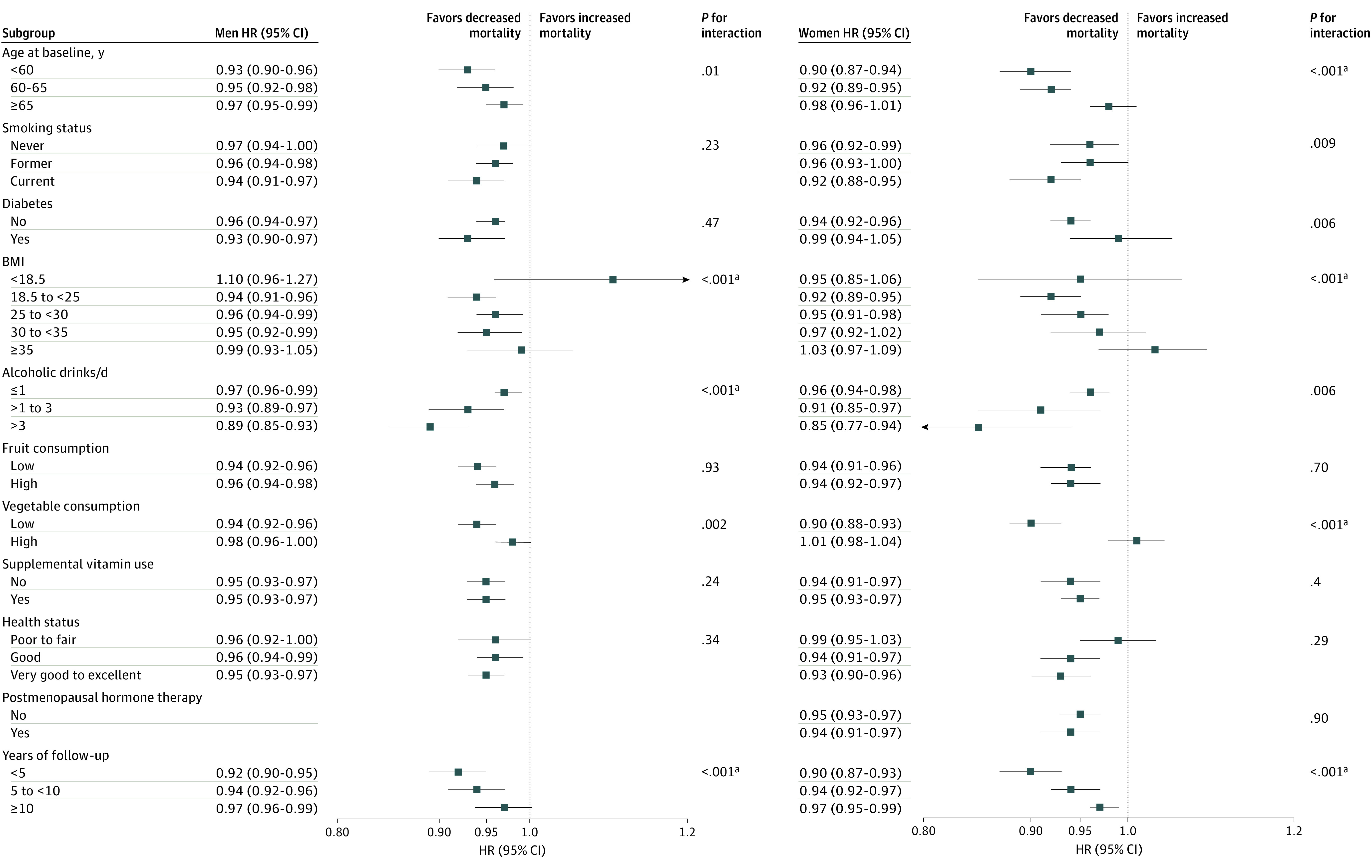
The illustrated analysis is detailed in the Results section at the first mention of the Figure. HR indicates hazard ratio; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
aAchieved Bonferroni-corrected threshold (.05/21 = .0024).
