Abstract

Polymeric membranes are used on very large scales for drinking water production and kidney dialysis, but they are nearly always prepared by using large quantities of unsustainable and toxic aprotic solvents. In this study, a water-based, sustainable, and simple way of making polymeric membranes is presented without the need for harmful solvents or extreme pH conditions. Membranes were prepared from water-insoluble polyelectrolyte complexes (PECs) via aqueous phase separation (APS). Strong polyelectrolytes (PEs), poly(sodium 4-styrenesulfonate) (PSS), and poly(diallyldimethylammonium chloride) (PDADMAC) were mixed in the presence of excess of salt, thereby preventing complexation. Immersing a thin film of this mixture into a low-salinity bath induces complexation and consequently the precipitation of a solid PEC-based membrane. This approach leads to asymmetric nanofiltration membranes, with thin dense top layers and porous, macrovoid-free support layers. While the PSS molecular weight and the total polymer concentrations of the casting mixture did not significantly affect the membrane structure, they did affect the film formation process, the resulting mechanical stability of the films, and the membrane separation properties. The salt concentration of the coagulation bath has a large effect on membrane structure and allows for control over the thickness of the separation layer. The nanofiltration membranes prepared by APS have a low molecular weight cutoff (<300 Da), a high MgSO4 retention (∼80%), and good stability even at high pressures (10 bar). PE complexation induced APS is a simple and sustainable way to prepare membranes where membrane structure and performance can be tuned with molecular weight, polymer concentration, and ionic strength.
Keywords: polyelectrolyte complexes, aqueous phase separation, nanofiltration, solvent-free, green fabrication
Introduction
Nonsolvent induced phase separation (NIPS) became the dominant technique to produce polymeric membranes shortly after its discovery in the 1960s. In this technique, a homogeneous polymer solution is cast and then immersed in a coagulation bath that contains nonsolvent which has a low affinity for the polymer. The nonsolvent, typically water, should be completely miscible with the solvent so that the nonsolvent will replace the solvent when the cast film is immersed in the coagulation bath. Immersion in the nonsolvent bath leads to the controlled precipitation of the polymer film into a porous membrane. The membrane structure and thus its performance depend on both the polymer/solvent/nonsolvent interactions (thermodynamics) and the mass transport through the system (kinetics). For example, fast and immediate precipitation of a polymer often leads to asymmetric membranes.1 These asymmetric membranes have thin, dense skin layers on top that provide selectivity and thicker porous parts that provide mechanical strength. The natural tendency of NIPS to form asymmetric structures is an important reason NIPS was a major breakthrough.2
NIPS is a simple and versatile technique; however, the solvents used for this process are nearly always dipolar aprotic solvents, and these are mostly reprotoxic and harmful to the environment.3 The most commonly used solvent is N-methyl-2-pyrrolidone (NMP), a solvent that has recently come under increased scrutiny, with the European Union introducing new regulations to restrict its usage from 2020.4 Figoli and co-workers reviewed publications using numerous nontoxic solvent replacements for NIPS and thermally induced phase separation (TIPS) processes.5 In their detailed review, several solvents were evaluated as promising candidates to replace these harmful solvents; however, most of the alternatives come with their own downsides. Specifically, some of the alternatives are unable to dissolve the desired polymers at high concentrations;6 others are expensive (e.g., ionic liquids), are produced by using limited natural resources (e.g., solvents containing phosphorus), or have problematic hazardous properties (e.g., ionic liquids and dimethyl sulfoxide).5 Finally, the solvents should ideally be recyclable and should thus be easy to separate from the nonsolvent water after membrane formation.
While greener organic solvents are promising, an even more sustainable approach would be to use water as both the solvent and the nonsolvent. To achieve this, a polymer is required that is soluble and insoluble in aqueous media depending on conditions such as pH, salinity, and temperature. For example, poly(vinyl alcohol)7 and hydroxypropylcellulose8 membranes have been prepared in aqueous media via TIPS, followed by chemical cross-linking for stability. The membranes were evaluated in terms of phase behavior, mechanical properties, swelling, and pure water permeabilities but were not studied for their separation behavior. A major downside of TIPS is that typically only symmetric membranes can be formed; for asymmetric membranes a concentration gradient throughout the casting film (from top to bottom) is required as is the case for traditional NIPS.9 Inspired by the traditional NIPS process, de Vos showed that aqueous phase separation can be achieved using weak polyelectrolytes whose solubility is pH-dependent.10 Using this approach, Willott et al. prepared porous poly(4-vinylpyridine) asymmetric and symmetric membranes by variations of casting solution and coagulation bath composition.11
Polyelectrolytes (PEs) are polymers that have charged repeating units accompanied by small counterions, and because of the charged nature of the repeating units they dissociate in a polar media (e.g., water). When two oppositely charged PEs are mixed, they interact and can form polyelectrolyte complexes (PECs) which are typically water-insoluble. An important point to understand, especially for this work, is that the driving force for complexation is the entropic gain via release of small counterions.12 PEs are classified as weak or strong depending on the pH response of the charges. If the monomers can dissociate or be neutralized depending on the pH of the medium, then the PE is classified as weak. On the other hand, for strong PEs the repeating units remain charged over entire pH range (i.e., pH 1–14). In this case, charges cannot be neutralized, but they can be screened by high salinity (i.e., excess counterions). The phase behavior of PECs as a function of salt concentration reveals that PECs exist in a homogeneous solution phase at high salinity while they are solid precipitates at low salinity.13,14 These two points suggests that a phase separation method utilizing polyelectrolyte complexation can be used to prepare membranes. Significantly, PE complexation can be performed entirely in aqueous media, thus eliminating the need for toxic solvents.
Coating layers of PECs sequentially, namely polyelectrolyte multilayers (PEMs) on porous support membranes, has been demonstrated to give the membranes with excellent performance such as selectivity,15−19 stability,20 resistance to fouling,21,22 and hydrophilicity.16,21,23,24 Of all systems studied, the combination of poly(sodium 4-styrenesulfonate) (PSS) and poly(diallyldimethylammonium chloride) (PDADMAC) was found to be especially promising due to the high resistance against chemical cleaning, showing a 100 times higher chemical stability against oxidants20 and better selectivity toward fluoride,25 sulfate,26 and phosphate27 ions compared to commercial membranes. The pH stability of this PE pair is also outstanding; PSS–PDADMAC multilayer films exhibited similar wetting properties between pH 4 and 11.28 More significantly, membranes coated with PSS–PDADMACshowed stable MgSO4 retention even at high acid concentrations such as 3 M HCl.29 Thus, polyelectrolyte multilayer based nanofiltration membranes, especially of PSS and PDADMAC, hold great promise. Unfortunately, the preparation of PEM membranes is time-consuming and laborious. Moreover, the support membranes are still produced with NIPS, which requires the harmful solvents.
For decades, using PEMs was the main way to facilitate polyelectrolyte complexes as functional materials since bulk PECs were widely considered to be unprocessable due to being infusible and very brittle in the dry state.30 In 2009, the concept of saloplastics was introduced, leading to the production of a new set of PEC based materials.31 The term saloplastic refers to polyelectrolyte complexes that can be processed after treatment with saline water. Schlenoff and co-workers demonstrated a great variety of possibile ways to prepare these materials.31−35 Porous saloplastic materials are obtained by centrifugation31,36 and electrospinning37 of coacervate phases or by desalting a homogeneous PE solution in between semipermeable membranes.38 In addition, doped PECs can be extruded to obtain dense materials in various shapes (tape, tube, rod, and fiber)32,33 or spin-coated,34,39 cast,40,41 or pressed in between templates42,43 to obtain transparent films. Moreover, some of the saloplastics show self-healing behavior which can operate under room temperature and tuned by type of salt.35,44 The majority of saloplastics reported in the literature are composed of PSS–PDADMAC,31−34,37−39,42,43,45 emphasizing its properties such as chemical and thermal stability, biocompatility, and no requirement for cross-linking agent.
