Abstract
Populus fremontii (Fremont cottonwood) is recognized as one of the most important foundation tree species in the southwestern USA and northern Mexico because of its ability to structure communities across multiple trophic levels, drive ecosystem processes and influence biodiversity via genetic-based functional trait variation. However, the areal extent of P. fremontii cover has declined dramatically over the last century due to the effects of surface water diversions, non-native species invasions and more recently climate change. Consequently, P. fremontii gallery forests are considered amongst the most threatened forest types in North America. In this paper, we unify four conceptual areas of genes to ecosystems research related to P. fremontii’s capacity to survive or even thrive under current and future environmental conditions: (i) hydraulic function related to canopy thermal regulation during heat waves; (ii) mycorrhizal mutualists in relation to resiliency to climate change and invasion by the non-native tree/shrub, Tamarix; (iii) phenotypic plasticity as a mechanism for coping with rapid changes in climate; and (iv) hybridization between P. fremontii and other closely related Populus species where enhanced vigour of hybrids may preserve the foundational capacity of Populus in the face of environmental change. We also discuss opportunities to scale these conceptual areas from genes to the ecosystem level via remote sensing. We anticipate that the exploration of these conceptual areas of research will facilitate solutions to climate change with a foundation species that is recognized as being critically important for biodiversity conservation and could serve as a model for adaptive management of arid regions in the southwestern USA and around the world.
Keywords: Canopy thermal regulation, hybridization, local adaptation, mycorrhizal mutualists, phenotypic plasticity, unmanned airborne remote sensing
Introduction
A foundation species is often defined as one that creates environmental conditions that are necessary for the survival of other species by stabilizing fundamental ecosystem processes (Ellison et al., 2005). Almost all foundation species are locally abundant, regionally common and are most often trees with morphological and physiological characteristics that define forest structure, microclimate and ecohydrology. However, foundation tree species are declining throughout the world due to a combination of interactive global change factors including changes in climate, outbreaks of pests and pathogens, logging and alterations in ecosystem hydrology (Ellison et al., 2005; Vörösmarty et al., 2010). In many ecosystems, community structure and ecosystem processes are largely controlled by a single foundation species (Ellison et al., 2005; Peters and Yao, 2012). Therefore, the loss of any one foundation species could have dramatic cascading impacts on a broad range of ecosystem services from forested landscapes, including nutrient and water cycles, food webs, biodiversity and habitat for threatened and endangered species.
Populus fremontii, S. Wats. (Fremont cottonwood) is a dominant riparian tree that occupies a broad climatic range across the southwestern USA. It is also recognized as a critically important foundation species in the southwest (Whitham et al., 2008) because of its ability to structure communities across multiple trophic levels, drive ecosystem processes (Whitham et al., 2008) and influence biodiversity via genetic-based functional trait variation (e.g. Schweitzer et al., 2008; Lamit et al., 2014, 2015, 2016; Keith et al., 2017). However, drought and altered flow regimes combined with land use changes (Merritt and Poff, 2010) have resulted in a 97% decline of pre-20th century habitat (Noss et al., 1995). Consequently, P. fremontii gallery forests, and more broadly, riparian ecosystems—which support a disproportionately high wildlife biodiversity in arid regions (Poff et al., 2012)—are particularly susceptible to both climate change (Gitlin et al., 2006) and invasive species (Merritt and Poff, 2010). The little remaining riparian habitat is faced with ongoing climate stress as the southwestern USA is becoming warmer and drier, experiencing decreased river flows, decreased soil moisture and increased evaporative demand (Garfin et al., 2013). Decreases in plant available water coupled with increases in the duration and frequency of episodic heat waves have resulted in recent P. fremontii die-offs along many major river reaches throughout the Southwest (Gitlin et al., 2006).
Compounding these climate-induced reductions in riparian habitat is the successful spread and establishment of Tamarix (a.k.a. tamarisk or salt cedar) into riparian areas throughout the western USA. Habitat once dominated by native P. fremontii and other Populus species has been replaced by T. ramosissima and/or T. chinensis and their hybrids, with the highest densities along dammed river systems (Busch and Smith, 1995; Merritt and Poff, 2010). Tamarix invasion has resulted in extensive monocultures along many major river corridors and tributaries. Once Tamarix is established along a river reach, it often increases soil salinity (Taylor et al., 1999; Merritt and Shafroth, 2012), alters hydrology (Graf, 1978) and reduces native vegetation cover (Busch and Smith, 1995) and wildlife communities (Ellis et al., 1997; Bateman et al., 2013). Tamarix also disrupts belowground mycorrhizal fungal communities upon which native trees like P. fremontii depend (Meinhardt and Gehring, 2012), leaving behind a legacy of low mycorrhizal abundance even when it is removed (Hultine et al., 2015). Despite considerable efforts to control Tamarix through biocontrol and mechanical removal campaigns (Hultine et al., 2010; González et al., 2018), there has been a continued decline in native riparian habitat with deleterious impacts on ecosystem function, biodiversity (Harms and Hiebert, 2006) and even agriculture (Zavaleta, 2000).
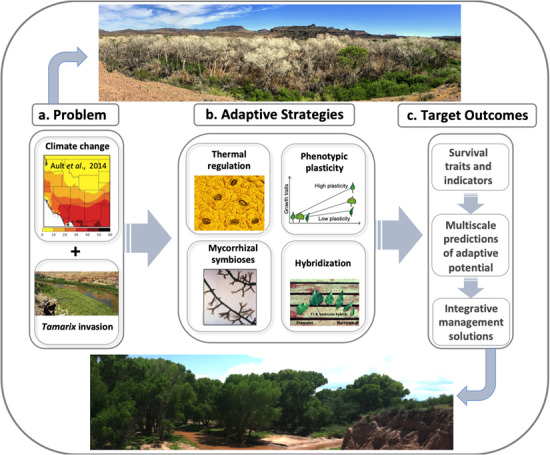
This review highlights the primary threats to P. fremontii and its capacity to serve as an important foundation species across its range in the face of climate change and the presence of non-native Tamarix. We start by reviewing the population genomics of P. fremontii and identify the key adaptive traits that underpin its current success across its range. Our review synthesizes previous investigations using experimental common gardens and greenhouse experiments to evaluate the expression of genetically based traits that largely govern resource uptake and stress tolerance. Our review will specifically focus on two key mechanisms to cope with the primary challenges of global environmental changes: (i) the expression of leaf/canopy traits required to balance trade-offs between minimizing plant hydraulic dysfunction and minimizing canopy thermal stress, and (ii) the maintenance of mycorrhizal symbionts in the presence of climate change and Tamarix presence that can synergistically disrupt mycorrhizal associations (Fig. 1). We argue that traits that best optimize the balance between water loss and canopy thermal regulation during climate stress, and traits that maximize mycorrhizal associations in Tamarix soil legacies are likely to be under some of the most significant current and future selection pressures. We therefore hypothesize that the expression of these traits will most likely be identified in (i) genotypes with the highest plasticity in trait expression across environmental conditions such that survival and adaptation to climate stress and Tamarix presence correlates with plasticity (Fig. 1) and (ii) hybrids between P. fremontii and other closely related Populus species yielding genotypes that are more adaptable to climate stress and Tamarix presence than either parental species. We anticipate that future restoration efforts of P. fremontii gallery forests will require selection of naturally occurring populations and genotypes in the wild and breeding programmes targeting a suite of traits that can best maximize resource use efficiency during periods of resource limitations and maximize resource uptake efficiency during brief resource pulses (Fig. 1). We suggest that high spatial and spectral resolution remote sensing data and associated methods can inform restoration by detecting key traits in the common gardens and upscaling trait detection to the landscape and ecosystem scales.
Figure 1. Overall approach to investigating adaptive survival strategies in Populus fremontii as responses to climate change and non-native species invasion. (a) Climate change and Tamarix invasion that can synergistically result in rapid mortality of P. fremontii forests (e.g. upper photo of deceased P. fremontii trees along the lower Bill Williams River in west central Arizona in 2017—photo credit: Hillary Cooper). (b) Four adaptive strategies that are subject to climate change and invasive species as selective agents. (c) Target outcomes of integrated management approaches that amplify adaptive potential of P. fremontii and riparian ecosystem structure and function (e.g. lower photo of vibrant P. fremontii gallery forest along the upper San Pedro River in southeastern Arizona—photo credit: Kevin Hultine).