The overall mortality association with a 1-SD increment of plant protein intake was largely similar across population subsets based on smoking status, diabetes, fruit consumption, vitamin supplement use, and self-reported health status in both men and women, and on use of postmenopausal hormone therapy for women. By contrast, significant differences were observed in subgroups of BMI and years of follow-up (in both sexes), alcohol consumption (men), and age and vegetable consumption (women) (Figure). We observed stronger inverse associations for those with BMI of 18.5 to 25 (men: HR, 0.94; 95% CI, 0.91-0.96; women: HR, 0.92; 95% CI, 0.89-0.95), follow-up time of less than 5 years (men: HR, 0.92; 95% CI, 0.90-0.95; women: HR, 0.90; 95% CI, 0.87-0.93), consumption of 3 drinks or more daily (HR, 0.89; 95% CI, 0.85-0.93) for men, and for women who were younger than 60 years of age (HR, 0.90; 95% CI, 0.87-0.94) or who reported lower dietary vegetable consumption (HR, 0.90; 95% CI, 0.88-0.93). Although we observed a stronger inverse plant protein intake–mortality association during the first 5 years of cohort follow-up, our primary findings were not materially altered by excluding the first 5 years of observation, with overall HRs among men of 0.95 (95% CI, 0.94-0.97) per 1-SD intake increment and 0.88 (95% CI, 0.84-0.95) per 10 g/1000 kcal and corresponding HRs of 0.96 (95% CI, 0.94-0.98) per 1 SD and 0.88 (95% CI, 0.84-0.93) per 10 g/1000 kcal for women (all P < .001).
Substitution of 3% Energy From Plant Protein for Specific Animal Protein Sources
Table 4 presents mortality data for substitution of 3% energy from plant protein for the equivalent amount of animal protein of various sources and shows a significant association with lower overall mortality (men: HR, 0.90; 95% CI, 0.88-0.93; women: HR, 0.90; 95% CI, 0.87-0.93; all P < .001), and CVD mortality (men: HR, 0.89; 95% CI, 0.85-0.94; women: HR, 0.88; 95% CI, 0.82-0.94; P < .001), including in men mortality from heart disease (HR, 0.91; 95% CI, 0.86-0.96; P < .001) and stroke (HR, 0.78; 95% CI, 0.68-0.90; P < .001).
Table 4. Risk of Overall and Cause-Specific Mortality Associated With Substitution of 3% Dietary Energy Intake From Plant Protein for Various Animal Protein Sources Among 237 036 Men and 179 068 Womena.
| Animal protein source | Men | Women | ||
|---|---|---|---|---|
| HR (95% CI)b | P value | HR (95% CI)b | P value | |
| Total animal protein | ||||
| Overall | 0.90 (0.88-0.93) | <.001c | 0.90 (0.87-0.93) | <.001c |
| Cancer | 0.97 (0.92-1.02) | .19 | 0.93 (0.88-0.99) | .02 |
| CVD | 0.89 (0.85-0.94) | <.001c | 0.88 (0.82-0.94) | <.001c |
| Heart disease | 0.91 (0.86-0.96) | <.001c | 0.90 (0.84-0.98) | .01 |
| Stroke | 0.78 (0.68-0.90) | <.001c | 0.81 (0.70-0.94) | .007 |
| Respiratory disease | 0.92 (0.82-1.03) | .14 | 0.92 (0.82-1.04) | .18 |
| Infection | 0.99 (0.85-1.14) | .86 | 0.81 (0.68-0.97) | .02 |
| Injury and accident | 0.82 (0.71-0.95) | .008 | 0.89 (0.72-1.11) | .30 |
| Other causes combined | 0.83 (0.78-0.88) | <.001c | 0.90 (0.83-0.97) | .005 |
| Red meat protein | ||||
| Overall | 0.87 (0.85-0.90) | <.001c | 0.85 (0.81-0.88) | <.001c |
| Cancer | 0.93 (0.88-0.98) | .004 | 0.89 (0.83-0.95) | <.001c |
| CVD | 0.88 (0.83-0.93) | <.001c | 0.82 (0.76-0.89) | <.001c |
