Abstract
The metastatic process of ovarian cancer (OC) is almost exclusively defined by direct shedding of tumor cells into the abdominal cavity, followed by clustering into multicellular aggregates and posterior peritoneal anchorage. This process relies on dynamic intercellular interactions which are modified by epithelial- mesenchymal interconversions and, therefore, E-cadherin expression variability. Although widely accepted as a tumor suppressor in many types of cancer, E-cadherin is currently known to have a dynamic expression and a much more complex role in OC. First, high E-cadherin expression is considered a sign of metaplasia in the normal ovarian epithelium, due to its association with epithelial growth factor receptor (EGFR) mediated cell proliferation. Subsequently, it is the decreased expression of E-cadherin that allows the acquisition of a more invasive phenotype, leading to the spread of primary tumor cells into the peritoneal fluid. This downregulation seems to depend on complex regulatory mechanisms, from molecular proteolysis to microenvironment interference and epigenetic regulation. E-cadherin cleavage and its resulting fragments appear to be essential to the process of dissemination and even to the formation of multicellular aggregates. Paradoxically, the maintenance of some E-cadherin expression seems to promote intercellular adhesion, resistance, and survival while decreasing cancer response to chemotherapy. Multiple studies have shown that reversing epithelial-mesenchymal transaction (EMT) and increasing E-cadherin expression prevents OC intraperitoneal dissemination, but findings that simultaneously correlate E-cadherin downregulation to higher chemotherapy sensitivity should not be ignored. Nevertheless, EMT and E-cadherin seem to have a potential interest as therapeutic targets in novel approaches to OC treatment.
Key words: Ovarian cancer, E-cadherin, epithelial-mesenchymal transition, peritoneal metastasis
Introduction
Most ovarian cancers (OC) are rapidly progressive and aggressive tumors, almost invariably associated with a poor prognosis, 1 explaining why this disease remains the deadliest gynecologic malignancy in developed countries and the fifth leading cause of cancer death among women.2,3 While extra-abdominal metastases are rare,4,5 the intraabdominal spread is present in almost two-thirds of patients at diagnosis, representing a late-stage disease.6
The pathological mechanisms for intraabdominal dissemination in OC require some critical steps (Figure 1). First, disaggregation of solid tumor tissue is required, causing tumor-cell shedding into the peritoneal fluid. Once in suspension, these cells form clusters as multicellular aggregates (MCAs) or spheroids, in order to overcome anoikis and survive in the abdominal cavity. Finally, the MCAs themselves disaggregate, promoting cellular adhesion to the peritoneal surface and thus forming secondary distant lesions.2,7-9
This unique metastatic process is only possible due to dynamic modifications in cell interactions, more specifically in cell adhesion mechanisms. E-cadherin is essential for an intercellular bond through adherents junctions and one of the molecules responsible for the acquisition of epithelial phenotype, defining cell shape and polarity. These intercellular junctions mediated by E-cadherin are formed by the extracellular connection of two cadherins from different cells, continued by the cell cytoskeleton in the intracellular domain, using catenin as a mediator. Moreover, E-cadherin also participates in intracellular signaling pathways, like the PI3K/AKT pathway.2,10
Epithelial-mesenchymal transition (EMT) is a physiological process by which differentiated epithelial cells (expressing epithelial markers including desmosomes, tight junctions, keratin and laminin) acquire mesenchymal characteristics, such as vimentin and interstitial collagen types I and III, and become more undifferentiated, allowing, for example, cell movement during tissue regeneration or tumor progression (Figure 2).The loss of E-cadherin expression, a typical epithelial marker, is the hallmark of this process and in many tumors it is followed by a gain of N-cadherin expression, the correspondent mesenchymal marker.9,11 However, some tissues, as the ovarian superficial epithelium (OSE), are not completely differentiated, and thus have inherent characteristics from both phenotypes.9,12 This explains why N-cadherin is the main responsible for intercellular junctions in OSE,5 while the expression of E-cadherin is not uniformly observed.13
Within the molecules responsible for cellular interaction, Ecadherin is not only one of the most studied, with increasing evidence for its relevant role in many tumors,14 but also one of the most consistently expressed in areas of transformed ovarian epithelia.15 As it promotes cellular differentiation and aggregation, we can easily understand why E-cadherin was for a long time considered a tumor suppressor agent, opposing effects on metastization, in most solid neoplasms.16 However, it has a more ambiguous and complex function in OC, being crucial to oncogenesis and its intraabdominal dissemination, because it is closely related to the dynamic process of epithelial-mesenchymal interconversions, as will be explained throughout this article.
