Abstract
Objective
To investigate disease spread in amyotrophic lateral sclerosis (ALS), and determine the influence of lower (LMN) and upper motor neuron (UMN) involvement.
Methods
We assessed disease spread in ALS in 1376 consecutively studied patients, from five European centers, applying an agreed proforma to assess LMN and UMN signs. We defined the pattern of disease onset and progression from predominant UMN or lower motor neuron (LMN) dysfunction in bulbar, upper limbs, lower limbs, and thoracic regions Non‐linear regression analysis was applied to fit the data to a model that described the relation between two random variables, graphically represented by an inverse exponential curve. We analyzed the probability, rate of spread, and both combined (area under the curve).
Results
We found that progression was more likely and quicker to or from the region of onset to close spinal regions. When the disease had a limb onset, bulbar motor neurons were more resistant. Furthermore, in the same time frame more patients progressed from bulbar to lower limbs than vice‐versa, whether predominantly UMN or LMN involvement. Patients with initial thoracic involvement had a higher probability for rapid change. The presence of predominant UMN signs was associated with a faster caudal progression.
Interpretation
Contiguous progression was leading pattern, and predominant UMN involvement is important in shortening the time for cranial‐caudal spread. Our results can best be fitted to a model of independent LMN and UMN degeneration, with regional progression of LMN degeneration mostly by contiguity. UMN lesion causes an acceleration of rostral‐caudal LMN loss.
Introduction
Amyotrophic lateral sclerosis (ALS) is characterized by the progressive development of abnormalities in upper (UMN) and lower (LMN) motor neurons, in a variable distribution. 1 The disorder progresses within these motor systems. Both UMN and LMN features are important in diagnosis, as well as for understanding the patterns of disease progression.
Previous studies of disease spreading have a number of limitations. Only three are prospective, 1 , 2 , 3 others rely on chart reviews, 4 , 5 , 6 , 7 patient self‐administered questionnaire at one time point, 8 or EMG studies. 9 Only four investigators 1 , 5 , 7 , 8 studied the impact and distribution of UMN dysfunction on the pattern and rate of progression of the disease. All these studies, except one 6 were carried out in single centers, and none included patients with frontotemporal dementia (FTD). Most excluded patients with the progressive muscular atrophy (PMA) syndrome, a disorder closely related to classical ALS in which pyramidal tract degeneration is commonly seen at autopsy. 9 , 10
There has been controversy regarding the possibility of a primary abnormality in the UMN, at motor cortex level, a concept derived from cortical excitability studies, 11 , 12 , 13 the LMN abnormality then being considered as secondary. Uncertainty regarding the role of the UMN in ALS, and the clinical pattern of UMN involvement itself, prompted us to study the development of both UMN and LMN involvement in the disease and their inter‐relationship, in a prospective European multicenter investigation (OnWebDuals Project).
Materials and Methods
Patients with ALS were studied during clinical practice in five European University Hospital centers – Akdeniz University, Antalya, Turkey; Hannover University Medical School, Germany; Jena University Hospital, Germany; Faculty of Medicine, University Hospital of Lisbon, Portugal; Medical University of Warsaw, Poland. The clinical assessments, at each center, were made by a designated senior neurologist with wide clinical experience of ALS. Ethical agreement for the study was granted by the respective Clinical Ethics Committees, and each patient gave written informed consent for collection and analysis of their medical data. The study was supported by an EU research grant (EU Joint Programme Neurodegenerative Diseases Research: OnWebDUALS).
A standardized clinical assessment protocol was developed that required recruitment of patients with definite, probable and possible ALS according to the Awaji revision of the El Escorial criteria. 14 Patients with PMA were included, 10 but not patients with primary lateral sclerosis or with monomelic disease (defined as sporadic single limb disease with follow‐up >12 months).
The clinical protocol defined UMN and LMN signs according to conventional neurological practice (Table 1), during detailed discussions at meetings of the investigators, and later validated by an international panel of neurologists in Europe, Japan, Australia, South Africa, South America, and the USA. 15 Disease onset was defined as the onset of symptomatic limb or bulbar weakness, axial weakness or primary respiratory muscle weakness. Other clinical features, such as fasciculations, muscle cramp, and fatigability, were usually present, but these were regarded as indefinite indications of disease onset. At the initial assessment patients were classified by region of onset, and by the predominance of UMN or LMN dysfunction. The focality and distribution of UMN and LMN signs was recorded in detail as part of the agreed protocol. Patients with two regions affected at onset were categorized as such and those with more than two regions affected at onset were classified as generalized onset. As more than 95% of ALS patients were right‐hand dominant, patients were not subdivided according to their predominant handedness. To avoid selection bias, the protocol required recruitment of consecutively diagnosed patients in each center.
Table 1.
Criteria for upper motor neurone (UMN) and lower motor neurone (LMN) signs in each region: agreed by consensus (see text). The signs are considered specific for each region (signs of diaphragm weakness were considered to indicate thoracic region involvement, since trunk and diaphragm lower motor neuron loss have a strong correlation – de Carvalho et al., 2010).
| Region | UMN | LMN |
|---|---|---|
| Bulbar |
Brisk jaw jerk Jaw clonus Tongue spasticity |
Tongue atrophy Tongue or facial fasciculations Atrophy of masseter muscle Weak orbicularis oris muscle Reduced jaw jerk |
|
Upper limbs |
Increased tendon reflexes Hoffmann’s sign Spasticity |
Weakness and muscle atrophy Fasciculations Reduced tendon reflexes |
|
Axial (thoracic) |
_ |
Weakness of neck Orthopnea Resting respiratory fatigue Exertional respiratory fatigue to minor efforts Paradoxical respiration Weak cough |
|
Lower limbs |
Increased tendon reflexes Babinski response Spasticity |
Weakness and muscle atrophy Fasciculations Reduced tendon reflexes |
Between March 2015 and December 2018, 1376 patients with ALS were recruited (Antalya = 235, Hannover = 236, Jena = 148, Lisbon = 471, Warsaw = 286). At each center patients were assessed every 3 months as part of their regular follow‐up care, to assess disease progression and functional status. Double pseudonymized data were entered in preconfigured Excel files restricting the input to valid entries after three rounds of monitoring for correctness, and stored in an OpenSSH secured storage space at the Jena University Hospital with access limited to investigators. The data were analyzed in Lisbon by the clinical researchers and biostatisticians (MG, MF, SP, SM, MS, and MdC) and then reviewed by all clinical investigators.