| Heart disease | 0.89 (0.84-0.94) | <.001c | 0.84 (0.77-0.92) | <.001c |
| Stroke | 0.79 (0.68-0.91) | .001c | 0.75 (0.63-0.89) | <.001c |
| Respiratory disease | 0.83 (0.74-0.94) | .003 | 0.80 (0.70-0.91) | .001c |
| Infection | 0.94 (0.81-1.10) | .47 | 0.75 (0.62-0.92) | .006 |
| Injury and accident | 0.80 (0.69-0.94) | .005 | 0.90 (0.71-1.16) | .42 |
| Other causes combined | 0.80 (0.75-0.86) | <.001c | 0.85 (0.78-0.92) | <.001c |
| White meat protein | ||||
| Overall | 0.98 (0.94-1.01) | .11 | 0.95 (0.91-0.98) | .005 |
| Cancer | 1.02 (0.97-1.08) | .40 | 0.93 (0.87-0.99) | .02 |
| CVD | 0.95 (0.90-1.01) | .07 | 0.94 (0.87-1.02) | .12 |
| Heart disease | 0.97 (0.91-1.03) | .27 | 0.97 (0.89-1.05) | .41 |
| Stroke | 0.83 (0.71-0.96) | .01 | 0.90 (0.76-1.06) | .20 |
| Respiratory disease | 1.17 (1.03-1.33) | .02 | 1.06 (0.93-1.21) | .40 |
| Infection | 1.06 (0.90-1.25) | .52 | 0.88 (0.72-1.07) | .20 |
| Injury and accident | 0.91 (0.77-1.07) | .23 | 0.99 (0.78-1.26) | .96 |
| Other causes combined | 0.90 (0.84-0.97) | .004 | 0.90 (0.84-0.97) | .004 |
| Dairy protein | ||||
| Overall | 0.92 (0.89-0.95) | <.001c | 0.92 (0.89-0.95) | <.001c |
| Cancer | 1.01 (0.96-1.06) | .73 | 0.97 (0.91-1.03) | .31 |
| CVD | 0.89 (0.84-0.94) | <.001c | 0.88 (0.82-0.95) | <.001c |
| Heart disease | 0.91 (0.86-0.97) | .003c | 0.92 (0.84-0.99) | .03 |
| Stroke | 0.77 (0.66-0.89) | <.001c | 0.80 (0.69-0.94) | .006 |
| Respiratory disease | 0.94 (0.83-1.06) | .31 | 0.97 (0.85-1.10) | .60 |
| Infection | 0.98 (0.84-1.15) | .79 | 0.80 (0.67-0.97) | .02 |
| Injury and accident | 0.83 (0.71-0.98) | .02 | 0.88 (0.70-1.10) | .25 |
| Other causes combined | 0.83 (0.78-0.89) | <.001c | 0.90 (0.83-0.97) | .008 |
| Egg protein | ||||
| Overall | 0.76 (0.72-0.80) | <.001c | 0.79 (0.73-0.85) | <.001c |
| Cancer | 0.85 (0.78-0.93) | <.001c | 0.83 (0.73-0.93) | .002c |
| CVD | 0.74 (0.67-0.82) | <.001c | 0.72 (0.63-0.83) | <.001c |
| Heart disease | 0.76 (0.69-0.85) | <.001c | 0.72 (0.62-0.85) | <.001c |
| Stroke | 0.67 (0.52-0.88) | .003 | 0.75 (0.55-1.03) | .08 |
| Respiratory disease | 0.61 (0.50-0.74) | <.001c | 0.66 (0.53-0.83) | <.001c |
| Infection | 0.94 (0.71-1.25) | .66 | 0.80 (0.55-1.17) | .25 |
| Injury and accident | 0.71 (0.53-0.94) | .02 | 0.67 (0.43-1.05) | .08 |
| Other causes combined | 0.71 (0.63-0.80) | <.001c | 0.92 (0.78-1.08) | .28 |
Abbreviations: CVD, cardiovascular disease; HR, hazard ratio.
Plant, animal and total protein were modeled as percentages of energy, using energy from the specific protein divided by total energy intake, and the leave-1-out model was used as the substitution approach: to evaluate the substitution of 3% energy from plant protein for an individual component of animal protein (red meat protein, white meat protein, dairy protein and egg protein), energy from plant protein, total protein, and other 3 components of animal protein were simultaneously included in the model. The HRs represent the relative risk of overall mortality with 3% energy from plant protein substituted for the excluded animal protein energy.
Adjusted as indicated in note c of Table 2, and for women, the risk estimates were additionally adjusted for postmenopausal hormone replacement therapy (yes vs no).
Achieved Bonferroni-corrected threshold (.05/18 = .0028).