Methods
Search and selection of qualified review and research Englishwritten articles published between January 2007 and November 2019 using two databases: Pubmed and ClinicalKey. The following keywords were used: ovarian cancer; E-cadherin; epithelialmesenchymal transition; peritoneal metastasis. Relevant studies about E-cadherin phenotypic expression and role in ovarian cancer abdominal metastization were included.
Cell differentiation and oncogenesis stimulation
The ambiguous role of E-cadherin in oncogenesis and OC dissemination
As in other tissues, EMT seems to play a physiological role in ovarian epithelial repair. As shown in Figure 3, after ovulation, the epithelial cells trapped inside inclusion cysts may become part of the stroma by acquiring mesenchymal features. If there is no EMT, epithelial inclusion cysts become a preferential location for oncogenesis in ovarian epithelial cancer,17 and there is fairly strong evidence that cells inside those inclusion cysts overexpressing E-cadherin are more prone to malignant transformation.9,12,18 The coexpression of N-cadherin and E-cadherin in OSE cells is nowadays considered a phenomenon of metaplasia and, therefore, OC are composed of cells with a phenotypic heterogeneity, combining different proportions of both cadherins.5
Despite previously considered a tumor suppressor agent, these recent findings suggest that E-cadherin has a pro-oncogenic function since the earliest events in tumor formation. In addition, for most carcinomas, there is a preconception that E-cadherin expression is inversely related to their aggressiveness and invasion capacity.6,11 This correlation may not apply to ovarian cancer, or at least not completely, since E-cadherin expression may promote metastasis formation, as shown by numerous studies.10 Indeed, large recent immunohistochemistry studies have demonstrated the expression of this molecule in metastatic lesions, and also in OC cells dispersed in the peritoneal fluid, justifying the possible Ecadherin influence in cancer development and resistance.2,9
Figure 1.
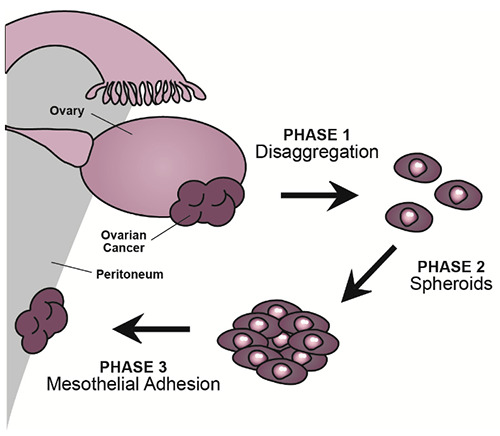
The three subsequent yet simultaneous phases of ovarian cancer intraabdominal dissemination. Cells disaggregate from the solid tumor (phase 1) and then circulate freely in the peritoneal fluid as spheroids (phase 2). This process allows tumor cells to survive programmed cell death that occurs when cells disconnect from the extracellular matrix. When in contact with the peritoneal surface, the spheroids undergo molecular changes, allowing their adhesion to the mesothelial cells of the peritoneum (phase 3).
Figure 2.
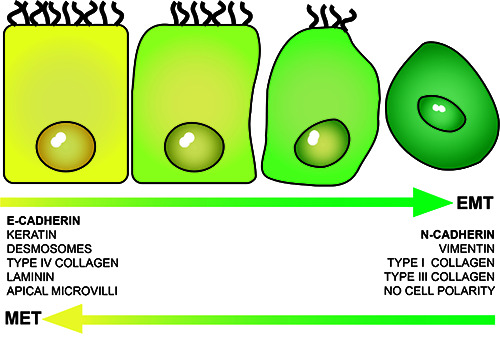
During epithelial-mesenchymal transition (EMT), the cells lose epithelial characteristics and markers and acquire a more mesenchymal phenotype. This is a gradual transformation process, from a highly differentiated state to an undifferentiated one. The opposite process or mesenchymal-epithelial transition (MET) is also possible. Therefore, in both EMT and MET, the cells can express intermediate phenotypes with both epithelial and mesenchymal features, like the ones from the ovarian superficial epithelium.
Figure 3.
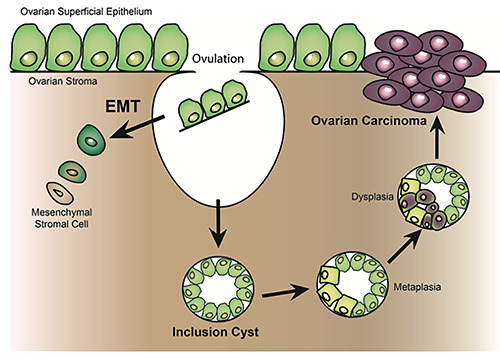
Epithelial-mesenchymal transition is essential to repair the ovarian superficial epithelium. If EMT does not occur after ovulation, the cells trapped inside ovarian stroma may form inclusion cysts and these cells are more prone to undergo MET. If epithelial markers are acquired, more specifically, E-cadherin, these cells may undergo metaplasia and evolve into a pro-oncogenic state.
