Abstract
Objective
To compare the effect of self-inflating bag (SIB) vs. T-piece resuscitator (TPR) on PaCO2 levels, when used for brief manual ventilation during administration of first dose of surfactant.
Methods
Preterm neonates were randomized to receive positive pressure ventilation with either self-inflating bag or T-piece resuscitator during administration of first dose of surfactant. Arterial blood gases were obtained at baseline and 1 h after the intervention. Primary outcome was the mean change in PaCO2 levels 1 h after the intervention.
Results
Eighty neonates were enrolled (40 in each group). The drop in PaCO2 at 1 h was significantly greater in the self inflating bag group as compared to the T-piece resuscitator group [8.96 ± 9.06 mmHg vs. 1.37 ± 9.06 mmHg, Mean difference = 7.58 mmHg, (95% CI: 3.78 to 11.4); P < 0.01]. The PaCO2 change was also statistically significant in the subgroup of infants that required only non-invasive ventilation. The need for second dose of surfactant was higher in the self-inflating bag group [77% vs. 55%, RR - 1.41 (95% CI: 1.02 to 1.94); P = 0.03].
Conclusions
T-piece resuscitator results in smaller reduction in PaCO2 levels compared to the self inflating bag, when used for brief manual ventilation during surfactant administration.
Keywords: Self inflating bag, T- piece resuscitator, Surfactant, Preterm, Arterial blood gas
Introduction
Self-inflating bag (SIB) and T-piece resuscitator (TPR) are the two most common manual ventilation devices (MVDs) used in neonates in the delivery room and intensive care unit.
Various studies on mechanical models have shown that TPR, as compared to SIB, generates inflation pressures closer to the targeted values and minimizes breath-to-breath variability in the inspiratory pressures [1–4]. Sustained lung inflations could be more easily achieved with TPR than SIB [5, 6]. TPR also delivers lower and less variable tidal volumes than SIB [7]. Currently, there is limited data on the pressures and volumes achieved in neonatal patients. Dawson et al. observed a lower tidal volume provided by TPR in preterm neonates less than 29 wk of gestation [8]. Whether this effect translates into reduced risk of hypocarbia in the early neonatal period has not yet been evaluated. Studies have also raised concerns regarding generation of inadvertent positive end expiratory pressures (PEEP) with TPR [9–11]. The effect of these two MVDs on PaCO2 levels still remains unclear.
Hypocarbia is defined by a PaCO2 level below 35 mmHg [12]. Two or more PaCO2 values less than 25 mmHg in first 48 h of life constitutes severe early hypocarbia [13]. Major fluctuations in PaCO2 can significantly affect systemic blood pressure and cerebral blood flow. A retrospective analysis of 314 preterm neonates <29 wk of gestation revealed that a single PaCO2 value <30 mmHg in first 48 h of life was associated with increased incidence of severe intraventricular hemorrhage and periventricular leukomalacia. Prolonged hypocarbia during this period also increased the odds of bronchopulmonary dysplasia [14]. Given the long term implications of hypocarbia, it is essential to assess the impact of these two MVDs on carbon dioxide elimination in preterm neonates. There is also a paucity of comparative studies on MVDs outside the context of delivery room resuscitation. In centres where minimally invasive surfactant therapy is not practiced, MVDs are regularly being used during surfactant administration while on non-invasive respiratory support.
Hence, the authors planned to conduct a randomized controlled trial (RCT) comparing the effect of SIB vs. TPR on PaCO2 levels, when used for brief manual ventilation during administration of first dose of surfactant. They hypothesized that manual ventilation with TPR, as compared to SIB, results in smaller reduction in PaCO2 levels.
Material and Methods
This single centre, parallel group RCT was conducted in the Neonatal Intensive Care Unit of Surya Children’s Hospital, Mumbai, Western India from August 2015 through February 2018. Preterm neonates having respiratory distress and/or FiO2 requirements of >35%, despite being on positive end expiratory pressure (PEEP) of 6 cm H2O within first 24 h of life, qualified for the first dose of surfactant and were considered eligible for enrolment into the study. Neonates with major congenital anomalies, complex congenital heart disease, pneumothoraces and those who had received first dose of surfactant prior to transfer to the hospital were excluded.