Examination of substitution of 3% energy from plant protein for specific sources of animal protein revealed that the mortality reduction appeared strongest with substitution for egg protein, with 15% to 39% lower risk in men and 17% to 34% lower risk in women for overall (24% lower risk in men and 21% lower risk in women), cancer, CVD, heart disease, and respiratory disease mortality (Table 4). Substitution of 3% energy from plant protein for red meat protein intake showed stronger mortality reductions of 11% to 21% in men and 11% to 25% in women for overall (13% lower risk in men and 15% lower risk in women), CVD, heart disease, and stroke mortality in both sexes, and cancer and respiratory disease mortality in women. A similar change from dairy protein to plant protein was associated with lower overall and CVD mortality in men and women, and heart disease and stroke mortality in men, with overall mortality HRs of 0.92 in both men and women (P < .001). However, substituting of 3% energy from plant protein for white meat protein was not associated with risk of mortality (Table 4).
Substitution of 3% Energy From Specific Dietary Plant Protein Sources for Egg Protein and Red Meat Protein Intake
In this sensitivity analysis of specific plant protein sources substituting 3% energy for egg protein and red meat protein, we observed lower overall and cause-specific mortality (with the exception of deaths from infection, injuries, and accidents) when exchanging bread, cereal, and pasta plant protein for egg protein, with risk reductions of 18% to 45% in men and 20% to 39% in women (eTable 5 in the Supplement). Similarly, we found 12% to 30% lower mortality in men, and 13% to 28% lower mortality in women, when substituting for red meat protein (again, except for deaths from infection in men and women, and from injuries and accidents in women) (eTable 6 in the Supplement).
Discussion
In this analysis of a large prospective cohort of men and women in the US with 16 years of observation, we found higher plant protein intake was associated with reduced risk of overall mortality, with men and women experiencing (respectively) 12% and 14% lower mortality per 10 g/1000 kcal intake increment (5% lower mortality per 1-SD increment). The inverse association was apparent for CVD and stroke mortality in both sexes, was independent of several risk factors, and was evident in most other cohort subgroups. Replacement of 3% energy from various animal protein sources with plant protein was associated with 10% decreased overall mortality in both sexes. Of note, substitution analyses suggested that replacement of egg protein and red meat protein with plant protein resulted in the most prominent protective associations for overall mortality, representing 24% and 21% lower risk for men and women, respectively, for egg protein replacement, and 13% and 15% lower risk for men and women for red meat protein replacement. The effect sizes of these risk estimates were small.
Relatively few prospective studies have examined the association between dietary protein sources and overall mortality. Concordant with the present findings, the NHS and HPFS cohorts found an inverse association for higher plant protein intake and overall and CVD mortality, and reduced overall mortality for greater substitution of plant protein for animal protein.12 Increased plant protein intake was also associated with lower risk of total and CVD mortality in a cohort in Japan,13 whereas a study of postmenopausal women in the US showed that replacement of animal protein with plant protein led to decreased coronary heart disease mortality.11 Collectively, the present and previous studies indicate the importance and diverse effects of primary dietary protein sources, including beneficial associations for higher plant protein intake in long-term health outcomes.
Considerable evidence has accumulated supporting a beneficial role for plant-based diets in the prevention of cardiovascular disease. Dietary plant protein has been associated with reduced CVD risk factors, including lower systolic and diastolic blood pressure, improved lipid and lipoprotein profiles, and decreased circulating concentrations of insulin-like growth factor-1.20,21,22 Consistent with our findings, a recent meta-analysis of randomized clinical trials has shown that substitution of red meat with protein from high-quality plant sources, including legumes, soy, nuts, and other foods, was associated with benefits on lipid profiles, including favorable reductions in blood total cholesterol and low-density lipoprotein cholesterol.23 Replacement of 1 standard serving of red meat with nuts and seeds daily reduced coronary heart disease risk by 30% in the NHS,24 and greater animal protein intake was associated with 25% higher risk of ischemic heart disease among healthy men in the HPFS.25 In addition, lower risks of coronary heart disease and stroke were associated with substituting other protein sources (ie, fish and poultry) for red meat in the analysis of the NHS and HPFS.24,26 Underlying mechanisms for the CVD mortality associations remain unclear, although different amino acid constituents, protein source-related endogenous responses, and coexisting bioactive nutrients in the plant food matrix have been suggested as contributory. For example, plant-based proteins contain higher concentrations of nonessential amino acids (eg, arginine and glycine) and fewer essential amino acids (eg, methionine, lysine, and tryptophan), which could potentially influence cardiovascular health through lower arterial stiffness or decreased systemic and vascular generation of reactive oxygen species.10,27,28,29 In addition, increased concentrations in circulation of the gut microbiome-generated metabolite trimethylamine N-oxide, which results from higher intake of L-carnitine and choline from animal-based diets,10 has been proposed to mediate the effect of protein sources on cardiovascular health associated with increased risk of major adverse cardiac events.30,31 Beyond such potential direct effects of protein per se, other bioactive nutrients in foods may influence health, including heme iron, nitrates and nitrites, and sodium in red and processed meat32,33 and unsaturated fatty acids, antioxidants, vitamins, and trace minerals in plant-based foods.34,35 Our findings of more pronounced inverse plant protein associations in women who were younger than 60 years, and in both men and women with a favorable BMI, suggest that those health effects can be overshadowed by, for example, metabolic impairment-associated oxidative stress and systemic chronic inflammation.