Paradoxically, despite participating actively in the dissemination process, as described in further detail below, more advanced cancers express low levels of E-cadherin or do not even express it at all. Therefore, there might be a correlation between low levels of E-cadherin and more undifferentiated and aggressive OC.11
E-cadherin-mediated tumor growing
In areas of altered OSE, the formation of adherents junctions interferes in intracellular communication, triggering mitogenic pathways. The connection between two E-cadherin molecules in the extracellular domain modifies the epithelial growth factor receptor (EGFR), in one way preventing its connection to its ligand (epithelial growth factor or EGF),2,19 and in another activating a ligand-independent upregulation of EGFR. The last one results in the activation of enzymes like AKT and MAPK,2,20 capable of initiating signaling cascades that promote cellular proliferation, such as PI3K/AKT cascade11 and the MEK/ERK signaling pathway.21
In normal polarized epithelial cells, E-cadherin acts as a growth suppressor factor, depending on the expression of a zonula adherens component, namely PLEKHA7. Low levels of this molecule were found in patients with high-grade serous ovarian Cancers and it appears to regulate the previously explained E-cadherin/ EGFR interplay. Thus, upon increased expression of PLEKHA7, E-cadherin’s influence on EGFR is lost and EGFR activation decreases.22
As previously explained, E-cadherin expression has been correlated to cellular mechanisms of growth inhibition, apoptosis, differentiation and low invasive capacity in multiple cancers,14,23 contrasting with multiple OC findings. Therefore, we can conclude that the inverse of EMT - in other others, MET - might be the key for the first steps of OC oncogenesis, but if E-cadherin expression is cause or effect of MET remains unclear.14
Cellular effusion: the reversing of EMT?
Wu et al. (2018) reported that adherens junctions, resulting from E-cadherin expression, can induce cellular cohesion and prevent cellular migration, as observed in a monolayer culture of human OC cells.12 This study made it clear that E-cadherin downregulation and EMT are extremely important in later events of tumor cell shedding into the peritoneum, leading to intraabdominal metastization. Several biologic processes contribute and are involved, such as molecular proteolysis, interference of microenvironmental compounds and epigenetic regulation.
Metalloproteinases (MMPs), such as MMP-9, have shown to induce cleavage of the extracellular domain of E-cadherin, breaking adherens junctions and inducing EMT in mammary epithelial cells of murine and cataract models in rats.24 The findings of higher levels of MMP-9 in ascitic fluid of OC patients, but also of soluble E-cadherin (sE-cadherin), an extracellular fragment of the molecule with 80 KDa,24 suggest that: first, there might be a relation between the levels of the first and the second molecules,25 as sE-cadherin results from proteolysis of E-cadherin by MMP-9 (Figure 4); second, it suggests a potential role for proteolysis in inducing EMT and starting the peritoneal spreading of tumor cells. To reinforce this, high levels of both molecules in the ascitic fluid have shown to be related to more aggressive OC with poorer prognosis, 2,11 higher tumor recurrence and low survival rates.24,25
Another protease, Calpain, breaks the intracellular end of Ecadherin, impairing its ability to connect to the cytoskeleton via β- Catenin, and thus creating an unstable cellular configuration, disrupting cell-cell contact6 (Figure 4). Also, released β-Catenin enters the cell nucleus and activates Wnt/β-catenin pathway, inhibiting E-cadherin gene transcription.
Figure 4.
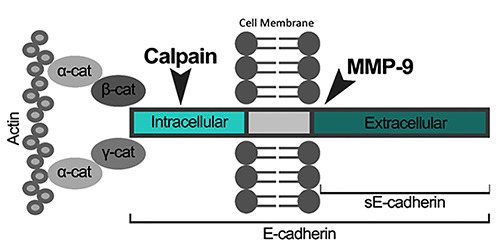
Schematic representation of an E-cadherin molecule and it’s proteolysis by two different enzymes. In the intracellular end, the molecule is connected to a cell’s cytoskeleton component – actin -, using catenin (cat) as a mediator. In the same intracellular end, E-cadherin is susceptible to Calpain’s action. In the extracellular domain, the cleavage of E-cadherin by MMP-9 creates an extracellular fragment of 80 KDa, also known as soluble E-cadherin (sE-Cadherin).
