Abstract
Immune function, height and resource accumulation comprise important life history traits in humans. Resource availability models arising from life history theory suggest that socioeconomic conditions influence immune function, growth and health status. In this study, we tested whether there are associations between family income during ontogeny, adult height, cortisol level and immune response in women. A hepatitis B vaccine was administered to 66 young Latvian women from different socioeconomic backgrounds, and blood samples were then collected to measure the level of antibodies that the women produced in response to the vaccination. Cortisol levels were measured from plasma samples pre- and post-vaccination. Women from wealthier families had lower cortisol levels, and women from the highest family income group had the highest levels of antibody titers against hepatitis B vaccine. No significant relationships were observed between cortisol level and immune function, nor between family income and height. The results show that income level during ontogeny is associated with the strength of immune response and with psychoneuroendocrine pathways underlying stress perception in early adulthood. The findings indicate that the quality of the developmental niche is associated with the condition-dependent expression of immune function and stress response.
Subject terms: Biological anthropology, Evolutionary ecology
Introduction
Life history theory focuses on how organisms allocate finite resources to maximize their evolutionary fitness, with the ultimate goal of passing their genes to the next generation. Life history theory is predicated on the idea that the principal functions of organismal growth, survival and reproduction require sufficient resources, parceled out from the finite energy that each organism can extract from its environment1,2. Scarcity of bioenergetic resources restrains the development of central life history functions such as somatic growth, immune function, reproduction and socioeconomic development3–10. It has been shown that growing up in poverty causes developmental stress11 and that exposure to poverty is linked with premature aging12. Resource availability is therefore an important dimension in life history models of human evolution and development9,10.
Each life stage brings a distinct set of adaptive challenges: responses to these challenges affect organismal development and function not only in childhood or adolescence2,13–15, but they also predict viability in adulthood10,16. Krams et al.9 have recently studied associations between socioeconomic status (SES), height and antibody titers against hepatitis B antigen (a measure of strength of immune response) in young Latvian men. The findings showed positive correlations between height and antibody response. The relationship between height and strength of immune response was indirect, and both variables were associated with family income9. The findings highlight the importance of childhood environment and nutrition to ensure that young people make the best possible start in life with regard to somatic and immunological development9.
Individual differences in family income, height and immune function can be conceptualized using a combination of developmental niche construction and life history theory9,13. Developmental niche construction recognizes the importance of environmental parameters in modifying the life cycle and in shaping the development of plastic phenotypes13,17. According to resource availability models based on life history theory, a high-quality environment can reduce somatic maintenance costs inasmuch as such an environment imposes fewer threats to the immune system; this, in turn, can lead to increased growth rates and earlier reproduction5. In contrast, worse socioeconomic conditions (particularly in Africa) are associated with declines in women’s height4. The correlation between wealth and height was positive (95% CI 0.05–1.16) in 96% of 54 countries observed4. Morisaki and colleagues note that better environmental and social conditions, e.g. nutrition and sanitation, are the reasons for increases in average adult height in the majority of European and Asian countries over the last century18. However, recent trends in reduced average adult height in both sexes in Japan have been linked with an increase in low birth weight prevalence because of undernutrition, infection and social factors such as increased competition18.
Sexual dimorphism and/or sex differences occur in various traits and life history strategies that comprise important components of fitness10,19–22. Phenotypic characteristics such as body size5,23–25, physical strength26,27, appearance28 and immune defense3,6,29 all show significant sex differences in humans. These differences are influenced by genetics21,30,31, but also by socioeconomic and environmental factors15,32,33. Because of its whole-organism focus on resource allocations and phenotypic plasticity guided by environmental conditions, life history theory has substantial utility for explaining these sex differences and the adaptive processes underlying them10,20,34,35.
Recent empirical findings support some of the main hypotheses arising from life history theory, namely that competing functions and processes cause bioenergetic trade-offs between life history traits36, and that the availability of bioenergetic resources can restrict the development of life history traits9,10,14,37. The findings also suggest that trait development might be at least moderately sex-specific. Georgiev and colleagues, for instance, detected that women appeared immunologically more sensitive to pathogen exposure early in life than men6. Exposure to early life psychosocial stress can perturb the development of hypothalamic–pituitary–adrenal (HPA) and hypothalamic–pituitary–gonadal (HPG) coupling, resulting in early sexual maturation and early reproduction in females15. Women made a higher relative investment toward innate immunity, not acquired immunity6. Stoehr and Kokko explained women’s greater investment in immune function as an investment in longevity3. The sex that makes a higher investment in survival and longevity—typically females—will have superior immune defenses, a prediction supported by many studies38–42.
There is a growing recognition in biomedical research that while sex is among the most important individual characteristics related to health and disease, women remain understudied relative to men21. In this study, we therefore sought to evaluate whether recent findings on the relationships between men’s socioeconomic background, height and immune function replicate in women9. We reanalyzed a part of the data sets from Krams et al.43 and Skrinda et al.44 and added data that have not been used before. We predicted that young women with taller stature and stronger immune response grew up in families that had higher income. We predicted negative correlations between family income and cortisol levels, and between cortisol levels and antibody titers (Fig. 1). Since increased adiposity is associated with impaired immune function45, we also expected that total fat, visceral fat and BMI would be negatively correlated with antibody titers (Fig. 1).
Figure 1.
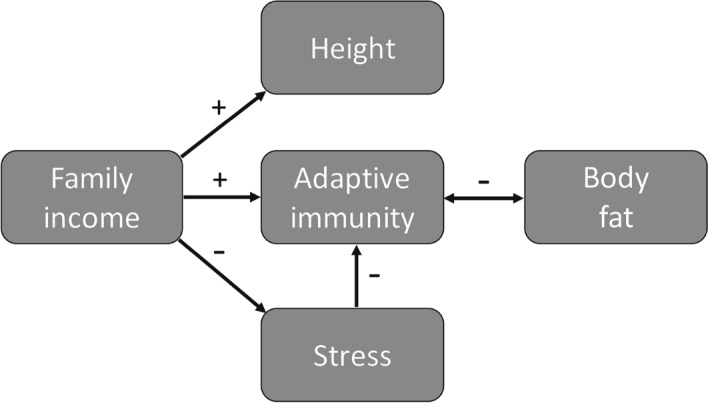
A conceptual framework of the study, showing the predicted relationships between the level of family income, the strength of adaptive immune response, body fat reserves, stress intensity and height in a sample of young Latvian women.
Results
Participants
We studied associations between socioeconomic status, cortisol levels, height and antibody titers against hepatitis B antigen in 66 young Latvian women (20.62 ± 0.86 years old, mean ± SD, range 19–22 years) (Table 1) in southeastern Latvia in 2010. Participants were students from Daugavpils University and Transportation College of Daugavpils. We measured participants’ height between 8.00 and 11.00 A.M. with a precision of 0.5 cm as described elsewhere9,43,44. None of the participants had any major disorders or infectious diseases either prior to or during the study. The average height in the current sample was 167.81 ± 6.14 cm, while the average height for 20–21-year-old women is 169.2 ± 0.7 cm in our population46. We measured each participant's body fat percentage (the total fat content and visceral fat) by using Omron Body Composition Monitor BF500. None of the participants was obese: body mass index (BMI) of 56 young women fell within the normal BMI range (18.5–24.9 25) while 10 participants were overweight (BMI between 25 and 30).