In addition to saloplastics mentioned above, PE complexation has been used by Sadman et al. to prepare porous membranes.46 In their work, PECs were obtained by mixing two strong PE solutions. After PECs were extruded and treated with salt water annealing, they were partially dissolved at varying salt solutions to obtain coacervates and then cast into thin films. The resultant membranes had high pure water permeability (75–400 L m–2 h–1 bar–1) and high rejection toward polystyrene beads (100 nm and larger), indicating that these membranes were in the ultrafiltration (UF) range. This study showed that making membranes on the basis of polyelectrolyte complexation is possible; however, there were a couple of drawbacks related to the membrane production procedure. Besides the multiple-step membrane preparation process, casting coacervates in different salinities instead of homogeneous solutions results in change in polymer concentration and the hydration of the coacervates. This leads to limited control over the membrane structure and performance. Indeed, only UF membranes were prepared. Our research group recently demonstrated that it is also possible to prepare membranes by casting homogeneous PE mixtures instead of coacervates.47 A switch between two extreme pH regimes was used to control the complexation of a strong polyanion and a weak polycation. Polymer molecular weight, polymer concentration, and coagulation bath salinity were the factors that gave a great control over the membrane structure and resulted in membranes ranging from microfiltration (MF) type to nanofiltration (NF) type.
In this work, we discuss the formation and performance of the PEC based nanofiltration membranes prepared by aqueous phase separation (APS). We use PSS and PDADMAC as the strong polyanion and strong polycation, respectively. Herein, a simple change in salt concentration is used to control complexation instead of switching between extreme pH values as was used in our earlier work.47 Therefore, by use of a salt switch, the membrane formation conditions are milder and the requirement for using a weak and strong PE pair is removed. In this study, a homogeneous mixture of the two strong PEs is prepared at a high salinity where the entropic driving force for complexation is eliminated. Therefore, the PE chains are in a dissolved and coiled form and surrounded by an excess of counterions (Figure 1). The homogeneous polyelectrolyte mixture is cast on a substrate and immersed in a low-salinity water bath. This dramatic decrease in sal concentration facilitates polyelectrolyte complexation based on counterion release. The oppositely charged PE chains interact with each other and form a water-insoluble polyelectrolyte complex. The precipitated polymer is considered to have an amorphous, entangled structure,30 and the charges are mostly compensated by an oppositely charged monomer rather than small counterions (see Figure 1). In preparing the PSS–PDADMAC membranes by complexation induced APS, we intend to combine the excellent separation performance of PSS–PDADMAC PEM-based nanofiltration membranes with a simple and sustainable production process. We investigate the effects of polymer concentration, molecular weight, and coagulation bath salinity on membrane structure and performance. We show that nanofiltration membranes are obtained, of which the structure and performance can be tuned with the salinity of the coagulation bath. The performance of the membranes is studied by pure water permeability, salt retention, and molecular weight cutoff measurements, at pressures up to 10 bar.
Figure 1.

Schematic illustration of membrane preparation procedure for salt triggered complexation induced aqueous phase separation. Homogenous mixture of two oppositely charged PEs are mixed at high salinity, casted on a substrate, and immersed in a low-salinity bath.
Experimental Section
Materials
Poly(sodium 4-styrenesulfonate) (PSS, Mw ∼ 70, 200, and 1000 kDa in aqueous solution), poly(diallyldimethylammonium chloride) (PDADMAC, Mw 200–350 kDa, 20 wt % in water), magnesium sulfate (MgSO4), and 2-propanol (IPA) were purchased from Sigma-Aldrich. Poly(ethylene glycol) (PEG) of different molecular weights (200, 400, 600, 1000, 1500, and 2000 Da) were purchased from Merck. Sodium chloride (NaCl, pharmaceutical grade, SanalP) was kindly supplied by Akzo Nobel.
Membrane Formation
Solution Preparation
The as-received PSS and PDADMAC solutions were used to prepare casting solutions containing 10, 12.5, and 15.4 wt % total polymer. First, water (if needed) and NaCl were added to PSS and PDADMAC solutions, separately. After the solutions became homogeneous, they were mixed such that the monomer ratio is stoichiometric. Here, a stoichiometric monomer ratio means that the ratio of PSS monomers to PDADMAC monomers is equal to 1. For the mixtures with 17.5 and 20 wt % polymer, stock solutions of PSS and PDADMAC were dried (separately) in an oven at 80 °C overnight. Then, the desired solutions were prepared by mixing the dried polymer, NaCl, and water. PSS and PDADMAC solutions were mixed and stirred over 16 h to form a stoichiometric, amber-colored, homogeneous mixture. The mixture was left overnight, without stirring, to remove air bubbles. Properties of PE casting mixtures used in this study are listed in Table 1.
Table 1. Properties of the Homogenous PE Mixtures Used for Membrane Preparation.
| PSS molecular weight (kDa) | PDADMAC molecular weight (kDa) | total polymer concentration (wt %) |
|---|---|---|
| 70 | 200–350 | 15.5 |
| 200 | 200–350 | 15.4 |
| 1000 | 200–350 | 15.5 |
| 200 | 200–350 | 10.0 |
| 200 | 200–350 | 12.5 |
| 200 | 200–350 | 17.5 |
| 200 | 200–350 | 20.0 |
Casting
Homogenous mixtures were cast at a thickness 0.3 mm with a casting knife on a transparent plastic sheet (termed acetate sheet by the provider, JEJE Produkt) and immediately immersed in a coagulation bath. The coagulation bath consisted of either Milli-Q water (resistivity at 25 °C is 18.2 MΩ·cm), called 0 M, or 0.5, 1.0, 1.5, and 2.0 M NaCl. After 10 min in the desired coagulation bath, the membranes were transferred to washing baths containing demineralized water, and the washing bath was refreshed four times in total to remove any remaining salt. PSS–PDADMAC complexes do not swell in many organic solvents like isopropanol.48 Therefore, after the washing steps, the membranes were immersed in 30 vol %, then 60 vol %, then 90 vol %, and last pure IPA baths so that membranes could be removed from the substrate without causing any deformation of the membrane. Membranes were stored in IPA prior to any filtration test.
Membrane Characterization
Structure
Membrane samples were taken from IPA and air-dried; then they were broken after immersion in liquid nitrogen. The samples were sputter-coated with a 5 nm chromium layer (Quorum Q150T ES). Then the cross section and surface morphologies of the membranes were investigated by scanning electron microscopy (SEM, JSM6010LA) integrated with an energy dispersive X-ray spectrometer (EDS).
Besides observing and comparing cross-section morphologies of the membranes, we also measured the average skin layer thicknesses. To do that, cross-section images at ×5000 magnification were analyzed with ImageJ software. The distance between the top edge of the cross section and the closest visible pore to the edge is defined as the skin layer thickness. Multiple positions were measured, and the average value is reported as the skin layer thickness of the membrane. A representative skin layer thickness measurement is given in Figure S1 of the Supporting Information.