On the other hand, sE-cadherin itself may have biological activity. When human OC cells’ reaction to a recombinant form of E-cadherin ectodomain was tested, there was a phenotypical shift towards the mesenchymal phenotype and dispersion of cells from the solid tumor to the peritoneal fluid.24 So, this sE-cadherin rich peritoneal microenvironment might play an important role regarding in situ OC dissemination into peritoneal fluid, but also in spheroids disaggregation, essential to peritoneal tumor-cell implantation, the final stages of OC intraabdominal metastization.11,24
The number of many transcription factors, such as Slug, Snail and, in some tumors, Twist, has shown to be increased in OC and to suppress the expression of E-cadherin.9,26-29 More precisely, Snail seems to be a key regulator of E-cadherin, and its upregulation seems to correlate with E-cadherin lower expression and EMT induction.29 Many tumor microenvironmental aspects influence the expression of these transcription factors, but Snail’s upregulation seems to be favored by hypoxia.29
According to Wang et al, reactive oxygen species (ROS) formed in the hypoxic tumor microenvironment activate the hypoxia-induced factor 1-alfa (HIF1-α), leading to a downregulation of E-cadherin expression30 and upregulation of AEG-1 (astrocyte elevated gene-1).31 Other studies also found that HIF1-α is a positive regulator of Snail, suggesting a HIF1-α - Snail - E-cadherin sequence initiated by hypoxia, responsible for OC development. 32,33 Besides, AEG-1 induces MMPs expression, like MMP- 9, and consequently also proteolysis, with both AEG-1 and HIF1- α linked to the migration capacity of OC cells.31 These pathological pathways explain why all these effects were observed by Wang et al when Emodin was used to increase ROS production by in vitro OC cells (30). These findings reinforce the importance of tumor microenvironment in the downregulation of E-cadherin9 and that a hypoxic microenvironment, typical of many fast-growing solid tumors,30 may promote EMT process in OC, through a complex network of molecular signaling.
Furthermore, E-cadherin downregulation can occur at the epigenetic level and some studies point epigenetics as co-responsible for E-cadherin expression.34,35 The activity of one histone methyltransferase, G9a, has been linked to epigenetic modification of tumor suppressor genes and diminished E-cadherin expression in OC.36
Despite apparently less important for the understanding of Ecadherin expression regulation, micro RNAs (miR) are indeed potential therapeutic targets and may also control this molecule at a post-transcriptional phase. For example, miR-200 and miR-145 apparently contribute to preventing the rapid proliferation of OC, by increasing E-cadherin levels.37-39 Also, miR-506 seems to inhibit multiple targets responsible for EMT.40,41 On the contrary, miR-9 acts as a direct inhibitor of E-cadherin mRNA translation, with opposing effects comparing to the other above mentioned micro RNAs.42
Spheroids: the ultimate survival mechanism
The protective role of E-cadherin
During tumor disaggregation, cells shed from the primary tumor site and lose their anchors to the extracellular matrices. To survive anoikis and apoptosis processes, they form multicellular aggregates (MCAs) or spheroids,11 in a process that depends on cell-cell adhesion. E-cadherin has already proved to be crucial to this phenomenon because a dominant mutant form of the molecule has shown to disrupt adherens junctions and prevent spheroids formation in human OC cell lines.12 Xu et al. demonstrated, using three-dimensional models of OC spheroids, that higher cellular levels of E-cadherin were found in more compact and intact MCAs, with longer survival, as opposed to cellular groups with a lower or absent expression of the molecule. Indeed, inhibition of the molecule led to spheroids disaggregation.16
As previously discussed, E-cadherin downregulation is essential to intraperitoneal dissemination, so its sustained expression must have an underlying molecular mechanism. The expression of E-cadherin in MCAs is already well established. Nevertheless, there are contradictory data showing it is either increased or reduced, when compared to the solid tumor.43 As a result, there is a theory according to which, like in some lines of breast cancer cells, the intercellular adhesion in spheroids might exist due to Ecadherin fragments that maintain its extracellular domain, rather than the complete molecule, like the fragment E-cad-85 produced by Calpain, as previously explained.6,44
Spheroids also have an important contribution to OC treatment resistance. On one hand, arrangements in MCAs themselves, despite E-cadherin levels, have proved to allow cells survival to chemotherapy agents.11,16 On the other hand, by mechanisms not yet understood, E-cadherin expression seems to increase chemotherapy resistance already provided by the three-dimensional arrangement of the cells. This was demonstrated by the lower cell death rate of OC spheroids with higher E-cadherin expression in three-dimensional models, after treatment with cisplatin.16 Additionally, when there is a downregulation of E-cadherin expression through phosphatase CDC25A in OC cell cultures, it is possible to reduce OC spheroids’ resistance to cisplatin and paclitaxel. 45
EMT induced shapeshifting