The trial was approved by the institutional ethics committee. The trial was prospectively registered with Clinical Trials Registry of India (CTRI/2015/08/006067) following which the first subject was enrolled.
Preterm neonates requiring respiratory support were transported to authors’ unit on Intermittent Mandatory Ventilation or nasal Continuous Positive Airway Pressure through a transport ventilator (T1500 Globe Trotter, Drager, Lubeck, Germany). Neonates with severe respiratory distress and/or FiO2 requirement of >50% were transported on Intermittent Mandatory Ventilation. Those infants requiring ≤40% FiO2 on admission were directly extubated to Continuous Positive Airway Pressure following administration of the first dose of surfactant. Neonates retrieved on Continuous Positive Airway Pressure and requiring >35% FiO2 were considered for surfactant treatment. They were continued on non-invasive ventilation unless they met previously specified ventilation criteria. Second dose of surfactant was given if the FiO2 requirement was ≥35% at 6 h after the first dose.
Random number sequence was generated in variable block sizes of two or four each, using Stata software. The random sequence was generated by a statistician who was not a part of the study. The random number codes were placed in sequentially numbered sealed opaque envelopes. Neonates were enrolled by a senior resident after obtaining written informed consent from the parents/guardians. The resident then opened the sealed envelope to determine the infant’s MVD assignment.
Masking the nurse and senior resident to allocated MVD was not possible due to the nature of the intervention. However, the study investigators and the laboratory technicians processing the blood gas were unaware of the allocation. Treating clinicians had to be informed the blood gas report since it was crucial for further management.
The intervention was brief manual ventilation with either SIB or TPR during administration of first dose of surfactant. Baseline preductal arterial blood gas was obtained prior to the intervention. The staff nurse involved in the care of the neonate, endotracheally administered the first dose of surfactant while the resident provided positive pressure ventilation using the appropriate MVD. All senior residents had formal training on the use of MVDs.
Bovine surfactant (25 mg phospholipids/ml) at the dose of 4 ml/kg was instilled in four aliquots through a 5 or 6 French infant feeding tube. After each aliquot, neonates were ventilated with SIB/TPR for either 30 s or the time to achieve target saturations of 92–95%, whichever was later. Infant’s vital signs (heart rate, respiratory rate) and saturations were continuously monitored during the procedure. Preductal arterial blood gas was obtained at 1 h and 6 h post intervention. Second dose of surfactant, if indicated, was administered after the 6 h ABG.
Infant silicone manual resuscitator (Zeal Medical, Mumbai, India) without a positive end expiratory valve was used in SIB group and Neopuff (Fisher & Paykel Healthcare, Auckland, New Zealand) was used in the TPR group. The SIB had a capacity of 250 ml with the pop off valve set at 35 cm H2O. In the TPR group, initial peak inspiratory pressure and PEEP was set at 20 cm H2O and 5 cm H2O respectively. Subsequently, peak pressure was adjusted to achieve optimal chest rise. Maximal pressure release valve was set at 40 cm H2O. Manual ventilation was provided at the rate of 40 breaths per min in both the groups. Initial FiO2 of 30% was titrated to a target saturation of 92–95%.
Neonates requiring ventilation were commenced on volume guarantee mode with a tidal volume of 4–6 ml/kg, inspiratory time of 0.3 s, PEEP of 5 cm H2O and FiO2 to target SpO2 of 92–95%. Extubation was considered if peak inspiratory pressure required to generate the set tidal volume was ≤18 cm H2O, FiO2 requirement ≤30% and blood gas was suggestive of a pH ≥ 7.25 with PaCO2 ≤ 55 mmHg.
The primary outcome was the difference between baseline and 1 h PaCO2. This was quantified as mean change 1.
Secondary outcomes were: (a) The difference between baseline PaCO2 and 6 h PaCO2 - mean change 2; (b) Blood gas variables (pH, PaCO2 and PaO2) at 1 and 6 h post intervention; (c) Clinical outcomes such as mortality prior to discharge, requirement of mechanical ventilation, need for second dose of surfactant, duration of invasive and non-invasive ventilation, duration of oxygen therapy and length of hospital stay; (d) Neonatal morbidities - severe intraventricular hemorrhage, patent ductus arteriosus requiring medical treatment, necrotizing enterocolitis, late onset sepsis, retinopathy of prematurity requiring treatment, and bronchopulmonary dysplasia.