Performance
Membrane samples were taken from IPA and rinsed with demineralized water to exchange IPA; then the membranes were tested in terms of their filtration performance. First, pure water permeability measurements were conducted. For that the membranes were placed in dead-end filtration cells in which pure water was pressurized toward the filtration system by nitrogen gas. The permeate was collected, and its weight was measured as a function of time, giving the pure water flux (J). The membrane active area for the cells operated at 4 bar was 3.0 cm2, while for the cells operated at 10 bar this was 15.3 cm2. The permeability P was calculated by taking the slope of flux over transmembrane pressure (TMP) as follows:
where J is the water flux and TMP is transmembrane pressure. After the pure water permeability measurements, the membranes were subjected to retention tests. For these tests, the same dead-end setup was used, and instead of pure water, a solution containing the carefully selected probe molecule (MgSO4 and PEG) was fed while stirring the feed solution within the dead-end cell. Retention of the membrane was calculated via the relationship
where CP, CF, and CR are the concentrations of the solute in the permeate, feed, and retentate, respectively. The feed is the solution that was in the cell prior to the rejection test, while the retentate is the solution that was left in the cell after the test (see Figure S2).
Pure water permeability and retention measurements were performed at the same transmembrane pressure for the same membrane piece (either 4 or 10 bar). Retention tests were performed for either a 5 mM MgSO4 solution or a mixture of PEG molecules to investigate the molecular weight cutoff (MWCO) of the membranes. For both cases, ∼20 mL of solution was fed to the filtration cell. The permeate was collected such that recovery ratio (permeate/feed, g/g) does not exceed 1/3 for MgSO4 retention tests and 1/20 for MWCO tests to keep concentration polarization to a minimum. For MgSO4 retention, concentrations of feed, permeate, and retentate samples were calculated by analyzing the conductivities of the samples. For MWCO measurements, a mixture of PEGs with molecular weights of 200, 400, 600, 1000, 1500, and 2000 Da was prepared so that each PEG was at a concentration of 1 g/L. Samples were analyzed by gel permeation chromatography with a size exclusion column (Agilent 1200/1260 Infinity GPC/SEC series, Polymer Standards Service column compartment and data center (UDC 810 Interface)). The sample solution flow rate was 1 mL/min, and the eluent was 50 mg/L NaN3 in Milli-Q water. Samples went through two Polymer Standards Service Suprema 8 × 300 mm2 columns in series: 1000 Å, 10 μm followed by 30 Å, 10 μm. Concentration vs molecular weight curves were obtained for feed, permeate, and retentate samples and converted into retention vs molecular weight graphs (i.e., a sieving curve). MWCO is defined as the molecular weight of the solute that the membrane retains by 90%, i.e., where the sieving curve reaches the 90% level.49 In this work, for some of these membranes, the sieving curve did not reach exactly 90% retention, although it reaches a plateau. This is an indication of defect(s) on the membrane, and therefore the maximum retention level that the curve reached was considered as the 100% rejection level. The MWCO value was estimated relative to the level considered for total rejection (see Figure S3).
Results and Discussion
In this work, porous polymeric films were prepared from polyelectrolyte complexes with the aqueous phase separation technique (see Figure 1). Diffusion of salt out of the film upon exposure to a low-salinity bath was used to trigger PE complexation. In this section, we first explain the selection of a suitable polyelectrolyte mixture; more specifically, we discuss the effect of PSS molecular weight and polymer concentration of the mixtures. Thereafter, we discuss the effect of coagulation bath salinity on the structure and the performance of the resulting membranes.
Selection of Suitable Polyelectrolyte Mixture
Effect of Molecular Weight
Films were prepared by immersing a thin layer of a homogeneous PE mixture into a coagulation bath containing Milli-Q water. These mixtures had the same polymer and salt concentrations (15.5 wt % polymer and 18 wt % NaCl), and only the PSS molecular weight was varied (70, 200, and 1000 kDa) which will be referred as 70 kDa PSS, 200 kDa PSS, and 1000 kDa PSS, respectively.
SEM images of the films are presented in Figure 2. Cross-section images of the films are shown in the left column, and the top surface images are shown in the right column. The cross-section images clearly show that all the films have an asymmetric structure with thin dense layers at the top and porous support layers below. Higher magnifications of the SEM images (Figures S4 and S5) show no visible pores on the surface (up to that magnification), indicating the density of the selective layers. Moreover, support layers have interconnected pore structure, with pores visible through other pores (i.e., open-cell spongy structure). Therefore, it is expected that the selective layers dominate the resistance to permeation, while the support layers are highly permeable. These SEM images immediately demonstrate that our approach successfully leads to the formation of membranes with the highly desired asymmetric structure. Also striking is that no macrovoids are observed in the support structure: macrovoids are a common and generally undesired feature when preparing membranes by NIPS. The viscosity of the casting solution is typically a major factor controlling the phase inversion kinetics. Solutions with different viscosities are expected to phase separate at different rates and therefore lead to membranes with different cross-section morphologies. Although, there is a substantial difference in the viscosities of the mixtures with 70 kDa, 200 kDa, and 1000 kDa PSS (see Table S1), all three membranes have essentially the same asymmetric structure. For all three asymmetric films, no defects and/or pores were observed from the surface SEM images at magnifications up to ×10000 (see Figure S4). Some of the surface SEM images in this paper show particle-like structures on the membranes (like the ones prepared with 70 kDa and 1000 kDa PSS). The exact cause of these structures could not be determined; however, they are considered to be polymer precipitates since no substantial difference from the bulk of the film can be observed from the elemental analysis (see Figure S6). Additionally, no effect of these impurities was observed on the membrane performance tests.
Figure 2.
SEM images of cross-section and top surfaces of the membranes prepared with mixtures of varying PSS molecular weight. All cross-section images are at ×1000 magnification while surface images are at ×5000 magnification.
No substantial differences between the films can be seen from the SEM images, but conversely there are distinct differences in the membrane performance. The pure water permeability (PWP) values of the membranes were measured at 4 bar with a dead-end filtration setup. While a stable flux could not be achieved with the 70 kDa PSS film, the 200 kDa and 1000 kDa PSS films had stable PWP values of approximately 1.0 and 0.4 L m–2 h–1 bar–1, respectively. Longer polymer chains (i.e., higher molecular weight) result in more chain entanglements, and this in turn provides films with a greater mechanical strength.50 A stable water flux was not observed for the 70 kDa PSS film, while the 200 kDa PSS and 1000 kDa PSS films showed good stability, indicating that higher molecular weights are needed to obtain enough entanglement for mechanical stability. Figure S7 shows that the skin layer thicknesses of membranes prepared with the 200 kDa PSS and the 1000 kDa PSS are very close to each other, while the PWP values are quite different. This indicates that the membrane skin layer becomes denser with more entanglements, leading to a lower permeability. Although the 1000 kDa PSS membrane is expected to show a better rejection, it has a much lower permeability than the 200 kDa PSS membrane, and the membrane formation procedure is much more difficult and time-consuming compared to the 200 kDa PSS membrane due to gelation of the 1000 kDa PSS solution (see Figure S8). Because the 70 kDa PSS film did not show a stable membrane performance and the 1000 kDa mixture has such a high viscosity, the 200 kDa PSS mixture was used for further experiments.
