Abstract
Introduction
OnabotulinumtoxinA (BoNT-A) was proved effective and safe in chronic migraine (CM) prevention by the Phase III Research Evaluating Migraine Prophylaxis (PREEMPT) and Phase IV Chronic migraine OnabotulinuMtoxinA Prolonged Efficacy open-Label (COMPEL) trials over 1 and 2 years of treatment, respectively. Real-life studies highlighted BoNT-A sustained benefits up to 3 years of administration. Aim of this retrospective real-life study was observing within a 4-year timeframe the progress of a consecutive series of CM patients treated with BoNT-A and evaluating whether long-term quarterly treatment (up to 16 cycles) confirms the outcomes of previous studies over shorter periods of therapy.
Methods
One hundred nine chronic migraineurs were quarterly treated with BoNT-A according to the PREEMPT paradigm. Headache days and hours, analgesics intake and latency time together with disability were analysed at baseline, thereafter bi-annually up to 48 months. Patient responsiveness (improvement in monthly headache days and hours versus baseline) was computed at each study timepoint.
Results
A significant overall decrease from baseline to the 48-month assessment (p < 0.001) was evidenced for the mean number of monthly headache days and hours, analgesics intake and latency time. Severe disability cases significantly decreased at 6 months (p < 0.001), and a progressive shift towards lower degrees of disability was observed at each subsequent timepoint. A gradual percentage increase of responsive cases was observed as treatment was repeated over time. Transitory neck pain was reported in 6 cases.
Conclusions
This study appears to reconfirm the benefits of long-lasting CM prevention with BoNT-A, thus supporting quarterly treatment with BoNT-A over several year.
Keywords: Chronic migraine, Headache, OnabotulinumtoxinA, Long-term treatment, Real-life, Botox®
Introduction
Chronic migraine (CM) is a complex neurological disorder constituting one of the ten leading causes of disability worldwide [1]. Each year approximately 2.5% of patients with episodic migraine develop new onset CM [2]. Compared with episodic migraineurs, CM patients more likely suffer also from depression, anxiety and other co-morbidities [3], which can act on migraine incrementing disability and an uncontrolled multiple need of analgesics, thus leading to medication overuse headache (MOH) as a complication of CM [4]. Abortive medications are overused in around 50–80% of chronic migraineurs [5] and are associated with a greater risk of adverse events (AE) [6, 7]. Therefore, preventing CM is crucial not only to lessen the frequency and severity of migraine attacks, but also to reduce the need of analgesics.
The efficacy and safety of onabotulinumtoxinA (BoNT-A) for the prevention of CM was established in the PREEMPT trials [8–11] demonstrating that repeated treatment with BoNT-A (≤ 5 injection cycles) could safely produce significant improvements in headache symptoms, analgesics intake and health-related quality of life in chronic migraineurs. Thereafter the phase IV COMPEL study expanded these results in migraineurs who received up to nine BoNT-A administrations over 2 years [12].
Clinical trial findings have been corroborated by several real-world studies. The efficacy of quarterly repeated BoNT-A cycles was shown beyond the first year of treatment in chronic migraineurs with MOH [13]. We proved the efficacy and safety of six quarterly cycles over a period of 18 months in 47 patients with CM, highlighting that efficacy significantly increases upon repeated administration [14]. Other real-life studies confirmed BoNT-A safety and effectiveness for periods of two [15–19] and three [20, 21] years, evidencing the sustained therapeutic benefits associated with long-term treatment. Moreover, a general worsening of patient conditions was reported upon abrupt discontinuation of treatment [13] or prolongation of the inter-injection time interval [22]. And as a result, BoNT-A is considered a long-lasting therapy for CM prevention by a remarkable number of clinicians [23].
With this in mind, we conducted the present retrospective study to analyse in real-life within a 4-year timeframe the progress of a cohort of chronic migraineurs regularly treated with BoNT-A and assess whether long-term term quarterly treatment (up to 16 cycles) reaffirms the sustained benefits and good tolerability reported by previous studies over shorter treatments.
Methods
Patients and study design
From March 2013 to April 2019 a total of 399 patients with CM received at least one cycle of BoNT-A at our centre, Fondazione IRCSS Casa Sollievo della Sofferenza, San Giovanni Rotondo, S.C. di Neurologia. Among the whole patient population, this retrospective study includes the consecutive series of patients who received the first treatment by 31 March 2015, thus potentially having a 4-year timeframe for treatment and follow-up when database was locked (31 March 2019). Included patients were adult males and females diagnosed as chronic migraineurs [24] with or without medication overuse, had tried and failed other migraine prophylactics, had received at least one BoNT-A treatment and had at least one usable post-treatment assessment. Exclusion criteria were pregnancy or breastfeeding, symptoms of psychiatric disease and any history of botulinum toxin use for other clinical purposes. To fully reflect a real-life population, patients receiving any preventive or symptomatic therapy were not excluded from this analysis.
The study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of IRCCS Istituto Tumori Giovanni Paolo II of Bari at Fondazione IRCCS Casa Sollievo della Sofferenza of San Giovanni Rotondo ICF: VI.0_07 APR 2015. Each patient signed an informed consent for the analysis and publication of data.
All data entered in the database were double-checked vs paper records to ensure all entries were correct and up-to-date. Phone interviews were attempted on patients who had discontinued treatment for unknown reasons.
Treatment
BoNT-A (BOTOX®; Allergan plc, Ireland) was administered every 3 months (± 10 days) in a day-hospital setting, following the PREEMPT paradigm (155–195 U, in 31–39 sites) [25]. In presence of CM with medication overuse, as defined by ICHD 3rd edition [24], detoxification with intramuscular betamethasone for 6 days was performed alongside the start of BoNT-A prophylactic treatment. After 15 days from detoxification patients were advised to take a maximum of 2 dosage units of NSAIDs (nonsteroidal anti-inflammatory drugs) per week.
Clinical assessment and outcome measures
The number of monthly headache days, cumulative hours of headache in headache days, abortive medication intakes (total number of dosage units) and related latency time (in hours) were daily recorded by patients on headache diary, then evaluated by the investigator at each quarterly visit. Patient quality of life, expressed as migraine-related disability, was evaluated using the Migraine Disability Assessment (MIDAS) questionnaire [26] that was administered to the patient at baseline and at each quarterly visit to explore disability during the previous 3 months. Baseline values for all parameters were referred to the month preceding the initiation of BoNT-A treatment.