Table 1.
Demographic parameters and the corresponding mean values (± SD) of height, cortisol, BMI and fat reserves in a sample of young Latvian women (n = 66).
| Age | Height (cm) | Cortisol (nmol/L) | BMI | Total fat (%) | Visceral fat (%) |
|---|---|---|---|---|---|
| 19, n = 4 | 167.1 ± 4.77 | 480.5 ± 263.22 | 20.7 ± 2.82 | 28.7 ± 7.30 | 2.8 ± 1.26 |
| 20, n = 29 | 167.1 ± 6.48 | 349.8 ± 169.25 | 21.7 ± 3.24 | 29.1 ± 8.21 | 3.2 ± 1.24 |
| 21, n = 21 | 169.4 ± 6.27 | 375.3 ± 158.09 | 21.3 ± 3.65 | 29.0 ± 7.95 | 3.1 ± 1.14 |
| 22, n = 12 | 167.1 ± 5.57 | 355.7 ± 162.33 | 22.2 ± 3.45 | 29.1 ± 9.17 | 3.3 ± 1.36 |
The participants visited the laboratory during the fertile phase of their menstrual cycle (20–14 days before the onset of their next period of menstrual bleeding). This method of fertility estimation is based on the assumption that the luteal phase lasts 14 days and that the fertile phase does not exceed 6 days47.
Immune response
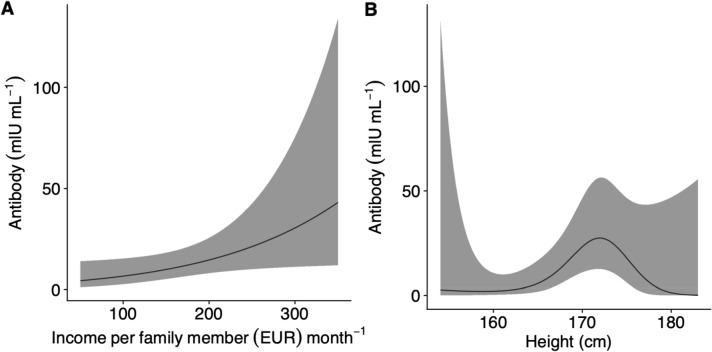
Frequency distribution of immune response of young women was right-skewed (mean = 3.83, SE = 0.3), with 39 of 63 (62%) women having 0 mIU ml−1 of anti-HBs (Fig. 2). The strength of immune response was found to be significantly different across income groups (Kruskal–Wallis H = 14.7, d.f. = 5, P = 0.012) (Fig. 3), with the highest level of immune response (median = 19.5) observed in the highest income group. Analyses done within the generalized additive model (GAM) framework showed a significant relationship between income and antibody titers (Fig. 4A, GAM Tweedie model, edf = 1, P = 0.043).
Figure 2.
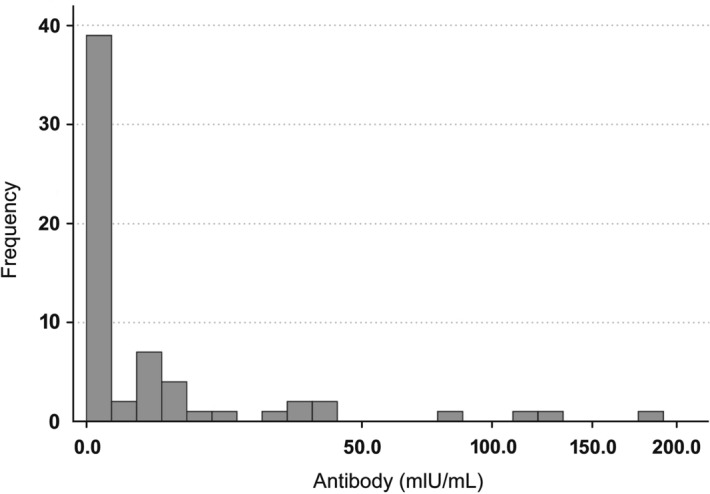
Frequency distribution of immune response in a sample of young Latvian women (n = 66), shown on power (0.5) scale.
Figure 3.
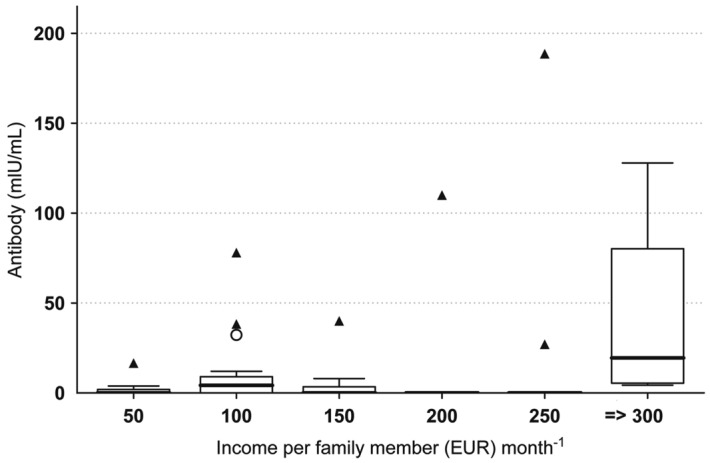
Median immune response across the range of family income in a sample of young Latvian women (n = 66). Thick lines indicate median, box—interquartile range, whiskers—nonoutlier range, circle—outlier, triangle—extreme outlier.
Figure 4.
(A) A non-linear relationship between income level and immune response and (B) a non-linear relationship between height and immune response in a sample of young Latvian women (n = 66).
Height
There were no significant correlations between family income and height (rs = 0.05, P = 0.69). There was a weak, non-significant linear (rs = 0.074, P = 0.56) and a marginally non-significant non-linear (GAM Tweedie model, edf = 2.8, P = 0.066) relationship between height and antibody titers (Fig. 4B).
Cortisol
A negative correlation was found between family income and cortisol level (rs = -0.481, P < 0.001; Fig. 5). There was no significant linear (rs = 0.102, P = 0.426) or non-linear (GAM Tweedie model, edf = 2.3, P = 0.114) relationship between cortisol level and antibody titers.
Figure 5.
