Abstract
Objective
Distal sensory polyneuropathy (DSP) and neuropathic pain are important clinical concerns in virally suppressed people with HIV. We determined how these conditions evolved, what factors influenced their evolution, and their clinical impact.
Methods
Ambulatory, community‐dwelling HIV seropositive individuals were recruited at six research centers. Clinical evaluations at baseline and 12 years later determined neuropathy signs and distal neuropathic pain (DNP). Additional assessments measured activities of daily living and quality of life (QOL). Factors potentially associated with DSP and DNP progression included disease severity, treatment, demographics, and co‐morbidities. Adjusted odds ratios were calculated for follow‐up neuropathy outcomes.
Results
Of 254 participants, 21.3% were women, 57.5% were non‐white. Mean baseline age was 43.5 years. Polyneuropathy prevalence increased from 25.7% to 43.7%. Of 173 participants initially pain‐free, 42 (24.3%) had incident neuropathic pain. Baseline risk factors for incident pain included unemployment (OR [95% CI], 5.86 [1.97, 17.4]) and higher baseline body mass index (BMI) (1.78 [1.03, 3.19] per 10‐units). Participants with neuropathic pain at follow‐up had significantly worse QOL and greater dependence in activities of daily living than those who remained pain‐free.
Interpretation
HIV DSP and neuropathic pain increased in prevalence and severity over 12 years despite high rates of viral suppression. The high burden of neuropathy included disability and poor life quality. However, substantial numbers remained pain‐free despite clear evidence of neuropathy on exam. Protective factors included being employed and having a lower BMI. Implications for clinical practice include promotion of lifestyle changes affecting reversible risk factors.
Introduction
Despite considerable research prior to the development of virally suppressive combination antiretroviral therapy (cART) regimens, comparatively little is known about the longitudinal evolution over a decade or more of distal sensory polyneuropathy (DSP) and neuropathic pain in virally suppressed older people with HIV (PWH), and what clinical factors influence this evolution. Notably, not all those with DSP have neuropathic pain, and whether these individuals will go on to develop it despite viral suppression is unknown.
The clinical impact of DSP is substantial. In previous cross‐sectional studies, distal neuropathic pain (DNP) in HIV‐DSP is associated with opioid use, depression, and low quality of life (QOL). 1 , 2 , 3 , 4 Sensory neuropathy is a major contributor to balance difficulties and falls. In one study, of 3379 PWH and HIV negative (HIV−) adults, balance problems were more common in those with DSP. 5 DNP is often treatment‐resistant. 6
This study sought to characterize the longitudinal evolution of DSP and DNP, evaluate predictors of these conditions and describe their impact on QOL over a 12‐year follow‐up. The CHARTER Aging cohort, which enrolled individuals shortly after the roll‐out of effective cART and brought them back 12 years later, provided a unique opportunity to assess these issues.
Methods
Participants
The participants underwent standardized clinical and laboratory evaluations at baseline and 12‐year follow‐up at six U.S. centers. Initial visits took place between 2003 and 2007, and follow‐up assessments between 2016 and 2019. Evaluations included clinical examination for neuropathy signs (bilateral distal vibration, sharp, and touch loss) and self‐reported neuropathy symptoms (pain, numbness, paresthesias). Moderate or worse neuropathy was defined as two or more clinical signs of neuropathy from the list above. DNP was defined as burning, aching, or shooting symptoms and classified into five categories of clinician‐rated pain severity: none, slight (occasional, fleeting), mild (frequent), moderate (frequent, disabling), and severe (constant, daily, disabling, requiring analgesic medication or other pain medication). All participants signed an IRB‐approved written consent.
Laboratory evaluations
HIV disease was diagnosed by enzyme‐linked immunosorbent assay with Western blot confirmation. HIV viral load in plasma was measured using commercial assays and deemed undetectable at a lower limit of quantitation of 50 copies/mL. CD4 was measured by flow cytometry and nadir CD4 was assessed by self‐report. Urine toxicology was assessed including detection of opioids and cannabis.