Statistical analyses
All subjects that received first treatment by 31 March 2015 and have at least one usable post-treatment assessment were included in the analysis. Subjects who discontinued treatment or were lost to follow-up were included up to the point of treatment discontinuation or last known post-treatment assessment.
Outcome measures were analysed bi-annually until the 48-month visit (i.e. after 16 injection cycles), in terms of changes in monthly days and hours of headache, acute medication consumption and latency time, and MIDAS grade distribution with respect to the baseline visit (T0) and to each previous timepoint. At the same timepoints patient responsiveness to BoNT-A treatment, expressed as percentage reduction of the number of headache days and hours with respect to baseline, was also computed to analyse over time patient distribution across four groups of increasing responsiveness, ranging from non-responders (< 30% reduction) to partial responders (≥30 and < 50% reduction), responders (≥ 50% and ≤ 75% reduction) and high responders (> 75% reduction), as previously described [14]. All continuous variables were expressed as mean ± standard deviation (SD), median along with interquartile range (IQR) and range (minimum-maximum), whilst categorical variables were expressed as frequencies and percentages. Changes of outcome measures and patients’ responsiveness over time were assessed by hierarchical generalised linear models (HGLMs) which included the follow-up time as main covariate, assuming Poisson and binomial distribution for continuous and categorical outcomes, respectively. The first-order autoregressive covariance structure was used to account for the correlation between repeated measurements over time. Estimated means (or percentages for categorical variables) were carried out from HGLMs and were reported along with their 95% confidence interval (95% CI), including the follow-up time variable into HGLMs as categorical covariate. The overall difference over time was assessed by looking at the significance of the type III test, whereas pairwise comparisons were assessed as statistical contrasts and p values were adjusted following Hochberg’s step-up procedure. The presence of linear trend was assessed by looking at the statistical significance of the regression coefficient estimated for the follow-up time variable when this was included into the HGLMs as continuous covariate. Longitudinal plots of the estimated means over time and histograms of the estimated percentages were further reported, along with error bars which represented 95% CI. Two-sided p values < 0.05 were considered for statistical significance. All analyses were performed using SAS Software, Release 9.4 (SAS Institute, Cary, NC, USA), and plots were produced by the computing environment R (R Development Core Team 2008, version 3.6, packages: ggplot2, gridExtra).
Results
Demographics, discontinuations and drop-outs
A consecutive series of 125 subjects started BoNT-A treatment by 31 March 2015. Sixteen patients quitted just after the first treatment. We tracked down twelve of them by phone and found that the reasons for discontinuation were concurrent pathologies preventing patient from coming back to our centre, relocation to another town, discomfort with forehead immobility or number of injections, and reclassification of migraine as secondary. Since no post-treatment assessment was available for these 16 patients, they were excluded for the present analysis as in no way they could contribute to achieving the study objective. Therefore, our study cohort consisted of 109 patients (82 females, 75.2%) with a mean age of 48.1 ± 13.5 years (range 18–76). Symptomatic drug withdrawal cycles were attempted in 20 patients (18.3%) alongside the start of BoNT-A treatment. Patient characteristics at baseline are summarised in Table 1. Within the study cohort BoNT-A treatment was stopped based on clinical judgement in 43 cases who reached the headache frequency threshold we had established for discontinuation. This was ≤ 4 headache days per month for 3 months in the beginning. Thereafter, we decided to set a higher threshold (≤ 4 headache days for 12 months) in order to prevent the risk of relapse after treatment interruption. Worsening was actually reported by 12 patients who came back to our centre after a time period ranging from 5 to 48 months from the interruption. Several discontinuations summarily defined as “lost to follow-up” in our previous study [14] could be better described after the mentioned database audit and phone interviews, which enabled us to identify the reason for discontinuation in a number of cases, as shown in Table 2. Overall, 29 patients were lost to follow-up, resulting in a study drop-out of 29/109 (26.6%). However, twenty-six of them had achieved a reduction of headache days ≥ 50% at the last available follow-up with respect to baseline, whilst 2 (1.8%) were not responding to BoNT-A treatment. Patient disposition through the study is showed in Fig. 1, whilst all treatment discontinuations that occurred before the 16th cycle are outlined in Table 2.
Table 1.
Demographic details of study cohort at baseline (109 patients)
| Age (years) |
Mean ± SD Median (IQR) Range |
48.1 ± 13.5 48 (39–58) 18–76 |
| Gender (females) | n (%) | 82 (75.2) |
| Years of chronic headache |
Mean ± SD Median (IQR) Range |
11.7 ± 9.8 10 (5–15) 1–60 |
| Patients assuming NSAIDs | n (%) | 72 (66.1) |
| Patients assuming triptans | n (%) | 46 (42.2) |
| Patients assuming other drugs | n (%) | 36 (33.0) |
| Patients assuming other preventive treatments | n (%) | 47 (43.1) |
SD, standard deviation; IQR, interquartile range (i.e. first-third quartiles); NSAIDS, nonsteroidal anti-inflammatory drugs
Table 2.
Detailed patient disposition within study cohort (109 patients) with respect to treatment discontinuation over time
| Assessment | T0 | T6 | T12 | T18 | T24 | T30 | T36 | T42 | T48 | ||
|
Ongoing patients n (%) |
109 |
101 (92.7) |
84 (77.1) |
50 (45.9) |
38 (34.9) |
33 (30.3) |
27 (24.8) |
22 (20.9) |
22 (20.2) |
||
| Total | |||||||||||
|
Discontinuers n (%) |
8 (7.3) |
17 (15.6) |
34 (31.2) |
12 (11.0) |
5 (4.6) |
6 (5.5) |
5 (4.6) |
87 (79.8) |
|||
| Reasons for discontinuation, n (%) |
Achievement of threshold set by clinician n (%) |
6 (5.5) |
19 (17.4) |
9 (8.3) |
1 (0.9) |
3 (2.8) |
5 (4.6) |
43 (39.4) |
|||
|
Pregnancy n (%) |
1 (0.9) |
1 (0.9) |
1 (0.9) |
3 (2.8) |
|||||||
|
Concurrent pathology n (%) |
1 (0.9) |
3 (2.8) |
4 (3.7) |
||||||||
|
Improvement not in line with patient expectations n (%) |
1 (0.9) |
2 (1.8) |
1 (0.9) |
4 (3.7) |
|||||||
|
Satisfactory status perceived by patient n (%) |
1 (0.9) |
1 (0.9) |
|||||||||
|
Financial limitations n (%) |
1 (0.9) |
1 (0.9) |
|||||||||
|
Secondary migraine n (%) |
1 (0.9) |
1 (0.9) |
|||||||||
|
Lack of efficacy n (%) |
1 (0.9) |
1 (0.9) |
|||||||||
|
Lost to FU (responders and high responders at the last available FU) n (%) |
5 (4.6) |
7 (6.4) |
9 (8.3) |
2 (1.8) |
1 (0.9) |
2 (1.8) |
26 (23.9) |
||||
|
Lost to FU (partial responders at the last available FU) n (%) |
1 (0.9) |
1 (0.9) |
|||||||||
|
Lost to FU (non-responders at the last available FU) n (%) |
1 (0.9) |
1 (0.9) |
2 (1.8) |
||||||||
Percentages were calculated on the total of 109 patients. “High responders” (> 75% reduction), “responders” (≥ 50 and ≤ 75% reduction), “partial responders” (≥ 30 and < 50% reduction) and “non-responders” (< 30% reduction)
Fig. 1.