Statistical analysis
The patients were categorized into seven single symptom‐onset regions (i.e., Bulbar UMN or LMN, Upper limbs UMN or LMN, Lumbo‐Sacral UMN or LMN, and Thoracic). Patients with respiratory symptoms were categorized in the thoracic region, since diaphragm involvement is closely correlated with intercostal and paraspinal muscle weakness. 16 The time to progression was calculated in months from the date of first symptom to the 2nd and 3rd region affected. Of the 1376 recruited ALS patients, 76 were later excluded from analysis; 48 because they were considered not to fulfil the entry criteria for revised‐El Escorial diagnosis or PMA, and 28 because there was missing data, leaving 1300 patients (Fig. 1). We further excluded patients with two or more onset regions (n = 43), onset with FTD (n = 14), and 140 patients in whom there was uncertain information concerning UMN or LMN predominant features. From this group (n = 1103), we included all patients who had already progressed from the onset region to a 2nd region at the time of evaluation, as well as those with a disease duration longer than 36 months, resulting in a total of 889 patients for analysis (Fig. 1). Next, we analyzed all subjects with UMN or LMN spread to a 3rd region within 36 months after onset (588 patients). The analysis focussed on spread from the first affected region to the next two affected regions, no more extensive analysis was undertaken because the permutation of possible outcomes would require a much larger cohort. The cumulative percentage of patients with a specific region affected (LMN and UMN) over time was graphed.
Figure 1.
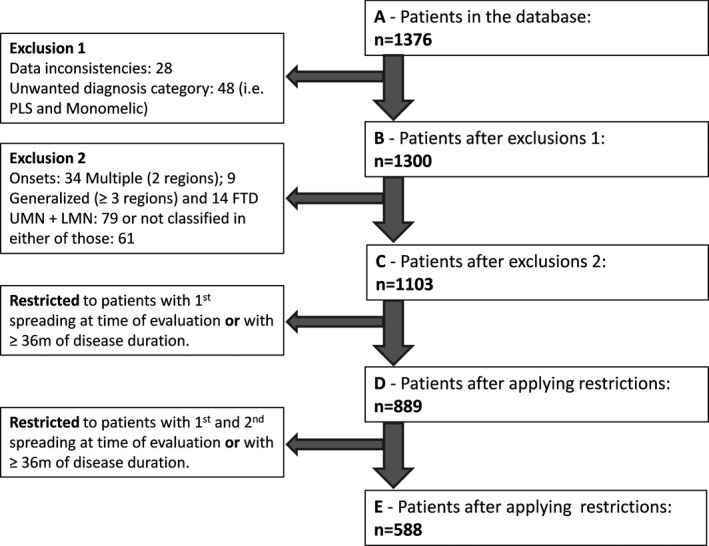
Flowchart showing recruitment of patients.
Nonlinear regression analysis was applied to fit the data to a model that described the relation between two random variables, graphically represented by the inverse exponential curve: Y = a * [1‐e(‐b*x)]. Data fitting was performed using the algorithm for general nonlinear least‐squares regression. Only curves with adjusted R2 values above 0.95 were accepted for the further analysis. This approach allowed us to compare the probability of spread derived from the corresponding estimated curves. The regression analysis also permitted evaluation of spreading rate taking into account different regions of onset. The parameter b (months−1) indicates spreading rate; the higher the “b value,” the faster the spreading rate. The plateau, that is, the “a value” at infinite times, expressed in the same units as Y, represents the maximum percentage of patients with spread to a particular region (given in results as percentage, and confidence interval, CI). The extra sum‐of‐squares F test was used to test if the best‐fit values of selected unshared parameters, that is, a and b, differed between data sets. Statistical analysis was performed using Prism software (Graph Pad, California, USA). The area under the curve (AUC) was calculated integrating spread probability and spread time to each region, applying a dedicated method (scipy.integrate.quad) with a Python function. This was calculated for the curves with R2 values >0.95 and normalized with the maximum value for comparison of the different spreading patterns. P values were adjusted using Bonferroni correction for multiple comparisons. P < 0.01 was considered significant.
Results
Of the total group of 1300 patients (Table 2 and Fig. 1), 58% were men. The median age at symptom onset was 61 years (interquartile range, 51‐69y). The median diagnostic delay was 11 months (first‐third interquartile range, 6‐21mo). Probable or definite ALS, 14 was recognized with equal frequency (30.9%, respectively), in 61.8% of the whole cohort. Patients with probable laboratory‐supported ALS comprised 13.1%, those with possible ALS 12.7%, and PMA 12.4%.
Table 2.