Populus fremontii population genomics and the role of experimental common gardens
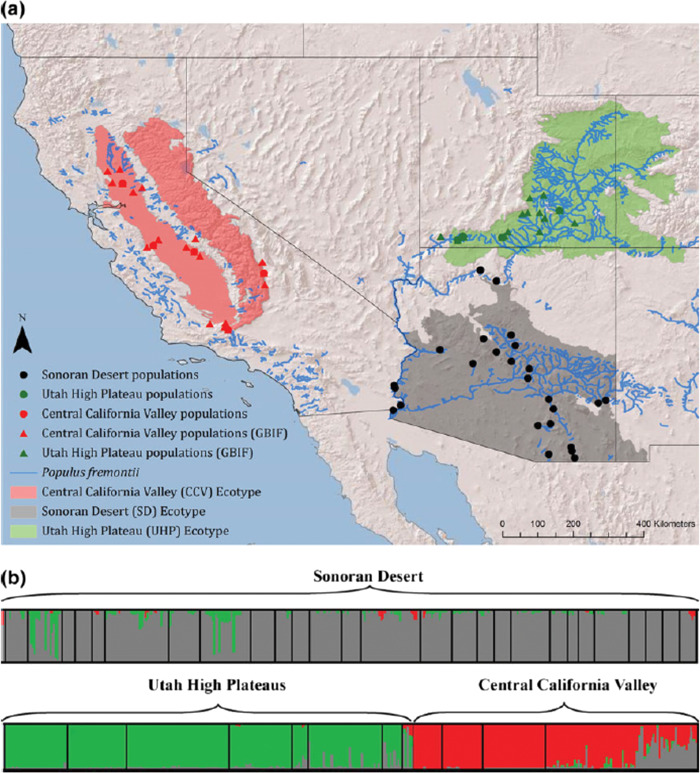
P. fremontii exhibits substantial genetic variation throughout its range (Cushman et al. 2014), which encompasses broad environmental gradients extending from Mexico, Arizona and California in the south and reaching northward into Nevada and northern Utah (Eckenwalder, 1977). Based on extensive field collections, Cushman et al. (2014) found that P. fremontii is strongly differentiated into three genetic groups (Fig. 2b), and genetic variation amongst populations is primarily driven by climate gradients and connectivity along river networks. Ikeda et al. (2017) utilized this genetic data coupled with additional occurrence records from the Global Biodiversity Information Facility (GBIF) to generate ecological niche models, which defined three climatically differentiated ecotypes comprising the Utah High Plateau (UHP), Sonoran Desert (SD) and California Central Valley (CCV) (Fig. 2a). Ikeda et al. (2017) also demonstrated that the inclusion of genetic marker data improved the predictive power of ecological niche models by 12-fold when compared to models that included no population genetic information, leading to more accurate forecasting of changes in P. fremontii’s distribution as a function of climate change. More recently, Bothwell et al. (unpublished data) examined genomic variation and structure in P. fremontii based on restriction site associated DNA sequencing (RADseq). Using ~9000 single nucleotide polymorphisms (SNPs), they found additional support for the three main ecoregions, as defined by Ikeda et al. (2017) showing that genetic structure in P. fremontii strongly correlates with variation in three primary environmental variables (minimum temperature of the coldest month, precipitation seasonality and mean temperature of the coldest quarter of the year). Together, these data support a hypothesis of strong niche differentiation as suggested by Ikeda et al. (2017) and provide the basis for investigating how environmental variation has shaped genetic variation and structure in P. fremontii.
Figure 2. From Ikeda et al. (2017). (a) Distribution of P. fremontii ecotypes. Genetic collections [circles, from Cushman et al. (2014)] and Global Biodiversity Information Facility (GBIF) occurrence locations (triangles) were used to construct ecological niche models for the three ecotypes: Central California Valley (red), Utah High Plateau (green) and Sonoran Desert (grey). (b) STRUCTURE diagram (Pritchard et al., 2000) showing P. fremontii population genetic structure. Each bar represents an individual tree; different colours indicate probability of belonging to a given genetic group.
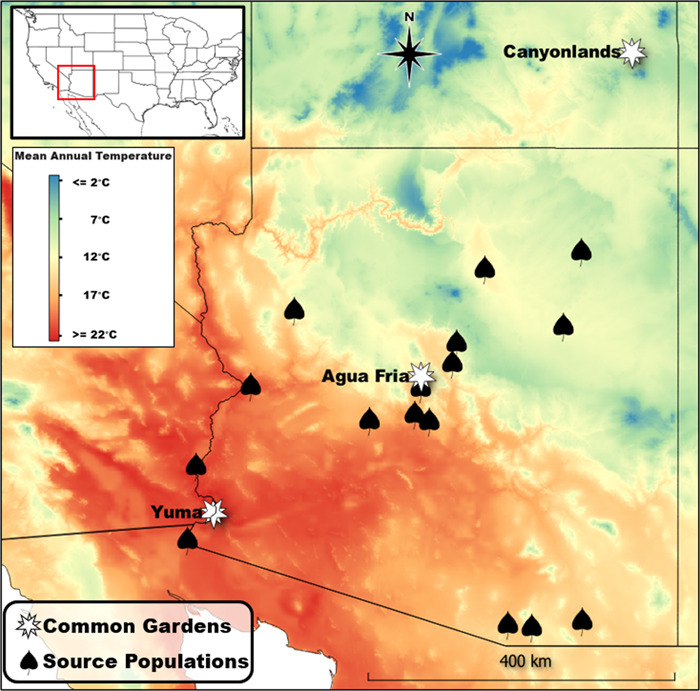
One powerful tool for investigating genetic variation, patterns of local adaptation and phenotypic plasticity is through the use of experimental common gardens. One example of a successfully constructed reciprocal common garden network is with P. fremontii in the southwestern USA (Fig. 3). The gardens were originally established in 2014 from cuttings collected from 12 genotypes per 16 source populations representing two of the three ecoregions defined by Ikeda et al. (2017; Fig. 2), including the UHP and SD ecoregions (Fig. 3). Each garden consists of over 4000 trees planted in four replicated blocks. The three gardens span an elevation gradient of almost 2000 m encompassing an extensive range of temperature extremes experienced by P. fremontii (Cooper et al., 2019). Mean annual temperature ranges from 10.7°C at the highest elevation garden near Canyonlands, UT, to 17.2°C at the mid-elevation garden north of Phoenix, AZ, to 22.8°C at the low-elevation garden near Yuma, AZ (Fig. 3). The summation of the three reciprocal gardens provides a robust tool for studying the impacts of climate change on phenotypic expression, productivity and fitness of P. fremontii in ways that were previously impossible at the landscape scale.
Figure 3. Map showing locations of P. fremontii source populations and network of reciprocal common garden mean annual temperatures and locations distributed across a broad climate, elevational and latitudinal gradient.
Trade-offs between thermal regulation and hydraulic risk
Episodic heat waves that are increasing in duration, frequency and intensity will likely amplify thermal stress, mortality and shifts in hydraulic trait expression. For many plant genotypes, especially those occurring on the warm edge of a species distribution, canopy thermal regulation is critical for maintaining leaf carbon balance. Extreme thermal stress not only increases both mitochondrial and photorespiration (at least in C3 plants) but can also irreversibly damage the electron transport capacity of Photosystem II. Thus, exposure to heat stress acts as a strong agent of selection in a broad range of taxa in terms of phenology, hydraulic architecture, xylem anatomy and stomatal regulation. In environments where thermal loads approach an upper limit above which leaf function is impaired (Tcrit), plants must transpire water to evaporatively cool leaves below Tcrit (i.e. Tleaf < Tcrit). Recent evidence indicates that leaf conductance in some species increases when leaf temperatures rise above 40°C, thereby increasing the difference between Tleaf < Tcrit (Slot et al., 2016). In some cases, leaf conductance may increase independent of changes in net photosynthesis, indicating there are alternative water-use strategies in some plants other than maximizing carbon gain for a fixed level of stomatal conductance (Urban et al., 2017; Drake et al., 2018; Aparecido et al., 2020). However, there is an inherent hydraulic risk of maintaining Tleaf < Tcrit under hot and dry conditions in that leaf conductance could drop plant water potential below a critical threshold (Ψcrit). Therefore, plants exposed to extreme thermal conditions must optimize traits to balance canopy thermal regulation with plant hydraulic limits.
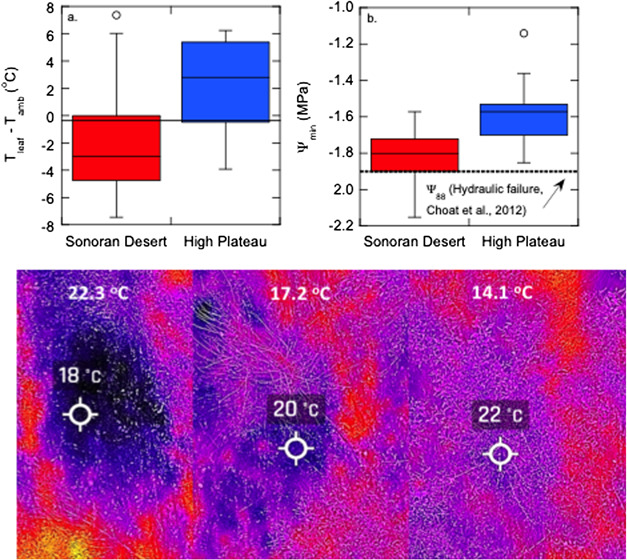
On the warm edge of its distribution, P. fremontii is exposed to some of the warmest mean annual temperatures in North America, often approaching 50°C in midsummer—temperatures that could far exceed Tcrit. A recent common garden experiment conducted at the mid-elevation common garden (Fig. 3) revealed that chronic exposure to intense heat waves could impose strong selection pressures on P. fremontii to maximize canopy thermal regulation via a suite of hydraulic strategies (Hultine et al., 2020). Genotypes sourced from the extremely warm SD ecoregion had midday leaf temperatures in midsummer that were on average 2.0°C (SE ± 0.58) below ambient temperature; while genotypes sourced from the higher elevation, cooler UHP ecoregion had leaf temperatures that were on average 2.1°C (SE ± 0.67) above ambient temperature (Fig. 4a). The cooler leaf temperatures corresponded with the warm-adapted, SD ecotypes having a 35% higher midday leaf transpiration rate (E) relative to the UHP ecotypes, resulting in substantially greater leaf evaporative cooling (Hultine et al., 2020). Although predawn leaf water potentials (Ψpd) were similar between the two ecotypes, minimum leaf water potentials (Ψmin) during midday were on average 0.2 MPa lower in the SD ecotypes (Fig. 4b). The higher leaf E coupled with the lower Ψmd suggests that the SD ecotypes have a reduced stomatal control over plant water potential than the UHP ecotypes perhaps as a consequence of having to cope with extreme thermal stress during midsummer. However, a reduced stomatal control over plant water potential is an inherently risky hydraulic strategy, illustrated in Fig. 4b. In many SD genotypes, Ψmin approached, or even fell below, the xylem pressure (−1.88 MPa) at which near complete hydraulic failure occurs in P. fremontii—defined as when the loss of maximum xylem conductivity reaches 88% (Ψ88: Choat et al., 2012). Conversely, Ψmin in the UHP genotypes never fell below the Ψ88 threshold (Fig. 3b), indicating that UHP genotypes have not been selected to take on the same ‘risky’ evaporative cooling strategy as the Sonoran Desert (SD) genotypes.