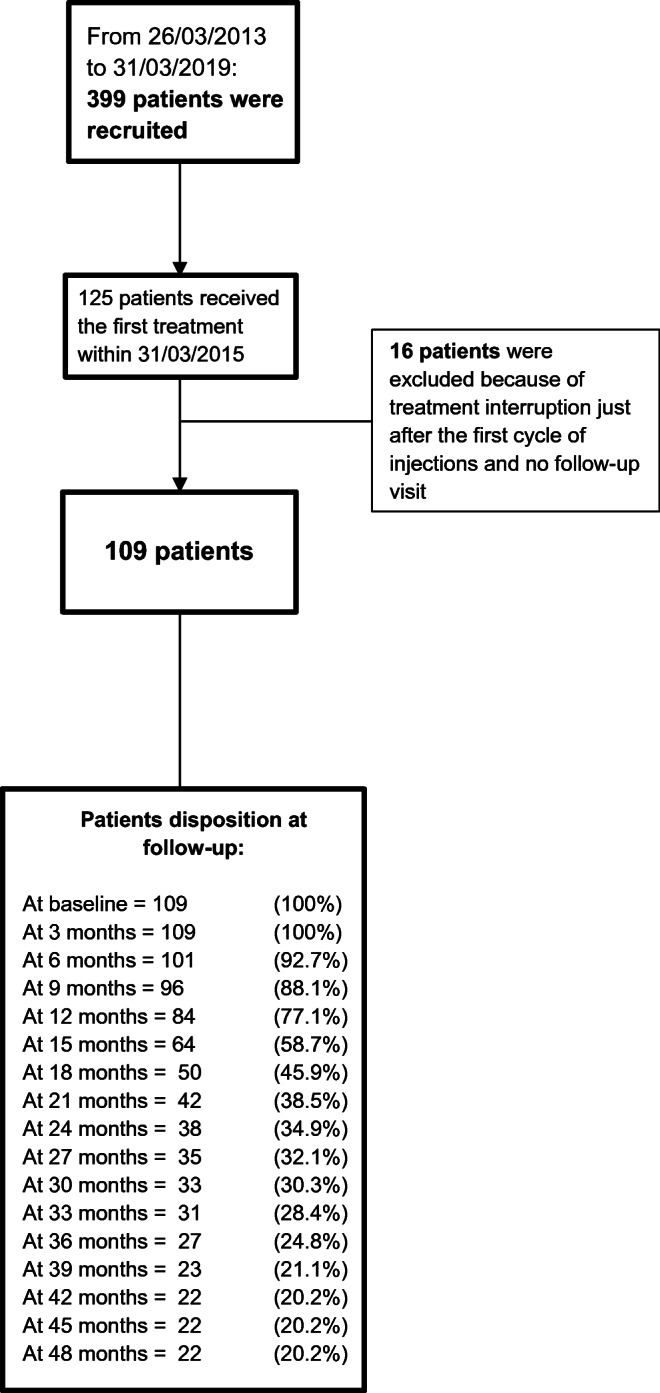
Flow diagram reporting the numbers of individuals at each stage of the study
Clinical outcomes
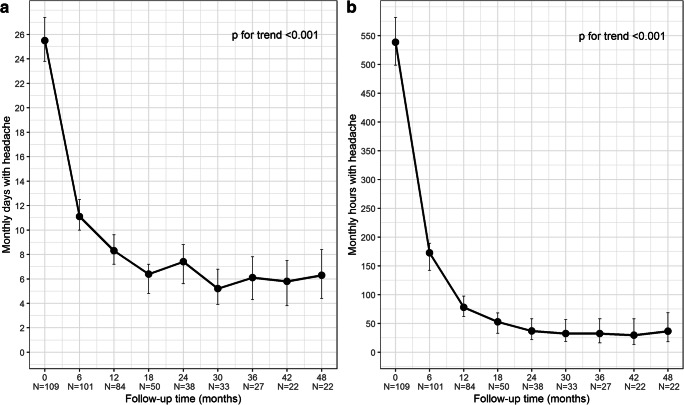
Mean numbers of headache days and hours per month at each individual timepoint are reported in Table 3. Mean number of monthly headache days showed a significant overall decrease (p for trend < 0.001) from 25.5 ± 5.8 at baseline assessment (T0) to 6.3 ± 3.3 at the 48-month assessment (T48) after the 16th BoNT-A cycle. A statistically significant decrease of monthly headache days was observed at the 6-month assessment (T6) vs T0 (p < 0.001), at 12 months (T12) vs T6 (p < 0.001) and at 18 months (T18) vs T12 (p = 0.004). Thereafter, the mean number of monthly headache days at each individual timepoint was always lower than T0, but not systematically lower than previous timepoint, and pairwise comparisons (each timepoint vs previous one) did not reveal statistically significant differences. Similar changes were observed for the mean number of monthly hours with headache, which significantly decreased over time from 538.6 ± 176.1 at T0 to 36.4 ± 29.0 at T48 (p for trend < 0.001) and showed statistically significant pairwise comparisons at the same timepoints, i.e. T6 vs T0 and T12 vs T6 (p < 0.001), and T18 vs T12 (p = 0.034). At each timepoint, the mean number of monthly hours with headache was always lower than T0 and lower than previous timepoint, with the only exception of T48 vs the 42-month assessment (T42). Plots of the estimated means of monthly headache days and hours over time are shown in Fig. 2a, b, respectively.
Table 3.