Observations from authors’ neonatal unit indicated that the average reduction in PaCO2 following a brief period of manual ventilation with SIB during surfactant administration was 12 mmHg (standard deviation of 8.5 mmHg). In order to detect a 6 mmHg difference in the change in PaCO2 between the two groups with an assumed standard deviation of 8 mmHg, a sample size of 39 neonates in each group was estimated for a study power of 90% and two tailed alpha of 0.05.
Statistical analysis was conducted using the Stata 13.1 software (Statacorp LP, 4905, Lakeway Drive, College Station, Texas, USA). Descriptive statistics were used to define the baseline and outcome variables in both groups. Categorical variables were compared using the Fisher’s exact test. Continuous measures were compared using two sample t-test or Mann-Whitney U-test as appropriate. Analysis was performed by applying intention to treat principle. A p value of <0.05 was considered statistically significant. Factorial analysis of variance was performed to check the dependence of the mean change in PaCO2 on the two factors- type of MVD and duration of respiratory support (hours of life) and interaction between them, if any. Line charts and box plots were constructed to provide a visual impression of the difference in the pre and post intervention PaCO2.
Results
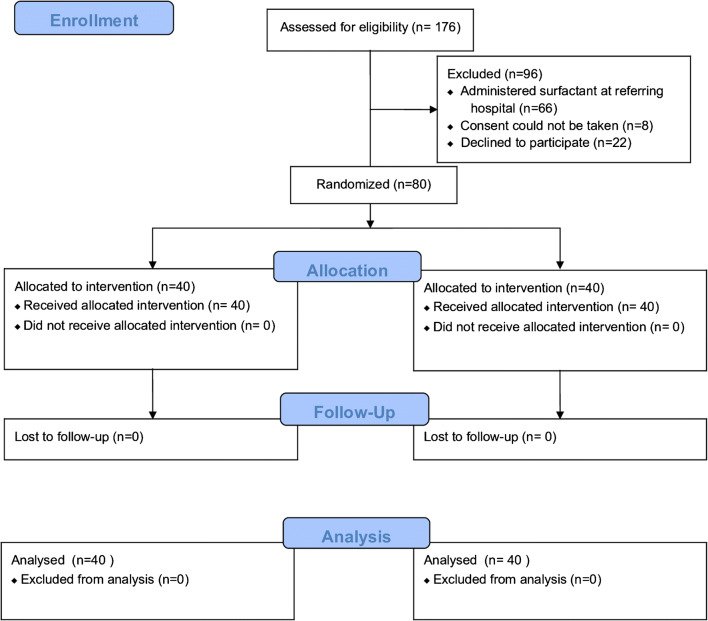
A total of 176 eligible neonates were admitted to authors’ unit from August 2015 through February 2018. Of these, 80 were enrolled in the study (40 in the TPR group and 40 in the SIB group) (Fig. 1). All the neonates were analyzed for the primary outcome. There were no adverse events associated with surfactant administration or manual ventilation.
Fig. 1.
CONSORT 2010 flow diagram
Majority of the preterm neonates were outborn (55%) and cesarean delivered (85%). The gestational age of the study patients ranged from 26 to 36 wk and 88% of them were > 28 wk. Most of them were vigorous (83%) at birth and were managed with non-invasive ventilatory support (80%). Neonates who were primarily commenced on non-invasive respiratory support did not require mechanical ventilation subsequently. Sixteen (20%) were on mechanical ventilation at the time of primary outcome assessment. Eleven (14%) continued to require ventilation at 6 h after intervention. Baseline maternal and neonatal characteristics were similar in the study groups (Table 1).
Table 1.