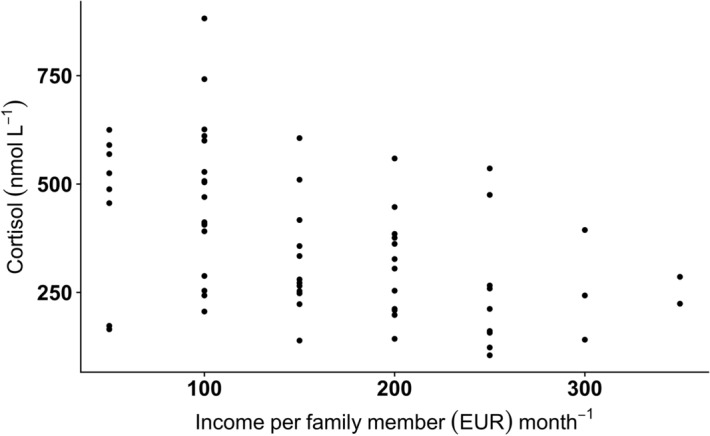
A relationship between cortisol level and income level in a sample of young Latvian women (n = 66) (rs = − 0.481, P < 0.001).
Adiposity
Family income was not associated with total fat nor visceral fat (both Ps > 0.05, Table 2). The relationship between family income and BMI was weak and non-significant (rs = 0.073, P = 0.558). The strength of immune response was not associated with BMI, total fat nor visceral fat (all Ps > 0.05, Table 2). There were no significant correlations between cortisol and total fat, visceral fat and BMI (all Ps > 0.05, Table 2).
Table 2.
Spearman’s rank correlation coefficients and P values (below) for the relationships between antibody titers, height, income, cortisol, BMI, total fat and visceral fat in a sample of young Latvian women (n = 66).
| Height | Income | Cortisol | BMI | Total fat | Visceral fat | |
|---|---|---|---|---|---|---|
| Antibody | 0.074 (P = 0.560) | − 0.002 (P = 0.988) | 0.102 (P = 0.426) | 0.005 (P = 0.972) | − 0.016 (P = 0.899) | − 0.008 (P = 0.948) |
| Height | 0.051 (P = 0.686) | 0.138 (P = 0.270) | 0.021 (P = 0.868) | 0.034 (P = 0.786) | − 0.056 (P = 0.653) | |
| Income | − 0.481*** (P < 0.001) | 0.073 (P = 0.558) | 0.039 (P = 0.759) | 0.062 (P = 0.621) | ||
| Cortisol | − 0.022 (P = 0.858) | − 0.024 (P = 0.847) | − 0.091 (P = 0.465) | |||
| BMI | 0.900*** (P < 0.001) | 0.880*** (P < 0.001) | ||||
| Total fat | 0.917*** (P < 0.001) |
Asterisks mark significant coefficients: ***P < 0.001.
Discussion
The results showed an association between the ability to produce antibodies against a novel antigen and resource availability during childhood and adolescence in a sample of young Latvian women. As predicted, family income was also negatively associated with cortisol levels. Contrary to predictions arising from existing research, family income was not associated with women’s height. The strength of women’s immune response was only weakly associated with their height and cortisol levels, not reaching standard levels of statistical significance in the current sample of 66 young Latvian women. Contrary to our prediction, women’s immune response was not impaired by higher total fat, visceral fat nor by higher BMI.
The current results are consistent with Krams et al.43, who reported no significant relationships between women’s height and the strength of their antibody response to a hepatitis B vaccine. However, as opposed to the results in women, a non-linear relationship has been shown between young men’s height and the strength of their antibody response to a hepatitis B vaccine, with a positive relationship in men up to 185 cm, but an inverse relationship in taller men14. Pawłowski et al.48 found no association between height and immune function parameters (testing both innate and adaptive immunity) in either sex. When testing for the simultaneous association between young men’s immune response, height and family income in ontogeny, the relationship between height and antibody levels was indirect and both were associated with family income9.
It is important to note that the large number of participants having 0 mIU ml−1 of anti-HBs may explain the lack of significant relationships between antibody response, height, BMI, total fat and visceral fat. The standard hepatitis B immunization protocol includes three vaccinations at months 0, 1 and 6. Previous research has shown a nearly exponential increase of anti-HBs levels towards the final vaccination event49–51. Interestingly, while some studies showed a negative association between stress and the strength of immune response50, another study failed to find a significant effect of stress on the levels of anti-HBs51. Unfortunately, these studies did not report the number of participants showing no antibody response because only participants with a detectable antibody level were included in the analyses or all participants with antibody levels below 10 IU/l were classified as non-responders50,51. This makes direct comparisons between the current study and previous studies impossible. Petri et al.49 reported a positive association between levels of psychosocial stressors and antibody response to hepatitis B vaccine. They note that all kinds of stress are equally detrimental to immune function and that a certain level of stress has the potential to mobilize the immune function. Petri et al.49 also pointed out that factors determining antibody formation and vaccine efficacy are not necessarily the same.
Prior research has explored the influence of environmental and psychosocial stressors on growth and immune system in both human and nonhuman species52. Poor environmental conditions affect the response to vaccinations, being weaker in African and Asian populations in developing countries than in populations from developed countries53. Studies on adolescents and adults54,55 showed that infections (helminths, Ascaris lumbricoides) affect responses to vaccines56 by reducing immune response. Blackwell and colleagues suggested that infection with helminths could impose hidden costs associated with immunological changes, and that such costs may affect somatic growth and other life history parameters57. Exposure to environmental toxicants during ontogeny directly or indirectly influences immune system and lung development, inducing adaptive responses in the immune and lung systems58. Importantly, the prevalence of soil-transmitted helminth infections is higher in communities with low household income59,60. Parasitic diseases, termed ‘neglected infections of poverty’, were shown to be widespread and associated with income level in Eastern Europe a decade ago61.
While socioeconomic status is recognized as an important predictor of health condition, the underlying molecular mechanisms linking low SES to poorer health outcomes are far from being understood. However, a recent genome-wide study showed that DNA methylation of a number of genes associated with immune function, cell communication and neurogenesis is higher in individuals with lower socioeconomic status62. Another study on several inflammation- and stress-related genes also found that low socioeconomic status is associated with higher levels of DNA methylation63. These findings indicate a role for epigenetic mechanisms in associations between socioeconomic conditions during childhood and adolescence and the development of immune phenotypes later in life. However, many other mechanisms and processes can be responsible for links between socioeconomic conditions during growth and the development of immunity64, and they need to be considered in future research.
Although socioeconomic factors influence height in women4,15,18, the current Latvian sample of women did not show similar associations between SES and height as found in other countries. One possible reason for this null finding can be the relatively decent national socioeconomic and psychosocial conditions during the study period. Importantly, while a positive relationship between height and socioeconomic conditions was reported in young Latvian males9, the current study was done two years later, which might have had a positive influence on the developmental conditions of the participants of this study because of improving national socioeconomic conditions, thus attenuating income-driven variation in height. Another reason for this sex difference might be that males appear to be more sensitive than women to developmental perturbations on growth65. Male height is a sexually selected trait, and it is thus possible that the development and expression of male height are condition-dependent65,66 in a similar way as with many other sexually selected traits10,67, therefore being more sensitive to resource availability than female height.