Monthly headache days and hours, from baseline (T0) up to 48 months (T48), at 6-month time intervals
|
T0 (n = 109) |
T6 (n = 101) |
T12 (n = 84) |
T18 (n = 50) |
T24 (n = 38) |
T30 (n = 33) |
T36 (n = 27) |
T42 (n = 22) |
T48 (n = 22) |
||
| Monthly days with headache | n | 109 | 101 | 84 | 50 | 38 | 33 | 27 | 22 | 22 |
| Mean ± SD | 25.5 ± 5.8 | 11.1 ± 8.6 | 8.3 ± 6.5 | 6.4 ± 5.6 | 7.4 ± 5.2 | 5.2 ± 4.0 | 6.1 ± 3.0 | 5.8 ± 2.5 | 6.3 ± 3.3 | |
| 95% CI | 23.6–27.4 | 10.0–12.5 | 7.2–9.6 | 4.8–7.2 | 5.6–8.8 | 3.9–6.8 | 4.3–7.8 | 3.8–7.5 | 4.4–8.4 | |
| Median (IQR) | 30 (20–30) | 9 (4–16) | 6.5 (3–12) | 5 (3–8) | 6 (4–10) | 4 (3–6) | 5 (4–8) | 5.5 (4–7) | 6 (4–10) | |
| Range | 15–30 | 0–30 | 0–30 | 0–30 | 0–25 | 0–18 | 2–12 | 1–11 | 0–11 | |
| Monthly hours with headache | n | 109 | 101 | 84 | 50 | 38 | 33 | 27 | 22 | 22 |
| Mean ± SD | 538.6 ± 176.1 | 162.8 ± 179.0 | 78.0 ± 91.3 | 52.8 ± 77.2 | 36.8 ± 26.5 | 32.4 ± 34.0 | 32.3 ± 23.5 | 29.7 ± 20.4 | 36.4 ± 29.0 | |
| 95% CI | 498.6–581.6 | 142.0–189.4 | 62.1–97.6 | 32.7–68.1 | 21.8–58.1 | 18.5–56.7 | 16.4–58.1 | 13.3–58.0 | 18.2–68.8 | |
| Median (IQR) | 600 (400–700) | 100 (30–200) | 42.5 (20–100) | 25 (15–50) | 32.5 (15–48) | 20 (10–48) | 25 (12–48) | 27.5 (10–45) | 34 (8–60) | |
| Range | 112–720 | 0–700 | 0–400 | 0–350 | 0–100 | 0–150 | 0–88 | 4–77 | 0–100 | |
| p values from HGLM and pairwise comparisons* | ||||||||||
| p for overall difference | T6 vs T0 | T12 vs T6 | T18 vs T12 | T24 vs T18 | T30 vs T24 | T36 vs T30 | T42 vs T36 | T48 vs T42 | p for trend | |
| Monthly days with headache | < 0.001 | < 0.001 | < 0.001 | 0.004 | 0.507 | 0.111 | 0.641 | 0.641 | 0.641 | < 0.001 |
| Monthly hours with headache | < 0.001 | < 0.001 | < 0.001 | 0.034 | 0.885 | 0.885 | 0.885 | 0.885 | 0.885 | < 0.001 |
SD, standard deviation; CI, confidence interval; IQR, interquartile range (i.e. first-third quartiles); *p values from hierarchical generalised linear model (HGLM) and adjusted following Hochberg’s step-up procedure
Fig. 2.
Plots of monthly headache days (a) and hours (b) means at baseline (T0) up to 48 months (T48), at 6-month time intervals. Error bars represent 95% confidence interval around estimated means
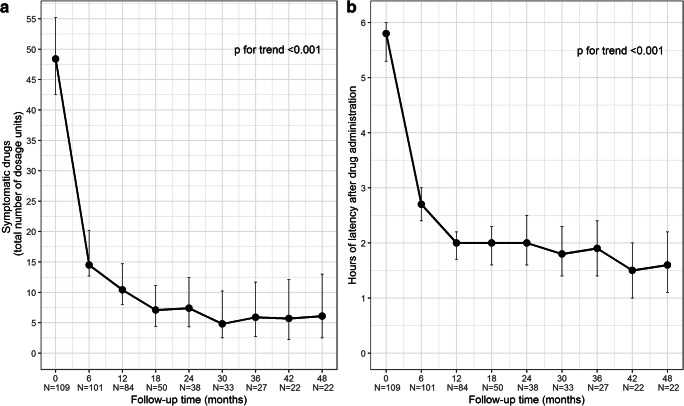
In line with the above outcomes, the consumption of painkillers (number of dosage units) significantly dropped over time (p for trend < 0.001) from a mean of 48.4 ± 46.4 at T0 to a mean of 6.1 ± 4.1 at T48, showing the sharpest decrease at T6 vs T0 (p < 0.001) and another significant decrease at T12 vs T6 (p = 0.028). Alongside consumption reduction, analgesics latency time (hours) significantly shrunk from a mean of 5.8 ± 0.9 at T0 to 2.7 ± 1.8 at T6 and 2.0 ± 1.6 at T12 (T6 vs T0 and at T12 vs T6 p < 0.001), revealing a significant trend over the entire period under consideration (p for trend < 0.001). The changes of analgesics intake and drug response latency time over follow-up are summarised in Table 4 and plotted in Fig. 3a, b, respectively.
Table 4.