Figure 4. Contrasts in leaf temperature and leaf water potentials between high- and low-elevation Populus fremontii genotypes measured in the mid-elevation common garden (Figure 3). (a) Box and whisker plots showing the median, 25th and 75th percentiles (box plots) and 10th and 90th percentiles (error bars) of leaf surface temperatures (Tleaf) subtracted from ambient temperature (Tamb). (b) Box and whisker plots of minimum daily leaf water potentials (Ψmin) measured monthly over the growing season with a Scholander type pressure chamber. Lower panels represent canopy temperatures measured with a thermal camera on three genotypes sourced from three populations varying in mean annual temperatures (shown in white text).
In order to mitigate the risks of hydraulic failure while maintaining Tleaf < Tcrit during midsummer heat waves, SD genotypes must optimize a suite of traits related to phenology, hydraulic architecture and xylem function. For example, leaf flush in the low and mid-elevation common gardens occurred as much as 2 months earlier in warm-adapted compared to cold-adapted genotypes (Cooper et al., 2019). The earlier leaf flush may be necessary to optimize photosynthetic carbon balance prior to midsummer when the difference between diurnal CO2 flux via photosynthesis and CO2 efflux via leaf respiration approaches zero due to heat exposure. In order to optimize leaf evaporative cooling, plants adapted to hot environments should also be constructed such that they maximize the supply of water from the soil and vascular system relative to the demand for water via transpiring leaves. (Martinez-Vilalta et al., 2009). At the mid-elevation garden, genotypes from the warmer SD ecoregion maintained a 38% higher sapwood area to whole-canopy leaf area ratio relative to cooler UHP ecoregion. Similarly, the same SD genotypes at the mid-elevation garden displayed a 89% higher petiole lumen area to cross-section petiole area fraction—a trait that reflects the maximum water conducting capacity of the petiole—to leaf surface area ratio (Blasini et al., unpublished data). Moreover, maximum rooting depth of riparian taxa, including P. fremontii is correlated with hydrologic regime (Stromberg, 2013). Experimental evidence indicates ephemeral river reaches are comprised of P. fremontii genotypes with deeper roots and larger root area to leaf area ratios than P. fremontii genotypes along perennially flowing river reaches (Cadmus et al., unpublished data). Thus, genotypes adapted to maintaining high daytime leaf-level transpiration fluxes are likely to also maintain higher root area to leaf area ratios.
Whether the expression of hydraulic architecture and leaf cooling traits is enough to overcome progressive warming and subsequent heat waves that are projected over the next century in the southwestern USA and northern Mexico is an open question. Warming temperatures may lead to significant range shifts in low-elevation SD ecotype plants, but potentially without an overall reduction in range distribution relative to other P. fremontii ecotypes (Ikeda et al., 2017). However, given the potential hydraulic risk of evaporative cooling, even small reductions in available water coupled with extreme thermal stress could lead to rapid episodic mortality events of P. fremontii gallery forests (e.g. upper photo of Fig. 1). Restoring and conserving P. fremontii gallery forests will therefore require targeted genetic and phenotypic information to overcome projected environmental constraints on riparian ecosystems.
P. fremontii root symbionts: coping with climate change and Tamarix
The results of recent reviews convincingly demonstrate that the mycorrhizal fungi associated with the roots of most plants (Brundrett, 2009) can buffer the effects of climate change, including warming temperatures and drought (Kivlin et al., 2013; Mohan et al., 2014; Augé et al., 2015). The importance of mycorrhizal fungi in the context of climate change is not surprising given that they improve host plant access to soil resources in exchange for fixed carbon (Smith and Read, 2008). In fact, there is evidence that mycorrhizal fungi are more beneficial in stressful situations. In the case of drought, the beneficial effects of mycorrhizal fungi are larger when drought is severe than when it is absent or moderate (Pena and Polle, 2013; Augé et al., 2015). However, invasive plants can alter soils in ways that negatively affect the mycorrhizal associations of native plants (Bunn et al., 2015, Grove et al., 2017). Although the mechanisms are not understood, Tamarix alters the mycorrhizal fungal abundance and community composition of P. fremontii, (Meinhardt and Gehring, 2012), leaving behind a legacy of low mycorrhizal abundance even after it is removed (Hultine et al., 2015). Given that most plant species form mycorrhizal associations, experience climate change and compete with exotic species, it is critical to understand if these fungi help plants overcome the physiological limits imposed by climate change, alone, and in conjunction with non-native competitors or their legacy.
Like many plant species in the western United States, P. fremontii faces warming temperatures punctuated by extreme drought, along with widespread non-native competitors such as Tamarix that negatively affect cottonwood survival as their densities increase (Gitlin et al., 2006). However, P. fremontii is unusual in that it simultaneously forms mycorrhizal associations with two major types of mycorrhizal fungi, the ectomycorrhizas (EM) that occur on many woody perennial plants and the arbuscular mycorrhizas (AM) that occur on the majority of grass, crop and herbaceous plants (Brundrett, 2009). This trait has been observed in a small percentage (~7%) of plant families (Teste et al., 2019) with many species only having these dual associations when they are young (Brundrett, 2009; Teste et al., 2019). However, P. fremontii, P. angustifolia and their hybrids form dual associations even when sexually mature (Gehring et al., 2006). While both AM and EM fungi enhance soil resource uptake, the two types of associations differ in the environments they occupy (Swaty et al., 2016, Soudzilovskaia et al., 2019), the soil resources they access (Smith and Read, 2008) and their effects on ecosystem properties (Phillips et al., 2013; Jo et al., 2019, Soudzilovskaia et al., 2019). Ectomycorrhizal fungi are more effective than AM fungi in reducing the effects of soil-borne pathogens in woody plants (Laliberté et al., 2015), which may contribute to the tendency for AM-associated trees to have negative plant-soil feedbacks, promoting plant diversity, while EM-associated trees tend to have positive plant-soil feedbacks, leading to monotypic stands (Bennett et al., 2017). The limited available data indicates that negative feedbacks are uncommon in dually colonized plants (Teste et al., 2019). Understanding the dynamics of AM and EM associations in dually colonized plants, particularly foundation species like P. fremontii, is thus important not only to the plant species themselves but has cascading effects on the ecosystem.
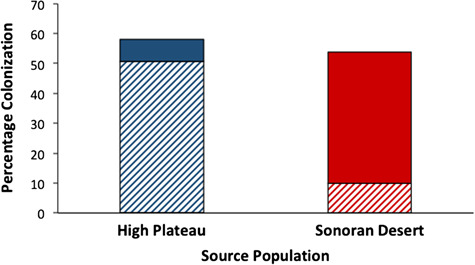
In the western cottonwoods, P. angustifolia and P. fremontii, root colonization by AM and EM fungi varies with soil moisture (Gehring et al., 2006) and/or cottonwood source population, demonstrating context dependency that could improve cottonwood responsiveness to environmental change. Six weeks of watering in the field during a hot, dry summer altered the dominant mycorrhizal association of juvenile cottonwoods from AM to EM (Gehring et al., 2006), a result consistent with studies of oak (Querejeta et al., 2009) and with the dominance of AM fungi in warmer, more arid landscapes (Swaty et al., 2016). In a mid-elevation common garden, P. fremontii from the warmer, more arid SD ecoregion were dominated by AM fungi while those from the UHP ecoregion were dominated by EM fungi (Fig. 5), suggesting a genetic component to dominant mycorrhizal association. Cottonwoods benefit from both AM and EM associations in terms of growth (Meinhardt and Gehring, 2012; Gehring et al., 2014), but it is unclear how the combined effects of climate change and Tamarix legacy will alter the abundance of these associations or if different cottonwood genotypes will respond similarly. For instance, both AM and EM have been observed to access and move water (e.g. Egerton-Warburton et al., 2007) and likely increase moisture retention in the soil (Querejeta et al., 2012). However, AM may increase plant water-use efficiency more than EM (e.g. Querejeta et al., 2006). Additionally, greenhouse experiments showed that the beneficial effects of mycorrhizas for P. fremontii were reduced in the presence of Tamarix with greater effects on EM (Meinhardt and Gehring, 2012). Current studies are demonstrating that adding live soil from the rhizosphere of P. fremontii from appropriately matched sites without a tamarisk history can improve survival and growth where Tamarix has been removed by 30–50% in the greenhouse and in the field (Hull et al., unpublished data, Markovchick et al., unpublished data). Yet, co-evolution between plants, mycorrhizal fungi and soil (e.g. Johnson et al., 1992) can set the stage for inappropriately matched mycorrhizal inoculation to result in neutral to negative plant effects (e.g. Rua et al., 2016). Thus, it is not always obvious how to best combine and apply research on mycorrhizal fungi with that on plant hydraulic traits, site adaptation, assisted migration and other management tools.
