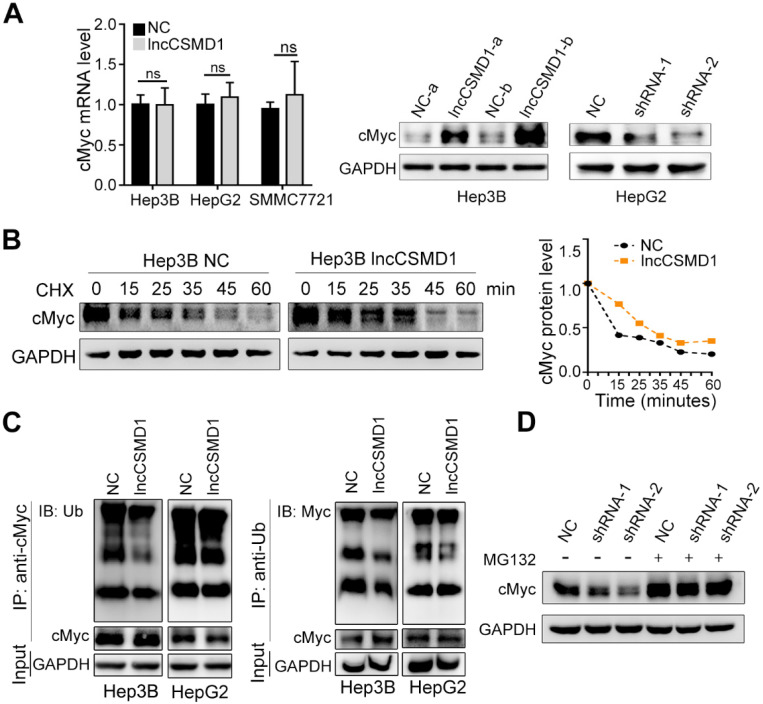
Figure 5.
LncCSMD1 stabilized MYC protein by inhibiting its ubiquitination. (A) MYC mRNA expression level in Hep3B cells stably expressing lncCSMD1 is not significantly higher than that in the control cells, as exhibited by qRT-PCR (left); however, MYC protein level is much higher in Hep3B cells stable overexpression of lncCSMD1 than in control cells (middle) or obviously lower in HepG2 cells with lncCSMD1 downregulation than in control cells (right). (B) Hep3B cells were treated with cycloheximide (CHX; 20 μg/ml) for the indicated times, and the half-life of MYC protein in HCC cells with overexpressing lncCSMD1 is prolonged when compared with that in control cells, as revealed by immunobloting (left); the curves were used to compare the half-life times of MYC protein in Hep3B cells with overexpression of lncCSMD1 or vector (right). (C) HCC cells overexpressing lncCSMD1 or vector were treated with MG132 (5 μM) for 24 h, and then cell lysates were immunoprecipitated with MYC antibody and the precipitated complexes were subjected to western blot with ubiquitin antibody (left); or the cell lysates were immunoprecipitated with ubiquitin antibody and the precipitated complexes were subjected to western blot with MYC antibody (right). The two experiments are aimed to detect the ubiquitination status of MYC protein. (D) MYC protein in Hep3B cells with downregulation of lncCSMD1 by shRNA is reduced compared with that in negative control cells, while after MG132 (20 μM) treatment for 24 h, MYC protein is restored to the same level as that in control cells, as shown by western blot assay.