Basic clinical features.
| Patients (n = 1300) | |
|---|---|
| Gender (male) (%) | 754 (58.0%) |
| Region of onset (n, %) | |
| Lower limbs | 443 (34.1%) |
| Right/ Left/ Both | 175 (39.5%)/ 171 (38.6%)/ 97 (21.9%) |
| Upper limbs | 438 (33.7%) |
| Right/ Left/ Both | 232 (53.0%)/ 154 (35.1%)/ 52 (11.9%) |
| Bulbar | 334 (25.7%) |
| Thoracic (respiratory and axial) | 28 (2.2%) |
| Fronto‐temporal dementia | 14 (1.1%) |
| Two affected regions at onset | 34 (2.6%) |
| Generalized (>2 affected regions at onset) | 9 (0.7%) |
| Diagnostic delay (months) (median, IQR) | 11.0 (6.0 – 21.0%) |
| Disease category (n, %) | |
| Definitive | 402 (30.9%) |
| Probable | 402 (30.9%) |
| Possible | 165 (12.7%) |
| Probable laboratory‐supported | 170 (13.1%) |
| PMA | 161 (12.4%) |
| UMN vs. LMN phenotype | |
| Fronto‐temporal dementia | 14 (1.1%) |
| UMN | 257 (19.8%) |
| LMN | 889 (68.4%) |
| UMN + LMN | 79 (6.1%) |
| Not classified | 61 (4.7%) |
The lower limbs were the predominant region of onset (34.1%), followed by the upper limbs region (33.7%), the bulbar region (25.7%), and the thoracic region (2.2%, including respiratory and axial onset). The right arm was more frequently first affected than the left (232 vs. 154, P < 0.001); in the lower limbs both sides were equally affected (175 vs. 171, P = 0.87). In 2.6% of patients, disease onset occurred in two regions and in 0.7% the disease began in more than two regions (generalized onset). In 1.1% of the population predominant UMN versus LMN dysfunction was not determined due to FTD presentation. In the remaining group, there was a predominant UMN phenotype (ALS‐UMN) at onset in 19.8%, a predominant LMN phenotype (ALS‐LMN) in 68.6%, and in 10.8% no UMN or LMN predominance could be determined (Table 2).
Disease Spread from 1st to 2nd region
There were 889 patients (Fig. 2 and Supplementary Table S1A).
Figure 2.
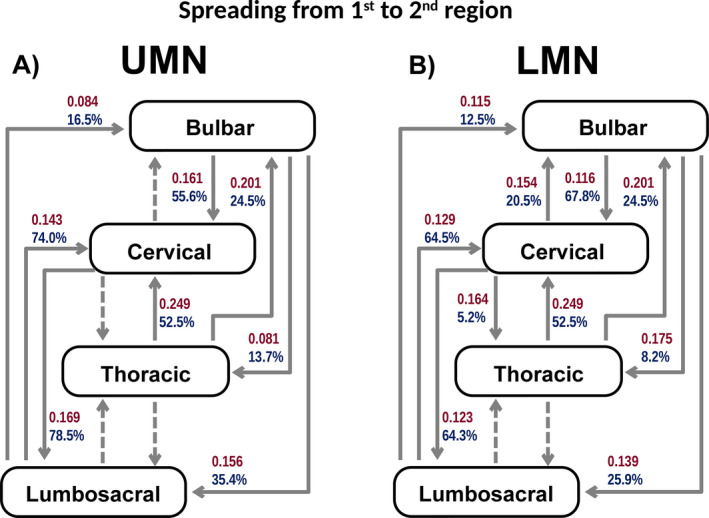
A and B, Illustrates the spread from the region of onset to the 2nd region affected in UMN onset (A) and LMN onset (B). Arrows with red numbers represent a unit related to the spreading rate (months‐1), and arrows with blue numbers represent the proportion of patients progressing to the next region (cumulative %) derived from the fitting curve equations. Dashed arrows represent progressions for which a good statistical model (adequate curve fit) was not attained.
Bulbar‐onset disease
Bulbar UMN onset: 111 patients
Progression occurred more frequently to the upper limbs region (Y = 55.6%, CI 55%‐56.2%) than to the lower limbs (Y = 35.4%, CI 34.8%‐36%) (P < 0.0025) and to lower limbs more frequently than to thoracic region (Y = 13.7%, CI 13.3%‐14.1%) (P < 0.0025). The spreading rate was similar from bulbar to upper limbs and lower limbs (P = 1.0), but slower to the thoracic region (P < 0.0025).
Bulbar LMN onset: 111 patients
Progression to the upper limbs region (Y = 67.8%, CI 66%‐69.6%) was more frequent than to the lower limbs (Y = 25.9%, CI 25.2%‐26.7%) (P < 0.0025) and to lower limbs more frequently than to thoracic region (Y = 8.2%, CI 8%‐8.4%) (P < 0.0025). The spreading rate was similar to these three regions (P = 0.28).
Upper limbs‐onset disease
Upper limbs UMN onset: 30 patients
Lack of numbers in this group restricted a good statistical model (adequate curve fit) to those that progressed to the lower limbs, precluding comparisons.
Upper limbs LMN onset: 284 patients
A greater proportion of these patients progressed to the lower limbs (Y = 64.3%, CI 63.1%‐65.6%) than to bulbar region (Y = 20.5%, CI 20.3%‐20.8%) (P < 0.0001) and to bulbar than to thoracic region (Y = 5.2%, CI 5.1%‐5.4%) (P < 0.0025). The rate of spread was similar to bulbar, lower limbs, and thoracic regions (P = 0.04).
Lower limbs‐onset disease
Lower limbs UMN onset: 68 patients
A greater proportion of these patients progressed to upper limbs (Y = 73.8%, CI 73.1%‐74.9%) than to bulbar region (Y = 16.5%, CI 15.3%‐17.7%) (P < 0.0025). The spread rate mirrored this pattern (P < 0.0025). Insufficient patients progressed to the thoracic region to permit an acceptable curve fit.
Lower limbs LMN onset: 260 patients
A greater proportion of patients progressed to upper limbs (Y = 64.5%, CI 63.7%‐65.3%) than to bulbar region (Y = 12.5%, CI 12.2%‐12.8%) (P < 0.0025). The spread rate was similar to bulbar and upper limbs regions (P = 1.0). Insufficient patients progressed to the thoracic region to permit an acceptable curve fit.
Thoracic‐onset disease
Thoracic onset: 25 patients
Spread from thoracic onset to upper limbs region (Y = 52.5%, CI 52%‐53%) was more frequent than to bulbar (Y = 24.5%, CI 23.8%‐25.1%) region (P < 0.0025), but occurred at similar rates to bulbar and upper limbs regions (P = 0.024). Insufficient patients progressed to the lower limbs to permit an acceptable curve fit. Absence of progression to other regions was more frequent in this group than in other onset groups (P < 0.001), except the bulbar LMN‐onset group (P = 1.0).