Other clinical evaluations
Factors potentially associated with DSP and DNP progression included HIV disease severity (nadir and current CD4+ cell counts), ART including cumulative exposure to neurotoxic ynucleoside antiretrovirals, zalcitabine (ddC), didanosine (ddI), and stavudine (d4T); aka “d‐drugs”), self‐reported employment, body mass index (BMI) and diabetes (self‐report and use of anti‐diabetic medications) (Table 1). Use of opioids was determined by urine toxicology, as noted above. The Medical Outcomes Study HIV Health Survey (MOSHIV) has been shown to be a reliable and valid tool for assessing overall QOL, daily functioning, and physical health. 7 , 8 The MOS‐HIV contains 36 questions that assess physical and mental dimensions of health. These are scored as summary percentile scales ranging from 0 to 100, with higher scores indicating better health. Current mood was assessed by the Beck Depression Inventory (BDI) II. 9 Dependence in instrumental activities of daily living (IADLs) was assessed with a modified version of the Lawton and Brody Scale 10 that asks participants to rate their current and best lifetime levels of independence for 13 major IADLs such as shopping, financial management, transportation, and medication management. 11 An employment questionnaire asked about job loss, decreases in work productivity, accuracy, and quality; increased effort required to do one's usual job; and increased fatigue with the usual workload. Major depressive disorder (MDD) was diagnosed using the CIDI. 12
Table 1.
Key predictor and outcome variable definitions.
| Outcomes | |
|---|---|
| Moderate or worse DSP | 2 or more signs: distal, symmetrical reduction or loss of vibration, pin or ankle reflexes |
| DNP | Distal, symmetrical pain with neuropathic quality |
| Incident DSP | DSP at follow‐up in a participant without DSP at baseline |
| Incident DNP | DNP at follow‐up in a participant without DNP at baseline |
| Worsened DNP | An increase in DNP of at least one grade in a participant with DNP at baseline |
| Incident or worse DNP | Either incident DNP or worsened DNP |
| Incident MDD | MDD at follow‐up in a participant who did not meet MDD criteria at baseline |
| Mood | Beck Depression Inventory (BDI) scores |
| IADLs | Lawton and Brody Scale instrumental activities of daily living |
| Quality of life | Medical Outcomes Study HIV Health Survey (MOSHIV) |
| Predictors | |
| HIV disease severity | Nadir and current CD4+ T cell counts |
| ART | Cumulative exposure to neurotoxic nucleosides – ddC, ddI, or d4T |
| Employment | Self‐reported employment |
| Obesity | Body mass index (BMI) |
| Diabetes | Self‐report and use of anti‐diabetic medications |
DSP, distal sensory polyneuropathy; DNP, distal neuropathic pain; MDD, major depressive disorder; IADLs, instrumental activities of daily living; ART, antiretroviral therapy.
Statistical analyses
Group differences on background characteristics (i.e., demographics, neuropsychiatric, and neuromedical characteristics) were examined using analysis of variance, Wilcoxon/Kruskal–Wallis tests, and Chi‐square statistics as appropriate. Odds ratios were calculated for the association of baseline variables to follow‐up neuropathy outcomes. We used multivariable linear regression models to test interaction effects. In the absence of an interaction, additive effects were tested. Analyses were conducted using JMP Pro® version 15.0.0 (SAS Institute Inc., Cary, NC, 2018).
Results
Baseline visits were between 2003 and 2007 and follow‐up visits were between 2013 and 2019. Table 2 presents the demographic and clinical characteristics of the study participants at their baseline visits. Of the 254 participants, Women comprised 54 (21.3%) and non‐whites 152 (57.5%). Mean baseline age was 43.5 (±8.01) years and median (interquartile range) nadir and current CD4 were 178 (IQR 29, 294) and 446 (282, 607), respectively. The number using combination ART increased from 191 (75.2%; 95% CI 69.5, 80.1) at baseline to 245 (96.5%; 93.4, 98.1) at follow‐up (during the interval, ART initiation guidelines changed to include all PWH). Viral suppression improved from 47.6% at baseline to 82.4% at follow‐up. Moderate or worse DSP (2 or more signs) increased from 65/253 (25.7%) at baseline to 110/253 (43.7%) at 12 years; 60 individuals (32.1%) had incident (new onset) DSP. DSP remitted in 14 (5.57%). Of 173 individuals without pain at baseline 42 (24.3%) had incident DNP. Of 80 with DNP at baseline, 23 (28.8; 95% CI [20.0, 39.5]) worsened (at least one grade higher) at 12 years. In the subset of 106 individuals never exposed to neurotoxic d‐drugs, the proportion with DSP increased from 14.2% to 34.0%. In the same subset, of 81 without baseline DNP, 17 (21.0%) had incident DNP.
