Abstract
Objectives
The data concerning the association between Tx and ADs remain unclear and are scarce. This study was undertaken to investigate whether people with Tx are more likely to develop ADs, compared to those without Tx.
Methods
Individuals who received Tx between 2002 and 2015 were identified and matched on age and sex with individuals without Tx. We performed multivariate and stratified analysis using the Kaplan–Meier method and Cox proportional hazards models in order to estimate the association between Tx and the risk of developing ADs.
Results
A total of 2550 thymectomized (Txd) patients and 24,664.941 non‐Txd comparison subjects were selected from NHIRD. Tx‐MG (myasthenia gravis) as compared with general population (nonTx‐nonMG), adjusted hazard ratio (aHR) were higher for incident Addison disease (aHR = 10.40, 95% CI 1.01–107), autoimmune hemolytic anemia (aHR = 21.54, 95% CI 2.06–14.8), Hashmoto thyroiditis (aHR = 5.52, 95% CI 1.34–34.7), ankylosing spondylitis (aHR = 2.73, 95% CI 1.09–6.84), rheumatoid arthritis (aHR = 5.25, 95% CI 1.79–15.47), primary Sjogren syndrome (pSS) (aHR = 3.77, 95% CI 1.30–11.0), and systemic lupus erythemtoasus (aHR = 10.40). Tx‐nonMG as compared with general population, aHR were higher for incident autoimmune hemolytic anemia (aHR = 25.50), Hashmoto thyroiditis (aHR = 6.75) and systemic lupus erythematosus (SLE) (aHR = 13.38). NonTx‐MG as compared with general population, aHR were higher for incident Hashmoto thyroiditis (aHR = 6.57), pSS (aHR = 4.50), SLE (aHR = 17.29), and systemic vasculitis (aHR = 25.86).
Interpretation
In conclusion, based on a retrospective cohort study throughout Taiwan, patients with Tx have a higher risk of new onset ADs than patients without Tx.
Introduction
The thymus plays a crucial role in the context of cell‐mediated immunity in the differentiation of T lymphocytes, not only during the embryogenesis and fetal period but also during the adulthood, even after its involution. 1 The thymus deletes self‐reactive T cells with high avidity T‐cell receptors for self antigens expressed in the thymus. 2 This, hence, means that thymus has a protective role against autoimmunity. It has been proved, indeed, that thymectomy (Tx) in adult rat entails a decrease in the T‐lymphocyte response to mitogens and eventually its abolition. 3 The removal of the thymus can decrease the activity of T‐helper cells but in the same time it might enhance the activity of T‐suppressor whose function is depressed in autoimmune diseases. 4
The surgical removal of the thymus gland is successfully used to manage myasthenia gravis (MG). 5 The goal of Tx was not only to prevent the spread of a thymoma, a well‐known associated disorder, but also to induce remission of the disease. 6 However, the precise mechanism by which Tx produces benefit in patients with MG is still unclear. This therapeutic option is indicated in young patients, usually <60 years old, 7 because there is uncertainty about the persistence of thymic tissue in elderly subjects. 8 However, it is advisable to delay Tx until puberty if possible because of the established role of the thymus in the development of the immune system. 9 Nevertheless, it is generally thought that Tx does not cause significant adverse effects. 8
In recent years, there has been growing evidence of systemic autoimmune diseases (AIDs) occurring many years after Tx in patients with MG or other immune‐mediated diseases. 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 The data reveled the long‐term effect of Tx on T cell and on T cell–dependent B‐cell activity in MG appear to be controversial. Our aim was to evaluate the role of Tx to clarify whether it may be regarded as therapeutic or a factor paving the way to the onset of ADs. No large cohort study has investigated the incidence of ADs among patients with Tx. We investigated this putative association utilizing a nationwide population‐based dataset of insurance claims.
Methods
Data sources
Medical claims data were obtained from the National Health Research Institutes (NHRI). After receiving approval for this study from the NHRI, we used scrambled patient identification numbers to assess the data, including inpatient care claims and the Registry for Beneficiaries. The NHRI maintains and updates the National Health Insurance (NHI) Research Database (NHIRD). The insurance program maintains contracts with 97% of the hospitals and clinics in Taiwan. 19 The accuracy and high validity of diagnoses in the NHIRD have been evaluated. 20 The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes were used as the diagnosis codes in the present study. The claims data for all 23,740,000 insured persons were used to establish the study cohorts. The research protocol was approved by the Taipei Medical University‐Joint Institutional Review Board (N201602014) and was performed in accordance with the approved guidelines. Informed consent from the study patients was not required because the dataset consisted of deidentified secondary data released for research purposes.
Study population and design
We identified 5418 patients (case group) who received Tx (Procedure Code: 07.8) from NHIR database between 1 January 2002, and 31 December 2015. Cases with unknown age and sex, with surveyed autoimmune diseases prior to study start, and diagnosis of all cancer (except thymoma and thymic carcinoma) were excluded from the study. Then case group (n = 2550) divided to (1) thymectomized (Txd) patients (n = 1183) with MG (ICD‐9‐CM 358) with a catastrophic illness certificate and (2) Txd patients (n = 1367) without MG. Subjects without Tx (comparison group) were selected from among the 23 million NHI beneficiaries recorded between 2002 and 2015 using the same exclusion. The comparison group was also divided to (3) nonTx‐MG group: nonTxd patients with MG and (4) nonTx‐nonMG group: non‐Txd patients without MG. Then we used frequency matching by sex and age to the four groups (1;1, 1;3, and 1;20, respectively).