Disease Spread from 1st to 3rd region
The analysis was based on the group of 588 patients (Fig. 3 and Supplementary Table S1B).
Figure 3.
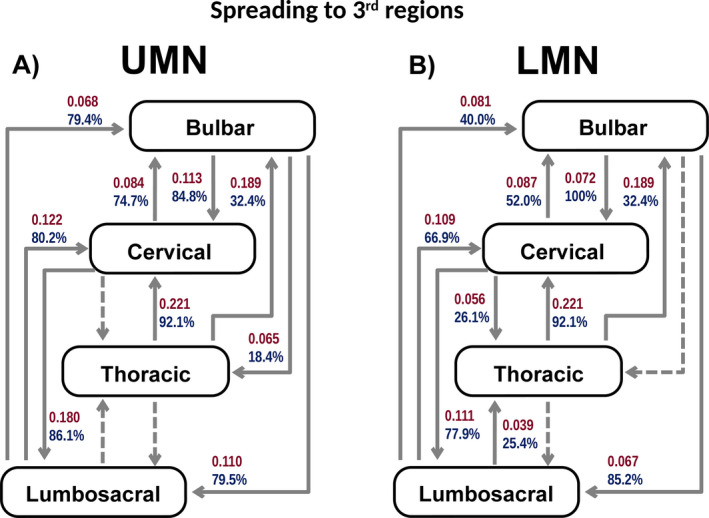
A and B, Illustrates the spread to the 3rd region affected in UMN onset (A) and LMN onset (B). Arrows, with red numbers represent a unit related to the spreading rate (months‐1), and arrows with blue numbers represent the proportion of patients progressing to the next region (cumulative %) derived from the fitting curve equations. Dashed arrows represent progressions for which a good statistical model (adequate curve fit) was not attained.
Bulbar‐onset disease
Bulbar UMN onset: 75 patients
Progression was more frequent to the upper limbs (Y = 84.8%, CI 83.6%‐85.9%) than to the lower limbs (Y = 79.5%, CI 78.4%‐80.7%) (P < 0.0025) and to lower limbs more frequently than to thoracic region (Y = 18.4%, CI 17.5%‐19.4%) (P < 0.0025). The spread rate was similar to upper limbs and lower limbs (P = 1.0), but slower to thoracic region (P < 0.0025).
Bulbar LMN onset: 63 patients
Spread to the upper limbs was more frequent than to the lower limbs (Y = 100.5%, CI 95.4%‐105.5%) (P < 0.0025). The rate of spread to upper limbs and lower limbs was similar (P = 0.38). A good statistical model (adequate curve fit) was not attained for patients progressing to the thoracic region.
Upper limbs‐onset disease
Upper limbs UMN onset: 22 patients
The proportion and spread rate to lower limbs (Y = 86.1%, CI 84.8%‐87.4%) was higher than to bulbar region (Y = 74.7%, CI 72.3%‐77%) (P < 0.0025). A good statistical model (adequate curve fit) was not attained for patients progressing to the thoracic region.
Upper limbs LMN onset: 194 patients
The proportion and rate of spreading was higher to lower limbs (Y = 77.9%, CI 76.4%‐79.5%) than to bulbar region (Y = 52%, CI 50.5%‐53.6%) (P < 0.0025), and to bulbar than to thoracic region (Y = 26.1%, CI 25.1%‐27.1%) (P < 0.0025).
Lower limbs‐onset disease
Lower limbs UMN onset: 47 patients
The proportion of patients progressing to upper limbs (Y = 80.2%, CI 78.5%‐82%) or bulbar (Y = 79.4%, CI 74.2%‐84.7%) regions was similar (P = 1.0). The speed of spread was greater to upper limbs than to bulbar regions (P < 0.0025). A good statistical model (adequate curve fit) was not attained for patients progressing to the thoracic region.
Lower limbs LMN onset: 168 patients
A greater proportion of these patients progressed to upper limbs (Y = 66.9%, CI 65.9%‐68%) than to bulbar region (Y = 40%, CI 39%‐41%) (P < 0.0025) and to bulbar than thoracic regions (Y = 25.4%, CI 22.3%‐28.5%) (P < 0.0025). The spread rate was higher to upper limbs than to bulbar region (P < 0.0025) and to bulbar than thoracic regions (P < 0.0025).
Thoracic‐onset disease
Thoracic: 19 patients
Progression in thoracic‐onset disease was more frequent than in any other group (P < 0.001), except bulbar LMN onset (P = 1.0). Thoracic onset progressed more to the upper limbs (Y = 92.1%, CI 90.3%‐93.8%) than bulbar region (Y = 32.3%, CI 31.6%‐33.2%) (P < 0.0025), but the spreading rate was similar (P = 1.0). Insufficient patients progressed to the lower limbs to permit an acceptable curve fit.
AUC analysis
We analyzed the area under the curve (AUC) of the cumulative occurrence of abnormality over time in the affected regions (Figs. 4 and 5), combining spreading rate and probability for each onset phenotype. These AUC values were normalized with the maximum value observed for an easier comparison of the different patterns of spreading.
Figure 4.