Table 2.
Participant demographic and clinical characteristics at the baseline visit, by DSP and DNP status.
| Characteristic | All | DSP‐free (0/1 sign) | Mod to severe DSP (≥2 signs) | P | Pain‐free with or without DSP | DNP with or without DSP | P |
|---|---|---|---|---|---|---|---|
| N | 253 | 188 | 65 | 173 | 80 | ||
| Age, years (mean ± SD) | 43.5 ± 8.01 | 41.8 ± 7.33 | 48.4 ± 7.94 | 0.0001 | 42.7 ± 8.02 | 45.2 ± 7.77 | 0.02 |
| Education, years (mean ± SD) | 13.1 ± 2.54 | 13.1 ± 2.62 | 13.4 ± 2.32 | 0.422 | 13.1 ± 2.49 | 13.2 ± 2.68 | 0.79 |
| Female sex, N (%) | 54 (21%) | 38 (20%) | 16 (25%) | 0.46 | 35 (20.2%) | 19 (23.8%) | 0.53 |
| non‐Hispanic white, N (%) | 108 (42%) | 81 (43%) | 38 (59%) | 0.82 | 70 (41%) | 38 (48%) | 0.29 |
| CD4 nadir (cells/μL; median, IQR) | 178 (29, 294) | 185 (40, 313) | 120 (19, 220) | 0.019 | 181 (27, 279) | 150 (41, 298) | 0.93 |
| Current CD4 (median, IQR) | 282 (446, 607) | 452 (279, 641) | 410 (314, 588) | 0.803 | 440 (261, 634) | 446 (336, 592) | 0.28 |
| On ART, N (%) | 191 (75%) | 132 (70.2%) | 58 (89.2%) | 0.001 | 128 (74%) | 63 (78.8%) | 0.41 |
| Virally suppressed | 120 (47.6%) | 80 (42.8%) | 40 (62.5%) | 0.006 | 78 (45.4%) | 42 (53.2%) | 0.25 |
| Cumulative d‐drug years, median (IQR) | 0.049 (0, 36.9) | 0 (0, 34.1) | 14.5 (0, 42.7) | 0.024 | 0 (0, 29.8) | 12.5 (0, 46.2) | 0.03 |
| Body mass index (mean ± SD) | 26.8 ± 5.97 | 26.8 ± 6.13 | 26.9 ± 5.57 | 0.87 | 27.1 ± 6.12 | 25.9 ± 5.33 | 0.15 |
| Employed, N (%) | 72 (28.3%) | 59 (31.4%) | 13 (20.0%) | 0.073 | 54 (31.2%) | 18 (22.5%) | 0.15 |
ART, antiretroviral therapy; DSP, distal sensory polyneuropathy; DNP, distal neuropathic pain. D‐drug, neurotoxic nucleoside antiretroviral – zalcitabine (ddC), didanosine (ddI), or stavudine (d4T).
Figure 1 shows baseline predictors of incident DSP at 12‐year follow‐up. Significant predictors were older age (OR 2.92 per decade [1.83, 4.86]), DNP (OR 3.93 [1.96, 8.02]) and height (OR 20.9 per cm [3.88, 128]). Non‐significant variables included sex, ethnicity, viral suppression, nadir and current CD4, diabetes mellitus, cumulative d‐drug exposure, BMI, employment, and lifetime alcohol abuse. Results were substantively similar for those with viral suppression at 12 years. The odds of developing moderate to severe incident neuropathy (2 signs) at 12 years increased by 2.59 (1.39, 4.89) for those with 1 sign of DSP at baseline, compared to those with no signs. For those with DNP at baseline, the odds of incident DSP were higher 3.92 (1.97, 7.94) compared to no DNP at baseline. The odds of incident DSP increased in rough proportion to the severity of DNP at baseline, from 3.84 (1.62, 9.09) for those with mild pain to 6.59 (1.16, 37.5) for those with severe pain. In a multivariable logistic regression that included each of the significant univariable predictors, age (OR 2.80 per decade [1.69, 4.83]), DNP (OR 4.03 [1.86, 8.98]) and height (OR 1.53 per cm [1.19, 2.0]) remained significant predictors. In the subset of PWH never exposed to neurotoxic d‐drugs, significant predictors were age (OR 3.28 per decade [1.60, 267]) and height (OR 2.40 [1.57, 4.00]).