Study endpoints
Each study patient was followed until one of the following outcomes occurred: an AD was diagnosed, the patient was lost to follow‐up, the patient died, the patient withdrew from the NHI system. We identified patients with ADs using ICD‐9‐CM codes. ADs in this study were categorized into two broad types: systemic and organ‐specific ADs. The systemic ADs included Sjögren syndrome (SS; ICD‐9‐CM code 710.2), psoriasis (ICD‐9‐CM codes 696.0 and 696.1), rheumatoid arthritis (RA; ICD‐9‐CM code 714.0), systemic lupus erythematosus (SLE; ICD‐9‐CM code 7100), scleroderma (ICD‐9‐CM code 710.1), polymyositis (PM; ICD‐9‐CM code 710.4), and systemic vasculitis (ICD‐9‐CM code 443.1, 446.4, 446.7). In Taiwan, patients with systemic ADs (except Ankylosing spondylitis ICD‐9‐CM code 712.0 and psoriasis ICD‐9‐CM codes 696.0, 696.1, and 694.3) are eligible for a catastrophic illness certificate after receiving the diagnosis from a rheumatology specialist based on their clinical manifestations, laboratory data, and international criteria; the certification requires the precise fulfillment of the related classification criteria. 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 The organ‐specific ADs included Addison's disease (ICD‐9‐CM code 255.4), autoimmune hemolytic anemia (ICD‐9‐CM code 283.0), diabetes mellitus type 1 (DM; ICD‐9‐CM code 250.0), Graves' disease (ICD‐9‐CM code 242.0), Hashimoto's thyroiditis (ICD‐9‐CM code 245.2), Henoch–Schönlein purpura (ICD‐9‐CM code 287.0), immune thrombocytopenic purpura (ICD‐9‐CM code 287.3), autoimmune hepatitis (ICD‐9‐CM code 571.49), MG (ICD‐9‐CM code 358.0), and inflammatory bowel disease (IBD; ICD‐9‐CM codes 555 and 556). A person was considered to have a new onset of an organic AD only if the condition occurred in an inpatient setting or was noted in three or more outpatient visits.
In addition, patients with the comorbidities of SLE, RA, scleroderma, PM, DM, a history of head and neck radiation treatment, hepatitis C infection, ADs, pre‐existing lymphoma, sarcoidosis, graft versus host disease, and anticholinergic drug use were excluded to limit our study sample to primary SS (pSS). Therefore, the catastrophic illness patient data are highly accurate and reliable. 30
Statistical analysis
We compared the frequency or mean of demographic status (age and sex) in the Tx‐MG, Tx‐nonMG, nonTx‐MG, and nonTx‐nonMG cohorts using the chi‐squared test or t test. The incidence rates of any type, organ‐specific and systemic ADs were estimated during the follow‐up duration in the Tx‐MG, Tx‐nonMG, nonTx‐MG, and nonTx‐nonMG cohorts. The Cox proportional hazards regression model was used to estimate the corresponding hazard ratios (HRs) and 95% confidence intervals (CIs). The HRs were adjusted for sex and age in the Cox regression model. Finally, the cumulative incidences of any type, organ‐specific and systemic ADs were estimated using the Kaplan–Meier estimator, known as the product limit estimator, in the Tx‐MG, Tx‐nonMG, nonTx‐MG, and nonTx‐nonMG cohorts. SAS (version 9.3, SAS Institute, Cary, NC) was used for all the data analyses, and P < 0.05 was considered statistically significant.
Patient and public involvement
There were no patients involved in developing the research question or the outcome measures, and no patients were involved in the design, recruitment, and conduct of the study. The data were collected from the National Health Insurance Research Database, and patients were anonymous. We were unable to disseminate the results of the research directly to study participants.
Results
Baseline characteristics of the study population
A total of 2550 Txd patients and 24,664.941 non‐Txd comparison subjects were selected from NHIRD. After individual frequency matching, patients were divided in to four group: (1) 807 Txd patients with MG (Tx‐MG) (2) 807 Tx patients without MG (Tx‐nonMG) (3) 2421 non‐Tx patients with MG (nonTx‐MG) and (4) 16,140 non‐Tx patients without MG (nonTx‐nonMG). The flowchart of study population selection is shown in Figure 1.
Figure 1.