Patients’ demographic and baseline characteristics
| Characteristics | SIB (n = 40) |
TPR (n = 40) |
P |
|---|---|---|---|
| Mothers | |||
| Cesarean section | 35 (87%) | 33 (82%) | 0.75 |
| Pregnancy induced hypertension | 10 (25%) | 9 (22%) | >0.99 |
| Gestational diabetes mellitus | 6 (15%) | 0 | 0.03 |
| Antenatal steroid exposure* | 37 (93%) | 33 (83%) | 0.31 |
| Neonates | |||
| Gestation, weeks | 31.57 (2.68) | 31.13 (2.4) | 0.43 |
| Gestation <28 wk | 5 (12.5%) | 5 (12.5%) | 0.63 |
| Birth weight, grams | 1605 (589) | 1498 (542) | 0.40 |
| Male sex | 27 (67.5%) | 22 (55%) | 0.36 |
| Outborn | 21 (52%) | 23 (57%) | 0.82 |
| Resuscitated at birth† | 7 (17%) | 7 (17%) | >0.99 |
| APGAR at 5 min | 8 (7–8) | 8 (7–8) | 0.1 |
| Hypoglycemia‡ | 8 (20%) | 4 (10%) | 0.35 |
| Duration of intervention (seconds)§ | 170 (150, 200) | 160 (148, 185) | 0.57 |
| Time to surfactant (minutes)§ | 50 (15, 120) | 60 (22, 160) | 0.48 |
| Ventilated till primary outcome | 10 (25%) | 6 (15%) | 0.40 |
| Minutes of ventilation (till primary outcome)§ | 112 (85, 180) | 135 (92, 188) | 0.89 |
| Baseline FiO2 | 40 (38, 45) | 40 (35, 42) | 0.17 |
| Baseline PEEP | 6 (5, 6) | 6 (5, 7) | 0.54 |
| Baseline pH | 7.27 (0.07) | 7.28 (0.07) | 0.42 |
| Baseline PaCO2 (mmHg) | 46.5 (9.8) | 45.3 (9.4) | 0.59 |
| Baseline PaO2 (mmHg)§ | 80.2 (61.7, 152.1) | 91.4 (70.4, 203.1) | 0.22 |
Data are presented as mean (SD) or n (%), unless indicated otherwise
PEEP Positive end expiratory pressure; RR Risk ratio; SIB Self-inflating bag; TPR T-piece resuscitator
*Received at least 1 dose of antenatal steroids 6 h prior to delivery
† Required more than 2 min of positive pressure ventilation in the delivery room
‡Blood sugar value less than 40 mg/dl
Variables with § are expressed as median (25th centile, 75th centile)
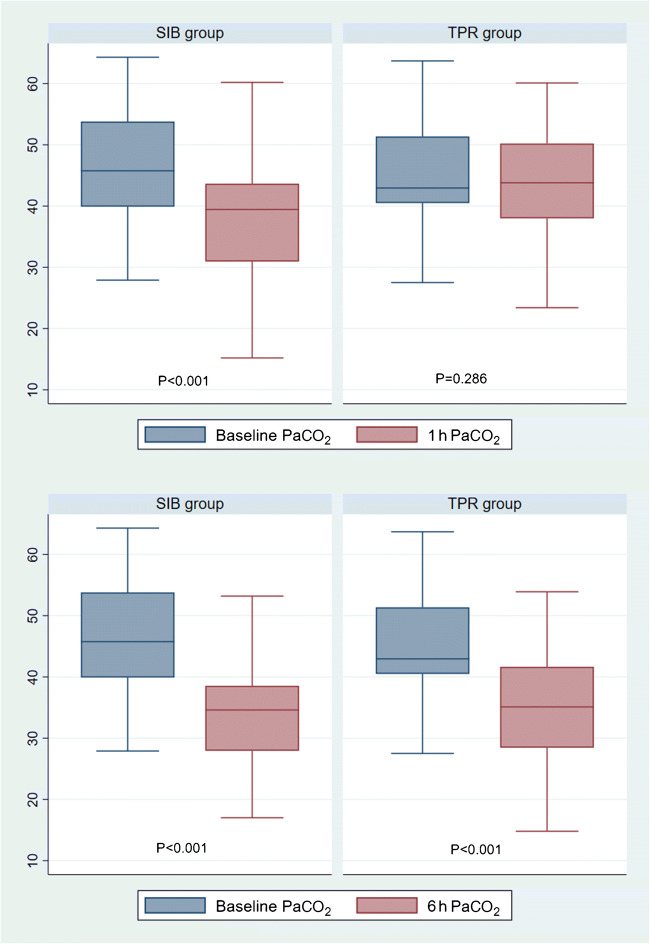
Blood gas variables of both groups are displayed in Table 2. A significant drop in PaCO2 at 1 h was found in the SIB group as compared to the TPR group [SIB: 8.96 ± 9.06 mmHg vs. TPR: 1.37 ± 9.06 mmHg, mean difference 7.58 mmHg, (95% CI: 3.78–11.4), p < 0.01]. However, the differential reduction did not persist at 6 h (Table 2).