Despite null findings between family income and women’s height, family income was associated with the strength of immune response also in women, but only at the highest income levels. This suggests the existence of an important relationship between income and immunity. However, our results need to be interpreted with caution because of the low number of participants in the highest family income category. To study variation in immune function more accurately, future studies may benefit from using larger data sets and a broader set of immune function parameters, as well as from analyzing how other factors such as nutrition, illnesses and/or psychosocial stress influence the strength of immune response16.
This study showed a negative correlation between income and cortisol level, consistent with prior research68–70. This finding can partially explain the association between lower socioeconomic status and adverse health outcomes, pointing to the role of psychoneuroendocrine pathways underlying stress perception and possible consequences of financial disadvantage for general health16,49–51, 71–75. This interpretation is consistent with many studies that indicate a negative effect of high stress and cortisol on health, causing cardiovascular diseases76–78, acute myocardial infarction72 and type 2 diabetes73 as well as predicting cancer survival81–83. Overall, a well-regulated cortisol stress response is an essential component of adaptive cognitive, emotional and behavioral responses to stress, which in turn influence long-term health outcomes10,84–87. A possible limitation of the current study was that cortisol was measured only within a narrow time period in a single day. Cortisol can substantially fluctuate during the day, and such fluctuations were not measured in this study. As cortisol levels may also fluctuate in response to transient stressors, cortisol measurements spread over longer periods of time or cortisol measurements taken from hair samples would be needed to more accurately assess chronic stress88,89.
This study tested for the trait development and possible trade-offs between growth and immune function in women with different income levels. While an earlier study9 found a positive correlation between family income and the strength of immune response in young men across different income groups, women’s immunity was better only in the highest income group. The possible cause of different immune function findings in men9 and women may be explained by more active innate immunity function in women6,41,90. Women’s immune system is more sensitive to early-life pathogen exposure compared with men6. Women also make greater relative investments toward innate, not acquired, immunity6. This process can be supported by the presence of estrogen receptors on most innate immune cells41,91, suppressing cytotoxicity natural killer cells41,92, increasing anti-inflammatory properties and decreasing the chemotactic activity of neutrophils41,93. It has been shown in birds that individuals with high innate immune response mount weaker antibody responses under stressful conditions, which suggests a competitive cross-regulation between the innate and the acquired branches of the immune system95. Interactions between the innate and the acquired immune systems have been blamed in maintaining the pathogenesis of metabolic diseases95. It is known that acquired immunity imposes high bioenergetic costs especially early in life96,97. The overall metabolic costs of the activation of adaptive immunity in its acute phase are substantial in humans98,99. Favorable conditions such as nutritional abundance, low mortality risk and high early-life socioeconomic status support the development of acquired immunity and high antibody response9,100–103. Under suboptimal developmental conditions, in contrast, it is to be expected that women invest more in their innate rather than adaptive immune system57,98. Thus, immunological studies in women may benefit from focusing on testing innate immune system function and/or possible competition between the two arms of immune function, instead of analyzing only adaptive immune system properties.
Conclusions
In summary, we found a relationship between socioeconomic conditions and the strength of immune response: the highest levels of antibody titers were found in young women who had the highest levels of family income during childhood and adolescence. However, family income was not associated with women’s height. Comparing with prior research in young Latvian men9, these findings indicate that there are sex differences in the covariation between family income and height. These sex differences are possibly based on different sexually selected traits in men and women10,66,104, with height being a condition-dependent sexual trait in human males but not necessarily in females. We also found a negative association between income level and plasma cortisol level, both of which are predictors of general health and fertility. Although we cannot rule out genetic influences underlying the relationships between income level, immune response and cortisol level9,21,105, our findings indicate the importance of the developmental niche13,17,106 in creating individual differences in the strength of immune response, which can be considered a key life history trait. Finally, the vaccination approach serves as a powerful eco-immunological tool, while antibody response to vaccination provides an estimate of total immune function. However, given the complexity of the immune system, vaccination and measurement of antibody response need to be carried out along with other immune function measurements107. The high number of non-seroconverters in our sample suggests that the antibody response might be a result of interactions between the innate and the acquired arms of the immune system, fluctuating environmental conditions and levels of physiological stress107,108.
Methods
Immune system and cortisol assays
We activated the immune system of the subjects using hepatitis B vaccine (Engerix-B, GlaxoSmithKline)9,43,44,109. Briefly, we collected venous blood in 6 ml vials to measure the presence of antibodies before the vaccination. This was done to ensure that none of the participants had hepatitis B-specific antibodies before the vaccination. One month after the vaccination, we collected 6 ml of venous blood again to measure antibodies produced. To quantitatively determine serum hepatitis B surface antigen (anti-HBs) levels, we used the commercially available AxSYM® AUSAB® microparticle enzyme immunoassay (MEIA). Anti-HBs concentrations were expressed in mIU/ml. Cortisol levels were measured from plasma samples taken during the first testing session (for more information, see Rantala et al.102). Cortisol was measured from the blood sampled between 9:00 and 10:00. All participants woke up between 2 and 2.5 h before the first sample was taken. We collected two cortisol samples (before vaccination and 30 min later) and calculated the average, which was used in the analyses.
Socioeconomic status
There are several important variables that characterize the socioeconomic status of an individual. The following parameters are central: age, education, job class and income (often represented as annual household income of the individual9,109. The participants were 19–22-year-old women; all were undergraduate students with no job class achieved, limited opportunities to work because of their full-time studies and largely dependent on parent income. All of the participants lived with their parents during the study. Thus, all socioeconomic parameters of the subjects were similar except for income. We interviewed the participants and their parents about current income of their families and their income since 1991, based on parents’ recall (similar to Krams et al.9). This is when most of the subjects were born and when Latvia regained its independence as a result of the economic crash and political crisis in the USSR. We divided the time since 1991 into five periods and assigned each family into one of seven income categories. The current analyses were done on recalled family income data divided by the number of family members in each family. We included only those families that remained in their income categories since 1991 or shifted away from the original socioeconomic status by a maximum of one category. In 2010, the first income group consisted of families with equivalent to or less than 50 EUR per family member/month (n = 8); the second group, 51–100 EUR per family member/month (n = 19); the third group, 101–150 EUR (n = 13); the fourth group, 151–200 EUR (n = 12); the fifth group, 201–250 EUR (n = 9); the sixth group, 251–300 EUR (n = 3); and the seventh group, 301–350 EUR (n = 2). This division of income per family member/month corresponds to those traditionally used by Latvian economists9,111. It is important to note that there were only a few families available in the area with more than 300 EUR per family member during the study period.
Statistics
Antibody level data were not normally distributed because of an excess of zero values. The data did not reach normality after logarithmic transformation. Therefore, Spearman’s rank correlation coefficient was used to test relationships between variables. A Kruskal–Wallis test was applied to compare immune response in different income groups. Further, the generalized additive model (GAM) framework was used to model the relationship between immune response (dependent variable) and height, income and cortisol level (independent variables). Specifically, a Tweedie (1.25) based GAM with a power (0.1) link function was implemented to model antibody levels. The analysis was done using the R 3.5.1. statistical package112 in conjunction with the ‘mgcv’ library113.