Our observation of stronger inverse mortality associations for plant protein from bread, cereal, and pasta substitution of red meat protein and egg protein do not stand alone in the literature. Higher red and processed meat consumption have been consistently associated with increased premature death, possibly owing to systemic oxidative stress, inflammation, heme iron, and endogenous formation of N-nitroso compounds.32,36,37,38 Grains and cereal foods have high contents of bioactive substances, including phenolic compounds, antioxidants, vitamins, minerals, and phytoestrogens,39 and increased whole grain consumption has been favorably associated with reduced cardiovascular disease risk factors, including hypertension, total cholesterol and low-density lipoprotein cholesterol.40,41,42 Moreover, a meta-analysis of 17 prospective cohorts that included 150 328 deaths found greater consumption of total red meat and processed meat was associated with excess risk of overall, CVD, and cancer mortality,38 and lower total mortality risk and cause-specific mortality risk in persons having greater consumption of plant protein–rich whole grains and cereal fiber.43,44 Consistent with our findings, it has been shown that replacement of 3% energy from egg protein with plant protein was associated with decreased risk of overall mortality, with mortality risk reductions of 19% and 18% in US cohorts12 and a Japanese cohort,13 respectively. In addition, an analysis of 6 prospective US cohorts, showed that higher egg consumption was associated with increased risk of incident CVD and all-cause mortality in a dose-response manner.45
Strengths and Limitations
There are several strengths of our study. The large cohort sample size and number of events as well as long-term follow-up afforded substantial power to detect moderate associations between substitution of plant protein for animal protein and mortality risk and to assess effect modification by other risk factors. The large number of deaths permitted a robust examination of cause-specific mortality. Of note, approximately 40% of daily dietary protein was from plant sources and 60% was animal protein (including 19% dairy protein), which are similar to the plant and animal protein intakes of the US population.46 In addition, 15.3% of total calories (median) from protein consumption in our study is very similar to that in the general US population (15%-16%),47,48 supporting the representativeness of our population. Study limitations include our use of a food frequency questionnaire to assess intake of plant protein and animal protein, with inherent measurement error (eg, underreporting of dietary intakes49) that would underestimate associations as a result of nondifferential misclassification and bias risk estimates toward the null. Although 24-hour dietary recalls are reasonable validation instruments but not criterion standards (eg, duplicate plate measurements), and they entail substantial measurement error, a previous validation study of the NIH-AARP DHQ and 24-hour dietary recalls reported correlations for total protein intake of 0.43 in men and 0.50 in women, reflecting moderate validity and reliability.15 Our analysis was based on a single dietary assessment at baseline, with possible unknown dietary changes during the follow-up period. Although the quantitative plant protein substitution interaction with follow-up time results were significant, the mortality risk estimates for the periods of 0 to fewer than 5 years, 5 to fewer than 10 years, and 10 or more years were essentially similar, suggesting consistency of the associations over time. The vast majority of our study participants were non-Hispanic white, which reduces the generalizability of the findings to other racial/ethnic populations. The latest linkage of the AARP-NIH cohort to the National Death Index contained cause-specific mortality through December 31, 2011, with the current analysis being based on 237 036 men, 179 068 women, and 77 614 deaths during 16 years of follow-up. The next linkage has been delayed logistically by the coronavirus disease 2019 pandemic, with no precise estimate of when we might have access to those data. In addition, because our findings are unlikely to be materially altered by the additional events, the present analysis was conducted with the currently available follow-up. Finally, we cannot preclude the possibility of residual confounding, including from unmeasured factors, which may have biased the observed associations. We did, however, control for a wide range of known potential confounding factors and used a propensity score to adjust for variation between comparison groups, and our main findings remained essentially unchanged.