Figure 1.
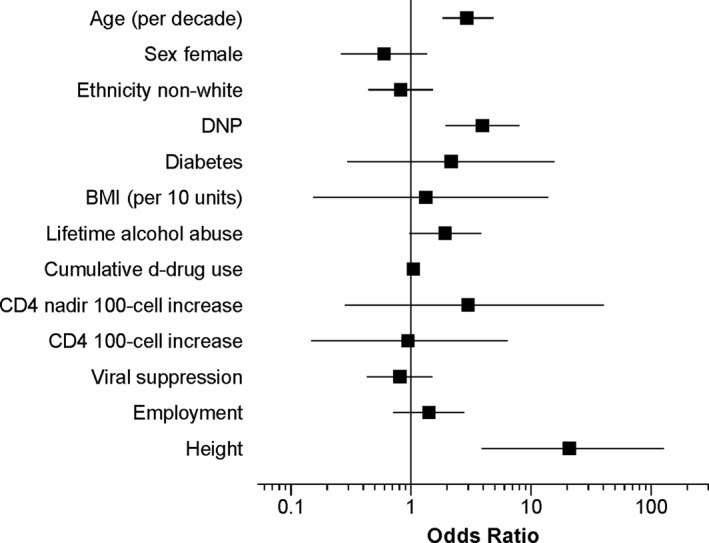
Univariate baseline predictors of incident DSP. DNP, distal neuropathic pain; BMI, body mass index. D‐drug, neurotoxic nucleoside antiretroviral – zalcitabine (ddC), didanosine (ddI), or stavudine (d4T).
Figure 2 shows baseline predictors of incident DNP at 12 years follow‐up. Age (OR 4.50 [0.595, 34.7]) was significantly associated with incident DNP; sex and ethnicity were not. Height, viral suppression and nadir and baseline CD4 did not predict incident DNP at follow‐up (Ps > 0.20). The presence of diabetes mellitus at baseline did not predict incident DNP (1.01 [0.196, 5.18]). Higher BMI was associated with incident DNP (OR 1.056 [1.00, 1.12] per unit increase in BMI). Individuals who were employed at baseline were much less likely to have incident DNP at 12 years than those who were unemployed (OR 0.166 [0.0561, 0.489]). Among those who already had DNP at baseline, worsening was more likely in those with lower CD4 nadir (OR 1.50 [1.09, 2.14] for every 100 cells decrease). Diabetes did not influence DNP worsening (OR 2.01 [0.695, 5.83]) and none of the other predictor variables was associated with DNP worsening. In a multivariable model with BMI and employment as predictors of incident DNP, only being employed remained significant (OR 0.186 [0.053, 0.501]). Forty of 80 (50%) PWH with DNP at baseline had improved or absent DNP at follow‐up. Improved DNP was not related to age, sex, ethnicity, BDI‐II, employment, IADLs, nadir or current CD4, viral suppression or BMI. In the subset of PWH never exposed to neurotoxic d‐drugs, no variables were significant predictors of incident DNP.
Figure 2.