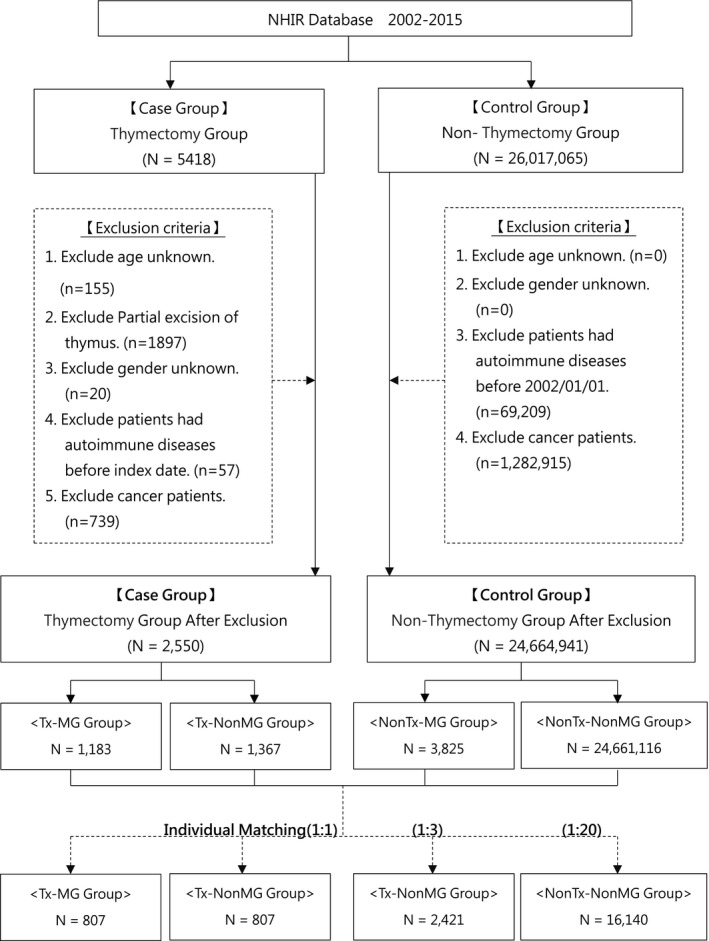
Flow chart for study design. MG, myasthenia gravis; Tx, thymectomy.
Table 1 presents the demographic characteristics and top five reason of Tx of the study cohorts. The mean ages in each group were Tx‐MG: 46.79 ± 14.80, Tx‐nonMG: 47.01 ± 17.78, nonTx‐MG: 46.86 ± 14.83, and nonTx‐nonMG: 46.75 ± 14.81, respectively, and the study population was dominated by women (58.98%). The mean follow‐up duration (year) in each group were Txd‐MG: 2.16 ± 1.47, Tx‐nonMG: 1.83 ± 1.358, nonTx‐MG: 1.56 ± 1.14, and nonTx‐nonMG: 2.35 ± 1.46, respectively. The top five reasons of Tx in order were benign neoplasm of thymus, malignant neoplasm of thymus, MG, other disorders from impaired renal function, other specified diseases of thymus gland.
Table 1.
Characteristic baseline of each group (Tx‐MG, Tx‐nonMG, nonTx‐MG, and nonTx‐nonMG).
| Variables | Tx‐MG (N = 807) | Tx‐nonMG (N = 807) | NonTx‐MG (N = 2421) | NonTx‐nonMG (N = 16,140) | P‐Value |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Gender | 1.0000 | ||||
| Female | 476 (58.98) | 476 (58.98) | 1428 (58.98) | 9520 (58.98) | |
| Male | 331 (41.02) | 331 (41.02) | 993 (41.02) | 6620 (41.02) | |
| Age | 0.9995 | ||||
| ≤30 | 122 (15.12) | 118 (14.62) | 346 (14.29) | 2405 (14.90) | |
| 31–40 | 145 (17.97) | 133 (16.48) | 456 (18.84) | 2942 (18.23) | |
| 41–50 | 201 (24.91) | 212 (26.27) | 605 (24.99) | 4079 (25.27) | |
| 51–60 | 202 (25.03) | 203 (25.15) | 603 (24.91) | 3980 (24.66) | |
| 61–70 | 87 (10.78) | 94 (11.65) | 264 (10.90) | 1756 (10.88) | |
| 71–80 | 44 (5.45) | 42 (5.2) | 135 (5.58) | 878 (5.44) | |
| ≥81 | 6 (0.74) | 5 (0.62) | 12 (0.50) | 100 (0.62) | |
| Mean (SD), year | 46.79 (14.80) | 47.01 (14.78) | 46.86 (14.83) | 46.75 (14.81) | 0.9525 |
| Follow‐up, year | |||||
| Mean (SD) | 2,16 (1,47) | 1,83 (1,35) | 1,56 (1,14) | 2,35 (1,46) | <0.0001 |
| Top five reasons of Tx | |||||
| Benign neoplasm of thymus | 18.77% | ||||
| Malignant neoplasm of thymus | 18.48% | ||||
| Myasthenia gravis | 17.78% | ||||
| Other disorders from impaired renal function | 5.92% | ||||
| Other specified diseases of thymus gland | 5.92% | ||||
Tx, thymectomy; MG, myasthenia gravis.