Conclusions
In conclusion, this large cohort investigation showed small but significant associations between higher intake of plant protein and lower overall and CVD mortality, with prominent inverse associations observed for replacement of egg protein and red meat protein with plant protein, particularly for plant protein derived from bread, cereal, and pasta. Findings from this and previous studies provide evidence that dietary modifications in choice of protein sources may promote health and longevity.
eTable 1. Baseline Characteristics of the Study Participants According to Fifths of Daily Dietary Animal Protein Intake
eTable 2. Risk of Overall and Cause-Specific Mortality Associated With Daily Dietary Plant Protein Intake (per 1-SD) Among 237,036 Men and 179,068 Women (Further Adjusted for Intake of Sugar-Sweetened Beverages and Carbohydrates)
eTable 3. Risk of Overall and Cause-Specific Mortality Associated With Daily Dietary Plant Protein Intake (per 1-SD) Among 237,036 Men and 179,068 Women (Further Adjusted for Median Household Income)
eTable 4. Risk of Overall and Cause-Specific Mortality Associated With Daily Dietary Animal Protein Intake Among 237,036 Men and 179,068 Women
eTable 5. Risk of Overall and Cause-Specific Mortality Associated With Substitution of 3% Energy From Different Sources of Plant Protein for Egg Protein Among 237,036 Men And 179,068 Women
eTable 6. Risk of Overall and Cause-Specific Mortality Associated With Substitution of 3% Energy From Different Sources of Plant Protein for Red Meat Protein Among 237,036 Men and 179,068 Women
eAppendix 1. Methods: Mortality Assessment and Statistical Analysis
eAppendix 2. Results: Animal Protein Intake and Dietary and Lifestyle Factors
References
- 1.Rodriguez NR. Introduction to Protein Summit 2.0: continued exploration of the impact of high-quality protein on optimal health. Am J Clin Nutr. 2015;101(6):1317S-1319S. doi: 10.3945/ajcn.114.083980 [DOI] [PubMed] [Google Scholar]
- 2.Leidy HJ, Clifton PM, Astrup A, et al. The role of protein in weight loss and maintenance. Am J Clin Nutr. 2015;101(6):1320S-1329S. doi: 10.3945/ajcn.114.084038 [DOI] [PubMed] [Google Scholar]
- 3.Westerterp-Plantenga MS, Nieuwenhuizen A, Tomé D, Soenen S, Westerterp KR. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21-41. doi: 10.1146/annurev-nutr-080508-141056 [DOI] [PubMed] [Google Scholar]
- 4.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281-1298. doi: 10.3945/ajcn.112.044321 [DOI] [PubMed] [Google Scholar]
- 5.Santesso N, Akl EA, Bianchi M, et al. Effects of higher- versus lower-protein diets on health outcomes: a systematic review and meta-analysis. Eur J Clin Nutr. 2012;66(7):780-788. doi: 10.1038/ejcn.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tielemans SM, Altorf-van der Kuil W, Engberink MF, et al. Intake of total protein, plant protein and animal protein in relation to blood pressure: a meta-analysis of observational and intervention studies. J Hum Hypertens. 2013;27(9):564-571. doi: 10.1038/jhh.2013.16 [DOI] [PubMed] [Google Scholar]
- 7.Rebholz CM, Friedman EE, Powers LJ, Arroyave WD, He J, Kelly TN. Dietary protein intake and blood pressure: a meta-analysis of randomized controlled trials. Am J Epidemiol. 2012;176(suppl 7):S27-S43. doi: 10.1093/aje/kws245 [DOI] [PubMed] [Google Scholar]
- 8.Dong JY, Zhang ZL, Wang PY, Qin LQ. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: meta-analysis of randomised controlled trials. Br J Nutr. 2013;110(5):781-789. doi: 10.1017/S0007114513002055 [DOI] [PubMed] [Google Scholar]
- 9.Wu G. Dietary protein intake and human health. Food Funct. 2016;7(3):1251-1265. doi: 10.1039/C5FO01530H [DOI] [PubMed] [Google Scholar]
- 10.Richter CK, Skulas-Ray AC, Champagne CM, Kris-Etherton PM. Plant protein and animal proteins: do they differentially affect cardiovascular disease risk? Adv Nutr. 2015;6(6):712-728. doi: 10.3945/an.115.009654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelemen LE, Kushi LH, Jacobs DR Jr, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am J Epidemiol. 2005;161(3):239-249. doi: 10.1093/aje/kwi038 [DOI] [PubMed] [Google Scholar]