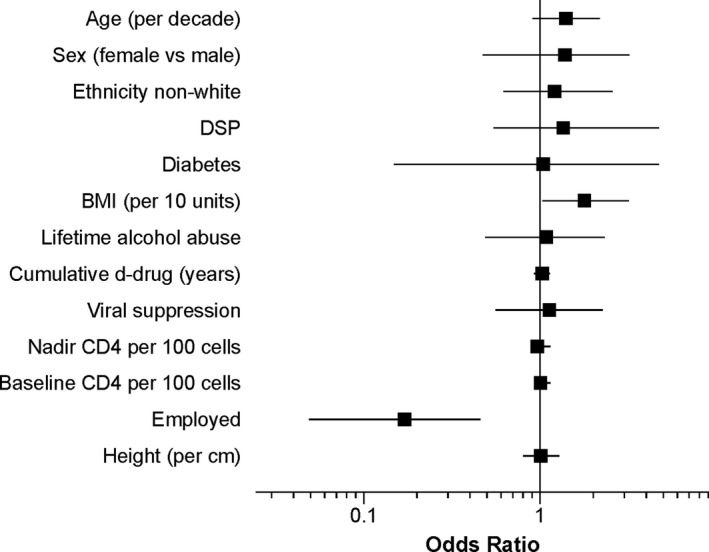
Univariate baseline predictors of incident DNP. DSP, distal sensory polyneuropathy; BMI, body mass index. D‐drug, neurotoxic nucleoside antiretroviral – zalcitabine (ddC), didanosine (ddI), or stavudine (d4T).
Relationship of DNP at follow‐up to depressed mood and other clinically relevant outcomes
PWH with DNP at follow‐up had significantly higher BDI‐II scores than those without DNP (11.7 ± 10.4 vs. 8.51 ± 8.89, P = 0.0089) but differences in the proportions with MDD were not significant (64.6% vs. 63.3%). Participants whose DNP worsened had nonsignificantly higher BDI‐II scores than those who did not worsen (13.8 ± 2.87 vs. 10.158 ± 9.76 P = 0.1631). Of 138 participants who were independent in IADLs at baseline, 37 (26.8%) became dependent. Participants with DNP at follow‐up were more likely to have become dependent in IADLs (OR 2.88 [1.30, 6.39]) and those with incident or worsening DNP at follow‐up were more likely to become dependent (OR 2.31 [0.950, 5.61]). DSP status did not influence IADL dependence (data not shown). Participants with DNP at V2 had significantly worse physical and mental health MOS scores than those without (39.1 ± 12.1 vs. 48.2 ± 10.8; P < 0.0001 and 46.8 ± 13.1 vs. 52.4 ± 9.6; P < 0.0002, respectively; Figure 3). PWH with DNP were more likely to have positive urine toxicology positive for opioids, in most cases prescribed (OR 3.63 [1.48, 8.93]).
Figure 3.
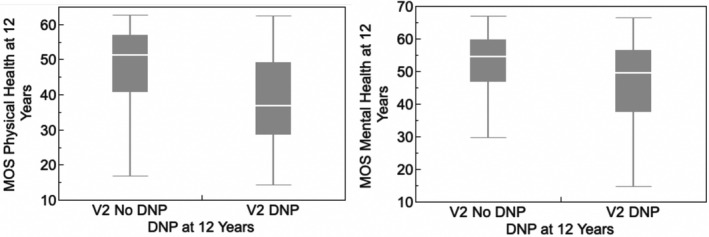
Distal neuropathic pain at 12 years was associated with poorer quality of life as measured by the MOS‐HIV Physical and Mental Health Summary Scores.
Discussion
Our findings highlight the high prevalence and increasing burden of DSP and neuropathic pain in older long‐term PWH survivors despite viral suppression on ART. HIV DSP and its symptoms worsened substantially with follow‐up over more than a decade. Even among those never exposed to neurotoxic d‐drugs, incident DNP occurred in 19.8% and incident DNP in 21%. Similar to other studies, advancing age and height were important risk factors for worsening of both DSP and DNP. 1 , 13 , 14 , 15 , 16 , 17 , 18 Opiates were more frequently used by individuals with DNP. Other studies have shown that DNP contributes to the burden of polypharmacy in older PWH. 19 Anticonvulsants, antidepressants, topical agents, and nonspecific analgesics may help relieve neuropathic pain, but their use is associated with drug‐drug interactions and substantial adverse side effects such as cognitive impairment.