While cellular aggregation into spheroids appears to be a random process, recent data prove that not only MCAs formation but also their morphology depend on the expression of adhesion molecules. While the cell clustering process seems to be dependent on an equal adhesion capacity, which is defined by cadherins expression profile, recent findings suggest that the same cell can express simultaneously E-cadherin and N-cadherin, hence creating hybrid MCAs, with both mesenchymal and epithelial phenotypes. Additionally, the same metastatic niche can originate MCAs with different cadherin configuration, thus creating spheroids with a heterogeneous cadherin expression.5,46
Klymenko et al. (2017) concluded that, in three-dimensional models, E-cadherin-mediated intercellular adhesion is more stable when compared to N-cadherin. Therefore, cells with more epithelial phenotypes led to more cohesive spheroids which tend to be more difficult to disaggregate. On the contrary, these cells expressing higher levels of E-cadherin were more spherical, limiting to a small area the contact between clustered cells and between MCAs and the peritoneum. This supports the importance of E-cadherin expression in free-floating MCAs, as previously explained. However, it seems to limit cellular adhesion to the peritoneum,5 while preserving MCAs morphology47 (Figure 5). Consequently, a metastatic advantage comes with N-cadherin expression gain, since a more mesothelial phenotype is correlated with a stronger peritoneal adhesion and greater submesothelial mobility, justifying a more invasive behavior.5,46
This dualistic role of E-cadherin in MCAs is yet to be fully understood. However it has already been suggested that hybrid spheroids (co-expressing both cadherins), may have the advantage of changing their differentiation state depending on the microenvironment. For this reason, they may adapt and become resistant to cancer treatments or more prone to mesothelial adhesion.5
Targeting EMT: boosting or preventing it?
Despite the role of E-cadherin during OC growth and metastization is not entirely known, it has become clear that the loss of Ecadherin plays a key role in intraabdominal OC dissemination. Although its clinical value as a molecular marker of disease progression in OC has not yet been considered,48 the correlations found between its expression and OC prognosis support this possible application. Therefore, lower levels of E-cadherin seem to be related to advanced stages of OC, lower-grade cell differentiation, higher recurrence rates48,49 and lower survival rates.20,48,50 In contrast, higher levels of E-cadherin have been related to a better prognosis. 51
Treatment of OC has always been considered a challenge, mainly because the majority of the patients have intraperitoneal spreading at diagnosis, and therefore they are treated for already advanced disease.52 Despite all the therapeutic improvements and clinical research findings, sustained remission of OC remains difficult to achieve.53 However, treatment advances probably justify the recent increase in survival rates.54
Using EMT and E-cadherin expression as potential therapeutic targets in OC may seem unreasonable due to various factors: the incomplete comprehension of intraabdominal metastatic spread mechanisms, the dualistic role of E-cadherin and the heterogeneous phenotype of different tumors and different spheroids originated from the same tumor. Nevertheless, the knowledge acquired from these biological and molecular mechanisms allows to understand how controlling EMT would contribute to the production of drugs capable of regulating the metastatic process of OC or to adjuvant therapy applications, by increasing cell sensitivity to chemotherapy.
Chen et al. (2019) demonstrated that treatment of OC cell lines with isoliquiritigenin (ISL) - a compound isolated from licorice roots that exhibits many pharmacological proprieties, such antiinflammatory and anticancer activity - has induced MET, increasing the levels of E-cadherin and changing cell morphology to an epithelial phenotype. Moreover, it has shown to suppress the formation of peritoneal secondary lesions and to increase the survival of mice bearing OC.55 Also, an EMT inhibitor found in multiple cancer types, miR-506, delivered by nanoparticles, has proved to inhibit OC growth and metastasis in orthotopic mouse models.40
Nowadays, first-line treatment for OC is optimal cytoreductive surgery associated or not with neoadjuvant or adjuvant platinumbased chemotherapy regimens, depending on tumor staging. Targeted therapies for OC consist mainly of vascular endothelial growth factor (VEGF) and poly ADP ribose polymerase (PARP) inhibitors.56-58 Therefore, in the light of the above-mentioned, like the correlation of E-cadherin expression and tumor prognosis, we can hypothesize that therapies interfering with EMT may become a new weapon against OC. However, previous studies showing that E-cadherin downregulation improves sensitivity to chemotherapy should not be disregarded, and more research is needed to understand the real value of E-cadherin. Moreover, an effort should be made, not only to find new drugs capable of interacting with EMT but also to understand how different drugs act and affect these pathological mechanisms in different stages of intraabdominal OC dissemination.