Effect of Overall Polymer Concentration
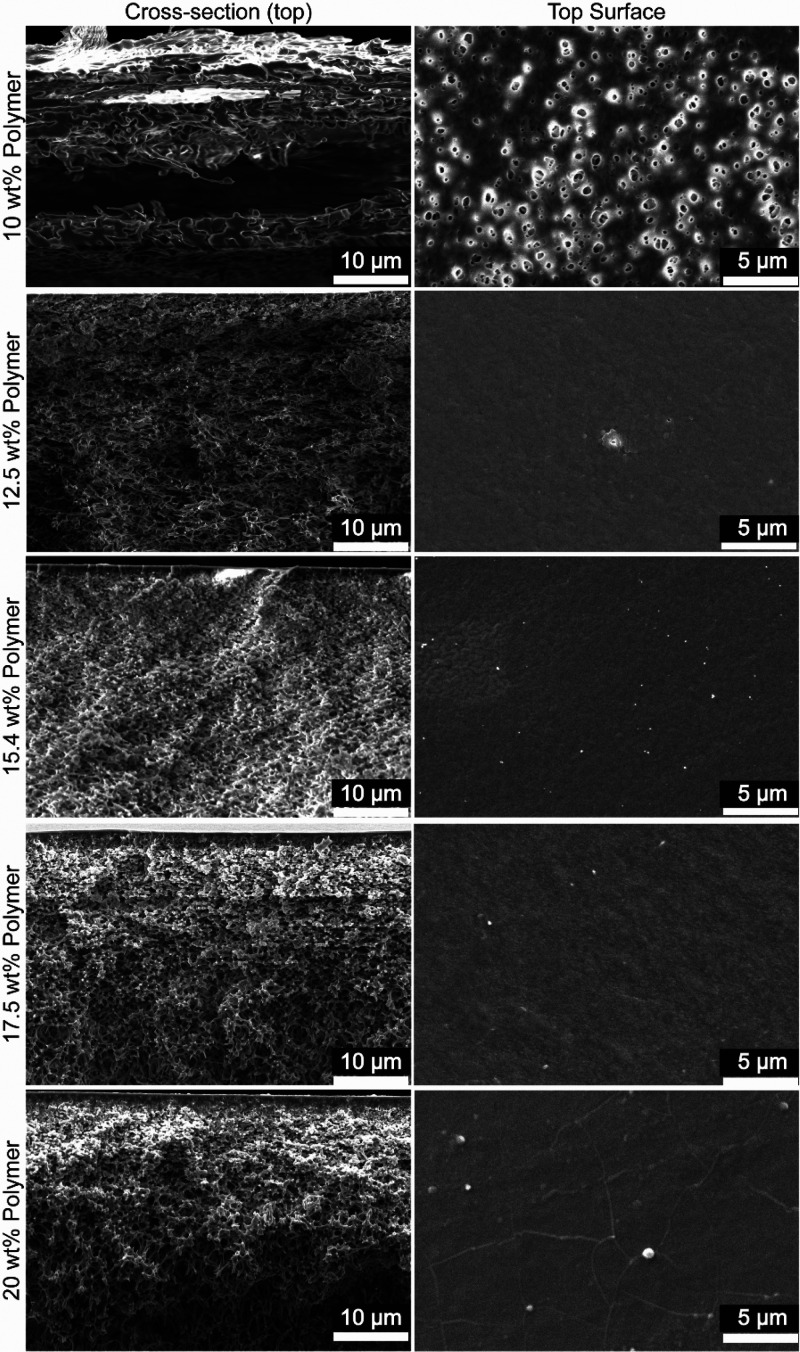
The polymer concentration of the casting solution is a widely used parameter for the NIPS process to control membrane structure.49 For APS, homogeneous PE mixtures with different polymer concentrations ranging from 10 to 20 wt % were prepared (see Table 1). The 10 wt % polyelectrolyte mixture with 18 wt % salt is slightly turbid compared to the other (see Figure S9); however, that mixture was cast and formed a continuous film. Figure 3 shows the cross-section and surface SEM images of the films prepared with mixtures at these different polymer concentrations. Films mentioned in this section will be referred to by the polymer concentration of the casting mixture.
Figure 3.
SEM images for cross-section and top surfaces of the membranes prepared with mixtures of varying total polymer concentration. All cross-section images are at ×2500 magnification and focused on the upper part of the membrane while surface images are at ×5000 magnification.
Except for the 10 wt % film, all these films again have an asymmetric structure with a thin skin layer and a porous and sponge-like support layer. The cross section of the 10 wt % film shows delamination within the entire structure, and the surface is porous (defects) unlike others. For the 12.5 wt % film there is a dense skin layer; however, the skin layer is very thin and has some defects on the surface. Both of these films were mechanically weak and therefore were not investigated further. Increasing the polymer concentration is known to reduce membrane pore size,49 and this has been observed for porous PSS–PDADMAC films in the literature.38 Therefore, polymer concentrations higher than 12.5 wt % are needed for mechanically stable membranes. SEM images of the 15.4, 17.5, and 20 wt % films are nearly identical. They have thicker skin layers (Figure S7) when compared to the 12.5 wt % film, and similar pore structure for the support layers is observed. Again, to investigate the membrane properties, pure water permeability tests were conducted at 4 bar with a dead-end filtration setup. Obtained PWP values are 1.0, 0.1, and 0.2 L m–2 h–1 bar–1 for 15.4, 17.5, and 20 wt % membranes, respectively. Skin layer thicknesses and the decrease in the PWP clearly show that increasing the polymer concentration densifies the skin layer, leading to a lower water transport. These three membranes are good candidates to investigate further, but importantly, the preparation procedure (see the Experimental Section) and high viscosity of the PE mixtures at 17.5 and 20 wt % polymer (see Table S1) make them nonideal for membrane production. For this reason the concentration of 15.4 wt % polymer mixture was chosen for all further experiments.
Effect of Coagulation Bath Salinity
So far, the different polyelectrolyte mixtures were prepared and evaluated in terms of the membranes that they form. Although increasing the molecular weight of PSS and increasing the total polymer concentration do not affect the membrane morphology significantly, these factors do affect the membrane performance. The homogeneous polyelectrolyte mixture prepared by using 200 kDa PSS and having 15.4 wt % polymer was chosen as the most promising mixture because it is easy to prepare and then cast, and it leads to reasonable pure water permeabilities. In the following, this mixture is used and the NaCl concentration in the coagulation bath is varied from 0 to 2 M to obtain membranes. The salinity of the coagulation bath will reduce the driving force for the complexation and thus slow down the diffusion of ions from the casting mixture to the bath. Therefore, it is expected that different membrane structures and performances can be obtained by varying the coagulation bath salinity.
Membrane Structure
Figure 4 shows SEM images of the films prepared with coagulation baths of different salinity. The left column gives images focused on the upper part of the cross section, and the right column gives the top surface images. It can be observed that all films have an asymmetric structure with a thin dense skin layer and porous support layer. The porous support layer is sponge-like in structure for the 0, 0.5, and 1 M films, with relatively small interconnected pores. However, for 1.5 and 2.0 M films, the internal pores are much larger, and this makes the films mechanically less stable. As in the traditional phase separation process, precipitation kinetics are expected to be the major factor in determining the membrane structure. As the NaCl concentration in the coagulation bath increases, the precipitation takes longer (see Video S1). Because the driving force for polyelectrolyte complexation is the entropic gain due to the release of counterions, the salt ions present in the coagulation bath limit this release, which leads to a slower complexation process and, consequently, a slower precipitation of the PEC. It can be seen in Video S1 that the precipitation rate of the 2.0 M film is much slower than the others; in fact, to solidify the film fully, it has to be transferred to a washing bath of demineralized water. Additionally, salt is known to be a plasticizer for PECs,30,51,52 and PSS–PDADMAC multilayers are known to undergo a so-called glass transition in salinities higher than 1.5 M NaCl;53,54 because of this, many studies use 2.0 M NaCl to anneal PSS–PDADMAC multilayers.53,55 Therefore, it is speculated that the combination of longer precipitation times and more mobile PE chains resulted in larger pores and/or defects for 1.5 and 2.0 M films. Besides the large defects in the skin layer seen in the 2.0 M film surface SEM image, large cavities in the cross section of the 1.5 and 2.0 M films indicate that these films will not be as mechanically stable. Indeed, during the PWP measurements on these films, the water flux was not stable and reproducible. Therefore, we did not continue characterizing these films further.