DNP was closely related to depressed mood, particularly MDD. We and others have observed this previously. 20 , 21 , 22 It is not clear whether treating depression can ameliorate DNP or vice‐versa, but this issue deserves exploration.
At the same time, substantial numbers of aging PWH remained free of DNP and disability. Some others improved, despite clear evidence of DSP on careful physical examination. Protective factors with respect to DNP included employment and lower BMI. It is not clear why employment was protective, but perhaps it is a surrogate for other, unmeasured factors such as better overall health status. Additionally, BMI may be a surrogate for vascular risk factors that may contribute to peripheral nerve injury. 23 , 24 , 25 , 26 Also, some individuals with DSP at baseline improved at 12 years. These results suggest partial reversibility of the neuropathy phenotype, and raise hope for the promise of therapies designed to promote neuroregeneration. For example, Calcutt et al recently made the novel observation that dermal muscarinic M3 receptors suppress regeneration of the epidermal nerve fiber layer, and that blockade of these receptors could restore the regenerative capacity of these fibers. 27 In addition, a recent Cochrane review 28 showed that acetyl‐l‐carnitine treatment produced a significant pain reduction with respect to baseline and showed beneficial effects on nerve conduction parameters and nerve fiber regeneration.
DNP was a predictor of incident DSP, suggesting that DNP is an early or subclinical manifestation of DSP. We have previously shown that the presence of DNP has high sensitivity for DSP on more comprehensive neuropathy evaluations, even in those for whom DSP is not found on routine clinical examination. 29 Thus, DNP, even in the absence of clinically documented DSP, may be used to identify individuals in whom neuroregenerative therapies might be most effective.
Before the introduction of ART, HIV neuropathy was closely associated with advanced immunosuppression, which is reflected in a lower current CD4 count and a higher viral load. In contrast, our findings in an ART‐treated cohort, similar to others, 13 , 17 show that that these factors are no longer associated with neuropathy or neuropathic pain. At the same time, ART has been associated with the development of DSP symptoms in some individuals despite reducing viral load. 30 , 31 Studies using animal models exposed to tenofovir disoproxil fumarate support a role for ART in DSP. 32 However, more work is needed to understand how DSP manifests despite reduced viral loads.
Limitations of this study include potential bias due to selective loss to follow‐up of participants from death or inability to return for research evaluations. We believe this is more likely to affect those with DSP and DNP, and thus represents a conservative bias. Our cohort may not be representative of the larger population of PWH. In particular, although rates of viral suppression increased very much from baseline to follow‐up, those rates were still lower than in many other cohorts. The number of women was relatively small, limiting any conclusions regarding sex differences.
A better understanding of risk factors for incident and worsening neuropathy and neuropathic symptoms are will promote the development of preventative strategies such as neuroprotective and neuroregenerative agents and lifestyle changes. Our findings suggest that obesity and unemployment may increase the risk of developing DSP.
Author Contributions
Ronald J. Ellis was a co‐investigator on the grant that funded the study, designed this project, contributed to data collection, performed the analyses, and wrote the manuscript. Monica Diaz, Ned Sacktor, Christina Marra, Ann C. Collier, and David B. Clifford contributed to data collection and reviewed and edited manuscript drafts. Nigel Calcutt and Jerel A. Fields reviewed and edited manuscript drafts. Robert K. Heaton and Scott L. Letendre was co‐principal investigator on the grant that funded the study and reviewed and edited manuscript drafts.
Conflict of Interest
The authors report no potential conflicts of interest.
Acknowledgment
The CNS HIV Anti‐Retroviral Therapy Effects Research was supported by awards N01 MH22005, HHSN271201000036C, HHSN271201000030C and R01 MH107345 from the National Institutes of Health.