Conclusions
Despite E-cadherin’s relevant role in OC oncogenesis and EGFR-related tumor growth, EMT subsequent to E-cadherin expression loss seems to be the most important mechanism in this tumor’s intraperitoneal dissemination. Multiple mechanisms have been reported to induce such process, alongside all the steps of protein synthesis: epigenetic control by methylation; transcriptional regulation by transcription factors according to the tumor microenvironment, like Snail relation with hypoxia; post-transcriptional interference of micro-RNAs; and molecular cleavage by metalloproteinases. On the other hand, E-cadherin expression, or to some extent its fragments, are crucial for maintaining spheroids aggregation and survival and thus contribute to the tumor chemoresistance.
Figure 5.
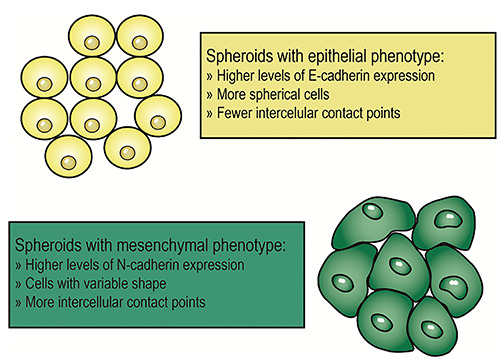
Two spheroids with different phenotypes. The spheroid represented in yellow is formed by cells with a more epithelial phenotype, hence with higher levels of E-cadherin expression. Therefore, the cells have a more regular shape and, despite there is cell-cell contact in fewer points, their connections may be stronger. Spheroids with these characteristics seem to have increased resistance to chemotherapy and longer survival. The spheroid represented in green has higher levels of N-cadherin expression. Its cells have irregular shapes and cell-cell contact is established in multiple points, due to a more mesenchymal phenotype. Spheroids with these characteristics seem to have a greater advantage in peritoneal anchorage.
Despite E-cadherin expression heterogeneity between different primary tumors and even between spheroids originated from the same solid neoplasm, the uncertain relation of cause-effect between EMT and E-cadherin downregulation, and the lack of knowledge about the biomolecular mechanisms that dictate epithelial to mesenchymal interconversions in OC, this review outstands the growing interest in exploring all the molecules and pathways interfering with E-cadherin expression and intercellular adhesion, for therapeutic proposes. Further investigation of the tumor microenvironment and the biochemical pathways involved in OC dissemination and spheroids chemoresistance is required to create targeted and effective therapies and improve patient survival in a disease with such unsatisfactory clinical outcomes.
Acknowledgments
We thank Helena Donado (Coimbra Universitary Hospital Center) for assistance with methodology of scientific literature selection.
References
- 1.Nezhat FR, Apostol R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol 2015;213:262-7. [DOI] [PubMed] [Google Scholar]
- 2.Francesca FR, Mezzanzanica D, Rea K, Tomassetti A. Guidance of signaling activations by cadherins and integrins in epithelial ovarian cancer cells. Int J Mol Sci 2016;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD. Cancer Treatment and Survivorship Statistics, 2019. CA CA Cancer J Clin 2019;69:7-34. [DOI] [PubMed] [Google Scholar]
- 4.Coffman LG, Burgos-Ojeda D, Wu R, et al. New models of hematogenous ovarian cancer metastasis demonstrate preferential spread to the ovary and a requirement for the ovary for abdominal dissemination. Transl Res 2015;175:92-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klymenko Y, Johnson J, Bos B, et al. Heterogeneous Cadherin Expression and Multicellular Aggregate Dynamics in Ovarian Cancer Dissemination. Neoplasia 2017;19:549-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trillsch F, Kuerti S, Eulenburg C, et al. E-Cadherin fragments as potential mediators for peritoneal metastasis in advanced epithelial ovarian cancer. Br J Cancer 2016;114:213-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed N, Stenvers KL. Getting to Know Ovarian Cancer Ascites: Opportunities for Targeted Therapy-Based Translational Research. Front Oncol 2013;3:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol 2010;177:1053-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N, Thompson EW, Quinn MA. Epithelial-mesenchymal interconversions in normal ovarian surface epithelium and ovarian carcinomas: An exception to the norm. J Cell Physiol 2007;213:581-8. [DOI] [PubMed] [Google Scholar]
- 10.Hu QP, Kuang JY, Yang QK, et al. Beyond a tumor suppressor: Soluble E-cadherin promotes the progression of cancer. Int J Cancer 2016;138:2804-12. [DOI] [PubMed] [Google Scholar]
- 11.Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: Unique cadherin profiles in tumor progression. Clin Exp Metastasis 2008;25:643-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Cipollone J, Maines-Bandiera S, et al. The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation 2008;76:193-205. [DOI] [PubMed] [Google Scholar]