Figure 5. Percent colonization of P. fremontii roots by two types of mycorrhizal fungi in mid-elevation common garden, where trees were proximate and conditions similar. AM colonization (solid bars) dominated in trees from the warmer, more arid SD ecoregion, while EM colonization (hatched bars) dominated in trees from the higher, cooler UHP ecoregion. Data represent averages of five root samples per ecoregion. Roots were processed as in Gehring et al. (2006).
Further research on combined environmental stressors (drought, heat, invasive species) and interacting management tools is necessary to determine the importance of AM and EM associations to the future resilience of P. fremontii as a foundation species. It is also important to understand the future distribution of AM and EM fungi in western US riparian areas because of their distinct influences on ecosystems processes such as carbon storage, nutrient cycling (Phillips et al., 2013, Jo et al., 2019, Soudzilovskaia et al., 2019), water access, plant survival under drought conditions (Egerton-Warburton et al., 2007, Teste and Simard, 2008) and plant diversity (Laliberté et al., 2015, Teste et al., 2019). Inoculation with appropriate mycorrhizal fungi in areas where these fungi are reduced due to the legacy of Tamarix invasion could be critical to restoring cottonwoods, their diverse associated aboveground and belowground communities and ecosystem functions.
The role of phenotypic plasticity in maximizing resilience to environmental change
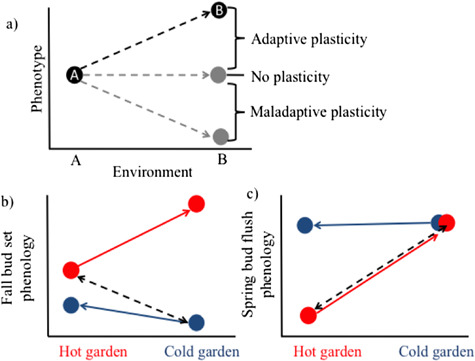
Phenotypic plasticity, the capacity of a genotype to express different phenotypes in response to different environments, can facilitate population persistence and influence the strength of selection and degree of genetic adaptation under rapid environmental change (Gibert et al., 2019). Plasticity can be expressed in an adaptive direction, where the phenotype shift is towards a local optimum and results in increased plant fitness, or in a non-adaptive direction, with traits shifting away from the local optimum and reducing fitness (Fig. 6a; Ghalambor et al., 2007, Gibert et al., 2019). Perhaps the most robust examples of phenotypic plasticity in plants revolve around growth and phenology traits and are best examined in multiple common garden experiments where genotypes are replicated across different environments (Franks et al., 2014). For example, common garden studies on P. fremontii (Fig. 3) show variation in the direction and magnitude of plasticity for two phenology traits, fall bud set and spring bud flush (Cooper et al., 2019). Here, the nature of plasticity that a population expressed (adaptive or non-adaptive) depended on both the trait and the climatic distance between population origin and common garden transplant distance. For example, populations from the hot, southern SD ecoregion exhibited non-adaptive bud set plasticity when they were transferred to the colder, northernmost garden in Utah, while populations from the coldest provenances showed limited adaptive plasticity when transferred into the hottest common garden in southern Arizona (Fig. 6b). Spring bud flush showed varied patterns, with hot populations exhibiting adaptive plasticity and cold populations showing little to no plasticity when grown in the common garden farthest from their source provenance (Fig. 6c). Plasticity in phenology is especially important as adjusting to earlier springs can increase growing season length, which will affect not only long-term growth and competitive ability of P. fremontii trees but will also cascade to the broader community of arthropods, microbes, birds, etc. that utilize these plants for habitat and food, as well as ecosystem processes such as carbon and nitrogen cycling (Walker et al., 2019). Interestingly, the populations in this study showed a significant correlation between climate of origin and magnitude of plasticity (Cooper et al. 2019). Trees from low elevation, hot provenances were much more plastic than trees from cold climates that regularly experience freezing. This suggests inherent limits to plasticity due to the selective abiotic pressures that trees have evolved to withstand.
Figure 6. (a) Theoretical example of phenotypic plasticity. Environments A and B each have a local genotype (A and B) representing the local optimum phenotype, coloured black. If genotype A is moved to Environment B, it can exhibit adaptive plasticity where the phenotype approaches B. If it matches B’s phenotype (black dashed line), it is perfectly adaptive (Ghalambor et al., 2007). If the phenotype does not change, there is no plasticity. If the phenotype changes in the opposite direction as the local optimum, this is considered non-adaptive plasticity. These plastic responses can then impact higher levels of biological organization. (b) Fall but set phenology shows non-adaptive plasticity of hot genotypes transferred from the hot garden to the cold garden and slightly adaptive plasticity for cold genotypes transferred from cold to hot gardens. (c) Spring bud flush phenology shows highly adaptive plasticity of hot genotypes transferred from hot to cold gardens, and no plasticity for cold genotypes transferred from cold to hot gardens. The dashed black lines show perfectly adaptive plasticity. (b) and (c) adapted from Cooper et al. 2019.
Common gardens have also been utilized to show significant leaf size and symmetry plasticity in P. fremontii as genotypes were transferred to garden climates increasingly different from their home provenance climates (Parker et al., in review). Fluctuating leaf asymmetry is commonly used as an indicator of genetic and environmental stress (Wilsey et al., 1998, Beasley et al. 2013). Leaf size and symmetry decreased with increasing climate transfer distance to both warmer and colder garden climates, and this non-adaptive plasticity was correlated with lower tree growth and higher mortality (Parker et al., in review).
Plasticity can be especially important in preventing long-lived tree species showing high levels of local adaptation from going extinct under rapid climate change events (Franks et al., 2014). The ability of plants to maintain functional water transport systems during periods of extreme climatic stress, such as heat waves or drought, is critical for survival, especially as the frequency of climate extremes is predicted to increase. Phenotypic plasticity has been documented for a range of hydraulic traits in plants subjected to water and temperature stress. For example, increased resistance to cavitation via plastic xylem traits was documented in hybrid popular saplings under drought treatment (Plavcová and Hacke, 2012). Likewise, in years with low water availability, vessel size and density shifted to balance hydraulic conductivity and embolism risk in oaks (Abrantes et al., 2013). A recent global review of plasticity in turgor loss point revealed substantial plasticity in wilting point across both wild and crop species (Bartlett et al., 2014). Although there are many studies documenting plasticity in important hydraulic traits in response to environmental change, there may be limits or costs to plasticity due to correlated traits or trade-offs inherent in balancing a functional hydraulic system. For instance, Hultine et al. 2020 documented a trade-off in arid-adapted phreatophytic plants, where they can withstand decreases in available groundwater or increases in atmospheric demand (temperature), but not both.
Hybridization and resiliency to environmental stress
Hybridization has long been recognized as a major evolutionary pathway in the formation of new species (Grant, 1971; Arnold, 1997), and it is estimated that up to 70% of all plants owe their evolutionary origins to ancient and modern hybridization events (Stace, 1987). Some plant groups so readily hybridize (e.g. pines, oaks, eucalypts, cottonwoods) that the total number of species that hybridize within a group is referred to as a syngameon (Grant, 1971). A long history of hybridization events in such species complexes often results in incomplete lineage sorting (Wu et al., 2018) and reticulate evolutionary trees (McKinnon, 2005). This commonality amongst many native syngameons provides evidence for the importance of hybridization as a key evolutionary process that contributes to their continued persistence. Although the conservation of hybrids suffered for many years due to their being considered maladaptive and resulting in the disintegration of pure species (e.g. O’Brien and Mayr, 1991), this issue is much less of a concern with naturally occurring hybrids between native species (Whitham et al., 1991, Whitham and Maschinski, 1996). These naturally occurring hybrids often exhibit hybrid vigour and, in some cases, naturally occurring hybrids have been included in the recovery plans of listed species to preserve their evolutionary potential (e.g. U.S. Fish and Wildlife Service, 1995). Eckenwalder (1984) showed that, wherever two Populus species come together in the USA and Canada, hybridization occurs, and the geographic distribution of hybrids can be as great as their parental species; e.g. the hybrids between Populus angustifolia and P. fremontii are found from Mexico to southern Idaho.
Hybrids possess three major attributes that make them especially important in evaluating their potential in conservation and mitigating the impacts of climate change. First, Schweitzer et al. (2002) found that both F1 (i.e. the first generation cross between two species) and backcross generations (F1s can backcross with their parental species to produce a continuum of intermediates between species) can be equally or more fit than the parent taxa. F1 hybrids between P. angustifolia and P. fremontii produced as many viable seeds as P. angustifolia (but fewer than P. fremontii), and backcross hybrids were equal to both parents. Asexual reproduction through stump sprouting (coppicing) or suckering from root sprouts to form clones was nearly twice as high in hybrids compared to P. angustifolia and far greater than P. fremontii, which can coppice from stumps, but we have not observed any root sprouting. Similarly, Gom and Rood (1999) found that 5 years after a fire event, 97% of the sprouting trunks and 98% of the root sprouts were from species in the section Tacamahaca, which includes P. angustifolia, while species in the section Aigeiros, which includes P. fremontii, accounted for only 3% of the sprouting trunks and 2% of the root sprouts. Associated with these findings they considered the quality of clonal regeneration in P. fremontii to be poor with sparse trunk sprouting. They also note that the lack of resprouting by P. fremontii allows other invasive clonal species like Tamarix to invade following fire. Another study by Schweitzer et al. (2002) did not find any resprouting or formation of clones by P. fremontii in the field under no fire conditions, but at the same study, sites they found prolific resprouting by P. angustifolia and naturally occurring hybrids with P. fremontii.