The CNS HIV Anti‐Retroviral Therapy Effects Research (CHARTER) group is affiliated with Johns Hopkins University; the Icahn School of Medicine at Mount Sinai; University of California, San Diego; University of Texas, Galveston; University of Washington, Seattle; Washington University, St. Louis; and is headquartered at the University of California, San Diego and includes: Directors: Robert K. Heaton, Ph.D., Scott L. Letendre, M.D.; Center Manager: Donald Franklin, Jr.; Coordinating Center: Brookie Best, Pharm.D., Debra Cookson, M.P.H, Clint Cushman, Matthew Dawson, Ronald J. Ellis, M.D., Ph.D., Christine Fennema Notestine, Ph.D., Sara Gianella Weibel, M.D., Igor Grant, M.D., Thomas D. Marcotte, Ph.D. Jennifer Marquie‐Beck, M.P.H., Florin Vaida, Ph.D.; Johns Hopkins University Site: Ned Sacktor, M.D. (P.I.), Vincent Rogalski; Icahn School of Medicine at Mount Sinai Site: Susan Morgello, M.D. (P.I.), Letty Mintz, N.P.; University of California, San Diego Site: J. Allen McCutchan, M.D. (P.I.); University of Washington, Seattle Site: Ann Collier, M.D. (Co‐P.I.) and Christina Marra, M.D. (Co‐P.I.), Sher Storey, PA‐C.; University of Texas, Galveston Site: Benjamin Gelman, M.D., Ph.D. (P.I.), Eleanor Head, R.N., B.S.N.; and Washington University, St. Louis Site: David Clifford, M.D. (P.I.), Mengesha Teshome, M.D.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
Funding Information
The CNS HIV Anti‐Retroviral Therapy Effects Research was supported by awards N01 MH22005, HHSN271201000036C, HHSN271201000030C and R01 MH107345 from the National Institutes of Health.
Funding Statement
This work was funded by National Institutes of Health grants HHSN271201000030C, HHSN271201000036C, N01 MH22005, and R01 MH107345.
Contributor Information
Ronald J. Ellis, Email: roellis@ucsd.edu.
for the CNS Antiretroviral Therapy Effects Research (CHARTER) Study Group:
Donald Franklin, Jr., Brookie Best, Debra Cookson, Clint Cushman, Matthew Dawson, Christine Fennema Notestine, Sara Gianella Weibel, Igor Grant, Thomas D. Marcotte, Jennifer Marquie‐Beck, Florin Vaida, Vincent Rogalski, Susan Morgello, Letty Mintz, J. Allen McCutchan, Sher Storey, Benjamin Gelman, Eleanor Head, and Mengesha Teshome
References
- 1. Ellis RJ, Rosario D, Clifford DB, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus‐associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol 2010;67:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uebelacker LA, Weisberg RB, Herman DS, et al. Chronic pain in HIV‐infected patients: relationship to depression, substance use, and mental health and pain treatment. Pain Med 2015;16:1870–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu B, Liu X, Tang SJ. Interactions of opioids and HIV infection in the pathogenesis of chronic pain. Front Microbiol 2016;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV‐related pain. Pain Physician 2012;15:ES157–ES168. [PubMed] [Google Scholar]
- 5. Sakabumi DZ, Moore RC, Tang B, et al. Chronic distal sensory polyneuropathy is a major contributor to balance disturbances in persons living with HIV. J Acquir Immune Defic Syndr 2019;80:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cysique LA, Vaida F, Letendre S, et al. Dynamics of cognitive change in impaired HIV‐positive patients initiating antiretroviral therapy. Neurology 2009;73:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wachtel T, Piette J, Mor V, et al. Quality of life in persons with human immunodeficiency virus infection: measurement by the Medical Outcomes Study instrument. Ann Intern Med 1992;116:129–137. [DOI] [PubMed] [Google Scholar]
- 8. Wu AW, Revicki DA, Jacobson D, Malitz FE. Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS‐HIV). Qual Life Res 1997;6:481–493. [DOI] [PubMed] [Google Scholar]
- 9. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories‐IA and ‐II in psychiatric outpatients. J Pers Assess 1996;67:588–597. [DOI] [PubMed] [Google Scholar]
- 10. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–186. [PubMed] [Google Scholar]
- 11. Heaton RK, Marcotte TD, Mindt MR, et al. The impact of HIV‐associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004;10:317–331. [DOI] [PubMed] [Google Scholar]
- 12. Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry 1988;45:1069–1077. [DOI] [PubMed] [Google Scholar]