Figure 4.
SEM images for upper part of cross-section and top surfaces of the membranes prepared with varying salt concentrations of coagulation bath. All images are at ×5000 magnification.
Membrane Performance
The morphology of a membrane is one of the factors that directly affects the membrane performances; however, especially for dense membranes, molecular properties such as charge and affinity toward solutes become very important. In this section we discuss the performance of the membranes prepared with the PE mixture with 200 kDa PSS and 15.4 wt % polymer, coagulated in either 0, 0.5, or 1.0 M NaCl baths. For asymmetric membranes, the dense part (i.e., skin layer) provides the membrane its selectivity while porous part is very permeable and gives the mechanical support to the membrane. Therefore, the resistance of the membrane toward water permeation is dominated by the skin layer, and its thickness is inversely correlated to the permeability.2 Upon comparison of the 0, 0.5, and 1.0 M films, the skin layer is the thickest for the 1.0 M film (see Table 2) and the thinnest for the 0 M film. Therefore, it is expected that the 1.0 M membrane will have the lowest permeability (the highest water resistance). The PWP values of membranes (Figure 5) range from 0.4 to 1.0 L m–2 h–1 bar–1. These values indicate that the membranes are in the dense nanofiltration (NF) range, and they are in agreement with what we expected from SEM images.
Table 2. Skin Layer Thickness and Molecular Weight Cutoff Values of Membranes.
| membrane | skin layer thicknessa (μm) | MWCOb (Da) |
|---|---|---|
| 0 M | 0.6 ± 0.2 | 250 ± 30 |
| 0.5 M | 1.0 ± 0.2 | 280 ± 40 |
| 1.0 M | 1.2 ± 0.4 | 250 ± 30 |
Skin layer thicknesses were measured for at least 10 different points from one SEM image.
MWCO values are average of three measurements.
Figure 5.

Pure water permeability and MgSO4 retention of the membranes. The left-hand side of the dashed line (plain bars) shows the results of the filtrations at 4 bar while the right-hand side of the line (patterned bars) is at 10 bar. Pure water permeability values are the average, and error bars are the standard deviation of at least five measurements. Retention values are from at least three different measurements.
To understand the application range of these membranes, molecular weight cutoff measurements were performed. We stress that the MWCO values reported here provide information about the separation properties of the active separation layer. Still, in many cases we also observed defects that increased the overall MWCO of the membranes. The high polydispersity of especially the PDADMAC studied in this work could play a major role in the occurrence of these defects, as polydispersity is known to affect phase separation behavior of the solution in relation to membrane formation.56 Reducing the occurrence of defects in these PSS–PDADMAC membranes will be critical in their further development.
Membranes with MWCO values between 200 and 1000 Da are considered as NF membranes,2 and as shown in Table 2, all three PEC membranes fall in this range and can be considered as dense NF membranes. Based on this criteria, these membranes would be very suitable for the removal or concentration of small organic molecules, for example in biorefineries or in the removal of organics from wastewater.2
To further evaluate the performance of these novel NF membranes, the separation performance of the membranes toward multivalent ions was studied. For that, 5 mM of MgSO4 is used as feed solution. The plain bars in Figure 5 show the performance of three different membranes at 4 bar in terms of pure water permeability (gray bars) and MgSO4 retention (white bars). Although the PWP values progressively decrease with increasing skin layer thickness, MgSO4 retentions of the membranes increase from approximately 62% to 81%.
The MWCO, PWP, and salt retention data show that these membranes perform in the NF range; however, NF membranes are preferably operated at pressures higher than 4 bar.49 To understand whether these membranes can operate under these more challenging conditions, filtration experiments were also performed at 10 bar for the 0 M membrane. The patterned bars in Figure 5 show the performance at 10 bar. First, the PWP at 4 bar is 1.0 ± 0.2 L m–2 h–1 bar–1, while the one at 10 bar is 1.0 ± 0.1 L m–2 h–1 bar–1. This constant PWP illustrates that the membrane flux is linearly proportional to the transmembrane pressure; moreover, the membranes can withstand 10 bar of pressure without structural compaction or defect formation. It needs to be noted that for this membrane 10 bar was found to be the limit of stability; above this pressure an unstable performance was observed. However, at 10 bar, the water flux was stable for over a continuous 20 h and over a total of 50 h during the PWP measurements. Figure 5 shows that the MgSO4 retention at 10 bar is approximately 83%, while at 4 bar the retention is around 63%. This is in line with the expectation that these membranes function as dense membranes that separate on the basis of solution diffusion. An increase in pressure leads to a higher driving force for water permeation but not for the salt molecules. With increased water transport and similar salt transport the effective retention increases.2
The PWP values reported here are consistently low relative to the ones of PSS–PDADMAC multilayer membranes in the literature (5–15 L m–2 h–1 bar–1).20,29,57−60 This variation might be due to differences in selective layer thickness and density. On the other hand, MgSO4 retentions are reasonably comparable; in the literature, best results vary from 60% to more than 90%. Besides experimental differences, the defects observed in MWCO tests may be the cause of being near the low end of this range. Although this comparison indicates there is still room for improvement, it also implies that membrane formation via the salinity change APS technique is a good direction to follow for the preparation of sustainable NF membranes.
The stability of the membranes was not only evaluated as a function of transmembrane pressure but also as a function of time. Structural compaction is typically indicated by a decrease in water flux over time and is generally seen for polymeric membranes at the beginning of a filtration. However, a stable membrane is expected to maintain a persistent flux after the initial compaction period. In Figure 6, the water permeabilities of 0, 0.5, and 1.0 M membranes are given. The pure water permeability tests were continued over 55 h for each membrane. After a compaction period of <5 h (not included in the graph), the membranes showed a stable permeability. It needs to be noted that the erratic flux behavior is not an indication of instability of the flux, but they are deviations resulting from the experimental setup. These membranes thus show excellent stability over time when exposed to pure water. In some cases, exposure to MgSO4 during the retention tests led to a drop in flux. We expect that the ions are taken up by the membrane leading to some plasticization of the membrane matrix.
Figure 6.

Pure water permeability values of 0, 0.5, and 1.0 M membranes. The test was performed once for all the three membranes for ∼55 h.
Together with the mechanical stability, these PSS–PDADMAC membranes are expected to be stable in organic solvents and under extreme pH conditions.29,48 Indeed, 0 M membranes showed no observable changes when they are immersed in 1 M NaOH and 1 M HCl for 40 days (see Figure S10).
In this work, PSS–PDADMAC polyelectrolyte complex membranes were prepared via a simple aqueous phase separation approach that does not use any organic solvents or harsh aqueous conditions like extreme pH values. This APS system has a natural tendency to form asymmetric, nanofiltration membranes. The membrane morphology can be tuned by coagulation bath salinity, and the resultant membranes are macrovoid-free, which is a highly beneficial feature for a phase separation approach. Separation performance and mechanical stability of the membranes indicate that they are promising candidates for NF applications. Moreover, after being used, the salts from the coagulation baths can easily be recycled, making the overall system much more sustainable compared to conventional phase separation membranes. Overall, this study shows a unique and sustainable way of making polymeric nanofiltration membranes with good separation properties.
