Abstract
A 19-year-old African American woman presented to the emergency department with a history of left upper quadrant pain for a week, associated with nausea, malaise, loss of appetite, subjective fevers and chills. Her family history is significant for thalassemia in her maternal aunt, and hereditary spherocytosis in her brother, sister and cousin. A contrast-enhanced CT scan of the abdomen and pelvis revealed massive splenomegaly and multiple splenic infarcts. On the second day of admission, she developed a fever of 103°F. Further evaluation revealed acute Epstein-Barr virus (EBV) infection and hereditary spherocytosis. Her condition improved after 4 days on piperacillin/tazobactam, intravenous fluids, analgesics and antipyretics. Our case report describes a thorough clinical evaluation of a patient with fever, anaemia, massive splenomegaly and multiple splenic infarcts. It highlights the need for careful interpretation of multiple positive IgM results on viral serological testing that often accompanies acute EBV infections.
Keywords: haematology (incl blood transfusion), infectious diseases
Background
Hereditary spherocytosis is the most common inherited haemolytic anaemia caused by defects in genes encoding the red cell membrane skeleton.1 It is most commonly seen in individuals of northern European ancestry, where the prevalence is around 1 in 2000 individuals.2 It is known to be inherited in an autosomal dominant (75%) or an autosomal recessive pattern. The main clinical features of hereditary spherocytosis, include anaemia, jaundice, splenomegaly and often cholelithiasis. The disease can be classified as a trait, mild, moderate or severe, depending on factors, such as haemoglobin, reticulocyte count, serum bilirubin and spectrin molecules per erythrocyte.3
Epstein-Barr virus (EBV) is a highly prevalent herpesvirus affecting more than 90% of individuals worldwide.4 It is predominantly transmitted through exposure to infective saliva but can also spread through blood transfusions,5 organ transplantations6 and sexual intercourse.7 Acute EBV infection has diverse clinical manifestations. While it is often asymptomatic in children, primary EBV infection in adolescences and adults is often symptomatic.8 They typically present with classic infectious mononucleosis with a triad of fever, lymphadenopathy and pharyngitis. Other presenting symptoms, include fatigue, headache, nausea, sore joints and muscles and rash.
Splenic infarction is an uncommon diagnosis that typically presents with pain in the left upper quadrant or epigastrium, and sometimes associated with leukocytosis and elevated serum lactate dehydrogenase.9 Cardiogenic emboli are the most common risk factors for splenic infarction. Other risk factors include autoimmune diseases, such as systemic lupus erythematosus and antiphospholipid syndrome; and infection-associated conditions, such as peripancreatic abscess and septicemia. Early imaging by contrast-enhanced CT (CECT) scan is diagnostic, even in atypical presentations, whereas ultrasound was found to be diagnostic only in 18% of patients.10
Here, we present a case of multiple splenic infarcts in association with hereditary spherocytosis and acute EBV infection.
Case presentation
A 19-year-old African American woman presented to the emergency department with a history of sharp, non-radiating left upper quadrant pain for a week, not related to food intake. She also complained of nausea, malaise, loss of appetite, subjective fevers and chills, but denied recent trauma, jaundice, sick contacts, bleeding, abnormal menstrual cycle, recent medication use and haematuria. Her family history is significant for thalassemia in her maternal aunt, and hereditary spherocytosis in her brother, sister and cousin. On physical examination, marked conjunctival pallor, severe tenderness in the left upper quadrant without rigidity or guarding and normal bowel sounds were noted.
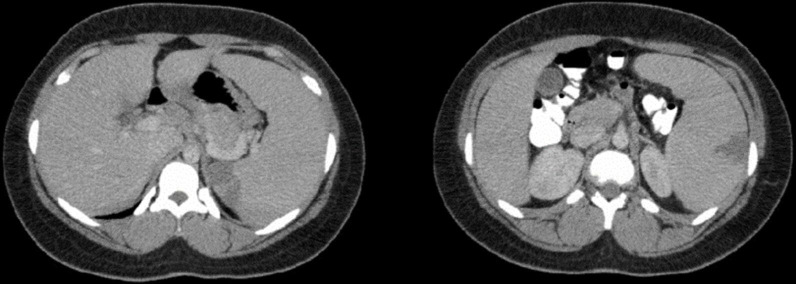
Initial laboratory investigations were consistent with normochromic normocytic anaemia, leukocytosis with a lymphocytic predominance, and elevated total bilirubin, aspartate transaminase (AST), urine bilirubin and urobilinogen (table 1). Although the chest X-ray was unremarkable, a CECT scan of the abdomen and pelvis revealed massive splenomegaly and several wedge-shaped areas within the periphery of the spleen consistent with multiple splenic infarcts (figure 1). Therefore, a provisional diagnosis of suspected hereditary spherocytosis with multiple splenic infarcts was made. Given that her vital signs were normal, and her pain was controlled with analgesics, she was initially discharged from the emergency department with a referral to the haematology clinic for further management.