Incidence rates, ratio and aHRs of ADs and subgroup ADs in each cohort
Table 2 presents the incidence rates and adjusted HRs (aHRs) of ADs and subgroup AIDs in each cohorts. During the study period, 3.3% Tx‐MG, 1.73% Tx‐nonMG, 1.98% nonTx‐MG, and 0.87% nonTx‐nonMG patients, respectively, developed any type of AIDs. The incidence rate of any‐type of AIDs was higher in the Txd‐MG cohort (524.38 per 100,000 person years), Tx‐nonMG cohort (347.02 per 100,000 person years), and nonTx‐MG (463.77 per 100,000 person years) than in the nonTx‐nonMG cohort (135.63 per 100,000 person‐years), with an aHR of the Tx‐MG cohort 3.85 (95% CI: 2.53–5.86), Tx‐nonMG cohort 2.51 (95% CI: 1.44–4.39), and nonTx‐MG cohort 3.32 (95% CI: 2.40–4.59) after adjustment for age and sex. For the organ‐specific AIDs, the incidence rate of overall organ‐specific AIDs was significantly higher in the Tx‐MG, Tx‐nonMG, and nonTx‐MG cohort than in the nonTx‐nonMG, with an aHR of the Tx‐MG cohort (3.53), Tx‐nonMG cohort (3.78), and nonTx‐MG cohort (3.30) after adjustment for age and sex (Table 2). Furthermore, for the respective organ‐specific ADs, the incidence rate of Hashmotos thyroiditis was significantly higher in the Tx‐MG, Txd‐non‐MG, and nonTx‐MG cohort than in the nonTx‐nonMG control cohort, with an aHR of the Tx‐MG cohort (5.52), Txd‐nonMG cohort (6.75), and nonTx‐MG cohort (6.57) after adjustment for age and sex. In addition, the incidence rate of autoimmune hemolytic anemia was significantly higher in the Tx‐non‐MG cohort than in the nonTx‐nonMG control cohort, with an aHR of the Tx‐MG cohort (25.50) after adjustment for age and sex.
Table 2.
Incidence rate and hazard rate of Autoimmune diseases and subgroup in each cohort.
| Type of ADs | Tx‐MG | Tx‐nonMG | NonTx‐MG | NonTx‐nonMG | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event (%) | IR | IRR | Adjusted‐HR (95% CI) | Event (%) | IR | IRR | Adjusted‐HR (95% CI) | Event (%) | IR | IRR | Adjusted‐HR (95% CI) | Event (%) | IR | IRR | Adjusted‐HR | |
| Organ specific‐AIDs | ||||||||||||||||
| Addison disease | 0.12 | 20.45 | 10.70 | 10.40* (1.01–107) | 0.00 | – | – | – | 0.00 | – | – | – | 0.01 | 1.91 | Ref | Ref |
| AHA | 0.12 | 20.44 | 21.40 | 21.54* (1.34–347) | 0.12 | 24.42 | 25.55 | 25.50* (1.59–410) | 0.04 | 9.53 | 9.97 | 9.01 (0.54–150) | 0.01 | 0.96 | Ref | Ref |
| DM (type 1) | 0.12 | 20.45 | 0.82 | 0.81 (0.11–6.01) | 0.12 | 24.8 | 0.98 | 0.95 (0.13–7.03) | 0.21 | 47.69 | 1.92 | 1.86 (0.69–4.97) | 0.16 | 24.89 | Ref | Ref |
| Hashmoto thyroiditis | 0.62 | 102.64 | 5.65 | 5.52** (2.06–14.8) | 0.62 | 122.62 | 6.75 | 6.75** (2.54–17.9) | 0.50 | 114.69 | 6.31 | 6.57*** (3.20–13.48) | 0.12 | 18.18 | Ref | Ref |
| HSP | 0.12 | 20.47 | 5.35 | 5.57 (0.62–50.4) | 0.12 | 24.43 | 6.39 | 6.28 (0.70–56.3) | 0.00 | – | – | – | 0.02 | 3.82 | Ref | Ref |
| Systemic ADs | ||||||||||||||||
| AS | 0.62 | 102.78 | 2.75 | 2.73* (1.09–6.84) | 0.12 | 48.87 | 1.31 | 1.23 (0.29–5.14) | 0.25 | 57.31 | 1.53 | 1.37 (0.58–3.28) | 0.24 | 37.35 | Ref | Ref |
| RA | 0.50 | 82.19 | 5.05 | 5.25** (1.79–15.4) | 0.00 | – | – | – | 0.04 | 19.07 | 1.17 | 1.20 (0.29–5.03) | 0.11 | 16.26 | Ref | Ref |
| PSS | 0.50 | 82.19 | 3.74 | 3.77* (1.30–11.0) | 0.12 | 24.40 | 1.11 | 1.07 (0.14–8.06) | 0.45 | 105.35 | 4.79 | 4.50*** (2.34–8.69) | 0.14 | 22.01 | Ref | Ref |
| SLE | 0.74 | 123.47 | 21.52 | 21.31*** (6.26–72.5) | 0.37 | 73.42 | 12.78 | 13.38** (3.10–57.8) | 0.37% | 85.95 | 14.98 | 17.29*** (6.23–48.0) | 0.04 | 5.74 | Ref | Ref |
| SSc | 0.00 | – | – | – | 0.00 | – | – | – | 0.04 | 9.53 | 9.97 | 8.80 (0.74–105) | 0.01 | 0.96 | Ref | Ref |
| Systemc vasculitis | 0.00 | – | – | – | 0.00 | – | – | – | 0.12 | 28.59 | 29.91 | 25.86** (3.70–181) | 0.01 | 0.96 | Ref | Ref |
IR, incidence rate: per 100,000 person year; IRR, incidence rate ratio; adjusted‐HR, adjusted hazards ratio was adjusted by gender, age group; AID, autoimmue disease; AHA, autoimmune hemolytic anemia; AS, ankylosing spondylitis; DM, diabetes mellitus; HSP, Henoch Scholen purpura; PSS, primary Sjogren syndrome; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; Tx, thymectomy; MG, myasthenia gravis.