The propensity of hybrids to reproduce asexually may be especially important in a changing environment where drought and altered stream flows due to the damming of rivers can prevent natural recruitment by cottonwoods that require flooding events to create bare mineral sand banks for seedling germination and establishment (Rood et al., 2005). Once a hybrid becomes established, it may asexually produce hundreds of progeny that initially receive parental care through their connected root systems, resulting in high survival rates. The clone may persist for 100 to 1000 s of years because regeneration is not dependent on flooding events. The fact that P. fremontii does not reproduce asexually via root sprouts could be a major detriment to its long-term survival in the arid Southwest. Even if restoration plantings are successful in altered river systems, lack of natural recruitment will eventually lead to failure of that population (Rood et al., 2005; Dixon et al., 2012; González et al., 2018).
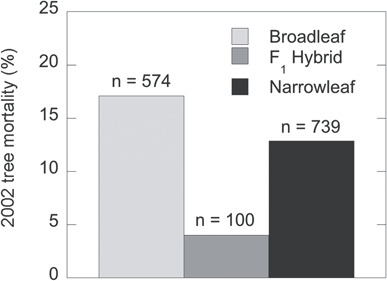
A second major adaptive feature of naturally occurring hybrids is their potential to tolerate drought events better than their parental species. After the Southwest’s record drought in 2002, mortality of F1 hybrids in 2003 on the Colorado Plateau was 1/4th and 1/3rd the mortality of P. fremontii and P. angustifolia, respectively (Fig. 7). Although the specific mechanism(s) that might account for these differences in reproduction and drought tolerance is unknown, molecular genetic analyses show that the hybrids, as expected, can exhibit far greater genetic diversity than their parental species as a result of recombination between the two parental genomes (Whitham et al., 1999; Martinsen et al., 2001). Such genetic variation may be especially important as it provides a wider range of novel variants for selection to act upon in a changing environment.
Figure 7. A survey of cottonwood mortality on the Colorado Plateau after the drought year of 2002 revealed higher survival of hybrids than parental species. Forty-six stands were chosen, including 20 narrow-leaf cottonwood stands, 15 Fremont cottonwood stands (broadleaf) and 11 stands in the hybrid zone where upper and lower elevation species are both found along with their F1 type hybrids (narrow-leaf n = 628 trees; F1 type n = 100; Fremont (broadleaf) cottonwood n = 574). The first 30 standing trees encountered when walking transects perpendicular to the river’s edge were counted and identified based on leaf morphology. Individuals were defined as being >2 m tall and included all resprouting that connected to the main trunk above the ground level. Death was defined as the complete mortality of a single individual, and a tree was considered live if there was evidence of basal resprouting from the trunk. The taxonomic status of dead trees was based upon dried leaves, tree structure and placement in relation to other trees. When a tree could not be clearly identified, it was not included in the survey. In order to capture the effect of the current drought on tree stands, only recently dead trees were counted (i.e. standing trees with intact bark and small diameter branches present). F1 type mortality never exceeded 8% for a single stand, while mortality for pure species ranged between 0 and >50% for individual stands. Average F1 mortality was 4% and differed significantly from mortality of parental species (χ2 = 14.889; P = 0.0006).
Third, naturally occurring hybrids affect communities in diverse ways. (i) They often host the different biota (e.g. arthropod communities) supported by both parental species (review by Whitham et al. 1999), which can greatly enhance their conservation importance in riparian habitat that is recognized as a hotspot of biodiversity (Bothwell et al., 2017). Importantly, hybrids also provide critical habitat for some arthropod species found nowhere else that have specifically evolved to live on hybrids (Evans et al., 2008, 2012). (ii) Recent studies have also shown that arthropod communities on hybrid versus parental cottonwoods differ significantly in their community phylogenetic structure, suggesting that hybrids are evolutionarily significant units of biodiversity that merit conservation management (Jarvis et al., 2017). (iii) Birds respond to the architectural differences of hybrids and some preferentially select hybrids as nesting habitat (Martinsen and Whitham, 1994). (iv) Cottonwoods are preferred forage for beaver (Castor canadensis), and their selective foraging can affect the species composition of riparian forests to favour less preferred species and hybrids that resprout prolifically following herbivory. In cafeteria choice experiments, Bailey et al. (2004) showed that beaver preferred P. fremontii more than hybrids and much more than P. angustifolia. Furthermore, over a 26-month study in the wild, they showed significantly more P. fremontii than hybrids were felled by beaver resulting in a significant shift in stand composition. This shift was driven by the combined effects of selective herbivory as well as the poorer resprouting abilities of P. fremontii relative to hybrids and P. angustifolia (Gom and Rood, 1999; Schweitzer et al., 2002). This is important because when hybrids are felled by beaver; they generally resprout from both the stump and roots to form clones that can be composed of 100 s of trees. Although McGinley and Whitham (1985) found that while small P. fremontii (about 3 m tall) stump sprouted following beaver herbivory, no root sprouting was observed and trees were transformed into shrubs with no formation of clones. At other sites with larger trees, no stump sprouting was observed, and the trees often died following beaver herbivory (Whitham personnel observation). (v) The fungal communities associated with hybrids can alter the performance of the trees themselves as well. For example, P. angustifolia cuttings grew significantly larger when inoculated with AM fungal spores from F1 hybrids than with spores from P. fremontii, or P. angustifolia (Gehring et al., 2014). Fungal pathogen communities also differ amongst P. fremontii, P. angustifolia and their F1 hybrid (Busby et al., 2013).
Future directions: scaling trait expression from genotypes to ecoregions
The common garden and greenhouse studies described above have identified key genomic, phenotypic and physiological variation that contributes to the capacity of P. fremontii and its hybrids to survive in the arid Southwest. Characterizing such variations at the landscape scale and understanding their interactions with climate change overtime requires scaling up measurements and observed relationships from genotypes to ecoregions. Remote sensing provides unique opportunities to measure phenotypic and physiological variation at various temporal and spatial scales. At the individual leaf level, ground-based, handheld spectroradiometer hyperspectral data can provide detailed estimates of plant chemical traits, carbon exchange, reflectance and moisture content. In comparison, unmanned and manned airborne hyperspectral sensors provide similar estimates at the scales of whole tree canopies, field plots or a landscape, whereas spaceborne satellite sensors can provide coarse resolution estimates of total ecosystem net primary productivity, vigor and moisture content at regional, continental and global scales. Taken together, these remote sensing data can provide detailed measurements at individual leaves and canopies, which can then be scaled up to satellite image-derived estimates at broader landscape scales.
Various sensors can be used to detect specific P. fremontii traits and their plasticity across environmental gradients. While hyperspectral sensors cover a large range of the electromagnetic spectrum (400–2500 nm) and can, therefore, detect variability in a wide range of plant traits, a suite of other sensors can be used to establish relationship with the hyperspectral measurements in high spatial resolution. In a hierarchical approach, Sankey et al. (2017a,b) used ground-based hyperspectral data as well as UAV hyperspectral and lidar data to characterize individual plant species and canopies over small areas covering 1–3-ha areas. Such hyperspectral measurements were further scaled up to include larger areas of 10–30 ha using unmanned aerial vehicle (UAV) multispectral sensors (Sankey et al., 2017a,b; Solazzo et al., 2018; Elkind et al., 2019) that only included four spectral bands centred at the visible and near-infrared regions of the electromagnetic spectrum.
Manned and unmanned airborne multispectral sensors with a few spectral bands can be used at the peak of the growing season to measure total green leaf abundance and biomass at the whole-canopy level across the landscape to determine if these traits differ amongst P. fremontii populations and genotypes, between hybrids versus parental species, and between P. fremontii trees growing with versus without Tamarix. For example, spectral band ratios including normalized difference vegetation index (NDVI) and optimized soil-adjusted vegetation index (OSAVI) from UAV multispectral images have been used to detect differences amongst genotypes within a single tree species (Santini et al., 2018). In similar applications for Populus trees, individual canopies with significantly greater leaf abundance, biomass, survival rates, NDVI and OSAVI values can be identified to determine genotypes that are best suited to cope with climate change, stress and impacts from Tamarix soil legacies. Furthermore, Bedford et al. (2018) demonstrated that manned airborne multispectral data can be used across entire river corridors to detect Tamarix invasion and subsequent defoliation by an introduced biocontrol agent, the Tamarix leaf beetle (Hultine et al., 2010). Using manned airborne lidar data in conjunction with multispectral data, Sankey et al. (2016) estimated changes in Tamarix biomass due to varying rates of defoliation. Similarly, unmanned and manned airborne lidar data (Sankey et al., 2013; Shin et al., 2018) can be used to detect differences in Populus aboveground plant biomass, canopy architecture, height and diameter amongst genotypes and hybrids versus parental species.