Painkiller consumption (number of dosage units) and latency time after intake (hours), from baseline (T0) up to 48 months (T48), at 6-month time intervals
|
T0 (n = 109) |
T6 (n = 101) |
T12 (n = 84) |
T18 (n = 50) |
T24 (n = 38) |
T30 (n = 33) |
T36 (n = 27) |
T42 (n = 22) |
T48 (n = 22) |
||
| Symptomatic drugs (number of dosage units) | n | 109 | 101 | 84 | 50 | 38 | 33 | 27 | 22 | 22 |
| Mean ± SD | 48.4 ± 46.4 | 14.5 ± 22.7 | 10.4 ± 11.6 | 7.1 ± 8.0 | 7.4 ± 6.4 | 4.8 ± 4.8 | 5.9 ± 3.3 | 5.7 ± 3.4 | 6.1 ± 4.1 | |
| 95% CI | 42.5–55.2 | 12.7–20.2 | 8.0–14.7 | 4.4–11.1 | 4.3–12.4 | 2.5–10.2 | 2.7–11.7 | 2.2–12.1 | 2.5–13.0 | |
| Median (IQR) | 34 (20–60) | 7 (3–15) | 7 (3–12.5) | 5 (2–8) | 6.5 (4–10) | 4 (2–6) | 5 (4–8) | 5 (4–8) | 5 (3–10) | |
| Range | 0–210 | 0–140 | 0–70 | 0–32 | 0–34 | 0–24 | 0–13 | 0–15 | 0–13 | |
| Latency time (h) | n | 109 | 101 | 84 | 50 | 38 | 33 | 27 | 22 | 22 |
| Mean ± SD | 5.8 ± 0.9 | 2.7 ± 1.8 | 2.0 ± 1.6 | 2.0 ± 1.7 | 2.0 ± 1.6 | 1.8 ± 1.7 | 1.9 ± 1.6 | 1.5 ± 1.0 | 1.6 ± 1.3 | |
| 95% CI | 5.3–6.0 | 2.4–3.0 | 1.7–2.2 | 1.6–2.3 | 1.6–2.5 | 1.4–2.3 | 1.4–2.4 | 1.0–2.0 (1–2) | 1.1–2.2 | |
| Median (IQR) | 6 (6–6) | 2 (1–4) | 1 (1–2) | 1 (1–2) | 2 (1–3) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | |
| Range | 0–6 | 0–6 | 0–6 | 0–6 | 0–6 | 0–6 | 0–6 | 0–4 | 0–5 | |
| p values from HGLM and pairwise comparisons* | ||||||||||
| p for overall difference | T6 vs T0 | T12 vs T6 | T18 vs T12 | T24 vs T18 | T30 vs T24 | T36 vs T30 | T42 vs T36 | T 48 vs T42 | p for linear trend | |
| Symptomatic drugs | < 0.001 | < 0.001 | 0.028 | 0.229 | 0.865 | 0.865 | 0.865 | 0.865 | 0.865 | < 0.001 |
| Latency time | < 0.001 | < 0.001 | < 0.001 | 0.968 | 0.968 | 0.968 | 0.968 | 0.968 | 0.968 | < 0.001 |
SD, standard deviation; IQR, interquartile range (i.e. first-third quartiles); *p values from hierarchical generalised linear model (HGLM) and adjusted following Hochberg’s step-up procedure
Fig. 3.
Plots of painkillers used (a) and latency time after intake (b) means at baseline (T0) up to 48 months (T48), at 6-month time intervals. Error bars represent 95% confidence interval around estimated means
Patient responsiveness to BoNT-A, in terms of improvement in monthly headache days, showed a progressive percentage increase of responsive cases as treatment was repeated over time, with a significant percentage increase from T6 (80.2%) to T12 (92.9%) (T12 vs T6 p = 0.029), alongside a mirrored progressive reduction of non-responders, which were totally absent from T30 on. When responsiveness was expressed by reduction of headache cumulative hours, total responders accounted for 88.1% of patients at T6, i.e. after two injection cycles, then significantly increased to represent the whole population under treatment at T12, after the 4th cycle (p = 0.002). Distribution of patients into the four responsiveness classes over time is reported in Table 5.
Table 5.
Distribution of patient response to treatment in terms of reduction of number of days and hours with headache at each study timepoint up to 48 months (T48) with respect to baseline (T0)
| Follow-up (months) | p values from HGLM (pairwise comparisons)# | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Responder groups (% reduction vs baseline*) | T6 (n = 101) |
T12 (n = 84) |
T18 (n = 50) |
T24 (n = 38) |
T30 (n = 33) |
T36 (n = 27) |
T42 (n = 22) |
T48 (n = 22) |
T12 vs T6 | T18 vs T12 | T24 vs T18 | T30 vs T24 | T36 vs T30 | T42 vs T36 | T48 vs T42 |
| Days with headache |
< 30% (non-responders) |
20 (19.8) |
6 (7.1) |
2 (4.0) |
2 (5.3) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0.029 | 0.656 | 0.805 | 1.000 | 1.000 | 1.000 | 1.000 |
| ≥ 30–< 50% | 12 (11.9) |
8 (9.5) |
4 (8.0) |
2 (5.3) |
1 (3.0) |
0 (0.0) |
0 (0.0) |
1 (4.5) |
0.749 | 0.749 | 0.749 | 0.749 | 1.000 | 1.000 | 1.000 | |
| ≥ 50–≤ 75% | 32 (31.7) | 33 (39.3) |
15 (30.0) |
16 (42.1) |
10 (30.3) |
13 (48.1) | 10 (45.5) |
10 (45.5) |
0.670 | 0.670 | 0.670 | 0.670 | 0.670 | 1.000 | 1.000 | |
| > 75% | 37 (36.6) | 37 (44.0) | 29 (58.0) | 18 (47.4) | 22 (66.7) | 14 (51.9) | 12 (54.5) | 11 (50.0) | 0.643 | 0.075 | 0.643 | 0.336 | 0.643 | 0.643 | 0.687 | |
|
≥ 30% (all responders) |
81 (80.2) |
78 (92.9) | 48 (96.0) | 36 (94.7) | 33 (100.0) | 27 (100.0) | 22 (100.0) | 22 (100.0) | 0.029 | 0.656 | 0.805 | 1.000 | 1.000 | 1.000 | 1.000 | |
| Hours with headache |
< 30% (non-responders) |
12 (11.9) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0.002§ | – | – | – | – | – | – |
| ≥ 30–< 50% |
9 (8.9) |
3 (3.6) |
0 (0.0) |
1 (2.6) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0.279 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |
| ≥ 50–≤ 75% |
24 (23.8) |
14 (16.7) |
5 (10.0) |
0 (0.0) |
2 (6.1) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0.467 | 0.467 | 1.000 | 0.591 | 1.000 | 1.000 | 1.000 | |
| > 75% | 56 (55.4) | 67 (79.8) | 45 (90.0) | 37 (97.4) | 31 (93.9) | 27 (100.0) | 22 (100.0) | 22 (100.0) | 0.001 | 0.206 | 0.533 | 0.533 | 0.533 | 1.000 | 1.000 | |
|
≥ 30% (all responders) |
89 (88.1) |
84 (100.0) | 50 (100.0) | 38 (100.0) | 33 (100.0) | 27 (100.0) | 22 (100.0) | 22 (100.0) | 0.002§ | – | – | – | – | – | – | |
Number of patients along with column percentages