Table 2.
Blood gas variables and other neonatal outcomes
| SIB Group (n = 40) |
TPR Group (n = 40) |
RR/ Mn diff./Md diff. | P | |
|---|---|---|---|---|
| Mean change in PaCO2 (1 h) | ||||
| Overall (n = 80) | 8.96 (9.06) | 1.37 (9.06) | 7.58 (3.78, 11.4) | <0.01 |
| NIV subgroup (n = 55) | 8.74 (9.22) | 0.71 (8.69) | 8.02 (3.18, 12.87) | <0.01 |
| Mean change in PaCO2 (6 h) | ||||
| Overall (n = 80) | 13.01 (11.59) | 10.72 (10.37) | 2.29 (−2.60, 7.18) | 0.35 |
| NIV subgroup (n = 55) | 14.41 (11.35) | 11.37 (9.86) | 3.05 (−2.69, 8.79) | 0.29 |
| 1 h after manual ventilation | ||||
| pH | 7.34 (0.08) | 7.31 (0.06) | 0.03 (−0.009, 0.06) | 0.06 |
| PaCO2 | 37.5 (9.74) | 44.0 (8.13) | −6.4 (−10.4, −2.4) | <0.01 |
| PaO2* | 105.9 (76, 158.3) | 99.9 (83,138.4) | 2.05 (−18.7, 23.8) | 0.85 |
| FiO2* | 35 (32, 36) | 34 (30, 36) | 0 (0, 2) | 0.39 |
| PEEP* | 5 (5, 6) | 5 (5, 6) | 0 (−1, 0) | 0.14 |
| 6 h after manual ventilation | ||||
| pH | 7.37 (0.09) | 7.38 (0.077) | −0.01 (−0.05,0.028) | 0.6 |
| PaCO2 | 33.50 (8.48) | 34.63 (8.67) | −1.12 (−4.94, 2.69) | 0.55 |
| PaO2* | 81.1 (67.1,118.4) | 92.3 (64.6,124) | −4.2 (−22.1, 16.7) | 0.63 |
| FiO2* | 31 (30–32) | 30 (25–32) | 1.5 (0, 4) | 0.06 |
| PEEP * | 5 (5, 6) | 5 (5, 6) | 0 | 0.70 |
| Need of 2nd dose of surfactant | 31 (77%) | 22 (55%) | 1.41 (1.02, 1.94) | 0.03 |
| Need of ventilation beyond 6 h | 7 (17%) | 4 (10%) | 1.75 (0.56, 5.43) | 0.52 |
| Days of invasive ventilation* | 0 (0, 0) | 0 (0, 10) | 0 | 0.15 |
| Days of non-invasive ventilation* | 5 (3, 9) | 4 (3, 12) | 0 (−1.0, 2.0) | 0.77 |
| Days of oxygen support* | 5.5 (4, 10) | 4 (3, 8) | 1.0 (−1.0, 3.0) | 0.36 |
| Days of hospital stay* | 25 (14, 35) | 29 (19, 36) | −2.0 (−10.0, 5.0) | 0.63 |
| Death | 2 (5%) | 2 (5%) | 1.0 | >0.99 |
| Severe IVH (grade 3 or 4) | 2 (5%) | 2 (5%) | 1.0 | >0.99 |
| PDA requiring medical treatment | 12 (30%) | 11 (27%) | 1.09 (0.54, 2.18) | >0.99 |
| ROP requiring treatment | 5 (12.5%) | 4 (10%) | 1.25 (0.36, 4.34) | >0.99 |
| BPD | 9 (22%) | 6 (15%) | 1.5 (0.58, 3.8) | 0.57 |
| NEC | 2 (5%) | 1 (2.5%) | 2 (0.19, 20.4) | >0.99 |
| Late onset sepsis | 6 (15%) | 3 (8.1%) | 0.5 (0.14, 1.8) | 0.29 |
Data are presented as mean (SD) or n (%), unless indicated otherwise, Variables with * are presented as median (25th centile, 75th centile)
BPD Bronchopulmonary dysplasia; IVH Intraventricular hemorrhage; Md diff. Median difference; Mn diff. Mean difference; NEC Necrotising enterocolitis; NIV Non-invasive ventilation; PDA Patent ductus arteriosus; PEEP Positive end expiratory pressure; ROP Retinopathy of prematurity; RR Risk ratio; SIB Self-inflating bag; TPR T-piece resuscitator
Neonates on NIV who received surfactant and then returned to NIV were analyzed as a separate subgroup (64 infants; 30 in SIB group and 34 in TPR group). The SIB group still demonstrated a significant drop in PaCO2 [SIB 8.74 mmHg, TPR 0.71 mmHg, Mean difference 8.02 mmHg, (95% CI: 3.18–12.87), p < 0.01].