Ethics statement
The study was approved by the Research Ethics Committee of the University of Daugavpils, Latvia (05/2012). All participants provided informed consent to participate in this study, and the ethics committee approved this informed consent procedure. The experiment was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). This paper is in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals.
Acknowledgements
We thank Sanita Kecko, Jolanta Vrublevska, Inese Kivleniece and Ljubova Sivacova for assistance. IK and TK were supported by the Latvian Council of Science (Grants 290/2012 and lzp-2018/1-0393) and the Estonian Ministry of Education and Science (Grant PUT1223). SL was supported by The Emil Aaltonen Foundation. MJR received support from the Academy of Finland.
Author contributions
Conceptualization of this study was done by I.A.K., M.J.R., T.K. and I.S. The methodology was designed by I.A.K., M.J.R., I.S., T.K., S.K., S.L., F.R.M. and J.C.-G. The formal analyses were performed by D.E., G.T., I.A.K. and A.R. S.K., I.A.K., I.S., M.J.R., R.K. collected data. A.R., S.L., I.A.K. wrote the original draft. A.R., S.L., I.A.K., F.R.M., T.K., R.K., M.J.R. and J.G.-C. wrote, reviewed and edited the submitted version. G.T. and D.E. designed figures. M.J.R., I.A.K., T.K. are responsible for funding acquisitions, supervision and administration of the project.
Data availability
All data sets are available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stearns, S. C. The evolution of life histories. (Oxford, 1992).
- 2.Ellison PT. Endocrinology, energetics, and human life history: a synthetic model. Horm. Behav. 2017;91:97–106. doi: 10.1016/j.yhbeh.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Stoehr AM, Kokko H. Sexual dimorphism in immunocompetence: what does life-history theory predict? Behav. Ecol. 2006;17:751–756. [Google Scholar]
- 4.Subramanian SV, Özaltin E, Finlay JE. Height of nations: a sioeconomic analysis of cohort differences and patterns among women in 54 low- to middle-income countries. PLoS ONE. 2011;6:e18962. doi: 10.1371/journal.pone.0018962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stulp G, Barrett L. Evolutionary perspectives on human height variation. Biol. Rev. 2016;91:206–234. doi: 10.1111/brv.12165. [DOI] [PubMed] [Google Scholar]
- 6.Georgiev AV, Kuzawa CW, McDade TW. Early developmental exposures shape trade-offs between acquired and innate immunity in humans. Evol. Med. Public Health. 2016;2016:256–269. doi: 10.1093/emph/eow022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krams I, Rumvolt K, Saks L, Krams R, Elferts D, Vrublevska J, Rantala M, Kecko S, Cīrule D, Luoto S, Krama T. Reproduction compromises adaptive immunity in a cyprinid fish. Ecol. Res. 2017;32:559–566. [Google Scholar]
- 8.Baumard N. Psychological origins of the industrial revolution. Behav. Brain Sci. 2018;1:1–47. doi: 10.1017/S0140525X1800211X. [DOI] [PubMed] [Google Scholar]
- 9.Krams I, Luoto S, Rubika A, Krama T, Elferts D, Krams R, Kecko S, Skrinda I, Moore FR, Rantala MJ. A head start for life history development? Family income mediates associations between height and immune response in men. Am. J. Phys. Anthropol. 2019;168:421–427. doi: 10.1002/ajpa.23754. [DOI] [PubMed] [Google Scholar]
- 10.Luoto S. An updated theoretical framework for human sexual selection: from ecology, genetics, and life history to extended phenotypes. Adapt. Hum. Behav. Physiol. 2019;5:48–102. [Google Scholar]
- 11.Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Zijl PV, Mostofsky S, Kaufmann WE, Kenet T, Dale AM, Jernigan TL, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nature Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeki A, Hazzouri A, Elfassy T, Sidney S, Jacobs D, Pérez Stable EJ, Yaffe K. Sustained economic hardship and cognitive function: The coronary artery risk development in young adults study. Am. J. Prev. Med. 2017;52:1–9. doi: 10.1016/j.amepre.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stotz K. Why developmental niche construction is not selective niche construction: and why it matters. Interface Focus. 2017;7:20160157. doi: 10.1098/rsfs.2016.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Said-Mohamed R, Pettifor JM, Norris SA. Life history theory hypotheses on child growth: potential implications for short and long-term child growth, development and health. Am. J. Phys. Anthropol. 2018;165:4–19. doi: 10.1002/ajpa.23340. [DOI] [PubMed] [Google Scholar]
- 15.Worthman CM, Trang K. Dynamics of body time, social time and life history at adolescence. Nature. 2018;554(7693):451–457. doi: 10.1038/nature25750. [DOI] [PubMed] [Google Scholar]
- 16.Cabeza de Baca T, Wahl RA, Barnett MA, Figueredo AJ, Ellis BJ. Adversity, adaptive calibration, and health: the case of disadvantaged families. Adapt. Hum. Behav. Physiol. 2016;2(2):93–115. doi: 10.1007/s40750-016-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateson P, Gluckman P. Plasticity, robustness, development and evolution. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 18.Morisaki N, Urayama KY, Yoshii K, et al. Ecological analysis of secular trends in low birth weight births and adult height in Japan. J. Epidemiol. Comm. Health. 2017;71:1014–1018. doi: 10.1136/jech-2017-209266. [DOI] [PubMed] [Google Scholar]
- 19.Puts DA. Beauty and the beast: mechanisms of sexual selection in humans. Evol. Hum. Behav. 2010;31:157–175. [Google Scholar]
- 20.Tarka M, Guenther A, Niemelä PT, Nakagawa S, Noble DW. Sex differences in life history, behavior, and physiology along a slow-fast continuum: a meta-analysis. Behav. Ecol. Sociobiol. 2018;72(8):132. doi: 10.1007/s00265-018-2534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khramtsova EA, Davis LK, Stranger BE. The role of sex in the genomics of human complex traits. Nature Rev. Genet. 2019;20:173–190. doi: 10.1038/s41576-018-0083-1. [DOI] [PubMed] [Google Scholar]