P < 0.01.
P < 0.001.
P < 0.0001.
For the systemic ADs, the incidence rate of overall systemic ADs was significantly higher in the Tx‐MG and nonTx‐MG cohort than in the nonTx‐nonMG, with an aHR of the Tx‐MG cohort (4.21) and nonTx‐MG cohort (3.51) after adjustment for age and sex. Furthermore, for the respective systemic AIDs, the incidence rate of ankylosing spondylitis and RA was significantly higher in the Tx‐MG than in the nonTx‐nonMG control cohort, with an aHR (2.73 and 5.25, respectively). In addition, the incidence rate of pSS was significantly higher in the Tx‐MG and nonTx‐MG cohort than in the nonTx‐nonMG control cohort, with an aHR of the Tx‐MG cohort (3.77) and nonTxd‐MG (4.50). The incidence rate of SLE was significantly higher in the Tx‐MG, Tx‐noMG, and nonTx‐MG, with an aHR of 21.31, 13.38, and 17.29, respectively. The incidence rate of systemic vasculitis was significantly higher in the nonTx‐MG, with an aHR of 25.86.
Higher incidence of incident autoimmune diseases among Txd patients than non‐Txd controls
For respective ADs, the incidence rate of any type ADs (aHR 2.65, 95% CI: 1.880–3.728, P < 0.001), organ specific ADs (aHR 3.00, 95% CI: 1.761–5.099, P < 0.001) and systemic ADs (aHR 2.56, 95% CI: 1.648–3.982, P < 0.001) were significantly higher in the Txd cohort than in the non‐Txd control cohort, as shown in Table 3. Kaplan–Meier analyses also revealed that patients with Tx had a higher risk for development of ADs (log‐rank test, P < 0.001, Fig. 2).
Table 3.
Incidence rate ratio and hazard rate of autoimmune diseases in patient with and without thymectomy.
|
Event (n/%) (any type ADs) |
Person Year | IR 1 | IRR | Adjusted HR 2 | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐Value | |||||
| Thymectomy | |||||||
| Yes | 39 (17.1) | 8803 | 443.04 | 2.68 | 2.65 | 1.880–3.728 | <0.0001 |
| No | 189 (82.9) | 114,306 | 165.35 | Ref. | Ref. | ||
|
Event (n/%) (organ specifc ADs) |
Person Year | IR 1 | IRR | Adjusted. HR 2 | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐Value | |||||
| Thymectomy | |||||||
| Yes | 17 (7.4) | 8919 | 190.60 | 3.04 | 3.00 | 1.761–5.099 | <0.0001 |
| No | 72 (31.6) | 114,815 | 62.71 | Ref. | Ref. | ||
|
Event (n/%) (systemic ADs) |
Person Year | IR 1 | IRR | Adjusted HR 2 | |||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐Value | |||||
| Thymectomy | |||||||
| Yes | 24 (10.5) | 8866 | 270.69 | 2.59 | 2.56 | 1.648–3.982 | <0.0001 |
| No | 120 (52.6) | 114,578 | 104.73 | Ref. | Ref. | ||
IR, incidence rate; IRR, incidence rate ratio; HR, hazard ratio.
Per 100,000 person year.
Adjusted HR was adjusted by gender, age group.
Figure 2.
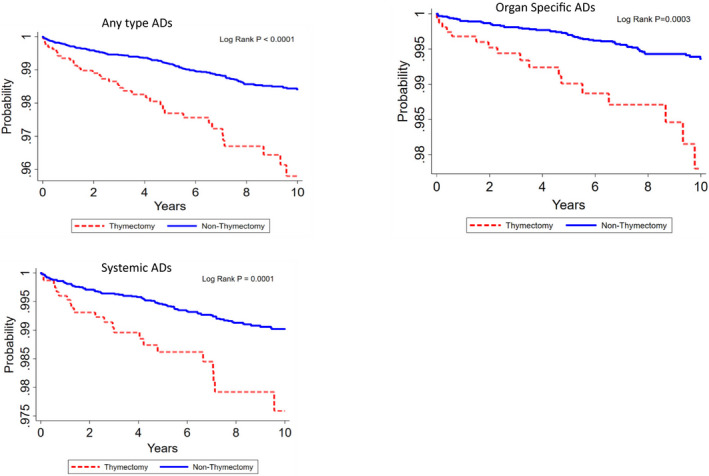
The overall survival of ADs outcomes in Thymectomy and non‐Thymectomy cohorts.
Kaplan–Meier survival of ADs and subgroup ADs in each cohort
Analysis of Kaplan–Meier survival of any type ADs, organ specific ADs and systemic ADs showed that the survival rate of Tx‐MG, Tx‐nonMG, and nonTx‐MG cohort was significantly lower than that of nonTx‐nonMG cohort (P < 0.0001; Fig. 3).
Figure 3.