- 13.Landen CN, Birrer MJ, Sood AK. Early events in the pathogenesis of epithelial ovarian cancer. J Clin Oncol 2008;26:995-1005. [DOI] [PubMed] [Google Scholar]
- 14.How S, Wong M, Fang CM, et al. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol 2018;121:11-22. [DOI] [PubMed] [Google Scholar]
- 15.Shield K, Ackland ML, Ahmed N, Rice GE. Multicellular spheroids in ovarian cancer metastases: biology and pathology. Gynecol Oncol 2009;113:143-8. [DOI] [PubMed] [Google Scholar]
- 16.Xu S, Yang Y, Dong L, et al. Construction and characteristics of an E-cadherin-related three-dimensional suspension growth model of ovarian cancer. Sci Rep 2014;4:5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Zheng T, Hong W, et al. Mechanism for the Decision of Ovarian Surface Epithelial Stem Cells to Undergo Neo- Oogenesis or Ovarian Tumorigenesis. Cell Physiol Biochem 2018;50:214-32. [DOI] [PubMed] [Google Scholar]
- 18.Choi P-W, Yang J, Ng S-K, et al. Loss of E-cadherin disrupts ovarian epithelial inclusion cyst formation and collective cell movement in ovarian cancer cells. Oncotarget 2016;7:4110-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawada K, Mitra AK, Radjabi AR, et al. Loss of E-Cadherin Promotes Ovarian Cancer Metastasis via α5 Integrin, which Is a Therapeutic Target. Cancer Res 2008;68:2329-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janesari-Ladani F, Hosein G, Monhasery N, et al. Wnt5a influences viability, migration, adhesion, colony formation, E- And N-cadherin expression of human ovarian cancer cell line SKOV-3. Folia Biol-Prague 2014;60:57-67. [DOI] [PubMed] [Google Scholar]
- 21.Dong L, Liu L, Ma C, et al. E-cadherin promotes proliferation of human ovarian cancer cells in vitro via activating MEK/ERK pathway. Acta Pharmacol Sin 2012;33:817-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rea K, Roggiani F, De Cecco L, et al. Simultaneous E-cadherin and PLEKHA7 expression negatively affects E-cadherin/ EGFR mediated ovarian cancer cell growth. J Exp Clin Cancer Res 2018;37:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendonsa AM, Na TY, Gumbiner BM. E-cadherin in contact inhibition and cancer. Oncogene 2018;37:4769-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Symowicz J, Adley BP, Gleason KJ, et al. Engagement of Collagen-Binding Integrins Promotes Matrix Metalloproteinase-9 - Dependent E-Cadherin Ectodomain Shedding in Ovarian Carcinoma Cells. Cancer Res 2007;67:2030-9. [DOI] [PubMed] [Google Scholar]
- 25.Grabowska MM, Day ML. Soluble E-cadherin: more than a symptom of disease. Front Biosci-Landmark 2012;17:1948-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai M, Terai Y, Kawaguchi H, et al. The EMT (epithelialmesenchymal- transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res 2014;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W-S, Yu S-L, Yang X-S, et al. Expression and significance of twist and E-cadherin in ovarian cancer tissues. Asian Pac J Cancer P 2013;14:669-72. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama K, Nakayama N, Katagiri H, Miyazaki K. Mechanisms of ovarian cancer metastasis: Biochemical pathways. Int J Mol Sci 2012;13:11705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou X mei, Zhang H, Han X. Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives. Tumor Biol 2014;35:9523-30. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Ma J, Shen H, et al. Reactive oxygen species promote ovarian cancer progression via the HIF-1α/LOX/E-cadherin pathway. Oncol Rep 2014;32:2150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao T, Zhao C, Zhou Y, et al. HIF-1α binding to AEG-1 promoter induced upregulated AEG-1 expression associated with metastasis in ovarian cancer. Cancer Med 2017;6:1072-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang P, Liu Y, Feng Y, Gao S. SNAIL gene inhibited by hypoxia-inducible factor 1alpha (HIF-1alpha) in epithelial ovarian cancer. Int J Immunopath Ph 2016;29:364-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Fan N, Yang J. Expression and clinical significance of hypoxia-inducible factor 1α, Snail and E-cadherin in human ovarian cancer cell lines. Mol Med Rep. 2015;12:3393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Zhuang YX, Hong CQ, et al. Clinical importance and therapeutic implication of E-cadherin gene methylation in human ovarian cancer. Med Oncol 2014;31. [DOI] [PubMed] [Google Scholar]