- 22.Luoto S, Krams I, Rantala MJ. A life history approach to the female sexual orientation spectrum: evolution, development, causal mechanisms, and health. Arch. Sex. Behav. 2019;48(5):1273–1308. doi: 10.1007/s10508-018-1261-0. [DOI] [PubMed] [Google Scholar]
- 23.Wells JCK. Sexual dimorphism in body composition across human populations: associations with climate and proxies for short- and long-term energy supply. Am. J. Hum. Biol. 2012;24:411–419. doi: 10.1002/ajhb.22223. [DOI] [PubMed] [Google Scholar]
- 24.García-Martínez D, Torres-Tamayo N, Torres-Sanchez I, García-Río F, Bastir M. Morphological and functional implications of sexual dimorphism in the human skeletal thorax. Am. J. Phys. Anthropol. 2016;161(3):467–477. doi: 10.1002/ajpa.23051. [DOI] [PubMed] [Google Scholar]
- 25.Fischer B, Mitteroecker P. Allometry and sexual dimorphism in the human pelvis. Anatomic. Record. 2017;300(4):698–705. doi: 10.1002/ar.23549. [DOI] [PubMed] [Google Scholar]
- 26.Lassek WD, Gaulin SJ. Costs and benefits of fat-free muscle mass in men: relationship to mating success, dietary requirements, and native immunity. Evol. Hum. Behav. 2009;30(5):322–328. [Google Scholar]
- 27.Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand grip strength: age and gender stratified normative data in a population-based study. BMC Res. Notes. 2011;4(1):127. doi: 10.1186/1756-0500-4-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samal A, Subramani V, Marx DB. An analysis of sexual dimorphism in the human face. J Vis. Comm Image Represent. 2007;18:453–463. [Google Scholar]
- 29.McDade TW. Life history theory and the immune system: Steps toward a human ecological immunology. Am. J. Phys. Anthropol. 2003;122:100–125. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- 30.Rigby N, Kulathinal RJ. Genetic architecture of sexual dimorphism in humans. J. Cell. Physiol. 2015;230(10):2304–2310. doi: 10.1002/jcp.24979. [DOI] [PubMed] [Google Scholar]
- 31.Stringer S, Polderman T, Posthuma D. Majority of human traits do not show evidence for sex-specific genetic and environmental effects. Sci. Rep. 2017;7(1):8688. doi: 10.1038/s41598-017-09249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grasgruber P, Sebera M, Hrazdíra E, Cacek J, Kalina T. Major correlates of male height: a study of 105 countries. Econom. Hum. Biol. 2016;21:172–195. doi: 10.1016/j.ehb.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Perkins JM, Subramanian SV, Davey Smith G, Özaltin E. Adult height, nutrition, and population health. Nutr. Rev. 2016;74:149–165. doi: 10.1093/nutrit/nuv105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hämäläinen A, Immonen E, Tarka M, Schuett W. Evolution of sex-specific pace-of-life syndromes: causes and consequences. Behav. Ecol. Sociobiol. 2018;72(3):50. doi: 10.1007/s00265-018-2462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Immonen E, Hämäläinen A, Schuett W, Tarka M. Evolution of sex-specific pace-of-life syndromes: genetic architecture and physiological mechanisms. Behav. Ecol. Sociobiol. 2018;72(3):60. doi: 10.1007/s00265-018-2462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phalane KG, Tribe C, Steel HC, Cholo MC, Coetzee V. Facial appearance reveals immunity in African men. Sci. Rep. 2017;7(1):7443. doi: 10.1038/s41598-017-08015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luoto S, Rantala MJ, Krams I. England first, America second: the ecological predictors of life history and innovation [Commentary] Behav. Brain Sci. 2019;42:1. doi: 10.1017/S0140525X19000165. [DOI] [PubMed] [Google Scholar]
- 38.Lourenço AM, Levy AM, Caetano LC, Carraro Abrahão AA, Prado JC. Influence sexual dimorphism on the persistence of blood parasites in infected Calomys callosus. Res. Vet. Sci. 2008;85:515–521. doi: 10.1016/j.rvsc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Klein, S. L. & Roberts, C. W. (Eds.) Sex Hormones and Immunity to Infection. (Springer Verlag, 2010).
- 40.Klein SL. Sex influences immune responses to viruses, and efficacy of prophylaxis and treatments for viral diseases. BioEssays. 2012;34:1050–1059. doi: 10.1002/bies.201200099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14(3):309–321. doi: 10.1111/acel.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xirocostas ZA, Everingham SE, Moles AT. The sex with the reduced sex chromosome dies earlier: a comparison across the tree of life. Bio. Lett. 2020;16:20190867. doi: 10.1098/rsbl.2019.0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krams IA, Skrinda I, Kecko S, Moore FR, Krama T, Kaasik A, Meija L, Lietuvietis V, Rantala MJ. Body height affects the strength of immune response in young men, but not young women. Sci. Rep. 2014;4:6223. doi: 10.1038/srep06223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skrinda I, Krama T, Kecko S, Moore FR, Kaasik A, Meija L, Lietuvietis V, Rantala MJ, Krams I. Body height, immunity, facial and vocal attractiveness in young men. Naturwissenschaften. 2014;101:1017. doi: 10.1007/s00114-014-1241-8. [DOI] [PubMed] [Google Scholar]
- 45.Rantala MJ, Coetzee V, Moore FR, Skrinda I, Kecko S, Krama T, Kivleniece I, Krams I. Adiposity, compared with masculinity, serves as a more valid cue to immunocompetence in human mate choice. Proc. R. Soc. B. 2013;280:20122495. doi: 10.1098/rspb.2012.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pļaviņa, L. & Kārkliņa, H. Sieviešu galveno antropometrisko parametru izvērtējums dažādos postnatālās ontoģenēzes periodos. Rīgas Stradiņa universitāte 2014. gada zinātniskā konference: Tēzes, Rīgā, 2014. gada 10. un 11. aprīlī. Rīga: RSU. 31. lpp. (2014).