Conclusions
In this study, we produced membranes by polyelectrolyte complexation induced aqueous phase separation (APS). Complexation is achieved by exposing a thin film of a homogeneous mixture of polyanion and polycation prepared at high salinity to a coagulation bath with a low salinity. Complexation of the two strong polyelectrolytes leads to phase separation and to the formation of asymmetric membranes with dense top layers and porous support layers. The effects of polyanion molecular weight, total polymer concentration of the mixture, and the coagulation bath salinity on membrane formation were investigated. It was seen that increasing both the molecular weight of PSS and the total polymer concentration led to denser membrane matrices and also improved the mechanical properties of the films, likely due to increased chain entanglements. The optimal casting mixture was found to be the one with 200 kDa PSS and 15.4 wt % polymer which combined the positives of ease of preparation with good mechanical properties of the resulting membranes.
In the second part of this work, the coagulation bath salinity was varied, and resultant membranes were studied in terms of their morphology, permeability, and retention performances. SEM cross-section images revealed that increasing the coagulation bath salinity resulted in membranes with thicker skin layers and larger pores in the support layers. At high bath salinities, namely 1.5 and 2.0 M NaCl, the combined effects of longer precipitation times and more mobile PE chains resulted in structures that could not be used as stable membranes. However, at lower salinities (0, 0.5, and 1.0 M NaCl), useful membrane structures were obtained. These membranes had water permeabilities ranging from 0.4 to 1.0 L m–2 h–1 bar–1 and MWCO values between 200 and 300 Da. Together with these low MWCO values, good MgSO4 retentions (>60%) were found, showing that these membranes indeed function as nanofiltration membranes. All of these membranes show good long-term stability at 4 bar of applied pressure, with the membrane produced at 0 M NaCl having good long-term stability even at 10 bar. An increase in transmembrane pressure led to significant increases in ion retention (from approximately 60 to 80%), as expected for dense membranes that separate species on the basis of solution diffusion mechanism.
We have demonstrated that it is possible to prepare novel nanofiltration membranes with good mechanical properties and separation performance in a simple single-step aqueous phase separation process. Further optimization of the APS process is expected to lead to higher water fluxes, selectivities, and better mechanical properties. This work thus demonstrates that APS is a very relevant and sustainable alternative to the traditional aprotic solvent based NIPS process. Interesting for future work is the fact that PSS–PDADMAC complexes are very stable in organic solvents, especially in nonpolar ones.48 Therefore, in further research these nanofiltration membranes will be investigated in terms of their organic solvent nanofiltration performance.
Glossary
Abbreviations
- PEC
polyelectrolyte complex
- APS
aqueous phase separation
- PSS
poly(sodium 4-styrenesulfonate)
- PDADMAC
poly(diallyldimethylammonium chloride)
- NIPS
nonsolvent induced phase separation
- NMP
n-methyl-2-pyrrolidone
- TIPS
thermally induced phase separation
- PE
polyelectrolyte
- PEM
polyelectrolyte multilayer
- UF
ultrafiltration
- MF
microfiltration
- NF
nanofiltration
- IPA
2-propanol
- PEG
poly(ethylene glycol)
- SEM
scanning electron microscopy
- EDS
energy dispersive X-ray spectrometer
- TMP
transmembrane pressure
- MWCO
molecular weight cutoff
- GPC
gel permeation chromatography
- PWP
pure water permeability.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsapm.0c00255.
An example of skin layer thickness measurement, a scheme for filtration setup, an example and explanation of sieving curve for MWCO measurement, a representative top surface SEM image at ×10000 magnification, a representative cross-section SEM image at ×10000 magnification, EDS mapping of 0 M membrane surface, table for mixture viscosities, figure of skin layer thicknesses, image for solution viscosities, image for turbid mixture, video for phase separation rates, figure for pH stability of the membranes (PDF)
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (ERC StG 714744 SAMBA). W.d.V. and J.D.W. acknowledge funding support from the “Vernieuwingsimpuls” program through project number VIDI 723.015.003 (financed by The Netherlands Organization for Scientific Research, NWO).
The authors declare no competing financial interest.
Supplementary Material
References
- Strathmann H.Introduction to Membrane Science and Technology, 1st ed.; Wiley-VCH Verlag & Co. KGaA: Weinheim, 2011. [Google Scholar]
- Baker R. W.Membrane Technology and Applications, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Prat D.; Hayler J.; Wells A. A Survey of Solvent Selection Guides. Green Chem. 2014, 16 (10), 4546–4551. 10.1039/C4GC01149J. [DOI] [Google Scholar]
- European Chemical Agency; https://echa.europa.eu/substance-information/-/substanceinfo/100.011.662 (accessed May 9, 2020).
- Figoli A.; Marino T.; Simone S.; Di Nicolò E.; Li X. M.; He T.; Tornaghi S.; Drioli E. Towards Non-Toxic Solvents for Membrane Preparation: A Review. Green Chem. 2014, 16 (9), 4034–4059. 10.1039/C4GC00613E. [DOI] [Google Scholar]
- Prézélus F.; Chabni D.; Barna L.; Guigui C.; Remigy J.-C. A Metrics-Based Approach to Preparing Sustainable Membranes: Application to Ultrafiltration. Green Chem. 2019, 21, 4457–4469. 10.1039/C9GC01178A. [DOI] [Google Scholar]
- M’barki O.; Hanafia A.; Bouyer D.; Faur C.; Sescousse R.; Delabre U.; Blot C.; Guenoun P.; Deratani A.; Quemener D.; Pochat-Bohatier C. Greener Method to Prepare Porous Polymer Membranes by Combining Thermally Induced Phase Separation and Crosslinking of Poly(Vinyl Alcohol) in Water. J. Membr. Sci. 2014, 458, 225–235. 10.1016/j.memsci.2013.12.013. [DOI] [Google Scholar]
- Hanafia A.; Faur C.; Guenoun P.; Garate H.; Deratani A.; Quemener D.; Pochat-Bohatier C.; Bouyer D. Fabrication of Novel Porous Membrane from Biobased Water-Soluble Polymer (Hydroxypropylcellulose). J. Membr. Sci. 2017, 526, 212–220. 10.1016/j.memsci.2016.12.037. [DOI] [Google Scholar]
- Matsuyama H.; Berghmans S.; Lloyd D. R. Formation of Anisotropic Membranes via Thermally Induced Phase Separation. Polymer 1999, 40, 2289–2301. 10.1016/S0032-3861(98)00040-8. [DOI] [Google Scholar]
- de Vos W. M. (Universiteit Twente), Aqueous phase separation method. U.S. Patent 20180318775 A1, Application no. 15/972,273, 2018.