The box plot shows that the magnitude of drop in the post intervention PaCO2 was significantly greater in the SIB group especially at 1 h (Fig. 2).
Fig. 2.
Drop in PaCO2 levels in SIB vs. TPR group. SIB Self-inflating bag; TPR T-piece resuscitator
Analysis of covariance model of linear regression was used to assess the effect of the MVD on the primary outcome after adjusting for the baseline PaCO2. That the regression lines for the SIB group/TPR group are nearly parallel and well separated (Fig. 3), confirms the drop in PaCO2 to be independent of the baseline values.
Fig. 3.
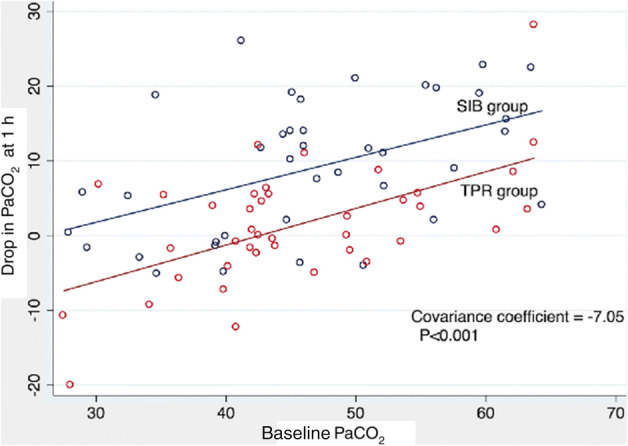
Analysis of covariance (Baseline PaCO2 vs. 1 h PaCO2)
Repeated measures of PaCO2 (baseline, 1 h and 6 h) were subjected to a factorial analysis of variance to determine the device effect (Type of MVD - SIB or TPR) and time effect (1 h and 6 h after manual ventilation). The change in PaCO2 was found to be significantly associated with time [F (1, 77) = 34.47, p < 0.01- time effect] rather than the device F (1, 77) = 3.31, p = 0.07- device effect]. However, the interaction effect (device x time) was significant [F (1, 77) = 3.69, p = 0.03], indicating the effect of the MVD on the mean change in PaCO2 at 1 h.
Outcomes related to neonatal morbidity were similar in both groups (Table 2). One neonate in the SIB group developed pneumothorax at 24 h of life requiring intercostal drainage.
Discussion
This clinical trial evaluates the effect of manual ventilation with a T-piece resuscitator or self-inflating bag during surfactant administration by measuring the mean change in arterial carbon dioxide levels after administration. The authors found that the fall in PaCO2 level was significantly greater with SIB as compared to TPR, 1 h post intervention. The strength of present study includes a robust randomized controlled trial design adequately powered for the primary outcome. To authors’ knowledge, there is no RCT until date comparing the effect of SIB/TPR on carbon dioxide elimination in neonates. Selection of an objective primary outcome and completeness of follow up eliminated detection and attrition bias.
Several studies performed on manikins have evaluated MVDs for tidal volume and peak inspiratory pressure. Dawson et al. randomized 80 neonates <29 wk of gestation to the two MVDs. A trend towards lower tidal volumes was reported in the TPR group (6.6 ml/kg vs. 9.2 ml/kg, p = 0.34) [8]. A multicenter crossover trial by Szyld et al. reported that TPR generated lower and less variable inflation pressures than SIB (26 vs. 28 cm H2O) in neonates >26 wk of gestation. Fewer neonates in the TPR group received peak inspiratory pressure > 25 cm H2O (5% vs. 33%) [15]. In the present study, authors chose to assess the effect of MVDs on the PaCO2 levels, especially since surfactant could exaggerate the response to manual ventilation.