- 47.Rantala, M., J., Coetzee, V., Moore, F. R., Skrinda, I., Kecko, S., Krama, T., Kivleniece, I. & Krams, I. Facial attractiveness is related to women’s cortisol and body fat, but not with immune responsiveness. Biol. Lett.9, 20130255 (2013). [DOI] [PMC free article] [PubMed]
- 48.Pawłowski B, Nowak J, Borkowska B, Augustyniak D, Drulis-Kawa Z. Body height and immune efficacy: testing body stature as a signal of biological quality. Proc. R. Soc. B. 2017;284:20171372. doi: 10.1098/rspb.2017.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petry LJ, Weems LB, Livingstone JN. Relationship of stress, distress, and the immunological response to a recombinant hepatitis-B vaccine. J Family Pract. 1991;32:481–486. [PubMed] [Google Scholar]
- 50.Jabaaij L, Grosheide PM, Heijtink RA, Duivenvoorden HJ, Ballieux RE, Vingerhoets AJ. Influence of perceived psychological stress and distress on antibody response to low dose rDNA hepatitis B vaccine. J. Psychosomat. Res. 1993;37(4):361–369. doi: 10.1016/0022-3999(93)90138-6. [DOI] [PubMed] [Google Scholar]
- 51.Jabaaij L, vanHattum J, Vingerhoets A, Oostveen FG, Duivenvoorden HJ, Ballieux RE. Modulation of immune response to rDNA hepatitis B vaccination by psychological stress. J. Psychosomat. Res. 1996;41:129–137. doi: 10.1016/0022-3999(96)00123-7. [DOI] [PubMed] [Google Scholar]
- 52.Ellis BJ, Del Giudice M. Developmental adaptation to stress: an evolutionary perspective. Ann. Rev. Psychol. 2019;70(1):111–139. doi: 10.1146/annurev-psych-122216-011732. [DOI] [PubMed] [Google Scholar]
- 53.LaBeaud AD, Malhotra I, King MJ, King CL, King CH. Do antenatal parasite infections devalue childhood vaccination? PLoS Negl. Trop. Diseases. 2009;3(5):e442. doi: 10.1371/journal.pntd.0000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect. Immun. 2001;69:1574–1580. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elias D, Britton S, Aseffa A, Engers H, Akuffo H. Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine. 2008;26:3897–3902. doi: 10.1016/j.vaccine.2008.04.083. [DOI] [PubMed] [Google Scholar]
- 56.Djuardi Y, Wammes LJ, Supali T, Sartono E, Yazdanbakhsh M. Immunological footprint: the development of a child's immune system in environments rich in microorganisms and parasites. Parasitology. 2011;138(12):1508–1518. doi: 10.1017/S0031182011000588. [DOI] [PubMed] [Google Scholar]
- 57.Blackwell AD, Snodgrass JJ, Madimenos FC, Sugiyama LS. Life history, immune function, and intestinal helminths: trade-offs among immunoglobulin E, C-reactive protein, and growth in an Amazonian population. Am. J. Hum. Biol. 2010;22(6):836–848. doi: 10.1002/ajhb.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao J, Xu X, Hylkema MN, Zeng EY, Sly PD, Suk WA, Huo X. Early-life exposure to widespread environmental toxicants and health risk: a focus on the immune and respiratory systems. Ann. Glob. Health. 2016;82(1):119–131. doi: 10.1016/j.aogh.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 59.Lander RL, Lander AG, Houghton L, Williams SM, Costa-Ribeiro H, Barreto DL, Mattos AP, Gibson RS. Factors influencing growth and intestinal parasitic infections in preschoolers attending philanthropic daycare centers in Salvador, Northeast Region of Brazil. Cadernos Saúde Pública, Rio de Janeiro. 2012;28(11):2177–2188. doi: 10.1590/s0102-311x2012001100017. [DOI] [PubMed] [Google Scholar]
- 60.Anuar TS, Salleh FM, Moktar N. Soil-transmitted helminth infections and associated risk factors in three Orang Asli tribes in Peninsular Malaysia. Sci. Rep. 2014;4:4101. doi: 10.1038/srep04101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hotez PJ, Gurwith M. Europe's neglected infections of poverty. Int. J. Infect. Diseas. 2011;15:e611–e619. doi: 10.1016/j.ijid.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 62.McDade TW, Ryan CP, Jones MJ, Hoke MK, Borja J, Miller GE, Kuzawa CW, Kobor MS. Genome-wide analysis of DNA methylation in relation to socioeconomic status during development and early adulthood. Am. J. Phys. Anthropol. 2019;1:1–9. doi: 10.1002/ajpa.23800. [DOI] [PubMed] [Google Scholar]
- 63.Needham BL, Smith JA, Zhao W, Wang X, Mukherjee B, Kardia SL, Diez Roux AV. Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: the multi-ethnic study of atherosclerosis. Epigenetics. 2015;10(10):958–969. doi: 10.1080/15592294.2015.1085139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kubzansky L, Seeman TE, Glymour MM. Biological pathways linking social conditions and health: plausible mechanisms and emerging puzzles. In: Berkman LF, Kawachi I, Glymour MM, editors. Social epidemiology. Oxford: Oxford University Press; 2014. pp. 512–561. [Google Scholar]
- 65.Gaulin SJ, Boster JS. Human marriage systems and sexual dimorphism in stature. Am. J. Phys. Anthropol. 1992;89(4):467–475. doi: 10.1002/ajpa.1330890408. [DOI] [PubMed] [Google Scholar]
- 66.Polo P, Fernandez A, Muñoz-Reyes JA, Dufey M, Buunk AP. Intrasexual competition and height in adolescents and adults. Evol. Psychol. 2018;16(1):1474704917749172. doi: 10.1177/1474704917749172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cornwallis CK, Uller T. Towards an evolutionary ecology of sexual traits. Trends Ecol. Evol. 2010;25(3):145–152. doi: 10.1016/j.tree.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 68.Jewell SL, Luecken LJ, Gress-Smith J, Crnic KA, Gonzales NA. Economic stress and cortisol among postpartum low-income Mexican American women: buffering influence of family support. Behav. Med. 2015;41(3):138–144. doi: 10.1080/08964289.2015.1024603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serwinski B, Salavecz G, Kirschbaum C, Steptoe A. Associations between hair cortisol concentration, income, income dynamics and status incongruity in healthy middle-aged women. Psychoneuroendocrinol. 2016;67:182–188. doi: 10.1016/j.psyneuen.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ursache A, Merz EC, Melvin S, Meyer J, Noble KG. Socioeconomic status, hair cortisol and internalizing symptoms in parents and children. Psychoneuroendocrinol. 2017;78:142–150. doi: 10.1016/j.psyneuen.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pepper GV, Nettle D. The behavioural constellation of deprivation: causes and consequences. Behav. Brain Sci. 2017;40:e314. doi: 10.1017/S0140525X1600234X. [DOI] [PubMed] [Google Scholar]
- 72.Burns VE, Carroll D, Ring C, Harrison LK, Drayson M. Stress, coping, and hepatitis B antibody status. Psychosom. Med. 2002;64(2):287–293. doi: 10.1097/00006842-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 73.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005;5(3):243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 74.O'Connor TG, Winter MA, Hunn J, Carnahan J, Pressman EK, Glover V, Robertson-Blackmore E, Moynihan JA, Lee FE-H, Caserta MT. Prenatal maternal anxiety predicts reduced adaptive immunity in infants. Brain Behav. Immun. 2013;32:21–28. doi: 10.1016/j.bbi.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayward SE, Dowd JB, Fletcher H, Nellums LB, Wurie F, Boccia D. A systematic review of the impact of psychosocial factors on immunity: implications for enhancing BCG response against tuberculosis. Soc. Sci. Med. Popul. Health. 2020;10:100522. doi: 10.1016/j.ssmph.2019.100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am. J. Hypertens. 2015;28(11):1295–1302. doi: 10.1093/ajh/hpv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golbidi S, Frisbee JC, Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am. J. Physiol. Heart Circ. Physiol. 2015;308(12):1476–1498. doi: 10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- 78.Cozma S, Dima-Cozma LC, Ghiciuc CM, Pasquali V, Saporano A, Patacchioli FR. Salivary cortisol and α-amylase: subclinical indicators of stress as cardiometabolic risk. Braz. J. Med. Biol. Res. 2017;50(2):e5577. doi: 10.1590/1414-431X20165577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steptoe A, Kiwimäki M. Stress and cardiovascular disease. Nature Rev. Cardiol. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 80.Kivimäki M, Kawachi I. Work stress as a risk factor for cardiovascular disease. Curr. Cardiol. Rep. 2015;17(9):630. doi: 10.1007/s11886-015-0630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN, Dhabhar FS, Spiegel D, Salmon P. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav. Immun. 2012;30(Suppl):S163–170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Spiegel D. Minding the body: psychotherapy and cancer survival. Br. J. Health Psychol. 2014;19(3):465–485. doi: 10.1111/bjhp.12061. [DOI] [PubMed] [Google Scholar]