- Willott J. D.; Nielen W. M.; de Vos W. M. Stimuli-Responsive Membranes through Sustainable Aqueous Phase Separation. ACS Appl. Polym. Mater. 2020, 2 (2), 659–667. 10.1021/acsapm.9b01006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v. Klitzing R. Internal Structure of Polyelectrolyte Multilayer Assemblies. Phys. Chem. Chem. Phys. 2006, 8 (43), 5012. 10.1039/b607760a. [DOI] [PubMed] [Google Scholar]
- Lindhoud S.; Stuart M. A. C.. Relaxation Phenomena During Polyelectrolyte Complex Formation. In Polyelectrolyte Complexes in the Dispersed and Solid State I: Principles and Theory; Müller M., Ed.; Springer: Berlin, 2014; pp 139–172. [Google Scholar]
- Ali S.; Prabhu V. Relaxation Behavior by Time-Salt and Time-Temperature Superpositions of Polyelectrolyte Complexes from Coacervate to Precipitate. Gels 2018, 4 (1), 11. 10.3390/gels4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adusumilli M.; Bruening M. L. Variation of Ion-Exchange Capacity, ζ Potential, and Ion-Transport Selectivities with the Number of Layers in a Multilayer Polyelectrolyte Film. Langmuir 2009, 25 (13), 7478–7485. 10.1021/la900391q. [DOI] [PubMed] [Google Scholar]
- Shan W.; Bacchin P.; Aimar P.; Bruening M. L.; Tarabara V. V. Polyelectrolyte Multilayer Films as Backflushable Nanofiltration Membranes with Tunable Hydrophilicity and Surface Charge. J. Membr. Sci. 2010, 349 (1–2), 268–278. 10.1016/j.memsci.2009.11.059. [DOI] [Google Scholar]
- Malaisamy R.; Bruening M. L. High-Flux Nanofiltration Membranes Prepared by Adsorption of Multilayer Polyelectrolyte Membranes on Polymeric Supports. Langmuir 2005, 21 (23), 10587–10592. 10.1021/la051669s. [DOI] [PubMed] [Google Scholar]
- Tieke B.; Van Ackern F.; Krasemann L.; Toutianoush A. Ultrathin Self-Assembled Polyelectrolyte Multilayer Membranes. Eur. Phys. J. E: Soft Matter Biol. Phys. 2001, 5 (1), 29–39. 10.1007/s101890170084. [DOI] [Google Scholar]
- Krasemann L.; Tieke B. Selective Ion Transport across Self-Assembled Alternating Multilayers of Cationic and Anionic Polyelectrolytes. Langmuir 2000, 16 (2), 287–290. 10.1021/la991240z. [DOI] [Google Scholar]
- de Grooth J.; Haakmeester B.; Wever C.; Potreck J.; de Vos W. M.; Nijmeijer K. Long Term Physical and Chemical Stability of Polyelectrolyte Multilayer Membranes. J. Membr. Sci. 2015, 489, 153–159. 10.1016/j.memsci.2015.04.031. [DOI] [Google Scholar]
- Wong S. Y.; Han L.; Timachova K.; Veselinovic J.; Hyder M. N.; Ortiz C.; Klibanov A. M.; Hammond P. T. Drastically Lowered Protein Adsorption on Microbicidal Hydrophobic/Hydrophilic Polyelectrolyte Multilayers. Biomacromolecules 2012, 13 (3), 719–726. 10.1021/bm201637e. [DOI] [PubMed] [Google Scholar]
- Yu W.; Koc J.; Finlay J. A.; Clarke J. L.; Clare A. S.; Rosenhahn A. Layer-by-Layer Constructed Hyaluronic Acid/Chitosan Multilayers as Antifouling and Fouling-Release Coatings. Biointerphases 2019, 14 (5), 051002. 10.1116/1.5110887. [DOI] [PubMed] [Google Scholar]
- Almodóvar J.; Place L. W.; Gogolski J.; Erickson K.; Kipper M. J. Layer-by-Layer Assembly of Polysaccharide-Based Polyelectrolyte Multilayers: A Spectroscopic Study of Hydrophilicity, Composition, and Ion Pairing. Biomacromolecules 2011, 12 (7), 2755–2765. 10.1021/bm200519y. [DOI] [PubMed] [Google Scholar]
- Pasco E. V.; Shi H.; Xagoraraki I.; Hashsham S. A.; Parent K. N.; Bruening M. L.; Tarabara V. V. Polyelectrolyte Multilayers as Anti-Adhesive Membrane Coatings for Virus Concentration and Recovery. J. Membr. Sci. 2014, 469, 140–150. 10.1016/j.memsci.2014.06.032. [DOI] [Google Scholar]
- Hong S. U.; Malaisamy R.; Bruening M. L. Separation of Fluoride from Other Monovalent Anions Using Multilayer Polyelectrolyte Nanofiltration Membranes. Langmuir 2007, 23 (4), 1716–1722. 10.1021/la061701y. [DOI] [PubMed] [Google Scholar]
- Hong S. U.; Malaisamy R.; Bruening M. L. Optimization of Flux and Selectivity in Cl-/SO42- Separations with Multilayer Polyelectrolyte Membranes. J. Membr. Sci. 2006, 283 (1–2), 366–372. 10.1016/j.memsci.2006.07.007. [DOI] [Google Scholar]
- Hong S. U.; Ouyang L.; Bruening M. L. Recovery of Phosphate Using Multilayer Polyelectrolyte Nanofiltration Membranes. J. Membr. Sci. 2009, 327 (1–2), 2–5. 10.1016/j.memsci.2008.11.035. [DOI] [Google Scholar]
- Elzbieciak M.; Kolasinska M.; Warszynski P. Characteristics of Polyelectrolyte Multilayers: The Effect of Polyion Charge on Thickness and Wetting Properties. Colloids Surf., A 2008, 321 (1–3), 258–261. 10.1016/j.colsurfa.2008.01.036. [DOI] [Google Scholar]
- Remmen K.; Schäfer R.; Hedwig S.; Wintgens T.; Wessling M.; Lenz M. Layer-by-Layer Membrane Modification Allows Scandium Recovery by Nanofiltration. Environ. Sci. Water Res. Technol. 2019, 5 (10), 1683–1688. 10.1039/C9EW00509A. [DOI] [Google Scholar]
- Schaaf P.; Schlenoff J. B. Saloplastics: Processing Compact Polyelectrolyte Complexes. Adv. Mater. 2015, 27 (15), 2420–2432. 10.1002/adma.201500176. [DOI] [PubMed] [Google Scholar]
- Porcel C.; Schlenoff J. Compact Polyelectrolyte Complexes:“Saloplastic” Candidates for Biomaterials. Biomacromolecules 2009, 10, 2968–2975. 10.1021/bm900373c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoun R. F.; Reisch A.; Schlenoff J. B. Extruded Saloplastic Polyelectrolyte Complexes. Adv. Funct. Mater. 2012, 22 (9), 1923–1931. 10.1002/adfm.201102787. [DOI] [Google Scholar]
- Wang Q.; Schlenoff J. B. Tough Strained Fibers of a Polyelectrolyte Complex: Pretensioned Polymers. RSC Adv. 2014, 4 (87), 46675–46679. 10.1039/C4RA08733J. [DOI] [Google Scholar]
- Kelly K. D.; Schlenoff J. B. Spin-Coated Polyelectrolyte Coacervate Films. ACS Appl. Mater. Interfaces 2015, 7 (25), 13980–13986. 10.1021/acsami.5b02988. [DOI] [PubMed] [Google Scholar]
- Reisch A.; Roger E.; Phoeung T.; Antheaume C.; Orthlieb C.; Boulmedais F.; Lavalle P.; Schlenoff J. B.; Frisch B.; Schaaf P. On the Benefits of Rubbing Salt in the Cut: Self-Healing of Saloplastic PAA/PAH Compact Polyelectrolyte Complexes. Adv. Mater. 2014, 26 (16), 2547–2551. 10.1002/adma.201304991. [DOI] [PubMed] [Google Scholar]