Thermal sensors spanning the 8000–12 000-nm spectral range can be used to evaluate patterns of canopy thermal regulation. Specifically, thermal sensors used at the peak of the growing season can measure whole-canopy-scale temperature of individual cottonwood trees (Sankey et al., unpublished data). Previous ground-based leaf temperature measurements in common gardens indicate up to 6°C midday difference amongst P. fremontii genotypes (Fig. 4), while some currently available thermal sensors are sensitive to differences within 1°C and can detect statistically significant differences amongst genotypes (Santini et al., 2019). Our UAV thermal data at a common garden demonstrates that P. fremontii populations have significantly different whole canopy-scale mean temperatures (Sankey et al., in review). The UAV thermal data further demonstrates that some of the P. fremontii genotypes also have significantly different canopy-scale mean temperatures (Sankey et al., in review). The temperature measurements can be further used to derive estimates of canopy transpiration and canopy stomatal conductance. While Landsat 8 OLI satellite thermal sensor and ECOSTRESS satellite sensors only provide large pixels of 100-m spatial resolution, the thermal bands can be similarly used to estimate whole P. fremontii stand temperature and derive stand-level transpiration and stomatal conductance. Canopy thermal regulation, transpiration and stomatal conductance can be measured across spatial scales by combining high-resolution manned or unmanned airborne images with small spatial extents with coarser resolution satellite images over larger areas.
Many remote sensing techniques can make consistent and repeated measurements at various phenological stages, providing a unique opportunity to detect differences in trait plasticity amongst genotypes. For example, early spring images can be used to detect differences in greening and leaf flush, whereas peak of the growing season images might be used to measure leaf area and survival/mortality to determine if these traits are significantly different amongst genotypes (Sankey et al., unpublished data). In such repeated measurements, the high resolution, fine-scale images can be correlated to coarser resolution images covering a much larger area to scale up the specific measurements from genotypes to whole landscapes and ecosystems. In such scaling efforts, the high-resolution images are critical for training and validating the coarser resolution image-based estimates.
Conclusions: management of P. fremontii forests for adaptation and resilience
The capacity for P. fremontii to continue supporting diverse biotic communities in aridland riparian areas may depend largely on identifying and exploiting adaptive traits that match future environmental conditions, and our research suggests that optimal genotypes for any given location will depend on trade-offs in their varying capacities to cope with the effects of drought, heat waves, the presence of Tamarix and other stressors. In some regions such as the seasonally hot SD, restoration projects will inevitably be limited to genotypes selected for extreme evaporative leaf cooling and locations where groundwater remains stable during the hottest periods of the year. Likewise, successful restoration and maintenance of P. fremontii forests may also require active management of plant microbial interactions through inoculation of AM and EM fungi, particularly in areas previously dominated by Tamarix. Because environmental conditions of riparian ecosystems are highly dynamic and are expected to experience greater climate extremes in the near future (Garfin et al., 2013), genotypes that express a high degree of plasticity in traits related to frost avoidance, heat avoidance and tolerance to low soil microbial abundance will inevitably be favoured. Finally, restoration ecologists have likely undervalued the potential of using Populus hybrids in restoration given evidence suggesting that hybrids are likely to have higher fitness and survivorship compared to parental species when exposed to episodic stress conditions.
Opportunities to better inform restoration of P. fremontii and other foundation tree species may be amplified by taking advantage of cutting-edge tools to evaluate the adaptive capacity of plant stress tolerance. Recent advances in Populus whole-genome sequencing to evaluate adaptive trait associations (Tuscan et al., 2006; Evans et al., 2014), high throughput phenotyping (Araus and Cairns, 2014), hyperspectral imaging of forest canopies (Asner et al., 2017) and coupled fluvial hydrologic/plant hydraulics modelling (Tai et al., 2018) amongst many other advances provide emerging opportunities to rapidly evaluate and predict survivorship and foundational capacity of P. fremontii in rapidly changing environmental conditions. Approaches that merge a broad suite of phenotypic traits with process-based models will provide a new way forward to studying and protecting groundwater dependent vegetation, riparian communities and ecosystem processes into the future.
Funding
This research was supported by the National Science Foundation MacroSystems Biology programme [DEB-1340852 and DEB-1340856], BEE grant DEB-1914433 and MRI-DBI-1126840.
References
- Abrantes J, Campelo F, García-González I, Nabais C (2013) Environmental control of vessel traits in Quercus ilex under Mediterranean climate: relating xylem anatomy to function. Trees 27: 655–662. [Google Scholar]
- Arnold ML. (1997) Natural Hybridization and Evolution. Oxford University Press, New York. [Google Scholar]
- Augé RM, Toler HD, Saxton AM (2015) Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25: 13–24. [DOI] [PubMed] [Google Scholar]
- Aparecido LMT, Woo S, Suazo C, Hultine KR, Blonder B (2020) High water use in desert plants exposed to extreme heat. Ecol Lett. 10.1111/ele.13516. [DOI] [PubMed] [Google Scholar]
- Araus LA, Cairns JE (2014) Field high-throughput phenotyping: the new crop breeding frontier. Trends Plant Sci 19: 52–61. [DOI] [PubMed] [Google Scholar]
- Asner GP, Martin RE, Knapp DE, Tupayachi R, Anderson CB, Sinca F, Vaught NR, Llactayo (2017) Airborne laser-guided imaging spectroscopy to map forest trait diversity and guide conservation. Science 355: 385–389. [DOI] [PubMed] [Google Scholar]
- Ault TR, Cole JE, Overpeck JT, Pederson GT, Meko DM (2014) Assessing the risk of persistent drought using climate model simulations and paleoclimate data. J Climate 27: 7529–7549. [Google Scholar]
- Bailey JK, Schweitzer JA, Rehill BJ, Lindroth RL, Martinsen GD, Whitham TJ (2004) Beavers as molecular geneticists: a genetic basis to the foraging of an ecosystem engineer. Ecology 85: 603–608. [Google Scholar]
- Bartlett MK, Zhang Y, Kreidler N, Sun S, Ardy R, Cao K, Sack L (2014) Global analysis of plasticity in turgor loss point, a key drought tolerance trait. Ecol Lett 17: 1580–1590. [DOI] [PubMed] [Google Scholar]
- Bateman HL, Nagler PL, Glenn EP (2013) Plot - and landscape – level changes in climate and vegetation following defoliation of exotic saltcedar (Tamarix sp.) from the biocontrol agent Diorhabda carinulata along a stream in the Mojave Desert (USA). J Arid Environ 89: 16–20. [Google Scholar]
- Beasley DAE, Bonisoli-alquati A, Mousseau TA (2013) The use of fluctuating asymmetry as a measure of environmentally induced developmental instability: a meta-analysis. Ecol Indic 30: 218–226. [Google Scholar]
- Bedford A, Sankey TT, Sankey JB, Durning L, Ralston BE (2018) Remote sensing of tamarisk beetle (Diorhabda carinulata) impacts along 412 km of the Colorado River in the Grand Canyon, Arizona, USA. Ecol Indic 89: 365–375. [Google Scholar]
- Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J (2017) Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355: 181–184. doi: 10.1126/science.aai8212. [DOI] [PubMed] [Google Scholar]
- Bothwell HM, Cushman SA, Woolbright SA, Hersch-Green EI, Evans LM, Whitham TG, Allan GJ (2017) Conserving threatened riparian ecosystems in the American West: precipitation gradients and river networks drive genetic connectivity and diversity in a foundation riparian tree (Populus angustifolia). Molec Ecol 26: 5114–5132. [DOI] [PubMed] [Google Scholar]
- Brundrett MC. (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320: 37–77. [Google Scholar]
- Busch DE, Smith SD (1995) Mechanisms associated with decline of woody species in riparian ecosystems of the southwestern U.S. Ecol Mon 65: 347–370. [Google Scholar]
- Bunn RA, Ramsey PW, Lekberg Y (2015) Do native and invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta-analysis. J Ecol 103: 1547–1556. doi: 10.1111/1365-2745.12456. [DOI] [Google Scholar]
- Busby PE, Newcombe G, Dirzo R, Whitham TG (2013) Genetic basis of pathogen community structure for foundation species in a common garden and in the wild. J Ecol 101: 867–877. [Google Scholar]
- Choat B, et al. (2012) Global convergence in the vulnerability of forests to drought. Nature 491: 752–756. [DOI] [PubMed] [Google Scholar]
- Cooper HF, Grady KC, Cowan JA, Best RJ, Allan GJ, Whitham TG (2019) Genotypic variation in phenological plasticity: reciprocal common gardens reveal adaptive responses to warmer springs but not to fall frost. Global Change Biol 25: 187–200. [DOI] [PubMed] [Google Scholar]
- Cushman SA, Max T, Meneses N, Evans LM, Ferrier S, Honchak B, Whitham TG, Allan GJ (2014) Landscape genetic connectivity in a riparian foundation tree is jointly driven by climatic gradients and river networks. Ecol App 24: 1000–1014. [DOI] [PubMed] [Google Scholar]
- Dixon MD, Johnson WC, Scott ML, Bowen DE, Rabbe LA (2012) Dynamics of plains cottonwood (Populus deltoides) forests and historical landscape change along unchannelized segments of the Missouri River, USA. Environ Manag 49: 990–1008. [DOI] [PubMed] [Google Scholar]
- Drake JE, et al. (2018) Trees tolerate an extreme heatwave via sustained transpirational cooling and increased leaf thermal tolerance. Global Change Biol 24: 2390–2402. [DOI] [PubMed] [Google Scholar]