Table 1.
Laboratory values on the first visit
| Investigations | Results | Normal range |
| Hb | 73 (L) | 120–160 g/L |
| Hct | 23.0 (L) | 36.0%–46.0% |
| MCV | 91.3 | 80.0–100.0 fL |
| MCH | 29.0 | 26.0–34.0 pg |
| MCHC | 317 | 314–358 g/L |
| RDW | 20.6 (H) | 11.5%–13.4% |
| White cell count | 12.82 (H) | 3.50–11.00×109/L |
| Neutrophils percentage | 33.3 (L) | 48.0%–82.0% |
| Lymphocyte percentage | 63.4 (H) | 19.0%–48.0% |
| Absolute neutrophil count | 4.27 | 1.70–9.00×109/L |
| Absolute lymphocyte count | 8.13 (H) | 1.20–3.50×109/L |
| Platelet | 233 | 150–440×109/L |
| Total bilirubin | 3.1 (H) | 0.1–1.5 mg/dL |
| Direct bilirubin | 0.4 | 0.0–1.5 mg/dL |
| AST | 49 (H) | 0–40 U/L |
| ALT | 18 | 0–45 U/L |
| ALP | 65 | 30–120 U/L |
| Serum amylase | 73 | 40–130 U/L |
| Serum lipase | 30 | 8–78 U/L |
| Urine bilirubin | Moderate (H) | Negative |
| Urine urobilinogen | ≥8.0 (H) | 0.1–1.0 EU/dL |
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; H, high; Hb, haemoglobin; Hct, hematocrit; L, low; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; RDW, red cell distribution width.
Figure 1.
The initial contrast-enhanced CT scan of the abdomen and pelvis showing massive splenomegaly and several wedge-shaped areas within the periphery of the spleen consistent with multiple splenic infarcts.
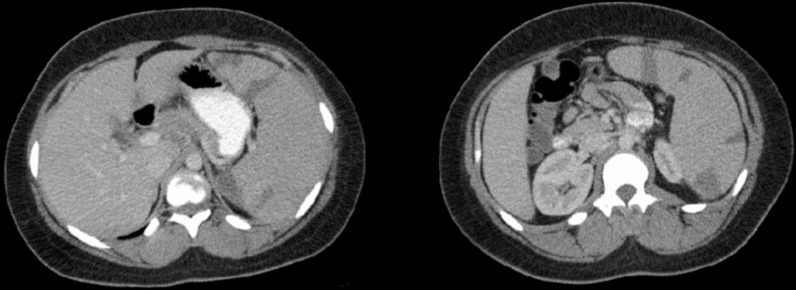
However, 5 days after discharge, the patient returned to the emergency department with worsening left upper quadrant pain. Repeated laboratory investigations showed results similar to the initial laboratory investigations. In addition, a CECT scan of the abdomen and pelvis revealed increases in the number and size of splenic infarcts compared with the previous CECT scan (figure 2). She was admitted to the general medical floor for pain control and further evaluation.
Figure 2.
The repeated contrast-enhanced CT scan of the abdomen and pelvis showing increases in the number and size of splenic infarcts compared with the previous contrast-enhanced CT scan.
Differential diagnosis
On the second day of admission, a fever of 103°F was noted along with chills. Based on the patient’s clinical presentation of multiple splenic infarcts, febrile episode and leukocytosis with lymphocytic predominance, differential diagnoses at that time included infective endocarditis, and viral infections, such as infectious mononucleosis, acute cytomegalovirus (CMV) infection and parvovirus B19 infection. Blood cultures and rapid respiratory panel were performed, then piperacillin/tazobactam was initiated along with analgesics, antipyretics and intravenous fluid. Subsequently, the results of blood cultures and rapid respiratory panel were negative. The transthoracic echocardiogram did not reveal vegetations.
Other laboratory findings included positive heterophile antibody test, EBV viral capsid antigen IgM and IgG antibodies and EBV early antigen (EA) IgG antibody, but negative EBV nuclear antigen IgG antibody, suggesting acute EBV infection (table 2). CMV IgM and IgG antibodies, and PCR test were negative. Although the patient had parvovirus B19 IgM antibody and negative parvovirus B19 IgG antibody, a transient aplastic crisis was unlikely given an elevated reticulocyte count of 9.59%. Repeat testing of parvovirus B19 IgM antibody a week later was equivocal. Therefore, her positive parvovirus B19 IgM antibody may have been due to an initial non-specific polyclonal IgM response.