*This percentage represents the amount of relative responsiveness during the follow-up and was calculated as follows:
[(outcome at baseline − outcome at follow-up)/outcome at baseline] × 100. Based on this percentage, patients were classified as follows:
“Non-responders” (< 30% reduction), “partial responders” (≥ 30 and < 50% reduction), “responders” (≥ 50 and ≤ 75% reduction) and “high responders” (> 75% reduction)
#p values from hierarchical generalised linear models (HGLM) and adjusted following Hochberg’s step-up procedure; §p values from exact McNemar test (HGLMs were not estimable because of degenerate distribution of responders/non-responders over time, i.e. they were all 0% or 100% after the sixth month)
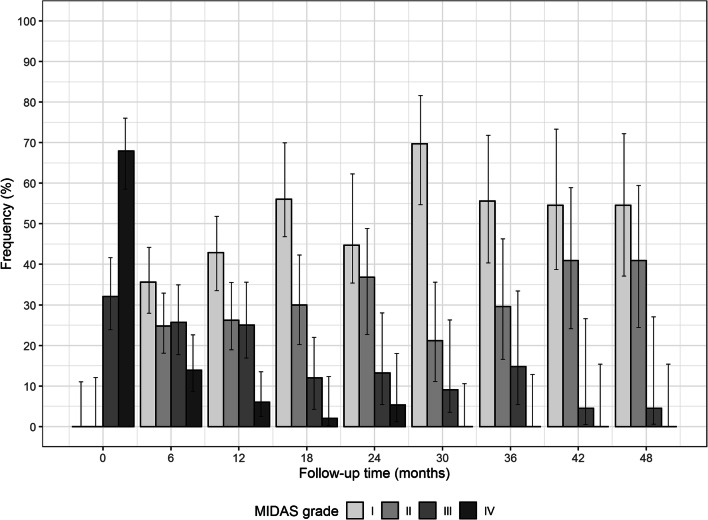
Patient headache-related disability showed a progressive improvement at each timepoint and over the entire 48-month period, as revealed by MIDAS grade distribution over time (Fig. 4). At T0 patients reported a high degree of disability ranging from Grade III, i.e. moderate disability (32.1%), to Grade IV, i.e. severe disability (67.9%), as shown in Table 6. At T6 severe cases significantly dropped to 13.9% (p < 0.001), mild to moderate cases accounted for about half of treated patients, and more than one-third exhibited little or no disability (Grade I, 35.6%). In general, as BoNT-A treatment was repeated over time, a progressive shift towards lower degrees of disability was observed at each subsequent timepoint (p < 0.001 for trend).
Fig. 4.
Frequency distribution for MIDAS grade at baseline (T0) and after 48 months (T48), at 6-month time intervals. Error bars represent 95% confidence interval around estimated percentages
Table 6.
Patient distribution as per MIDAS grades, from baseline (T0) up to 48 months (T48) at 6-month time intervals
|
T0 (n = 109) |
T6 (n = 101) |
T12 (n = 84) |
T18 (n = 50) |
T24 (n = 38) |
T30 (n = 33) |
T36 (n = 27) |
T42 (n = 22) |
T48 (n = 22) |
||
| MIDAS–n (%) | n | 109 | 101 | 84 | 50 | 38 | 33 | 27 | 22 | 22 |
| Grade I | 0 (0.0) | 36 (35.6) | 36 (42.9) | 28 (56.0) | 17 (44.7) | 23 (69.7) | 15 (55.6) | 12 (54.5) | 12 (54.5) | |
| Grade II | 0 (0.0) | 25 (24.8) | 22 (26.2) | 15 (30.0) | 14 (36.8) | 7 (21.2) | 8 (29.6) | 9 (40.9) | 9 (40.9) | |
| Grade III | 35 (32.1) | 26 (25.7) | 21 (25.0) | 6 (12.0) | 5 (13.2) | 3 (9.1) | 4 (14.8) | 1 (4.5) | 1 (4.5) | |
| Grade IV | 74 (67.9) | 14 (13.9) | 5 (6.0) | 1 (2.0) | 2 (5.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| p values from HGLM and pairwise comparisons* | ||||||||||
| p for overall difference | T6 vs T0 | T12 vs T6 | T18 vs T12 | T24 vs T18 | T30 vs T24 | T36 vs T30 | T42 vs T36 | T48 vs T42 | p for linear trend | |
| MIDAS I | 0.002 | 0.085 | 0.586 | 0.085 | 0.586 | 0.085 | 0.586 | 0.990 | 0.990 | < 0.001 |
| MIDAS II | 0.182 | 0.190 | 0.966 | 0.966 | 0.966 | 0.738 | 0.966 | 0.966 | 0.966 | < 0.001 |
| MIDAS III | 0.035 | 0.970 | 0.970 | 0.181 | 0.970 | 0.970 | 0.970 | 0.970 | 0.970 | < 0.001 |
| MIDAS IV | < 0.001 | < 0.001 | 0.083 | 0.358 | 0.358 | 1.000 | 1.000 | 1.000 | 1.000 | < 0.001 |
*p values from hierarchical generalised linear model (HGLM) and adjusted following Hochberg’s step-up procedure
Safety
Few patients (6/109, 5.8%) reported neck pain during the first year of therapy with BoNT-A. This AE was transitory and never caused treatment discontinuation.
Discussion
A successful migraine preventive therapy should reduce the frequency and burden of attacks, thus stopping drug use escalation and improving patient quality of life whilst causing limited side effects. Moreover, improvements should be stable and long-lasting and this benefit should be the most sought-after goal of such therapy. Several studies have been evaluating BoNT-A use in chronic migraineurs since its approval for CM prevention, which was based on PREEMPT results over a 56-week period [11]. PREEMPT findings were then extended by COMPEL study showing persistent clinical benefits during 2 years of therapy [12]. CM prevention with BoNT-A has been further validated by real-world studies reporting a consistently positive trend of improvement beyond the first year of treatment [13], over an 18-month period [14], and over 2 years [15–19]. Two real-life studies on a 3-year treatment [20, 21] reported, on top of good tolerability, continuous improvements of headache frequency [20, 21], migraine severity, headache days with acute medication use [20], pain and analgesics consumption [21], thus supporting the strategy of consistent BoNT-A administration over long time.
A survey revealed that almost 60% of the interviewed clinicians looked at BoNT-A as a long-lasting therapy [23]. This was deemed to reflect practical experience in observing increased benefits over time and rebound worsening upon interruption [27]. A general worsening of patient conditions was indeed reported after therapy discontinuation or prolonged inter-injection time interval even when patient is a stable responder [13, 22]. Nevertheless, the European Headache Federation recommended to stop BoNT-A if patient has less than 10 headache days per month for 3 months [28] and a group of experts suggested to prolong the inter-injection interval based on patient responsiveness [27].