- 83.Garland EL, Beck AC, Lipschitz DL, Nakamura Y. Dispositional mindfulness predicts attenuated waking salivary cortisol levels in cancer survivors: a latent growth curve analysis. J. Cancer Survivorship. 2015;9:215. doi: 10.1007/s11764-014-0402-2. [DOI] [PubMed] [Google Scholar]
- 84.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann. N. Y. Acad. Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neurosci. Biobehav. Rev. 2011;35(7):1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rantala MJ, Luoto S, Krams I, Karlsson H. Depression subtyping based on evolutionary psychiatry: proximate mechanisms and ultimate functions. Brain Behav. Immun. 2018;69:603–617. doi: 10.1016/j.bbi.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 87.Rantala MJ, Luoto S, Krama T, Krams I. Eating disorders: an evolutionary psychoneuroimmunological approach. Front. Psychol. 2019;10:2200. doi: 10.3389/fpsyg.2019.02200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greff MJ, Levine JM, Abuzgaia AM, Elzagallaai AA, Rieder MJ, van Uum SH. Hair cortisol analysis: an update on methodological considerations and clinical applications. Clin. Biochem. 2018;63:1–9. doi: 10.1016/j.clinbiochem.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 89.Khoury JE, Enlow MB, Plamondon A, Lyons-Ruth K. The Association between adversity and hair cortisol levels in humans: a meta-analysis. Psychoneuroendocrinol. 2019;103:104–117. doi: 10.1016/j.psyneuen.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doyle HH, Murphy AZ. Sex differences in innate immunity and its impact on opioid pharmacology. J. Neurosci. Res. 2017;95(1–2):487–489. doi: 10.1002/jnr.23852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nature Rev. Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hao S, Zhao J, Zhou J, Zhao S, Hu Y, Hou Y. Modulation of 17b-estradiol on the number and cytotoxicity of NK cells in vivo related to MCM and activating receptors. Int. Immunopharmacol. 2007;7:1765–1775. doi: 10.1016/j.intimp.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 93.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am. J. Pathol. 1999;155:1137–1146. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krams I, Vrublevska J, Cirule D, Kivleniece I, Krama T, Rantala MJ, Sild E, Hõrak P. Heterophil/lymphocyte ratios predict the magnitude of humoral immune response to a novel antigen in great tits (Parus major) Comp. Biochem. Physiol. A: Mol. Integrat. Physiol. 2012;161:422–428. doi: 10.1016/j.cbpa.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 95.Stoll ML. Interactions of the innate and adaptive arms of the immune system in the pathogenesis of spondyloarthritis. Clin. Exp. Rheumatol. 2011;29:322–330. [PMC free article] [PubMed] [Google Scholar]
- 96.Klasing KC, Leshchinsky TV. Functions, costs, and bene- fits of the immune system during development and growth. Ostrich. 1999;69:2817–2832. [Google Scholar]
- 97.McDade TW, Georgiev AV, Kuzawa CV. Trade-offs between acquired and innate immune defenses in humans. Evol. Med. Publ. Health. 2016;2016(1):1–16. doi: 10.1093/emph/eov033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elia, M. Organ and tissue contribution to metabolic rate. in Energy Metabolism: Tissue Determinants and Cellular Corollaries (eds. McKinney, J. M. & Tucker, H. N.) 61–80. (Raven, 1992).
- 99.Muehlenbein MP, Hirschtick JL, Bonner JZ. Toward quantifying the usage costs of human immunity: altered metabolic rates and hormone levels during acute immune activation in men. Am. J. Human Biol. 2010;22:546–556. doi: 10.1002/ajhb.21045. [DOI] [PubMed] [Google Scholar]
- 100.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol. Psych. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 101.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch. Pediatr. Adolesc. Med. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol. Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Archer J. The reality and evolutionary significance of human psychological sex differences. Biol. Rev. 2019;94(4):1381–1415. doi: 10.1111/brv.12507. [DOI] [PubMed] [Google Scholar]
- 105.Hartling C, Fan Y, Weigand A, Trilla I, Gärtner M, Bajbouj M, Grimm S. Interaction of HPA axis genetics and early life stress shapes emotion recognition in healthy adults. Psychoneuroendocrinol. 2019;99:28–37. doi: 10.1016/j.psyneuen.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 106.Frankenhuis WE, Nettle D, Dall SR. A case for environmental statistics of early-life effects. Phil. Trans. R. Soc. B. 2019;374(1770):20180110. doi: 10.1098/rstb.2018.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Foo YZ, Simmons LW, Perrett D, Holt PG, Eastwood PR, Rhodes G. Immune function during early adolescence positively predicts adult facial sexual dimorphism in both men and women. Evol. Hum. Behav. 2020;1:1. doi: 10.1016/j.evolhumbehav.2020.02.002. [DOI] [Google Scholar]
- 108.Cohen S, Miller GE, Rabin BS. Psychological stress and antibody response to immunization: a critical review of the human literature. Psychosom. Med. 2001;63:7–18. doi: 10.1097/00006842-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 109.Rantala MJ, Moore FR, Skrinda I, Krama T, Kivleniece I, Kecko S, Krams I. Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nature Comm. 2012;3:694. doi: 10.1038/ncomms1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tyrrell J, Jones SE, Beaumont R, Astley CM, Lovell R, Yaghootkar H, Tuke M, Ruth SR, Freathy RM, Hirschhorn JN, Wood AR, Murray A, Weedon MN, Frayling TM. Height, body mass index, and socioeconomic status: mendelian randomisation study in UK Biobank. Br. Med. J. 2016;352:582. doi: 10.1136/bmj.i582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lavrinoviča, I., Lavriņenko, O., & Teivāns-Treinovskis, J. Population income differentiation and its influence on the crime. in Proceedings of the XIII International Scientific Conference Sustainable Business under Changing Economic Conditions (Dotkus, W., Holger, B., Žilys, J., Rozīte, M., Rumpīte, D., & Vīksne, I., Eds.) Rīga, Latvia: School of Business Administration Turība, pp. 242–251. Retrieved from: https://aurora.turiba.lv/bti/Editor/Manuscript/Proceeding/ (2012).
- 112.R Core Team. R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/ (2018).
- 113.Wood SN. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B. 2011;73:3–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data sets are available upon request.