Table 2.
Viral serological and PCR results
| Investigations | Results | Interpretation | Normal range |
| Heterophile Ab | Positive | Positive | Negative |
| EBV VCA IgM EIA | >160.0 | Positive | ≤35.9 U/mL (negative) |
| EBV VCA IgG EIA | 99.9 | Positive | ≤17.9 U/mL (negative) |
| EBV EA Ab EIA | >150.0 | Positive | ≤8.9 U/mL (negative) |
| EBNA IgG EIA | <3.0 | Negative | ≤17.9 U/mL (negative) |
| CMV IgM Ab | <8.0 | Negative | ≤29.9 AU/mL (negative) |
| CMV IgG Ab | 0.23 | Negative | ≤0.59 U/mL (negative) |
| CMV PCR | Not detected | Negative | Not detected |
| Parvovirus B19 IgM Ab | 1.8 | Positive | ≤0.8 index (negative) |
| Parvovirus B19 IgG Ab | 0.2 | Negative | ≤0.8 index (negative) |
| Parvovirus B19 IgM Ab* | 1.0 | Negative | ≤0.8 index (negative) |
| Parvovirus B19 IgG Ab* | 0.4 | Equivocal | ≤0.8 index (negative) |
*Parvovirus serological tests were repeated 1 week after the initial tests due to the possibility of an initial non-specific polyclonal IgM response.
Ab, antibody; CMV, cytomegalovirus; EBV EA, Epstein-Barr virus early antigen; EBNA, Epstein-Barr virus nuclear antigen; EBV VCA, Epstein-Barr virus viral capsid antigen; EIA, enzyme immunoassay.
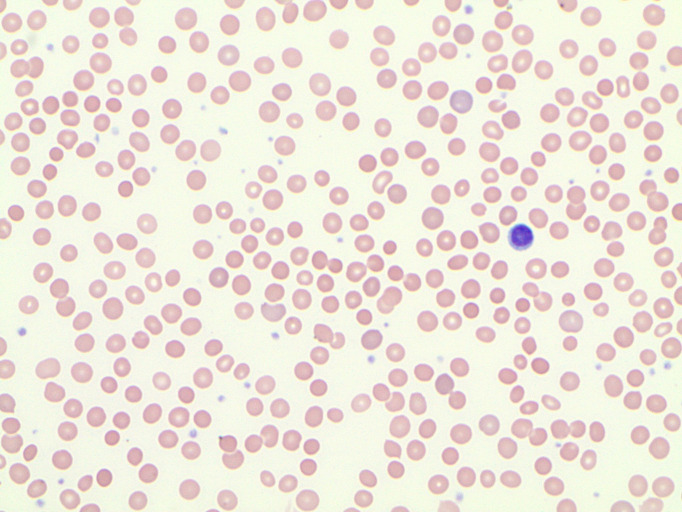
Mean corpuscular haemoglobin concentration (MCHC) is typically elevated in patients with hereditary spherocytosis. Although our patient has a strong family history of hereditary spherocytosis, her MCHC was within normal limits. Further evaluation revealed evidence of haemolysis, such as reduced haptoglobin of <20 mg/dL, elevated reticulocyte count and lactate dehydrogenase of 889 U/L. A peripheral blood smear showed increased polychromasia, nucleated red blood cells, increased mature lymphocytes and microspherocytes/spherocytes (figure 3). Haemoglobinopathies were excluded by normal haemoglobin electrophoresis, negative alpha-globin common mutation analysis and nucleotide sequence analysis of the beta-globin gene.
Figure 3.
Peripheral blood smear showing spherocytosis along with increased polychromasia, nucleated red blood cells and increased mature lymphocytes.
Spherocytes on peripheral blood smear are often due to hereditary spherocytosis and autoimmune haemolytic anaemia (AIHA). These conditions may be differentiated by a direct antiglobulin (Coombs) test—positive in AIHA but negative in hereditary spherocytosis. Further evaluation demonstrated a positive osmotic fragility test, an equivocal eosin-5′-maleimide (EMA) binding test (Band 3 essay) and a negative direct antiglobulin test. It is important to note that the EMA binding test may be masked by reticulocytosis.11 Given her clinical features, a strong family history of hereditary spherocytosis, combined with anaemia, reticulocytosis, hyperbilirubinemia, spherocytes on the peripheral blood smear, a positive osmotic fragility test and a negative direct antiglobulin test, a diagnosis of moderate hereditary spherocytosis was made.