The difference in 1 h PaCO2 values in present study could be attributed only to the type of MVD used during surfactant administration. Factors that could affect alveolar ventilation such as, extent of lung disease, initial ventilatory requirements, timing and dose of surfactant administered, were similar in the study groups. Furthermore, the main outcome was uninfluenced by the magnitude of individual PaCO2 reading. Significant drop in PaCO2 (in SIB group) even after restricting the analysis to the NIV subgroup strengthens the findings and captures the impact of MVD on the PaCO2 values. The lack of effect on the 6 h PaCO2 values could be explained by the ongoing non-invasive respiratory support in both groups.
Very few studies have evaluated the effects of MVDs on clinical outcomes in neonates. Thakur et al. randomized 90 neonates >26 wk of gestation requiring delivery room resuscitation to TPR or SIB. They demonstrated that neonates in TPR group required positive pressure ventilation for a lesser duration and had lower intubation rates [16]. A Brazilian prospective cohort study of 1962 inborn preterm infants showed that TPR, as compared to SIB, increased the odds of survival to discharge without major morbidities [17]. In the present study, the requirement of second dose of surfactant was significantly lower in the TPR group. Consistent delivery of positive end expiratory pressure and better lung recruitment with the TPR could possibly explain this outcome. A trend towards lower FiO2 requirement at 6 h in the TPR group is in line with these findings. The arterial oxygen concentration in both groups, especially in the early phase, were high from a comparison with current practice. However, significant hyperoxia has also been reported in preterm neonates transported on non-synchronized mechanical ventilation [18].
The present study has certain limitations. Clinicians and nurses could not be blinded to the intervention. Real time monitoring and regulation of pressures generated by the SIB was not done in the study patients. In the absence of a manometer to monitor the peak inspiratory pressure generated, a bias towards administration of higher positive pressure in the SIB group could have existed. Minor group differences due to operator variability, especially with SIB, also could not be ruled out. A blood gas immediately after surfactant administration would have provided information specific to the effect of MVD. Only 12% of neonates in this study were below 28 wk of gestation and a large proportion of infants were outborn (55%). This could limit the generalizabilty of present study results.
The present study highlights the need to regulate pressures generated by MVDs even during brief periods of manual ventilation. RCTs with large sample size addressing clinically important outcomes in high risk neonates (gestation <28 wk) and follow up beyond the neonatal period would be required to assess the implications of the findings from the present study.
Conclusions
T-piece resuscitator results in smaller reduction in PaCO2 levels compared to self inflating bag, when used for brief manual ventilation during surfactant administration.
Authors’ Contributions
NK, HB conceptualized and designed the study and carried out the data analysis. AJ performed the literature search, collected the data and drafted the initial manuscript. All authors critically reviewed and approved the final manuscript as submitted. NK will act as Guarantor for this paper.
Compliance with Ethical Standards
Conflict of Interest
None.