- Hariri H. H.; Schlenoff J. B. Saloplastic Macroporous Polyelectrolyte Complexes: Cartilage Mimics. Macromolecules 2010, 43 (20), 8656–8663. 10.1021/ma1012978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X.; Perry S. L.; Schiffman J. D. Complex Coacervation: Chemically Stable Fibers Electrospun from Aqueous Polyelectrolyte Solutions. ACS Macro Lett. 2017, 6, 505–511. 10.1021/acsmacrolett.7b00173. [DOI] [PubMed] [Google Scholar]
- Murakawa K.; King D. R.; Sun T.; Guo H.; Kurokawa T.; Gong J. P. Polyelectrolyte Complexation via Viscoelastic Phase Separation Results in Tough and Self-Recovering Porous Hydrogels. J. Mater. Chem. B 2019, 7 (35), 5296–5305. 10.1039/C9TB01376H. [DOI] [PubMed] [Google Scholar]
- Kurtz I. S.; Sui S.; Hao X.; Huang M.; Perry S. L.; Schiffman J. D. Bacteria-Resistant, Transparent, Free-Standing Films Prepared from Complex Coacervates. ACS Appl. Bio Mater. 2019, 2 (9), 3926–3933. 10.1021/acsabm.9b00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile M.; Sarwar O.; Henderson R.; Smith R.; Grunlan J. C. Polyelectrolyte Coacervates Deposited as High Gas Barrier Thin Films. Macromol. Rapid Commun. 2017, 38 (1), 1–5. 10.1002/marc.201600594. [DOI] [PubMed] [Google Scholar]
- Smith R. J.; Long C. T.; Grunlan J. C. Transparent Polyelectrolyte Complex Thin Films with Ultralow Oxygen Transmission Rate. Langmuir 2018, 34, 11086–11091. 10.1021/acs.langmuir.8b02391. [DOI] [PubMed] [Google Scholar]
- Nikolaev K. G.; Ulasevich S. A.; Luneva O.; Orlova O. Y.; Vasileva D.; Vasilev S.; Novikov A. S.; Skorb E. V. Humidity-Driven Transparent Holographic Free-Standing Polyelectrolyte Films. ACS Appl. Polym. Mater. 2020, 2 (2), 105–112. 10.1021/acsapm.9b01151. [DOI] [Google Scholar]
- Gai M.; Frueh J.; Kudryavtseva V. L.; Mao R.; Kiryukhin M. V.; Sukhorukov G. B. Patterned Microstructure Fabrication: Polyelectrolyte Complexes vs Polyelectrolyte Multilayers. Sci. Rep. 2016, 6 (July), 1–11. 10.1038/srep37000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Wang C.; Zhu G.; Zacharia N. S. Self-Healing of Bulk Polyelectrolyte Complex Material as a Function of PH and Salt. ACS Appl. Mater. Interfaces 2016, 8 (39), 26258–26265. 10.1021/acsami.6b06776. [DOI] [PubMed] [Google Scholar]
- Fu J.; Wang Q.; Schlenoff J. B. Extruded Superparamagnetic Saloplastic Polyelectrolyte Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7 (1), 895–901. 10.1021/am5074694. [DOI] [PubMed] [Google Scholar]
- Sadman K.; Delgado D. E.; Won Y.; Wang Q.; Gray K. A.; Shull K. R. Versatile and High-Throughput Polyelectrolyte Complex Membranes via Phase Inversion. ACS Appl. Mater. Interfaces 2019, 11 (17), 16018–16026. 10.1021/acsami.9b02115. [DOI] [PubMed] [Google Scholar]
- Baig M. I.; Durmaz E. N.; Willott J. D.; Vos W. M. Sustainable Membrane Production through Polyelectrolyte Complexation Induced Aqueous Phase Separation. Adv. Funct. Mater. 2020, 30 (5), 1907344. 10.1002/adfm.201907344. [DOI] [Google Scholar]
- Fares H. M.; Wang Q.; Yang M.; Schlenoff J. B. Swelling and Inflation in Polyelectrolyte Complexes. Macromolecules 2019, 52 (2), 610–619. 10.1021/acs.macromol.8b01838. [DOI] [Google Scholar]
- Mulder M.Basic Principles of Membrane Technology, 2nd ed.; Kluwer Academic Publishers: Dordrecht, 1996. [Google Scholar]
- Seymour R.; Carraher C.. Structure-Property Relationships In Polymer, 1st ed.; Plenum Press: New York, 1984. [Google Scholar]
- Zhang R.; Zhang Y.; Antila H. S.; Lutkenhaus J. L.; Sammalkorpi M. Role of Salt and Water in the Plasticization of PDAC/PSS Polyelectrolyte Assemblies. J. Phys. Chem. B 2017, 121 (1), 322–333. 10.1021/acs.jpcb.6b12315. [DOI] [PubMed] [Google Scholar]
- Han L.; Mao Z.; Wuliyasu H.; Wu J.; Gong X.; Yang Y.; Gao C. Modulating the Structure and Properties of Poly(Sodium 4-Styrenesulfonate)/ Poly(Diallyldimethylammonium Chloride) Multilayers with Concentrated Salt Solutions. Langmuir 2012, 28 (1), 193–199. 10.1021/la2040533. [DOI] [PubMed] [Google Scholar]
- Reurink D. M.; Haven J. P.; Achterhuis I.; Lindhoud S.; Roesink E. H. D. W.; de Vos W. M. Annealing of Polyelectrolyte Multilayers for Control over Ion Permeation. Adv. Mater. Interfaces 2018, 5 (20), 1800651. 10.1002/admi.201800651. [DOI] [Google Scholar]
- Shamoun R. F.; Hariri H. H.; Ghostine R. A.; Schlenoff J. B. Thermal Transformations in Extruded Saloplastic Polyelectrolyte Complexes. Macromolecules 2012, 45 (24), 9759–9767. 10.1021/ma302075p. [DOI] [Google Scholar]
- Fares H. M.; Ghoussoub Y. E.; Surmaitis R. L.; Schlenoff J. B. Toward Ion-Free Polyelectrolyte Multilayers: Cyclic Salt Annealing. Langmuir 2015, 31 (21), 5787–5795. 10.1021/la504910y. [DOI] [PubMed] [Google Scholar]
- Van De Witte P.; Dijkstra P. J.; Van Den Berg J. W. A.; Feijen J. Phase Separation Processes in Polymer Solutions in Relation to Membrane Formation. J. Membr. Sci. 1996, 117 (1–2), 1–31. 10.1016/0376-7388(96)00088-9. [DOI] [Google Scholar]
- Gherasim C. V.; Luelf T.; Roth H.; Wessling M. Dual-Charged Hollow Fiber Membranes for Low-Pressure Nanofiltration Based on Polyelectrolyte Complexes: One-Step Fabrication with Tailored Functionalities. ACS Appl. Mater. Interfaces 2016, 8 (29), 19145–19157. 10.1021/acsami.6b05706. [DOI] [PubMed] [Google Scholar]
- Menne D.; Kamp J.; Erik Wong J.; Wessling M. Precise Tuning of Salt Retention of Backwashable Polyelectrolyte Multilayer Hollow Fiber Nanofiltration Membranes. J. Membr. Sci. 2016, 499, 396–405. 10.1016/j.memsci.2015.10.058. [DOI] [Google Scholar]
- Irigoyen J.; Laakso T.; Politakos N.; Dahne L.; Pihlajamäki A.; Mänttäri M.; Moya S. E. Design and Performance Evaluation of Hybrid Nanofiltration Membranes Based on Multiwalled Carbon Nanotubes and Polyelectrolyte Multilayers for Larger Ion Rejection and Separation. Macromol. Chem. Phys. 2016, 217 (6), 804–811. 10.1002/macp.201500433. [DOI] [Google Scholar]
- Ng L. Y.; Mohammad A. W.; Ng C. Y.; Rohani R. Optimization of Polymeric Membrane Characteristics through Thermal Treatment and Deposition of Polyelectrolyte Layers Using Response Surface Modeling. Adv. Polym. Technol. 2015, 34 (1), 1–15. 10.1002/adv.21472. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.