Outcome and follow-up
During her hospital stay, the patient received piperacillin/tazobactam, intravenous fluids, analgesics and antipyretics. Her symptoms improved after 4 days, and she was discharged with folic acid supplements, an instruction to avoid contact sports, and a follow-up appointment in the haematology clinic. Three weeks after discharge, the patient presented completely asymptomatic. A reassessment of the blood tests revealed haemoglobin improvement from 73 to 95 g/L, lactate dehydrogenase decreased from 889 to 320 U/L and no leukocytosis with lymphocytic predominance. She was recommended pneumococcal (PCV13 and PPSV23), Haemophilus influenzae type B (Hib) and meningococcal vaccinations for functional asplenia.
Discussion
According to the 2011 Guidelines on hereditary spherocytosis,12 patients do not require additional tests for a diagnosis of hereditary spherocytosis if they have a family history of hereditary spherocytosis, typical clinical features and laboratory investigations, such as spherocytes, elevated MCHC and reticulocytosis. However, if the diagnosis is equivocal, a screening test with high predictive value, such as the cryohemolysis test and the EMA binding test may be done. Patients with moderate and severe hereditary spherocytosis are recommended to take folic acid supplements. Splenectomy should be performed in patients with severe hereditary spherocytosis and should be considered in those with moderate hereditary spherocytosis.
Of the previously reported cases of hereditary spherocytosis patients with splenic infarctions, some of them had an underlying prothrombotic disorder, such as sickle cell trait.13–16 In other patients, splenic infarctions occurred during infectious mononucleosis.17 18 It has been suggested that splenic oxygen supply may be insufficient during a rapid enlargement of spleen in these patients. In one patient with hereditary spherocytosis, infectious mononucleosis and protein C deficiency, it was postulated that associated protein C deficiency could have facilitated arterial occlusion and diminished splenic oxygen supply during infectious mononucleosis. Others have suggested that infectious mononucleosis may induce a transient hypercoagulable state.19
Splenic infarction may be the first clinical manifestation in some patients.9 In addition, it should be considered in hereditary spherocytosis patients presenting with left upper quadrant pain, and prompt evaluation by a CECT scan should be performed. Appropriate management includes adequate hydration, pain control and treatment of the underlying cause. Causes, such as cardiogenic emboli, and hypercoagulable conditions, such as antiphospholipid syndrome, may warrant anticoagulation therapy. Symptomatic management may be sufficient in splenic infarction secondary to acute viral infections, such as infectious mononucleosis.20 21
During serological screening tests for viral infections, multiple positive IgMs can be misleading.22 It is important to recognise the possibility of an initial non-specific polyclonal IgM response, and the results should be confirmed by PCR testing and follow-up samples for specific evolution of serological responses over time.
Our case report describes a thorough clinical evaluation of a patient with fever, anaemia, massive splenomegaly and multiple splenic infarcts. It highlights the need for careful interpretation of multiple positive IgM results on viral serological testing that often accompanies acute EBV infections.
Learning points.
Splenic infarction should be considered in hereditary spherocytosis patients presenting with left upper quadrant pain.
Early evaluation of splenic infarction by contrast-enhanced CT scan is diagnostic.
Recognising the common causes of splenic infarction is crucial to guide specific treatment of the underlying cause.
Multiple positive IgMs can be misleading during serological screening tests for viral infections. It is crucial to consider the possibility of an initial non-specific polyclonal IgM response, and the results should be confirmed by PCR testing and follow-up samples for specific evolution of serological responses over time.
Footnotes
Contributors: AMT acquired the data from the patient’s electronic medical records, obtained the patient consent and authored the manuscript. II acquired the data from the patient’s electronic medical records and edited the manuscript. PG was instrumental in the guidance and editing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mariani M, Barcellini W, Vercellati C, et al. Clinical and hematologic features of 300 patients affected by hereditary spherocytosis grouped according to the type of the membrane protein defect. Haematologica 2008;93:1310–7. 10.3324/haematol.12546 [DOI] [PubMed] [Google Scholar]
- 2.Hereditary spherocytosis Genetics Home Reference 2020. Available: https://ghr.nlm.nih.gov/condition/hereditary-spherocytosis [Accessed 22 May 2020].