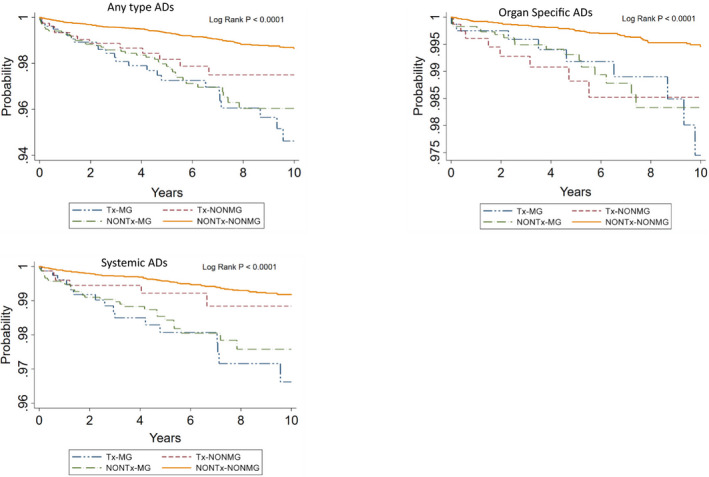
The overall survival of subgroup AIDs outcomes in each cohorts.
Discussion
According to our review of the relevant literature, this study is the first nationwide population‐based work to evaluate the relationship between Tx and ADs. In this study, the overall incidence rate of any type ADs was 2.68 times higher in the Txd cohort than in the non‐Txd cohort, with an aHR of 2.65 after adjustment for age and sex. Furthermore, we found that patients underwent Tx also had an increased risk of organ‐specific and systemic ADs, respectively.
In this study, we found that patients underwent Tx had an increased risk of any type ADs. Tx may exert contrasting effects on the course of distinct autoimmune diseases in humans. 31 Tx may induce clinical deterioration or even create a new disease, suggesting a protective role of the thymus against autoimmunity.
Although the results of Tx vary considerably from one species to another. Experimental animal models may help in understanding the effect of Tx in humans. For example, the extent of T‐cell depletion that follows neonatal Tx can range from moderate reduction of total T‐cell number in sheep to dramatic T‐cell depletion in mice. 32 In addition, the immunologic perturbation after Tx may be dependent on the time in which the gland is surgically removed. Recent studies show that autoreactive T cells, which are able to elicit organ‐specific autoimmune diseases, develop in both the neonatal and adult thymuses, and they persist in the normal peripheral immune system. 33 In adult mice these autoreactive T cells are under the control of suppressor T cells, which maintain peripheral tolerance. 33 , 34 When the clonal balance of these T cells is tipped in favor of pathogenic T cells, autoimmune disease could ensue. Thus autoimmunity may be induced by Tx in adults as a result of a T‐cell imbalance of autoreactive and regulatory T cells. In animal models of autoimmune disorder, surgical removal of the thymus can be associated with prevention or improvement of the autoimmune diseases. 31 , 35 More often, however, Tx is associated with induction or acceleration of the autoimmune process. 36
In humans, long‐term Txd MG patients display mild T‐cell lymphopenia, which is associated with hypergammaglobulinemia and evidence of B‐cell hyperreactivity. In addition, many of these patients have high titers of a variety of autoantibodies, including anti‐dsDNA and anti‐cardiolipin antibodies. 1
Our previous study revealed an association between MG and incident autoimmune rheumatic diseases. The MG cohort who underwent Tx had an increased risk of RA, pSS, and SLE. 23 The present study further confirmed such cohort increased more spectrum ADs. ADs mainly develops after the onset of MG, whereas occurrence of MG after SLE has been described only sporadically. 16 These observations seem to support the concept that MG, as an organ‐specific autoimmune disease, may predispose to the development of systemic autoimmunity. However, this study showed higher risk of ADs in Txd patients with MG compared with non‐Txd patients with MG. Furthermore, the Tx‐nonMG cohort also had higher risk compared to nonTx‐nonMG. These observation suggest an important role of the Tx, rather than the MG.
The therapeutic role of Tx is proved in MG even if the exact mechanism underlying its effect remains largely unknown. However, in recent years, evidences emerged of systemic autoimmune disorders including SLE, Hashimoto's thyroiditis, cutaneous vasculitis, and antiphospholipid syndrome occurring many years after Tx in patients with MG or other immunological diseases 1 seem to be consistent with this hypothesis.
This study showed that Tx in patients with MG or without MG both increase the risk of SLE. The proof that Tx can facilitate the development of SLE can be traced in the cases reported by the literature. 37 Txd patients revealed T‐helper subset and T‐helper/T‐suppressor ratio decreased. Suppressor cell function was also low. The authors proposed that Tx decreases the number and function of some lymphocyte subpopulations. Tx may be followed by the development of autoimmune diseases such as SLE. The possibility of secondary autoimmune pathologies following Tx should be highly considered.