- 35.Moselhy SS, Kumosani T, Kamal IH, et al. Hypermethylation of P15, P16, and E-cadherin genes in ovarian cancer. Toxicol Ind Health 2013;31:924-30. [DOI] [PubMed] [Google Scholar]
- 36.Hua K-T, Wang M-Y, Chen M-W, et al. The H3K9 methyltransferase G9a is a marker of aggressive ovarian cancer that promotes peritoneal metastasis. Mol Cancer 2014;13:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 2008;22:894-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu YM, Shang C, Ou YL, et al. miR-200c modulates ovarian cancer cell metastasis potential by targeting zinc finger E-boxbinding homeobox 2 (ZEB2) expression. Med Oncol 2014;31. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Wu X, Wang B, et al. Mechanisms of miR-145 regulating invasion and metastasis of ovarian carcinoma. Am J Transl Res 2017;9:3443-51. [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, Hu L, Zheng H, et al. MiR-506 inhibits multiple targets in the epithelial-to-mesenchymal transition network and is associated with good prognosis in epithelial ovarian cancer. J Pathol 2015;235:25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y, Mezzanzanica D, Zhang W. MiR-506: A Multitasker in Suppression of the Epithelial-to-Mesenchymal Transition. RNA Dis 2014;1:e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou B, Xu H, Xia M, et al. Overexpressed miR-9 promotes tumor metastasis via targeting E-cadherin in serous ovarian cancer. Front Med 2017;1-9. [DOI] [PubMed] [Google Scholar]
- 43.Ko SY, Naora H. HOXA9 promotes homotypic and heterotypic cell interactions that facilitate ovarian cancer dissemination via its induction of P-cadherin. Mol Cancer 2014;13:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye Y, Tian H, Lange AR, et al. The genesis and unique properties of the lymphovascular tumor embolus are because of calpain- regulated proteolysis of E-cadherin. Oncogene 2013; 32:1702-13. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Li S, Yang L, et al. CDC25A Facilitates Chemo-resistance in Ovarian Cancer Multicellular Spheroids by Promoting E-cadherin Expression and Arresting Cell Cycles. J Cancer 2019;10:2874-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klymenko Y, Kim O, Loughran E, et al. Cadherin composition and multicellular aggregate invasion in organotypic models of epithelial ovarian cancer intraperitoneal metastasis. Oncogene 2017;36:5840-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elloul S, Vaksman O, Stavnes HT, et al. Mesenchymal-toepithelial transition determinants as characteristics of ovarian carcinoma effusions. Clin Exp Metastasis 2010;27:161-72. [DOI] [PubMed] [Google Scholar]
- 48.Dai C, Cao J, Zeng Y, et al. E-cadherin expression as a prognostic factor in patients with ovarian cancer : a meta-analysis. Oncotarget 2017;8:81052-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryabtseva OD, Lukianova NY, Shmurakov YA, et al. Significance of adhesion molecules expression for estimation of serous ovarian cancer prognosis. Exp Oncol 2013;35:211-8. [PubMed] [Google Scholar]
- 50.Yu LL, Hua X, Yang Y, et al. An updated meta-analysis of the prognostic value of decreased E-cadherin expression in ovarian cancer. Oncotarget 2017;8:81176-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carduner L, Leroy-Dudal J, Picot CR, et al. Ascites-induced shift along epithelial-mesenchymal spectrum in ovarian cancer cells: Enhancement of their invasive behavior partly dependant on αv integrins. Clin Exp Metastasis 2014;31:675-88. [DOI] [PubMed] [Google Scholar]
- 52.Abd MA, Aziz E, Agarwal K, et al. Productive Cross-Talk with the Microenvironment: A Critical Step in Ovarian Cancer Metastasis. Cancers (Basel) 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coward JI, Middleton K, Murphy F. New perspectives on targeted therapy in ovarian cancer. Int J Womens Health 2015; 7:189-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eisenhauer EA. Real-world evidence in the treatment of ovarian cancer. 2017;28:61-5. [DOI] [PubMed] [Google Scholar]
- 55.Chen C, Huang S, Chen C, et al. Isoliquiritigenin Inhibits Ovarian Cancer Metastasis by Reversing Epithelial-to- Mesenchymal Transition. Molecules 2019;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim JY, Cho CH, Song HS. Targeted therapy of ovarian cancer including immune check point inhibitor. Korean J Intern Med 2017;32:798-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grunewald T, Ledermann JA. Targeted Therapies for Ovarian Cancer. Best Pract Res Cl OB 2017;41:139-52. [DOI] [PubMed] [Google Scholar]
- 58.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet 2019;393:1240-53. [DOI] [PubMed] [Google Scholar]