- 3.Eber SW, Armbrust R, Schröter W. Variable clinical severity of hereditary spherocytosis: relation to erythrocytic spectrin concentration, osmotic fragility, and autohemolysis. J Pediatr 1990;117:409–16. 10.1016/S0022-3476(05)81081-9 [DOI] [PubMed] [Google Scholar]
- 4.Smatti MK, Al-Sadeq DW, Ali NH, et al. Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol 2018;8:211. 10.3389/fonc.2018.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trottier H, Buteau C, Robitaille N, et al. Transfusion-related Epstein-Barr virus infection among stem cell transplant recipients: a retrospective cohort study in children. Transfusion 2012;52:2653–63. 10.1111/j.1537-2995.2012.03611.x [DOI] [PubMed] [Google Scholar]
- 6.Hanto DW, Frizzera G, Purtilo DT, et al. Clinical spectrum of lymphoproliferative disorders in renal transplant recipients and evidence for the role of Epstein-Barr virus. Cancer Res 1981;41:4253–61. [PubMed] [Google Scholar]
- 7.Crawford DH, Macsween KF, Higgins CD, et al. A cohort study among university students: identification of risk factors for Epstein-Barr virus seroconversion and infectious mononucleosis. Clin Infect Dis 2006;43:276–82. 10.1086/505400 [DOI] [PubMed] [Google Scholar]
- 8.Rea TD, Russo JE, Katon W, et al. Prospective study of the natural history of infectious mononucleosis caused by Epstein-Barr virus. J Am Board Fam Pract 2001;14:234–42. [PubMed] [Google Scholar]
- 9.Schattner A, Ami S, Adi M, Kitroser E, et al. Acute splenic infarction at an academic General Hospital over 10 years: presentation, etiology, and outcome. Medicine 2015;94:e1363. 10.1097/MD.0000000000001363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antopolsky M, Hiller N, Salameh S, et al. Splenic infarction: 10 years of experience. Am J Emerg Med 2009;27:262–5. 10.1016/j.ajem.2008.02.014 [DOI] [PubMed] [Google Scholar]
- 11.RBCME - Clinical: Red Blood Cell Membrane Evaluation, Blood. Mayo Clinic Laboratories. Available: https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/64897 [Accessed 22 May 2020].
- 12.Bolton-Maggs PHB, Langer JC, Iolascon A, et al. Guidelines for the diagnosis and management of hereditary spherocytosis--2011 update. Br J Haematol 2012;156:37–49. 10.1111/j.1365-2141.2011.08921.x [DOI] [PubMed] [Google Scholar]
- 13.Yang YM, Donnell C, Wilborn W, et al. Splenic sequestration associated with sickle cell trait and hereditary spherocytosis. Am J Hematol 1992;40:110–6. 10.1002/ajh.2830400207 [DOI] [PubMed] [Google Scholar]
- 14.Ustun C, Kutlar F, Holley L, et al. Interaction of sickle cell trait with hereditary spherocytosis: splenic infarcts and sequestration. Acta Haematol 2003;109:46–9. 10.1159/000067273 [DOI] [PubMed] [Google Scholar]
- 15.Dulman RY, Buchanan GR, Ginsburg H, et al. Splenic infarction due to concomitant hereditary spherocytosis and sickle cell trait. J Pediatr Surg 2007;42:2129–31. 10.1016/j.jpedsurg.2007.07.057 [DOI] [PubMed] [Google Scholar]
- 16.Tso ACY, Roper DR, Wong CL, et al. Splenic infarction in a patient with sickle cell trait and hereditary spherocytosis. Am J Hematol 2011;86:695–6. 10.1002/ajh.22015 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki Y, Shichishima T, Mukae M, et al. Splenic infarction after Epstein-Barr virus infection in a patient with hereditary spherocytosis. Int J Hematol 2007;85:380–3. 10.1532/IJH97.07208 [DOI] [PubMed] [Google Scholar]
- 18.Breuer C, Janssen G, Laws H-J, et al. Splenic infarction in a patient hereditary spherocytosis, protein C deficiency and acute infectious mononucleosis. Eur J Pediatr 2008;167:1449–52. 10.1007/s00431-008-0781-3 [DOI] [PubMed] [Google Scholar]
- 19.Machado C, Melo Salgado J, Monjardino L. The unexpected finding of a splenic infarction in a patient with infectious mononucleosis due to Epstein-Barr virus. BMJ Case Rep 2015;2015:bcr2015212428. 10.1136/bcr-2015-212428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician 2015;91:372–6. [PubMed] [Google Scholar]
- 21.Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004;350:586–97. 10.1056/NEJMra030840 [DOI] [PubMed] [Google Scholar]
- 22.Forde DG, Cope A, Stone B. Acute parvovirus B19 infection in identical twins unmasking previously unidentified hereditary spherocytosis. BMJ Case Rep 2014;2014. 10.1136/bcr-2013-202957. [Epub ahead of print: 29 Jul 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]