- 13. Anziska Y, Helzner EP, Crystal H, et al. The relationship between race and HIV‐distal sensory polyneuropathy in a large cohort of US women. J Neurol Sci 2012;315:129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cherry CL, Affandi JS, Imran D, et al. Age and height predict neuropathy risk in patients with HIV prescribed stavudine. Neurology 2009;73:315–320. [DOI] [PubMed] [Google Scholar]
- 15. Evans SR, Ellis RJ, Chen H, et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS 2011;25:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maritz J, Benatar M, Dave JA, et al. HIV neuropathy in South Africans: frequency, characteristics, and risk factors. Muscle Nerve 2010;41:599–606. [DOI] [PubMed] [Google Scholar]
- 17. Smyth K, Affandi JS, McArthur JC, et al. Prevalence of and risk factors for HIV‐associated neuropathy in Melbourne, Australia 1993–2006. HIV Med 2007;8:367–373. [DOI] [PubMed] [Google Scholar]
- 18. Wadley AL, Cherry CL, Price P, Kamerman PR. HIV neuropathy risk factors and symptom characterization in stavudine‐exposed South Africans. J Pain Symptom Manage 2011;41:700–706. [DOI] [PubMed] [Google Scholar]
- 19. Siefried KJ, Mao L, Cysique LA, et al. Concomitant medication polypharmacy, interactions and imperfect adherence are common in Australian adults on suppressive antiretroviral therapy. AIDS 2018;32:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status Scale. An examination of its reliability and validity in a research setting. Cancer 1984;53:2002–2007. [DOI] [PubMed] [Google Scholar]
- 21. Pillay P, Wadley AL, Cherry CL, et al. Psychological factors associated with painful versus non‐painful HIV‐associated sensory neuropathy. AIDS Behav 2018;22:1584–1595. [DOI] [PubMed] [Google Scholar]
- 22. Vecchio AC, Marra CM, Schouten J, et al. Distal sensory peripheral neuropathy in human immunodeficiency virus type 1‐positive individuals before and after antiretroviral therapy initiation in diverse resource‐limited settings. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roustit M, Loader J, Deusenbery C, et al. Endothelial dysfunction as a link between cardiovascular risk factors and peripheral neuropathy in diabetes. J Clin Endocrinol Metab 2016;101:3401–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350. [DOI] [PubMed] [Google Scholar]
- 25. Miscio G, Guastamacchia G, Brunani A, et al. Obesity and peripheral neuropathy risk: a dangerous liaison. J Peripher Nerv Syst 2005;10:354–358. [DOI] [PubMed] [Google Scholar]
- 26. Ylitalo KR, Sowers M, Heeringa S. Peripheral vascular disease and peripheral neuropathy in individuals with cardiometabolic clustering and obesity: National Health and Nutrition Examination Survey 2001–2004. Diabetes Care 2011;34:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sabbir MG, Calcutt NA, Fernyhough P. Muscarinic acetylcholine type 1 receptor activity constrains neurite outgrowth by inhibiting microtubule polymerization and mitochondrial trafficking in adult sensory neurons. Front Neurosci 2018;12:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Stefano G, Di Lionardo A, Galosi E, et al. Acetyl‐L‐carnitine in painful peripheral neuropathy: a systematic review. J Pain Res 2019;12:1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robinson‐Papp J, Morgello S, Vaida F, et al. Association of self‐reported painful symptoms with clinical and neurophysiologic signs in HIV‐associated sensory neuropathy. Pain 2010;151:732–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ndakala FN, Oyugi JO, Oluka MN, et al. Prevalent neuropathy in a cohort of HIV‐infected Kenyan sex workers using antiretroviral drugs. Pan Afr Med J 2016;25:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weldegebreal F, Mitiku H, Teklemariam Z. Magnitude of adverse drug reaction and associated factors among HIV‐infected adults on antiretroviral therapy in Hiwot Fana specialized university hospital, eastern Ethiopia. Pan Afr Med J 2016;24:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fields JA, Swinton MK, Carson A, et al. Tenofovir disoproxil fumarate induces peripheral neuropathy and alters inflammation and mitochondrial biogenesis in the brains of mice. Sci Rep 2019;9:17158. [DOI] [PMC free article] [PubMed] [Google Scholar]