- 12.Song M, Fung TT, Hu FB, et al. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern Med. 2016;176(10):1453-1463. doi: 10.1001/jamainternmed.2016.4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Budhathoki S, Sawada N, Iwasaki M, et al. ; Japan Public Health Center–based Prospective Study Group . Association of animal and plant protein intake with all-cause and cause-specific mortality in a Japanese cohort. JAMA Intern Med. 2019;179(11):1509-1518. doi: 10.1001/jamainternmed.2019.2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119-1125. doi: 10.1093/aje/154.12.1119 [DOI] [PubMed] [Google Scholar]
- 15.Thompson FE, Kipnis V, Midthune D, et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11(2):183-195. doi: 10.1017/S1368980007000419 [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4)(suppl):1220S-1228S. doi: 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- 17.Brown CC, Kipnis V, Freedman LS, Hartman AM, Schatzkin A, Wacholder S. Energy adjustment methods for nutritional epidemiology: the effect of categorization. Am J Epidemiol. 1994;139(3):323-338. doi: 10.1093/oxfordjournals.aje.a117000 [DOI] [PubMed] [Google Scholar]
- 18.Willcox BJ, Yano K, Chen R, et al. How much should we eat? the association between energy intake and mortality in a 36-year follow-up study of Japanese-American men. J Gerontol A Biol Sci Med Sci. 2004;59(8):789-795. doi: 10.1093/gerona/59.8.B789 [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281. doi: [DOI] [PubMed] [Google Scholar]
- 20.Chalvon-Demersay T, Azzout-Marniche D, Arfsten J, et al. A systematic review of the effects of plant compared with animal protein sources on features of metabolic syndrome. J Nutr. 2017;147(3):281-292. doi: 10.3945/jn.116.239574 [DOI] [PubMed] [Google Scholar]
- 21.Holmes MD, Pollak MN, Willett WC, Hankinson SE. Dietary correlates of plasma insulin-like growth factor I and insulin-like growth factor binding protein 3 concentrations. Cancer Epidemiol Biomarkers Prev. 2002;11(9):852-861. [PubMed] [Google Scholar]
- 22.Allen NE, Appleby PN, Davey GK, Kaaks R, Rinaldi S, Key TJ. The associations of diet with serum insulin-like growth factor I and its main binding proteins in 292 women meat-eaters, vegetarians, and vegans. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1441-1448. [PubMed] [Google Scholar]
- 23.Guasch-Ferré M, Satija A, Blondin SA, et al. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation. 2019;139(15):1828-1845. doi: 10.1161/CIRCULATIONAHA.118.035225 [DOI] [PubMed] [Google Scholar]
- 24.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876-883. doi: 10.1161/CIRCULATIONAHA.109.915165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Dietary protein and risk of ischemic heart disease in middle-aged men. Am J Clin Nutr. 2010;92(5):1265-1272. doi: 10.3945/ajcn.2010.29626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernstein AM, Pan A, Rexrode KM, et al. Dietary protein sources and the risk of stroke in men and women. Stroke. 2012;43(3):637-644. doi: 10.1161/STROKEAHA.111.633404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariotti F, Huneau JF. Plant and animal protein intakes are differentially associated with large clusters of nutrient intake that may explain part of their complex relation with CVD risk. Adv Nutr. 2016;7(3):559-560. doi: 10.3945/an.115.011932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jennings A, MacGregor A, Welch A, Chowienczyk P, Spector T, Cassidy A. Amino acid intakes are inversely associated with arterial stiffness and central blood pressure in women. J Nutr. 2015;145(9):2130-2138. doi: 10.3945/jn.115.214700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magné J, Huneau JF, Tsikas D, et al. Rapeseed protein in a high-fat mixed meal alleviates postprandial systemic and vascular oxidative stress and prevents vascular endothelial dysfunction in healthy rats. J Nutr. 2009;139(9):1660-1666. doi: 10.3945/jn.109.107441 [DOI] [PubMed] [Google Scholar]