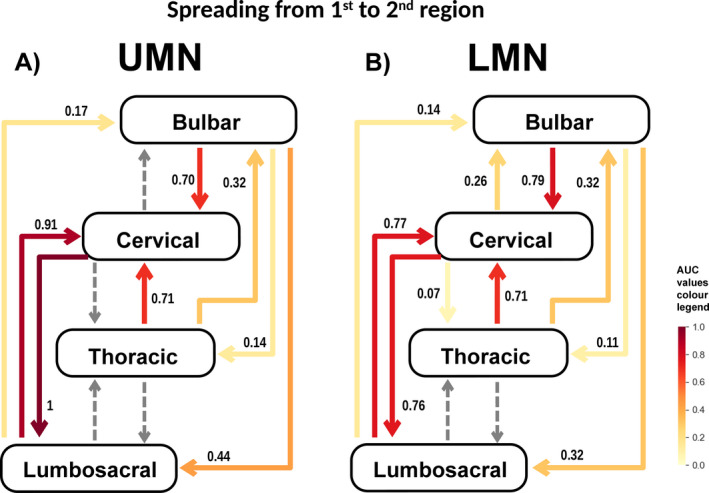
A and B, Illustrates the spread from the region of onset to the 2nd region affected in UMN onset (A) and LMN onset (B). The numbers are the normalized area under the curve (AUC) values. The AUC is represented graphically as the area between the % of patients versus time curve and the contralateral axis (x) and was calculated by computing a definite integral between the two points (x = 0 and x = 36) with the Python function, “scipy.integrate.quad” derived. Values were then normalized dividing all values for the maximum (the cervical UMN to lumbosacral region value). Dashed arrows represent progressions for which a good statistical model (adequate curve fit) was not attained.
Figure 5.
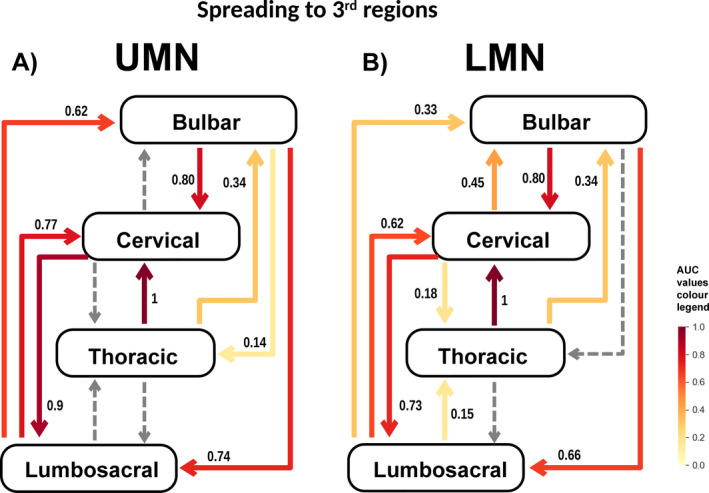
A and B, Illustrates the spread to the 3rd region affected in UMN onset (A) and LMN onset (B). The numbers are the normalized area under the curve (AUC) values. The AUC is represented graphically as the area between the % of patients versus time curve and the contralateral axis (x) and was calculated by computing a definite integral between the two points (x = 0 and x = 36) with the Python function, “scipy.integrate.quad” derived. Values were then normalized dividing all values for the maximum (the thoracic to cervical region value). Dashed arrows represent progressions for which a good statistical model (adequate curve fit) was not attained.
Spread to 2nd region
Spread from upper limbs UMN onset to lower limbs and its converse (lower limbs UMN onset to upper limbs) were the patterns of spread with the highest AUC, and lower limbs LMN onset to bulbar the lowest (Fig. 4). The spread of bulbar and lower limbs onsets to adjacent regions had higher AUCs than to more distant regions. The spread of upper limbs onset toward the caudal region had higher AUCs than to the rostral region. A good curve fit was not attained for spreading from upper limbs UMN to bulbar region. In general, UMN onsets had higher AUCs than LMN, except that disease spread from bulbar to upper limbs region had a higher AUC for LMN than for UMN onset.
Spread to 3rd regions
The spread patterns were similar to spread to the 2nd region; again, the highest AUC value was upper limbs UMN onset to lower limbs (Fig. 5). The main differences were the AUC increase in lower limbs UMN onset to bulbar and bulbar LMN onset to lower limbs, and the slight decrease in both lower limbs UMN onset and lower limbs LMN onset to upper limbs.
Contralateral Progression and the role of UMN lesion
In our population, the group of 30 patients with predominant UMN signs in arms progressed to ipsilateral leg in 27% of the patients as opposed to contralateral arm in 43% and contralateral leg in 14% (crossed pattern). From the group of patients with predominant LMN in upper limbs, 21% progressed to the ipsilateral leg, 55% to the contralateral arm, crossed progression occurred in 7%, less frequent than progression to bulbar region (11%). Predominant UMN involvement was not determinant of the pattern of progression (P = 0.56). As in arms, the onset in one leg was associated with more frequent contralateral progression (59% and 64%, for predominant UMN and LMN phenotype, respectively) followed by ipsilateral arm in 24% of the patients with spastic legs and in 21% of those with LMN phenotype, crossed pattern was also similar (7‐8%).
Patterns of descending versus ascending spread
Bulbar UMN onset progressed faster than bulbar LMN onset (P < 0.0025), but in both the rate of spread to upper limbs or lower limbs was similar (P = 1.0). Both bulbar UMN onset and bulbar LMN‐onset patients progressed more to upper limbs than to lower limbs (P < 0.0025). Upper limbs UMN‐ and LMN‐onset syndromes were more likely to progress to lower limbs than to bulbar region (P < 0.0025), but rostral‐caudal progression was faster in upper limbs UMN onset than upper limbs LMN‐onset disease (P < 0.0025) although caudal‐rostral progression was similar for both upper limbs UMN and LMN onset (P = 1.0). Lower limbs UMN‐onset disease progressed more frequently to upper limbs and bulbar regions than LMN onset (P < 0.0025 to both bulbar and upper limbs), but the speed of spread to those regions was similar for both lower limbs LMN and UMN onset (to bulbar P = 1.0: to upper limbs P = 0.363) and for both groups was faster to upper limbs than to bulbar region (lower limbs UMN onset, P < 0.0025 and lower limbs LMN onset, P < 0.0025). In general, lower limbs LMN onset progressed more by anatomical contiguity than lower limbs UMN onset (lower limbs UMN onset to upper limbs vs. bulbar, P = 1.0: lower limbs LMN onset to upper limbs vs. bulbar, P < 0.0025).