Our study allowed to further explore this highly debated topic. In fact, by observing the progress of a consecutive series of CM patients quarterly treated with BoNT-A within a 4-year timeframe, the study enabled us to evaluate whether the long-term regular treatment (up to 16 injection cycles) confirms the findings of previous studies over shorter periods of therapy.
We assessed BoNT-A efficacy bi-annually over the course of therapy, comparing each timepoint with T0 and previous timepoint. Results confirmed the progressive improvements at each study timepoint compared with T0 for monthly headache days and hours, with an overall significant difference at T48, after the 16th injection session, compared with T0 (p < 0.001). The most striking improvements occurred in the first year, as shown in Fig. 2a and b. It is worth to highlight that the significant drop of monthly headache days and hours observed also at T18, which was consistent with our previous study [14], was followed by a more gradual decline and, eventually, by a condition which appears nearly stable over time. This seems to indicate durable efficacy on BoNT-A even after many repeated administrations, as outlined by other authors after 2 [12, 15–19] or 3 [20, 21] years of therapy.
The overall significant reduction of painkillers consumption and latency time (p < 0.001) seems to confirm that BoNT-A long-term treatment can also successfully address medication overuse, as suggested by our previous study [14]. In fact, at T48 after 16 treatments analgesics intake and latency time are around one-tenth and one-third of those reported at baseline, respectively. Moreover, the remarkable decrease observed in the first 6 to 12 months was followed by gradual reductions with few fluctuations in the subsequent years. In line with the findings of Guerzoni et al. [21] on BoNT-A use in CM with MOH, these results seem to confirm the stability of BoNT-A effect long after the first year of therapy.
Analysis of responsiveness to BoNT-A confirms that chronic migraineurs who do not have the desired response after the first injection cycle may indeed experience clinical improvement after repeated treatments, as originally highlighted by Silberstein et al. [29]. We observed this “first time response” trend in our previous study focused on a subset of patients that received 6 injection cycles over 18 months [14]. The present study investigated this phenomenon beyond that timepoint in a larger consecutive series of patients over a 4-year period. It should be noted that BoNT-A treatment was consistently administered for several cycles (≥ 4) in patients not responding to treatment but willing to continue it and response started to be observed during the second or the third year of treatment. These cases would have been classified as “lack of response” if we had interrupted treatment after the 3rd cycle or earlier. When responsiveness was expressed as decrease in monthly headache days, our study showed a progressive reduction of non-responders, which disappeared after ten injection cycles, alongside a progressive increase of total responders upon regularly repeated treatment over time.
Efficacy results are fully reflected by the quality of life improvement. Patient distribution through MIDAS Grade [26] revealed a striking improvement at T6, after two cycles, with a significant decrease of severe cases (Grade IV), which thereafter continued to decrease and totally disappeared at T30, after ten treatments. On the other hand, cases with little to no disability (Grade I) and mild disability (Grade II), which were absent at baseline, altogether became more than half of the patients at T6, then progressively increased over time, up to representing 95% of the patients at T48. All in all, the consistent trend of improvement of MIDAS distribution observed in our previous study [14] appears to be extended over 4 years of treatment.
Some limitations of our study are intrinsic to its real-life nature, i.e. our course of action was driven by day-to-day experience and not by an a priori designed study protocol. The decision to stop treatment was indeed not always consistent throughout the study, since our requirements for treatment discontinuation became more stringent over time. Furthermore, at the beginning of our experience, we did not dedicate enough time to talk to our patients as we currently do and, as a result, several patients discontinued treatment, sometimes because disappointed with the outcome. Over the years, by improving our organisation, we have been able to more extensively communicate with our patients, in order to set realistic expectations and explain that CM requires sustained and possibly continuous treatment.
Conclusions
Our study confirmed that BoNT-A quarterly administration up to 4 years is a viable therapeutic approach in the prevention of CM. On top of eliciting significant overall improvements of chronic migraineurs conditions, BoNT-A looks persistently effective and well tolerated even upon many repeated administrations, appearing thus able to “stabilise” patient in a comfortable status which is maintained over time. Our study also confirmed the risk of relapse upon treatment discontinuation. These findings altogether seem to corroborate the strategy of CM long-lasting prevention through quarterly treatment with BoNT-A over several years.
Availability of data and materials
The dataset supporting the conclusions of this article is available on request through the corresponding author as appropriate.
Abbreviations
- AE
Adverse event
- BoNT-A
OnabotulinumtoxinA
- CM
Chronic migraine
- COMPEL
Chronic migraine OnabotulinuMtoxinA Prolonged Efficacy open-Label
- MIDAS
Migraine Disability Assessment
- MOH
Medication overuse headache
- PREEMPT
Phase III Research Evaluating Migraine Prophylaxis Therapy
Authors’ contributions
AS: study conception and design, patient diagnoses and follow-up assessments, BoNT-A injections, data acquisition/interpretation, coordination of activities and manuscript preparation. AMM: data collection, phone interviews and coordination of some activities. MC and AF: statistical analyses, interpretation of data and manuscript drafting/revision. MAL: study design, interpretation of data and manuscript drafting/revision. All the authors have read and approved the final manuscript.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflicts of interest.