- 30.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575-1584. doi: 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57-63. doi: 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etemadi A, Sinha R, Ward MH, et al. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ. 2017;357:j1957. doi: 10.1136/bmj.j1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010;362(7):590-599. doi: 10.1056/NEJMoa0907355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70(3)(suppl):475S-490S. doi: 10.1093/ajcn/70.3.475s [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Weinstein SJ, Yu K, Männistö S, Albanes D. Serum beta carotene and overall and cause-specific mortality. Circ Res. 2018;123(12):1339-1349. doi: 10.1161/CIRCRESAHA.118.313409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Brandt PA. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in The Netherlands Cohort Study. Eur J Epidemiol. 2019;34(4):351-369. doi: 10.1007/s10654-019-00483-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172(7):555-563. doi: 10.1001/archinternmed.2011.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Lin X, Ouyang YY, et al. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr. 2016;19(5):893-905. doi: 10.1017/S1368980015002062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr. 2000;19(3)(suppl):300S-307S. doi: 10.1080/07315724.2000.10718964 [DOI] [PubMed] [Google Scholar]
- 40.Flint AJ, Hu FB, Glynn RJ, et al. Whole grains and incident hypertension in men. Am J Clin Nutr. 2009;90(3):493-498. doi: 10.3945/ajcn.2009.27460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr. 2002;76(2):390-398. doi: 10.1093/ajcn/76.2.390 [DOI] [PubMed] [Google Scholar]
- 42.Hollænder PL, Ross AB, Kristensen M. Whole-grain and blood lipid changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr. 2015;102(3):556-572. doi: 10.3945/ajcn.115.109165 [DOI] [PubMed] [Google Scholar]
- 43.Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. doi: 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med. 2015;13:59. doi: 10.1186/s12916-015-0294-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong VW, Van Horn L, Cornelis MC, et al. Associations of dietary cholesterol or egg consumption with incident cardiovascular disease and mortality. JAMA. 2019;321(11):1081-1095. doi: 10.1001/jama.2019.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pasiakos SM, Agarwal S, Lieberman HR, Fulgoni VL III. Sources and amounts of animal, dairy, and plant protein intake of US adults in 2007-2010. Nutrients. 2015;7(8):7058-7069. doi: 10.3390/nu7085322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yancy WS Jr, Wang CC, Maciejewski ML. Trends in energy and macronutrient intakes by weight status over four decades. Public Health Nutr. 2014;17(2):256-265. doi: 10.1017/S1368980012005423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ford ES, Dietz WH. Trends in energy intake among adults in the United States: findings from NHANES. Am J Clin Nutr. 2013;97(4):848-853. doi: 10.3945/ajcn.112.052662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Macdiarmid J, Blundell J. Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev. 1998;11(2):231-253. doi: 10.1079/NRR19980017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics of the Study Participants According to Fifths of Daily Dietary Animal Protein Intake
eTable 2. Risk of Overall and Cause-Specific Mortality Associated With Daily Dietary Plant Protein Intake (per 1-SD) Among 237,036 Men and 179,068 Women (Further Adjusted for Intake of Sugar-Sweetened Beverages and Carbohydrates)
eTable 3. Risk of Overall and Cause-Specific Mortality Associated With Daily Dietary Plant Protein Intake (per 1-SD) Among 237,036 Men and 179,068 Women (Further Adjusted for Median Household Income)
eTable 4. Risk of Overall and Cause-Specific Mortality Associated With Daily Dietary Animal Protein Intake Among 237,036 Men and 179,068 Women
eTable 5. Risk of Overall and Cause-Specific Mortality Associated With Substitution of 3% Energy From Different Sources of Plant Protein for Egg Protein Among 237,036 Men And 179,068 Women
eTable 6. Risk of Overall and Cause-Specific Mortality Associated With Substitution of 3% Energy From Different Sources of Plant Protein for Red Meat Protein Among 237,036 Men and 179,068 Women
eAppendix 1. Methods: Mortality Assessment and Statistical Analysis
eAppendix 2. Results: Animal Protein Intake and Dietary and Lifestyle Factors