Contiguity versus multiple hits
The proportion of bulbar UMN‐onset cases spreading to lower limbs was similar to that in the reverse direction (P = 1.0), but bulbar LMN‐onset disease progressed more rapidly to the lower limbs than from the lower limbs LMN onset to bulbar region (P < 0.0025). The rate of spread from bulbar LMN onset to lower limbs was similar to lower limbs LMN onset to bulbar (P = 0.407), but the rate of bulbar UMN onset spread to lower limbs was faster than lower limbs UMN onset to bulbar (P < 0.0025). The proportion of patients with bulbar UMN and bulbar LMN‐onset progressing to the upper limbs region was greater than the converse – from upper limbs UMN or LMN onset to bulbar (P < 0.0025). The rate of spread from bulbar LMN onset to upper limbs region was similar to upper limbs LMN onset to bulbar (P = 0.559), but bulbar UMN‐onset patients progressed faster to the upper limbs region than upper limbs UMN onset to the bulbar region (P < 0.0025). Rostral‐caudal progression from upper limbs UMN and upper limbs LMN onset to lower limbs was more frequent than caudal‐rostral progression from lower limbs UMN or LMN onset to upper limbs (P < 0.0025). The rate of progression of rostral‐caudal upper limbs LMN onset to lower limbs was similar to caudal‐rostral progression of lower limbs LMN onset to upper limbs region (P = 1.0), but upper limbs UMN‐onset disease progressed faster to the lower limbs than caudal‐rostral lower limbs UMN‐onset to upper limbs (P < 0.0025).
Rate of spread
In general, for LMN‐onset the rate of rostral‐caudal spread was similar to caudal‐rostral spread (bulbar LMN‐onset to upper limbs vs. upper limbs LMN‐onset to bulbar, P = 0.56; bulbar LMN onset to lower limbs vs. lower limbs LMN onset to bulbar, P = 0.41; upper limbs LMN onset to lower limbs vs. lower limbs LMN onset to upper limbs, P = 1.0), but UMN onset was associated with faster rostral‐caudal than caudal‐rostral progression (bulbar UMN onset to upper limbs vs. upper limbs UMN onset to bulbar, P < 0.0025; bulbar UMN onset to lower limbs vs. lower limbs UMN onset to bulbar, P < 0.0025; upper limbs UMN onset to lower limbs vs. lower limbs UMN onset to upper limbs, P < 0.0025). As a rule both UMN and LMN predominant cases were more likely to progress caudally than rostrally (bulbar LMN onset to upper limbs vs. upper limbs LMN onset to bulbar, P < 0.0025; bulbar LMN onset to lower limbs vs. lower limbs LMN onset to bulbar, P < 0.0025; upper limbs LMN onset to lower limbs vs. lower limbs LMN onset to upper limbs, P < 0.0025; bulbar UMN onset to upper limbs vs. upper limbs UMN onset to bulbar, P < 0.0025; upper limbs UMN onset to lower limbs vs. lower limbs UMN onset to upper limbs, P < 0.0025;) except that the proportion of bulbar UMN onset patients progressing to lower limbs was similar to lower limbs UMN‐onset progressing to bulbar (P = 1.0). The rate of spread for UMN and LMN dysfunction is shown graphically using an inverse exponential curve fitting statistic in Supplementary Fig. S1A‐S1N.
Absence of spread
Spread of disease to another region was an almost inevitable feature of the disease: only a few patients did not show this pattern of disease spread. Regarding spread from onset to the second region (Fig. 6A and Supplementary Table S1C), lower limbs LMN‐onset cases had the highest proportion of patients without spread (21.3%, 95%CI: 20.4 –22.19%) followed by upper limbs LMN onset (11.47%, 95% CI: 10.22–12.72%, P < 0.001). Thoracic onset (0.11%, 95% CI: 0‐1.44%) and bulbar LMN onset (1.67%, 95% CI: 0‐3.71%) were the groups with the lowest proportion of patients without disease spread (thoracic vs. bulbar LMN onset, P = 1.0). In some patients there was no spread from the second region (Fig. 6B and Supplementary Table S1D); the highest proportion occurred in lower limbs LMN‐onset disease (33.0%, 95% CI: 32.03‐34.05%) followed by upper limbs LMN onset (18.38%, 95% CI: 17.36–19.41%, P < 0.001). Thoracic onset (1.04%, 95% CI: 0–2.58%) and bulbar LMN onset (2.14%, 95% CI: 0–4.53%) had the lowest proportion of nondisease spreading patients (thoracic vs. bulbar LMN onset, P = 1.0).
Figure 6.
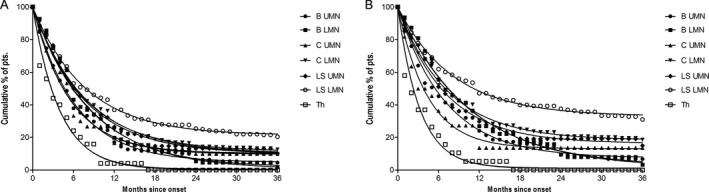
A, Cumulative % of patients that did not spread from onset to a 2nd region within 36 months of disease duration. B, Cumulative % of patients that did not spread to a 3rd region within 36 months of disease duration.