In addition, in animal models, the thymus has been demonstrated to be necessary for development of regulatory T cells (Tregs) and continued postnatal production is required to prevent autoimmunity. 38 The impact of incidental Tx and maintenance of functional Treg is unknown. Halnon et al, 39 found that there are long‐term immune effects after cardiothoracic surgeries and incidental Tx. While total Treg number is maintained, homeostasis of this population is affected with unclear affects on autoimmunity. Treg cells are a subset of CD4+ T cells that maintain self‐tolerance by suppressing autoreactive lymphocytes; Defects in Treg cells, or a lack of Treg cells, are therefore thought to contribute to SLE pathogenesis. 40
The strengths of the present study include large sample size, a large validation cohort, and the long‐term ascertainment of concurrent autoimmune diseases. However, the present study has some limitations. First, although the Bureau of NHI routinely and randomly checks patient charts to ensure the quality of claims from all medical institutions, the possibility of miscoding or misclassification cannot be completely ruled out. However, such bias would apply to both Tx and control cohorts, and therefore the present findings are expected to underestimate, rather than overestimate, the magnitude of the association between Tx and AIDs. Second, the relationship between disease activity and the severity of AIDs and Tx could not be analyzed. Third, although we employed many methods to prevent potential confounders, there is still a likelihood of unmeasurable bias due to the observational nature of our study design. Fourth, some important information regarding laboratory or clinical data was not readily available in the administrative database, namely ADs subtype data. Additional studies are warranted to explore this association. Finally, all thymomas are malignant. Although the ICD codes with "benign Tumors" of the thymus and some of this is a hangover from old terminology.
In conclusion, although Tx as a treatment of autoimmune diseases other than MG may be effective, our findings support the view that this surgical option may be a precipitating factor for other autoimmune diseases, such as SLE. Further investigations will need to elucidate the effects of Tx on the immune system in humans. Nonetheless, the possibility that novel autoimmune diseases emerge following Tx cannot be ignored.
Author Contributions
Tzu‐Min Lin and Jin‐Hua Chen contributed to study conception and design, article drafting, critical article revision for crucial intellectual content, and the final approval of the submitted version. Sheng‐Hung, Lin and Yu‐Sheng Chang contributed to data interpretation, critical article revision for crucial intellectual content, and the final approval of the submitted version. Wei‐Sheng Chen, Pei‐i Kuo, Tsung‐Yun Hou, and Hui‐Ching Hsu contributed to data analysis, critical article revision for crucial intellectual content, and the final approval of the submitted version. Yi‐Chun Lin contributed to data analysis, article drafting, and the final approval of the submitted version. Chi‐Ching Chang was responsible for study conception and design, complete data analysis, critical article revision for crucial intellectual content, and correspondence for the final approval of the submitted version.
Conflict of Interests
All authors declare no conflicts of interest.
Supporting information
Data S1. Causes of thymectomy.
Funding Information
This study did not receive any funding.
References
- 1. Gerli R, Paganelli R, Cossarizza A, et al. Long‐term immunologic effects of thymectomy in patients with myasthenia gravis. J Allergy Clin Immunol 1999;103:865–872. [DOI] [PubMed] [Google Scholar]
- 2. Omar HA, Alzahrani MA, Al Bshabshe AA, et al. Systenic lupus erythematosus after thymectomy for myastbenia gravis: a case report and review of the literature. Clin Exp Nephrol 2010;14:272–276. [DOI] [PubMed] [Google Scholar]
- 3. Bagavant H, Thompson C, Ohno K, et al. Differential effect of neonatal thymectomy on systemic and organ‐specific autoimmune disease. Int Immunol 2002;14:1397–1406. [DOI] [PubMed] [Google Scholar]
- 4. Tellez‐Zenteno JF, Remes‐Troche JM, Mimenza‐Alvarado A, et al. The association of myasthenia gravis and connective tissue diseases. Effects of thymectomy in six cases with rheumatoid arthritis and one case with systemic lupus erythematosus. Neurologia 2003;18:54–58. [PubMed] [Google Scholar]
- 5. Lewis RA, Selwa JF, Lisak RP. Myasthenia gravis: immunological mechanisms and immunotherapy. Ann Neurol 1995;37:51–62. [DOI] [PubMed] [Google Scholar]
- 6. Oosterhuis H. Observations of the natural history of myasthenia gravis and effect of thymectomy. Ann N Y Acad Sci 1981;377:678–684. [DOI] [PubMed] [Google Scholar]
- 7. Rowland LP. General discussion on therapy in myasthenia gravis. Ann N Y Acad Sci 1987;505:607–609. [DOI] [PubMed] [Google Scholar]
- 8. Olanow CW, Wechsler AS, Sirotkin‐Roses M, et al. Thymectomy as primary therapy in myasthenia gravis. Ann N Y Acad Sci 1987;505:595–606. [DOI] [PubMed] [Google Scholar]
- 9. Perlo VP, Arnason B, Castleman B. The thymus gland in elderly patients with myasthenia gravis. Neurology 1975;25:294–295. [DOI] [PubMed] [Google Scholar]