- Eckenwalder JE. (1977) North American cottonwoods (Populus, Salicaceae) of sections Abaso and Aigeiros. J Arnold Arb 58: 193–208. [Google Scholar]
- Eckenwalder JE. (1984) Natural intersectional hybridization between North American species of Populus (Salicaceae) in sections Aigeiros and Tacamahaca II. Taxonomy. Can J Bot 62: 325–335. [Google Scholar]
- Egerton-Warburton LM, Querejeta JI, Allen MF (2007) Common mycorrhizal networks provide a potential pathway for the transfer of hydraulically lifted water between plants. J Exp Bot 58: 1473–1483. [DOI] [PubMed] [Google Scholar]
- Elkind K, Sankey T, Munson S, Aslan C (2019) Invasive buffelgrass detection using high-resolution satellite and UAV imagery on Google Earth Engine. Rem Sen in Ecol Conser. doi: 10.1002/rse2.116. [DOI] [Google Scholar]
- Ellis LM, Crawford CS, Molles MC Jr (1997) Rodent communities in native and exotic riparian vegetation in the Middle Rio Grande Valley of Central New Mexico. Southwest Nat 42:13–19. [Google Scholar]
- Ellison AM, et al. (2005) Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3: 479–486. [Google Scholar]
- Evans L, et al. (2014) Population genomics of Populus trichocarpa identifies signatures of selection and adaptive trait associations. Nat Gen 46: 1089–1096. [DOI] [PubMed] [Google Scholar]
- Evans LM, Allan GJ, Shuster SM, Woolbright WA, Whitham TG (2008) Tree hybridization and genotypic variation drive cryptic speciation of a specialist mite herbivore. Evolution 62: 3027–3040. [DOI] [PubMed] [Google Scholar]
- Evans LM, Allan GJ, Whitham TG (2012) Populus hybrid hosts drive divergence in the herbivorous mite, Aceria parapopuli: implications for conservation of plant hybrid zones as essential habitat. Conserv Gen 13: 1601–1609. [Google Scholar]
- Franks SJ, Weber JJ, Aitken SN (2014) Evolutionary and plastic responses to climate change in terrestrial plant populations. Evol App 7: 123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfin G, Jardine A, Merideth R, Black M, LeRoy S (2013) Assessment of climate change in the Southwest United States: a report prepared for the National Climatic Assessment A report by the Southwest Climate Alliance Island Press, Washington. [Google Scholar]
- Gehring CA, Mueller RC, Whitham TG (2006) Environmental and genetic effects on the formation of ectomycorrhizal and arbuscular mycorrhizal associations in cottonwoods. Oecologia 149: 158–164. [DOI] [PubMed] [Google Scholar]
- Gehring CA, Ji B, Fong S, Whitham TG (2014) Differential feedback effects of arbuscular mycorrhizal fungi and other root endophytes in a Populus hybrid system. Botany 92: 287–293. [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21: 394–407. [Google Scholar]
- Gibert P, Debat V, Ghalambor CK (2019) Phenotypic plasticity, global change, and the speed of adaptive evolution. Current Op Ins Sci 35: 34–40. [DOI] [PubMed] [Google Scholar]
- Gitlin AR, Sthultz CM, Bowker MA, Stumpf S, Paxton KL, Kennedy K, Munoz A, Bailey JK, Whitham TG (2006) Mortality gradients within and among dominant plant populations as barometers of ecosystem change during extreme drought. Conserv Biol 20: 1477–1486. [DOI] [PubMed] [Google Scholar]
- González E, Martínez-Fernández V, Shafroth PB, Sher AA, Henry AL, Garófano-Gómez V, Corenblit D (2018) Regeneration of Salicaceae riparian forests in the northern hemisphere: a new framework and management tool. J Environ Manag 218: 374–387. [DOI] [PubMed] [Google Scholar]
- Gom L, Rood SB (1999) Patterns of clonal occurrence in a mature cottonwood grove along the Oldman River, Alberta. Canadian Journal of Botany 77: 1095–1105. [Google Scholar]
- Graf WL. (1978) Fluvial adjustments to the spread of tamarisk (Tamarix chinensis) in the Colorado Plateau region. Geol Soc Am Bull 89: 1491–1501. [Google Scholar]
- Grant V. (1971) Plant Speciation. Vol 1, 435 pages. Columbia University Press, New York. [Google Scholar]
- Grove SE, Haubensak KA, Gehring CA, Parker IM (2017) Mycorrhizae, invasions, and the temporal dynamics of mutualism disruption. J Ecol. doi: 10.1111/1365-2745.12853. [DOI] [Google Scholar]
- Harms RS, Hiebert RD (2006) Vegetation response following invasive tamarisk (Tamarix spp.) removal and implication for riparian restoration. Restoration Ecology 14: 461–472. [Google Scholar]
- Hultine KR, Belnap J, Riper IIIC, Dennison PE, Ehleringer JR, Lee ME, Nagler PL, Snyder KA, Uselman SM, West JB (2010) Tamarisk biocontrol in the western United States: ecological and societal implications. Fron Ecol Envir 8: 467–474. [Google Scholar]
- Hultine KR, Bean DW, Dudley TL, Gehring CA (2015) Species introductions and their cascading impacts on biotic interactions in desert riparian ecosystems. Integr Comp Biol 55: 587–601. [DOI] [PubMed] [Google Scholar]
- Hultine KR, Froend R, Blasini D, Bush SE, Karlinski M, Koepke DF (2020) Hydraulic traits that buffer deep-rooted plants from changes in hydrology and climate. Hydrol. Proc. 10.1002/hyp.13587. [DOI] [Google Scholar]
- Ikeda DH, Max TL, Allan GJ, Lau MK, Shuster SM, Whitham TG (2017) Genetically informed ecological niche models improve climate change predictions. Global Change Biol 23: 164–176. [DOI] [PubMed] [Google Scholar]
- Jarvis K, Allan GJ, Craig A, Beressic-Perrins R, Wimp G, Gehring C, Whitham TG (2017) Community phylogenetics of arthropods on cottonwoods: evolutionary implications for plant hybrid zones. Ecol Evol 15: 5909–5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo I, Fei S, Oswalt CM, Domke GM, Phillips RP (2019) Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Sci Adv 5: eaav6358. doi: 10.1126/sciadv.aav6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NC, Tilman D, Wedin D (1992) Plant and soil controls on mycorrhizal fungal communities. Ecology 73: 2034–2042. [Google Scholar]
- Keith AR, Bailey JK, Lau MK, Whitham TG (2017) Genetics-based interactions of foundation species affect community diversity, stability, and network structure. Proc Royal Soc B. 284: 20162703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivlin SN, Emery SM, Rudgers JA (2013) Fungal symbionts alter plant responses to global chan ge. Am J Bot 100: 1445–1457. [DOI] [PubMed] [Google Scholar]
- Laliberté E, Lambers H, Burgess TI, Wright SJ (2015) Phosphorus limitation, soilborne pathogens and the coexistence of plant species in hyperdiverse forests and shrublands. New Phytol 206: 507–521. [DOI] [PubMed] [Google Scholar]
- Lamit LJ, Holeski LM, Flores-Rentería L, Whitham TG, Gehring CA (2016) Tree genotype influences ectomycorrhizal fungal community structure: ecological and evolutionary implications. Fung Ecol 24: 124–134. [Google Scholar]
- Lamit LJ, Lau MK, Reese Næsborg R, Wojtowicz T, Whitham TG, Gehring CA (2015) Tree genetic and environmental regulation of the early stages of epiphytic lichen succession. Ecology 96: 960–971. [DOI] [PubMed] [Google Scholar]
- Lamit LJ, Lau MK, Sthultz CM, Wooley SC, Whitham TG, Gehring CA (2014) Tree genotype and genetically based growth traits structure twig endophyte communities. Am J Bot 101: 467–478. [DOI] [PubMed] [Google Scholar]
- Martinez-Vilalta J, et al. (2009) Hydraulic adjustment of scots pine across Europe. New Phytol 184: 353–364. [DOI] [PubMed] [Google Scholar]
- Martinsen GD, Whitham TG (1994) More birds nest in hybrid cottonwoods. Wilson Bul 106: 474–481. [Google Scholar]
- Martinsen GD, Whitham TG, Turek RJ, Keim P, (2001) Hybrid populations selectively filter gene introgression between species. Evolution 55: 1325–1335. [DOI] [PubMed] [Google Scholar]
- McGinley MA, Whitham TG (1985) Central place foraging by beavers (Castor canadensis): a test of foraging predictions and the impact of selective feeding on Populus fremontii. Oecologia 66: 558–562. [DOI] [PubMed] [Google Scholar]
- McKinnon G. (2005) Reticulate evolution in higher plants In Henry RJ, ed, Plant Diversity and Evolution: Genotypic and Phenotypic Variation in Higher Plants. CAB International, pp. 81–96. Vol 1, Wallingford, UK. [Google Scholar]
- Meinhardt KA, Gehring CA (2012) Disrupting mycorrhizal mutualisms: a potential mechanism by which exotic tamarisk outcompetes native cottonwoods. Ecol App 22: 532–549. [DOI] [PubMed] [Google Scholar]
- Merritt DM, Poff NL (2010) Shifting dominance of riparian Populus and Tamarix along gradients of flow alteration in western North American rivers. Ecol App 20: 135–152. [DOI] [PubMed] [Google Scholar]
- Merritt DM, Shafroth PB (2012) Edaphic, salinity, and stand structural trends in chronosequences of native and non-native dominated riparian forests along the Colorado River, USA. Biol Inv 14: 2665–2685. [Google Scholar]
- Mohan JE, et al. (2014) Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fung Ecol 10: 3–19. [Google Scholar]
- Noss RF, Laroe ET III, Scott JM (1995) Endangered ecosystems of the United States: a preliminary assessment of loss and degradation In Biological report 28. U.S. National Biological Service, Washington. [Google Scholar]
- O'Brien SJ, Mayr E (1991) Bureaucratic misschief: recognizing endangered species and subspecies. Science 251: 1187–1188. [DOI] [PubMed] [Google Scholar]
- Pena R, Polle A (2013) Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J 8: 321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DPC, Yao J (2012) Long-term experimental loss of foundation species: consequences for dynamics at ecotones across heterogeneous landscapes. Ecosphere 3: 27 10.1890/ES11-00273.1. [DOI] [Google Scholar]
- Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199: 41–51. [DOI] [PubMed] [Google Scholar]
- Plavcová L, Hacke UG (2012) Phenotypic and developmental plasticity of xylem n hybrid poplar saplings subjected to experimental drought, nitrogen fertilization, and shading. J Ex Bot 63: 6481–6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poff B, Koestner KA, Neary DG, Merritt D (2012) Threats to western United States riparian ecosystems. RMRS-GTR-269. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fort Collins.
- Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querejeta JI, Allen MF, Caravaca F, Roldan A (2006) Differential modulation of host plant δ13C and δ18O by native and nonnative arbuscular mycorrhizal fungi in a semiarid environment. New Phytol 169: 379–387. [DOI] [PubMed] [Google Scholar]
- Querejeta JI, Egerton-Warburton LM, Allen MF (2009) Topographic position modulates the mycorrhizal response of oak trees to interannual rainfall variability. Ecology 90: 649–662. [DOI] [PubMed] [Google Scholar]
- Querejeta JI, Egerton-Warburton LM, Prieto I, Vargas R, Allen MF (2012) Changes in soil hyphal abundance and viability can alter the patterns of hydraulic redistribution by plant roots. Plant Soil 355: 63–73. [Google Scholar]
- Rood SB, Samuelson GM, Braatne JH, Gourley CR, Hughes FMR, Mahoney JM (2005) Managing river flows to restore floodplain forests. Front Ecol Environ 3: 193–201. [Google Scholar]
- Rua MA, et al. (2016) Home-field advantage? Evidence of local adaptation among plants, soil, and arbuscular mycorrhizal fungi through meta-analysis. BMC Evol Biol 16: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankey T, Shreshtha R, Sankey J, Hardegree S (2013) Lidar-derived carbon estimates in woody encroachment. J Geophys Res 118: 1144–1155. [Google Scholar]
- Sankey T, Horne R, Bedford A, Sankey J (2016) Remote sensing of tamarisk biomass, insect herbivory, and defoliation: novel methods in the Grand Canyon region, Arizona, USA. Photog Eng Rem Sens 82: 645–652. [Google Scholar]
- Sankey T, Donager J, McVay J, Sankey J (2017a) UAV lidar, hyperspectral, and multispectral measurement capabilities in forested and ecotone environments in the USA. Rem Sens Environ 195: 30–43. [Google Scholar]
- Sankey T, McVay J, Swetnam T, McClaran M, Heilman P, Nicols M (2017b) UAV lidar and hyperspectral fusion: a new research tool for biogeoscience. Rem Sens Ecol Conser 4: 1–12. [Google Scholar]
- Santini F, Kefauver S, Resco de Dios V, Araus J, Voltas J (2018) Using unmanned aerial vehicle-based multispectral, RGB and thermal imagery for phenotyping of forest genetic trials: a case study in Pinus halepensis. Ann App Biol 2019: 1–5. [Google Scholar]
- Schweitzer JA, Martinsen GD, Whitham TG (2002) Cottonwood hybrids gain fitness traits of both parents: a mechanism for their long-term persistence? Am J Bot 89: 981–990. [DOI] [PubMed] [Google Scholar]
- Schweitzer JA, Bailey JK, Fischer DG, LeRoy CJ, Lonsdorf EV, Whitham TG, Hart SC (2008) Plant-soil-microorganism interactions: heritable relationship between plant genotype and associated soil microorganisms. Ecology 89: 773–781. [DOI] [PubMed] [Google Scholar]
- Shin P, Sankey T, Moore M, Thode A (2018) Evaluating unmanned aerial vehicle images for estimating forest canopy fuels in a ponderosa pine stand. Remote Sens Environ 10: 1266–1288. [Google Scholar]
- Slot M, Garcia MN, Winter K (2016) Temperature response of CO2 exchange in three tropical tree species. Func Plant Biol 43: 468–478. [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ (2008) Mycorrhizal Symbiosis. 3rd Edition, 800 pages. Academic Press, New York. [Google Scholar]
- Soudzilovskaia NA, et al. (2019) Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat Comm 10: 5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solazzo D, Sankey T, Sankey J, Munson S (2018) Mapping and measuring sand dunes with photogrammetry and lidar from unmanned aerial vehicles and multispectral satellite imagery on the Paria Plateau, AZ, USA. Geomorphology 319: 174–185. [Google Scholar]
- Stace CA. (1987) Hybridization and the plant species In Urbanska KM, ed, Differentiation Patterns in Higher Plants. Academic Press, NY, pp. 115–127. [Google Scholar]
- Stromberg JC. (2013) Root patterns and hydrogeomorphic niches of riparian planta in the American Southwest. J Arid Environ 94: 1–9. [Google Scholar]
- Swaty R, Michael HM, Deckert R, Gehring CA (2016) Mapping the potential mycorrhizal associations of the conterminous United States of America. Fung Ecol 24: 139–147. [Google Scholar]
- Tai X, Mackay DS, Sperry JS, Brooks P, Andregg WRL, Flanagan LB, Rood SB, Hopkinson C (2018) Distributed plant hydraulic and hydrological modeling to understand the susceptibility of riparian woodland trees to drought-induced mortality. Water Res Res. doi: 10.1029/2018WR022801. [DOI] [Google Scholar]
- Taylor JP, Wester DB, Smith LM (1999) Soil disturbance, flood management, and riparian woody plant establishment in the Rio Grande floodplain. Wetlands 19: 372–382. [Google Scholar]
- Teste FP, Simard SW (2008) Mycorrhizal networks and distance from mature trees alter patterns of competition and facilitation in dry Douglas-fir forests. Oecologia 158: 193–203. [DOI] [PubMed] [Google Scholar]
- Teste FP, Jones MD, Dickie IA (2019) Dual – mycorrhizal plants: their ecology and relevance. New Phytol 225: 1835–1851. [DOI] [PubMed] [Google Scholar]
- Tuscan GA, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, Putnam N, Ralph S, Rombauts SA et al. (2006) The genome of black cottonwood, Populus trichocarpa (Torr. and Gray). Science 313: 1596–1604. [DOI] [PubMed] [Google Scholar]
- Urban J, Ingwers MW, McGuire MA, Teskey R (2017) Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. J Exp Bot 68: 1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U. S. Fish and Wildlife Service (1995) Arizona cliffrose (Purshia subintegra) Recovery Plan USDA Fish and Wildlife Service, Arizona Ecological Services State Office, Phoenix, p 90.
- Vörösmarty CJ, et al. (2010) Global threats to human water security and river biodiversity. Nature 467: 555–561. [DOI] [PubMed] [Google Scholar]
- Walker TWN, Weckwerth W, Bragazza L, Fragner L, Forde BG, Ostle NJ, Signarbieux C, Sun X, Ward SE, Bardgett RD (2019) Plastic and genetic responses of a common sedge to warming have contrasting effects on carbon cycle processes. Ecol Lett 22: 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham TG, Morrow PA, Potts BM (1991) Conservation of hybrid plants. Science 254: 779–780. [DOI] [PubMed] [Google Scholar]
- Whitham TG, Maschinski J (1996) Current hybrid policy and the importance of hybrid plants in conservation In AZ Flagstaff, J Maschinski, DH Hammond, L Holter, eds, Southwestern Rare and Endangered Plants: Proceedings of the Second Conference; 1995 September 11–14. USDA Forest Service, Rocky Mountain Forest and Range Expt. Station, RM-GTR-283, Fort Collins, pp 103–112.
- Whitham TG, Martinsen GD, Floate KD, Dungey HS, Potts BM, Keim P (1999) Plant hybrid zones affect biodiversity: tools for a genetic-based understanding of community structure. Ecology 80: 416–428. [Google Scholar]
- Whitham TG, DiFazio SP, Schweitzer JA, Shuster SM, Allan GJ, Bailey JK, Woolbright SA (2008) Extending genomics to natural communities and ecosystems. Science 320: 492–495. [DOI] [PubMed] [Google Scholar]
- Wilsey BJ, Haukioja E, Koricheva J, Sulkinoja M (1998) Leaf fluctuating asymmetry increases with hybridization and elevation in tree-line birches. Ecology 79: 2092–2099. [Google Scholar]
- Wu M, Kostyun JL, Hahn MW, Moyle LC (2018) Dissecting the basis of novel trait evolution in a radiation with widespread phylogenetic discordance. Molec Ecol 27: 3301–3316. [DOI] [PubMed] [Google Scholar]
- Zavaleta E. (2000) The economic value of controlling an invasive shrub. Ambio 29: 462–467. [Google Scholar]