Discussion
We studied consecutive patients with ALS prospectively, using a structured datasheet derived from an agreed protocol. 15 The data were scrutinized for errors both by the investigators and by an independent statistical group (LASIGE) and most of the statistical analyses were done independently by the latter group. (Table 1). We found that progression was more probable and quickest to or from the region of onset to close spinal regions, as described elsewhere. 3 , 4 , 5 , 6 When the disease had a limb onset, bulbar neurons were more resistant to involvement compared with spinal regions, as illustrated by upper limb LMN‐onset and thoracic‐onset disease, which progressed more to lower limbs and upper limbs, respectively, than to bulbar region. Furthermore, both lower limb UMN‐ and LMN‐onset ALS progressed more to upper limbs than to bulbar region. This specific bulbar region resistance to progression has not been properly stressed before. In the same time‐frame more patients progressed rostral‐caudal from bulbar to lower limbs than vice‐versa, whatever the predominant UMN or LMN involvement. Moreover, UMN involvement seems important in shortening the time for cranial‐caudal spread, but not in caudal‐cranial progression. This is contrary to Hu et al. 7 observation that UMN signs had no influence on the rate of progression. Ravits et al., 1 described preferential involvement of the ipsilateral leg following arm onset in patients with predominant UMN signs, but we did confirm this observation. In our population, the next involved body part is the contralateral limb 3 , 5 , 6 (contralateral spread), in both patients with predominant UMN or LMN signs. Crossed‐progression, from one limb to the contralateral limb in another body region, was rarely observed as reported in another study. 6
In thoracic‐onset disease (including patients with respiratory onset) there was a lower probability of no spread, involving the upper limb region first, a finding consistent with the poor prognosis of thoracic‐onset disease. Patients with lower limb LMN and upper limb LMN presentations had a lower chance of spread within the same time‐frame, consistent with the better prognosis of patients with flail leg and flail arm syndrome. However, faster spread of LMN weakness has been correlated with shorter survival. 3 , 17
Contiguous spreading is the typical progression pattern, taking into account the more probable involvement of the upper limb region after bulbar onset. 3 , 4 , 5 , 6 However, as there are no sensitive clinical and functional signs of thoracic region involvement, we cannot reject a skipping of this region in patients progressing from lumbo‐sacral to upper limb region and vice‐versa. Sekiguchi et al., 9 used EMG to test “contiguous” versus “multifocal hits” progression in 39 patients they envisaged contiguous progression as prion‐like. 18 Noncontiguous progression has been associated with a poor prognosis. 6 , 7
Analysing the AUC, which incorporates both of disease spread and rate, spread of bulbar and lower limb onsets to adjacent regions had higher probabilities than to more distant regions. The disease tended to follow a caudal progression; the AUC from upper limbs to lower limbs was higher than its converse, and this was most influenced by UMN involvement. Brooks 2 studied 702 patients who had completed at least two follow‐up questionnaires. Some of his observations are confirmed in our study. In particular, he noted “limb symptom development occurred faster subsequent to bulbar onset than bulbar symptom development following limb onset,” and that “symptom accrual within the spinal cord occurred faster than bulbar symptom accrual.” He did not consider the possible role of UMN lesion in this process, which we found relevant. In an additional study of 145 patients, Brooks 4 reported more rapid respiratory involvement in bulbar than spinal disease as evaluated by forced vital capacity. However, our findings do not support earlier respiratory (thoracic) involvement in bulbar‐onset patients, supporting previous evidence. 19
Ravits et al., 1 studied UMN and LMN involvement of other anatomical regions in bulbar, upper limb, and lower limb‐onset disease. They used retrospective data from 100 patient records, but did not apply statistical analysis. They concluded that “outward spread of both UMN and LMN signs seemed to be weighted toward caudal body regions over rostral ones, suggesting underlying motor neuron degeneration had preferential directions of outward spread rather than simple radial or centrifugal directions.” Our analysis refines this suggestion since we found that faster caudal progression from initial bulbar or upper limb onset was related to UMN involvement, but the probability of involvement of a contiguous spinal region was similar in rostral‐caudal or caudal‐rostral directions. This pattern of longitudinal progression in ALS was further supported by a study of the relationship between spreading time and survival in 150 ALS patients. 3 There was greater progression from the bulbar to upper limb region, and from upper limbs to lower limbs and vice‐versa, as opposed to progression to bulbar region, but no statistical analysis was done for these comparisons.
Our study has some limitations: in order to keep the number of statistical models within a reasonable limit, and because the predominance of contralateral progression in ALS has been extensively reported, 2 , 6 , 7 , 20 contralateral spread was analyzed with a simpler approach. We did not stratify the population for age and gender, but these features are not likely relevant regarding disease spread. 1 , 4 , 6 , 7 Genetic analysis was not included, since the number of 10% of patients with C9orf72 mutation is insufficient for this statistical model. However, by integrating rate of progression, anatomical region and UMN and LMN signs, we have been able to reconsider the three‐dimensional anatomy of progression described by Ravits and LaSpada. 20 Contiguous, perhaps prion‐like, contralateral and longitudinal progression is an observation common to most studies. This segmental and intersegmental progression in the neuraxis is consistent with contiguous spread of the disease process in ALS. 21 We found that bulbar neurons seemed more resistant to spread in spinal‐onset disease, but spinal LMNs are susceptible in bulbar‐onset rostral‐caudal disease progression. In addition, rostral‐caudal progression was faster in patients with predominant UMN signs. We suggest that descending motor pathways modulate the pattern and rate of disease progression in ALS. This clinical evidence is in accord, for example, with the neuropathological concept in ALS of dissemination of TDP‐43 pathology from a cortical motor neuronal onset, via axonal transport, through synaptic contacts to the LMNs in the spinal cord, with early involvement of the dorsolateral motor nuclei columns of the upper limbs and lower limbs anterior horn. 22 , 23 , 24 However, the pattern of spread in caudal‐onset ALS and in “skip lesion” disease is not consistent with this, perhaps over‐simplified, model.
In conclusion our results can best be fitted to a model of independent LMN and UMN degeneration, with regional progression of LMN degeneration mostly by contiguity. UMN disease causes an acceleration of rostral‐caudal LMN loss in brain‐stem and spinal cord. This would explain the slower progression in flail‐arm and flail‐leg disease, in which there is no major UMN lesion. However, in primary lateral sclerosis, with slow progression, there seems to be resistance of LMNs in the spinal‐cord and bulbar regions, suggesting a different underlying process in this syndrome.