- 10. Alarcon‐Segovia D, Gallbraith RF, Maldonado JE. Systemic lupus erythematosus following thymectomy for myasthenia gravis: report of two cases. Lancet 1963;2:662–665. [DOI] [PubMed] [Google Scholar]
- 11. Simpson JA. Immunological disturbances in myasthenia gravis with a report of Hashimoto’s disease developing after thymectomy. J Neurol Neurosurg Psychiatry 1964;27:485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gallbraith RF, Summerskill WHJ, Murray J. Systemic lupus erythematosus, cirrhosis and ulcerative colitis after thymectomy for myasthenia gravis. N Engl J Med 1964;278:229–232. [DOI] [PubMed] [Google Scholar]
- 13. Mizon JP, Morcamp D, Lefebvre P, et al. Associated myasthenia and systemic lupus erythematosus: a report of two cases and review of the literature. Ann Med Interne (Paris) 1979;11:489–500. [PubMed] [Google Scholar]
- 14. Calabrese LH, Bach JF, Currie T. Development of systemic lupus erythematosus after thymectomy for myasthenia gravis. Studies of suppressor cell function. Arch Intern Med 1981;141:253–255. [PubMed] [Google Scholar]
- 15. Szobor A. Benefit of thymectomy in immune diseases other than myasthenia. Lancet 1984;1:277–278. [DOI] [PubMed] [Google Scholar]
- 16. Mevorach D, Perrot S, Buchanan NM, et al. Appearance of systemic lupus erythematosus after thymectomy: four case reports and review of the literature. Lupus 1995;4:33–37. [DOI] [PubMed] [Google Scholar]
- 17. Shoenfeld Y, Lorber M, Yucel T, Yazici H. Primary antiphospholipid syndrome emerging following thymectomy for myasthenia gravis: additional evidence for the kaleidoscope of autoimmunity. Lupus 1997;6:474–476. [DOI] [PubMed] [Google Scholar]
- 18. Chang CC, Lin TM, Chang YS, et al. Thymectomy in patients with myasthenia gravis increases the risk of autoimmune rheumatic diseases: a nationwide cohort study. Rheumatology (Oxford) 2019;58:135–143. [DOI] [PubMed] [Google Scholar]
- 19. Cheng TM. Taiwan’s National Health Insurance system: high value for the dollar In: Okma KGH. and Crivelli L, eds. Six countries, six reform models: the health reform experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. pp. 71–204. Taipei, NJ: World Scientific, 2009. [Google Scholar]
- 20. Chang CC, Chang YS, Chen WS, et al. Effects of annual influenza vaccination on morbidity and mortality in patients with systemic lupus erythematosus: a nationwide cohort study. Sci Rep 2016;6:37817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 22. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–324. [DOI] [PubMed] [Google Scholar]
- 23. American Rheumatism Association Diagnostic and Therapeutic Criteria Committee . Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980;23:581–590. [DOI] [PubMed] [Google Scholar]
- 24. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American‐European Consensus Group. Ann Rheum Dis 2002;61:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403–407. [DOI] [PubMed] [Google Scholar]
- 26. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–347. [DOI] [PubMed] [Google Scholar]
- 27. Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122–1128. [DOI] [PubMed] [Google Scholar]
- 28. Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener’s granulomatosis. Arthritis Rheum 1990;33:1101–1107. [DOI] [PubMed] [Google Scholar]
- 29. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–1134. [DOI] [PubMed] [Google Scholar]
- 30. Kuo CF, Grainge MJ, Valdes AM, et al. Familial aggregation of systemic lupus erythematosus and coaggregation of autoimmune diseases in affected families. JAMA Intern Med 2015;175:1518–1526. [DOI] [PubMed] [Google Scholar]
- 31. Sherer Y, Bar‐Dayan Y, Shoenfeld Y. Thymectomy and autoimmune diseases. The dual connection. Semin Clin Immunol 1997;1:5–11. [Google Scholar]
- 32. Miller JFAP, Osoba D. Current concept of the immunological function of the thymus. Physiol Rev 1967;47:437–520. [DOI] [PubMed] [Google Scholar]
- 33. Tungs KS. Mechanism of self‐tolerance and events leading to autoimmune disease and autoantibody response. Clin Immunol Immunopathol 1994;73:275–282. [DOI] [PubMed] [Google Scholar]
- 34. Tungs KS. Elucidation of autoimmune disease mechanism based on testicular and ovarian autoimmune disease models. Horm Metab Res 1995;27:539–543. [DOI] [PubMed] [Google Scholar]
- 35. Tsuchiya M, Asakura H, Yoshimatsu H. Thymic abnormalities and autoimmune diseases. Keito J Med 1989;38:383–402. [DOI] [PubMed] [Google Scholar]
- 36. Bonomo A, Kehn PJ, Shevach EM. Post‐thymectomy autoimmunity: abnormal T‐cell homeostasis. Immunol Today 1995;16:61–67. [DOI] [PubMed] [Google Scholar]
- 37. Le Loet X, Pouyol F, Lees O, et al. Acute systemic lupus erythematosus after thymectomy for myasthenia. Sequential study of lymphocyte subpopulations. Rev Med Interne 1986;7:425–432. [DOI] [PubMed] [Google Scholar]
- 38. Itoh M, Takahashi T, Sakaguchi N, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self‐tolerance. J Immunol 1999;162:5317–5326. [PubMed] [Google Scholar]
- 39. Halnon NJ, Cooper P, Chen DY, et al. Immune dysregulation after cardiothoracic surgery and incidental thymectomy: maintenance of regulatory T cells despite impaired thymopoiesis. Clin Dev Immunol 2011;91:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scheinecker C, Bonelli M, Smolen JS. Pathogenetic aspects of systemic lupus erythematosus with an emphasis on regulatory T cells. J Autoimmun 2010;35:269–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Causes of thymectomy.