Footnotes
Clinical Trials Registry of India (CTRI/2015/08/006067)
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hussey S, Ryan C, Murphy B. Comparison of three manual ventilation devices using an intubated mannequin. Arch Dis Child Fetal Neonatal Ed. 2004;89:F490–F493. doi: 10.1136/adc.2003.047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett S, Finer NN, Rich W, Vaucher Y. A comparison of three neonatal resuscitation devices. Resuscitation. 2005;67:113–118. doi: 10.1016/j.resuscitation.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Roehr CC, Kelm M, Fischer HS, Bührer C, Schmalisch G, Proquitté H. Manual ventilation devices in neonatal resuscitation: tidal volume and positive pressure-provision. Resuscitation. 2010;81:202–5. [DOI] [PubMed]
- 4.Nimbalkar SM, Suman Rao PN, Saudamini VN, et al. Comparison of efficacy of three devices of manual positive pressure ventilation: a mannequin-based study. Ital J Pediatr. 2015;41:25. doi: 10.1186/s13052-015-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klingenberg C, Dawson JA, Gerber A, et.al. Sustained inflations: comparing three neonatal resuscitation devices. Neonatology. 2011;100:78–84. [DOI] [PubMed]
- 6.do Prado C, Guinsburg R, de Almeida MF, et al. Manual ventilation and sustained lung inflation in an experimental model: influence of equipment type and operator's training. PLoS One. 2016;11:e0148475. [DOI] [PMC free article] [PubMed]
- 7.Hawkes CP, Ryan CA, Dempsey EM. Comparison of the T-piece resuscitator with other neonatal manual ventilation devices: a qualitative review. Resuscitation. 2012;83:797–802. doi: 10.1016/j.resuscitation.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Dawson JA, Schmolzer GM, Kamlin CO, et al. Oxygenation with T-piece versus self-inflating bag for ventilation of extremely preterm neonates at birth: a randomized controlled trial. J Pediatr. 2011;158:912–918. doi: 10.1016/j.jpeds.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Hinder M, Jani P, Priyadarshi A, McEwan A, Tracy M. Neopuff T-piece resuscitator: does device design affect delivered ventilation? Arch Dis Child Fetal Neonatal Ed. 2017;102:F220–F224. doi: 10.1136/archdischild-2016-311164. [DOI] [PubMed] [Google Scholar]
- 10.Finer NN, Rich WD. Unintentional variation in positive end expiratory pressure during resuscitation with a T-piece resuscitator. Resuscitation. 2011;82:717–719. doi: 10.1016/j.resuscitation.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 11.McHale S, Thomas M, Hayden E, Bergin K, McCallion N, Molloy EJ. Variation in inspiratory time and tidal volume with T-piece neonatal resuscitator: association with operator experience and distraction. Resuscitation. 2008;79:230–233. doi: 10.1016/j.resuscitation.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 12.Collins MP, Lorenz JM, Jetton JR, Paneth N. Hypocapnia and other ventilation-related risk factors for cerebral palsy in low birth weight infants. Pediatr Res. 2001;50:712–719. doi: 10.1203/00006450-200112000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Murase M, Ishida A. Early hypocarbia of preterm infants: its relationship to periventricular leukomalacia and cerebral palsy, and its perinatal risk factors. Acta Paediatr. 2005;94:85–91. doi: 10.1080/08035250410033015. [DOI] [PubMed] [Google Scholar]
- 14.Erickson SJ, Grauaug A, Gurrin L, Swaminathan M. Hypocarbia in the ventilated preterm infant and its effect on intraventricular haemorrhage and bronchopulmonary dysplasia. J Paediatr Child Health. 2002;38:560–562. doi: 10.1046/j.1440-1754.2002.00041.x. [DOI] [PubMed] [Google Scholar]
- 15.Szyld E, Aguilar A, Musante GA, Vain N, Prudent L, Fabres J, Carlo WA, Delivery Room Ventilation Devices Trial Group Comparison of devices for newborn ventilation in the delivery room. J Pediatr. 2014;165:234–239. doi: 10.1016/j.jpeds.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 16.Thakur A, Saluja S, Modi M, Kler N, Garg P, Soni A, Kaur A, Chetri S. T-piece or self inflating bag for positive pressure ventilation during delivery room resuscitation: an RCT. Resuscitation. 2015;90:21–24. doi: 10.1016/j.resuscitation.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Guinsburg R, de Almeida MFB, de Castro JS, Gonçalves-Ferri WA, Marques PF, Caldas JPS, Krebs VLJ, Souza Rugolo LMS, de Almeida JHCL, Luz JH, Procianoy RS, Duarte JLMB, Penido MG, Ferreira DMLM, Alves Filho N, Diniz EMA, Santos JP, Acquesta AL, Santos CN, Gonzalez MRC, da Silva RPGVC, Meneses J, Lopes JMA, Martinez FE. T-piece versus self-inflating bag ventilation in preterm neonates at birth. Arch Dis Child Fetal Neonatal Ed. 2018;103:F49–F55. doi: 10.1136/archdischild-2016-312360. [DOI] [PubMed] [Google Scholar]
- 18.Tracy M, Downe L, Holberton J. How safe is intermittent positive pressure ventilation in preterm babies ventilated from delivery to newborn intensive care unit? Arch Dis Child Fetal Neonatal Ed. 2004;89:F84–F87. doi: 10.1136/fn.89.1.F84. [DOI] [PMC free article] [PubMed] [Google Scholar]