Funding Information
This is an EU Joint Programme ‐ Neurodegenerative Disease Research (JPND) project. The project is supported through national funding organizations under the aegis of JPND ‐ www.jpnd.eu. This project was also partially supported by FCT funding to Neuroclinomics2 (PTDC/EEI‐SII/1937/2014).
Conflicts of Interest
None of the authors disclose any conflict of interest concerning this manuscript.
Authors' Contributions
JG, M K‐K, MdC, HU, and SPetri involved in conception and design of the study and acquisition of data. SP, MG, MF, and SM carried out analysis of data. MG also performed drafting a significant portion of the manuscript or figures. MF also involved in drafting a significant portion of the figures. MS and MG involved in conception and design of the study. MS and MdC involved in drafting a significant portion of the manuscript.
Supporting information
Figure S1. Graphic representation of the curve fitting of the spread progression to different regions.
Table S1. Equation model results of the curve fitting for the spread progression to different regions.
Michael Swash and Mamede de Carvalho were senior authors.
Funding Statement
This work was funded by EU Joint Programme ‐ Neurodegenerative Disease Research grant OnWebDuals.
References
- 1. Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology 2007;68:1571–1575. [DOI] [PubMed] [Google Scholar]
- 2. Brooks BR. The role of axonal transport in neurodegenerative disease spread: a meta‐analysis of experimental and clinical poliomyelitis compares with amyotrophic lateral sclerosis. Can J Neurol Sci / J Can des Sci Neurol 1991;18:435–438. [DOI] [PubMed] [Google Scholar]
- 3. Fujimura‐Kiyono C, Kimura F, Ishida S, et al. Onset and spreading patterns of lower motor neuron involvements predict survival in sporadic amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2011;82:1244–1249. [DOI] [PubMed] [Google Scholar]
- 4. Brooks BR. Natural history of ALS:symptoms, strength, pulmonary function, and disability. Neurology 1996;47:71S–82S. [DOI] [PubMed] [Google Scholar]
- 5. Körner S, Kollewe K, Fahlbusch M, et al. Onset and spreading patterns of upper and lower motor neuron symptoms in amyotrophic lateral sclerosis. Muscle Nerve 2011;43:636–642. [DOI] [PubMed] [Google Scholar]
- 6. Gargiulo‐Monachelli GM, Janota F, Bettini M, et al. Regional spread pattern predicts survival in patients with sporadic amyotrophic lateral sclerosis. Eur J Neurol 2012;19:834–841. [DOI] [PubMed] [Google Scholar]
- 7. Hu F, Jin J, Jia R, et al. Spread direction and prognostic factors in limb‐onset sporadic amyotrophic lateral sclerosis. Eur Neurol 2016;75:244–250. [DOI] [PubMed] [Google Scholar]
- 8. Walhout R, Verstraete E, van den Heuvel MP, et al. Patterns of symptom development in patients with motor neuron disease. Amyotroph Lateral Scler Front Degener 2018;19:21–28. [DOI] [PubMed] [Google Scholar]
- 9. Sekiguchi T, Kanouchi T, Shibuya K, et al. Spreading of amyotrophic lateral sclerosis lesions–multifocal hits and local propagation? J Neurol Neurosurg Psychiatry 2014;85:85–91. [DOI] [PubMed] [Google Scholar]
- 10. Ince PG, Evans J, Knopp M, et al. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 2003;60:1252–1258. [DOI] [PubMed] [Google Scholar]
- 11. Eisen A, Kim S, Pant B. Amyotrophic lateral sclerosis (ALS): a phylogenetic disease of the corticomotoneuron? Muscle Nerve 1992;15:219–224. [DOI] [PubMed] [Google Scholar]
- 12. Swash M. Amyotrophic lateral sclerosis: a phylogenetic disease of the corticomotoneuron? Comments on the hypothesis. Muscle Nerve 1992;15:226–228. [DOI] [PubMed] [Google Scholar]
- 13. Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet 2011;377:942–955. [DOI] [PubMed] [Google Scholar]
- 14. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 15. de Carvalho M, Ryczkowski A, Andersen P, et al. International survey of ALS experts about critical questions for assessing patients with ALS. Amyotroph Lateral Scler Front Degener 2017;18:1–6. [DOI] [PubMed] [Google Scholar]
- 16. de Carvalho M, Pinto S, Swash M. Association between paraspinal muscles and diaphragm denervation in ALS. Amyotr Lat Scler 2010;11:63–66. [DOI] [PubMed] [Google Scholar]
- 17. Tortelli R, Copetti M, Panza F, et al. Time to generalization and prediction of survival in patients with amyotrophic lateral sclerosis: a retrospective observational study. Eur J Neurol 2016;23:1117–1125. [DOI] [PubMed] [Google Scholar]
- 18. Pinto S, Pinto A, De Carvalho M. Do bulbar‐onset amyotrophic lateral sclerosis patients have an earlier respiratory involvement than spinal‐onset amyotrophic lateral sclerosis patients? Eura Medicophys 2007;43:505–509. [PubMed] [Google Scholar]
- 19. Swash M. How does ALS spread between neurones in the CNS? J Neurol Neurosurg Psychiatry 2013;84:116–117. [DOI] [PubMed] [Google Scholar]
- 20. Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: Deconstructing motor neuron degeneration. Neurology 2009;73:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maniatis S, Äijö T, Vickovic S, et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 2019;364:89–93. [DOI] [PubMed] [Google Scholar]
- 22. Braak H, Brettschneider J, Ludolph AC, et al. Amyotrophic lateral sclerosis—a model of corticofugal axonal spread. Nat Rev Neurol 2013;9:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brettschneider J, Arai K, Del Tredici K, et al. TDP‐43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol 2014;128:423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riku Y. Reappraisal of the anatomical spreading and propagation hypothesis about TDP‐43 aggregation in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Neuropathology 2020; 10.1111/neup.12644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Graphic representation of the curve fitting of the spread progression to different regions.
Table S1. Equation model results of the curve fitting for the spread progression to different regions.