Ethics approval and informed consent
The study was approved by the Ethics Committee Section of IRCCS Istituto Tumori Giovanni Paolo II of Bari at Fondazione IRCCS Casa Sollievo della Sofferenza of San Giovanni Rotondo ICF: VI.0_07 APR 2015. All procedures performed in the study were in accordance with the Declaration of Helsinki. Each patient signed an informed consent for the inclusion of clinical and demographic data in the study and for their analysis and publication in aggregated form.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.GBD Disease and injury incidence and prevalence collaborators (2016) global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2015;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manack AN, Buse DC, Lipton RB. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep. 2011;15(1):70–78. doi: 10.1007/s11916-010-0157-z. [DOI] [PubMed] [Google Scholar]
- 3.Buse DC, Manack A, Serrano D, Turkel C, Lipton RB. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81(4):428–432. doi: 10.1136/jnnp.2009.192492. [DOI] [PubMed] [Google Scholar]
- 4.Martelletti P. The journey from genetic predisposition to medication overuse headache to its acquisition as sequela of chronic migraine. J Headache Pain. 2018;19(1):2. doi: 10.1186/s10194-017-0830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diener HC, Limmroth V. Medication-overuse headache: a worldwide problem. Lancet Neurol. 2004;8:475–483. doi: 10.1016/S1474-4422(04)00824-5. [DOI] [PubMed] [Google Scholar]
- 6.Tajti J, Majláth Z, Szok D, Csáti A, Vécsei L. Drug safety in acute migraine treatment. Expert Opin Drug Saf. 2015;14(6):891–909. doi: 10.1517/14740338.2015.1026325. [DOI] [PubMed] [Google Scholar]
- 7.Bellei E, Monari E, Bergamini S, Cuoghi A, Tomasi A, Guerzoni S, Ciccarese M, Pini LA. Validation of potential candidate biomarkers of drug-induced nephrotoxicity and allodynia in medication-overuse headache. J Headache Pain. 2015;16:559. doi: 10.1186/s10194-015-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, Diener HC, Brin MF. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30:793–803. doi: 10.1177/0333102410364676. [DOI] [PubMed] [Google Scholar]
- 9.Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, Silberstein SD, Brin MF. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30:804–814. doi: 10.1177/0333102410364677. [DOI] [PubMed] [Google Scholar]
- 10.Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, Diener HC, Brin MF. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache. 2010;50:921–936. doi: 10.1111/j.1526-4610.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- 11.Aurora SK, Winner P, Freeman MC, Spierings EL, Heiring JO, DeGryse RE, VanDenburgh AM, Nolan ME, Turkel CC. OnabotulinumtoxinA for treatment of chronic migraine: pooled analyses of the 56-week PREEMPT clinical program. Headache. 2011;51:1358–1373. doi: 10.1111/j.1526-4610.2011.01990.x. [DOI] [PubMed] [Google Scholar]
- 12.Blumenfeld AM, Stark RJ, Freeman MC, Orejudos A, Adams AM. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19(1):13. doi: 10.1186/s10194-018-0840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerzoni S, Pellesi L, Baraldi C, Pini LA. Increased efficacy of regularly repeated cycles with OnabotulinumtoxinA in MOH patients beyond the first year of treatment. J Headache Pain. 2016;17:48. doi: 10.1186/s10194-016-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santoro A, Fontana A, Miscio AM, Zarrelli MM, Copetti M, Leone MA. Quarterly repeat cycles of onabotulinumtoxinA in chronic migraine patients: the benefits of the prolonged treatment on the continuous responders and quality-of-life conversion rate in a real-life setting. Neurol Sci. 2017;38(10):1779–1789. doi: 10.1007/s10072-017-3054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negro A, Curto M, Lionetto L, Crialesi D, Martelletti P. OnabotulinumtoxinA 155U in medication overuse headache: a two years prospective study. Springerplus. 2015;4:826. doi: 10.1186/s40064-015-1636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negro A, Curto M, Lionetto L, Martelletti P. A two years open-label prospective study of onabotulinumtoxinA 195 in medication overuse headache: a real-world experience. J Headache Pain. 2015;17:1. doi: 10.1186/s10194-016-0591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil M, Zafar HW, Quarshie V, Ahmed F. Prospective analysis of the use of OnabotulinumtoxinA (BOTOX) in the treatment of chronic migraine; real-life data in 254 patients from Hull, U.K. J Headache Pain. 2014;15:54. doi: 10.1186/1129-2377-15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buture A, Nimeri R, Khalil M, Ahmed F. Long term outcome for onabotulinumtoxinA in chronic migraine; two year follow up of 302 patients from the Hull migraine clinic [abstract no. EHMTC-0328] Cephalalgia. 2016;36(Suppl 1):16–17. [Google Scholar]
- 19.Ahmed F, Gaul C, García-Moncó JC, Sommer K, Martelletti P, Principal Investigators REPOSE. An open-label prospective study of the real-life use of onabotulinumtoxinA for the treatment of chronic migraine: the REPOSE study. J Headache Pain. 2019;20(1):26. doi: 10.1186/s10194-019-0976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vikelis M, Argyriou AA, Dermitzakis EV, Spingos KC, Makris N, Kararizou E. Sustained onabotulinumtoxinA therapeutic benefits in patients with chronic migraine over 3 years of treatment. J Headache Pain. 2018;19(1):87.15. doi: 10.1186/s10194-018-0918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerzoni S, Pellesi L, Baraldi C, Cainazzo MM, Negro A, Martelletti P, Pini LA. Long-term treatment benefits and prolonged efficacy of onabotulinumtoxinA in patients affected by chronic migraine and medication overuse headache over 3 years of therapy. Front Neurol. 2017;8:586. doi: 10.3389/fneur.2017.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cernuda-Morollón E, Ramón C, Larrosa D, Alvarez R, Riesco N. Pascual J (2015) long-term experience with onabotulinumtoxinA in the treatment of chronic migraine: what happens after one year? Cephalalgia. 2015;35(10):864–868. doi: 10.1177/0333102414561873. [DOI] [PubMed] [Google Scholar]
- 23.Tassorelli C, Aguggia M, De Tommaso M, Geppetti P, Grazzi L, Pini LA, Sarchielli P, Tedeschi G, Martelletti P, Cortelli P. Onabotulinumtoxin A for the management of chronic migraine in current clinical practice: results of a survey of sixty-three Italian headache centers. J Headache Pain. 2017;18(1):66. doi: 10.1186/s10194-017-0773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Headache Classification Committee of the International Headache Society (IHS) The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 25.Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: a safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50(9):1406–1418. doi: 10.1111/j.1526-4610.2010.01766.x. [DOI] [PubMed] [Google Scholar]
- 26.Stewart WF, Lipton RB, Kolodner K, Liberman J, Sawyer J. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia. 1999;19(2):107–113. doi: 10.1046/j.1468-2982.1999.019002107.x. [DOI] [PubMed] [Google Scholar]
- 27.Tassorelli C, Tedeschi G, Sarchielli P, Pini LA, Grazzi L, Geppetti P, De Tommaso M, Aguggia M, Cortelli P, Martelletti P. Optimizing the long-term management of chronic migraine with onabotulinumtoxinA in real life. Expert Rev Neurother. 2018;18(2):167–176. doi: 10.1080/14737175.2018.1419867. [DOI] [PubMed] [Google Scholar]
- 28.Bendtsen L, Sacco S, Ashina M, Mitsikostas D, Ahmed F, Pozo-Rosich P, Martelletti P. Guideline on the use of onabotulinumtoxinA in chronic migraine: a consensus statement from the European Headache Federation. J Headache Pain. 2018;19(1):91. doi: 10.1186/s10194-018-0921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silberstein SD, Dodick DW, Aurora SK, Diener HC, DeGryse RE, Lipton RB, Turkel CC. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86(9):996–1001. doi: 10.1136/jnnp-2013-307149. [DOI] [PMC free article] [PubMed] [Google Scholar]