Abstract
Background
Despite advances in vaccination and case management, pneumonia remains the single largest contributor to early child mortality worldwide. Zinc has immune-enhancing properties, but its role in adjunctive treatment of pneumonia in low-income and middle-income countries (LMICs) is controversial and research still active.
Methods
Systematic review and meta-analysis of randomised controlled trials of zinc and placebo in pneumonia in children aged 2 to 60 months in LMICs. Databases included MEDLINE, the Cochrane Library, EMBASE, LILACS, SciELO, the WHO portal, Scopus, Google Scholar and ClinicalTrials.gov. Inclusion criteria included accepted signs of pneumonia and clear measure of outcome. Risk of bias was independently assessed by two authors. ORs with 95% CI were used for calculating the pooled estimate of dichotomous outcomes including treatment failure and mortality. Time to recovery was expressed as HRs. Sensitivity analyses considering risk of bias and subgroup analyses for pneumonia severity were performed.
Results
We identified 11 trials published between 2004 and 2019 fulfilling the a priori defined criteria, 7 from South Asia and 3 from Africa and 1 from South America. Proportional treatment failure was comparable in both zinc and placebo groups when analysed for all patients (OR 0.95 (95% CI 0.80 to 1.14)) and only for those with severe pneumonia (OR 0.93 (95% CI 0.75 to 1.14)). No difference was seen in mortality between zinc and placebo groups (OR 0.64 (95% CI 0.31 to 1.31)). Time to recovery from severe pneumonia did not differ between the treatment and control groups for patients with severe pneumonia (HR 1.01 (95% CI 0.89 to 1.14)). Removal of four studies with high risk of bias made no difference to the conclusions.
Conclusion
There is no evidence that adjunctive zinc treatment improves recovery from pneumonia in children in LMICs.
Trial registration number
CRD42019141602.
Keywords: epidemiology, infectious diseases, paediatric practice
What is known about the subject?
Pneumonia is globally the single largest cause of childhood mortality.
Zinc is an important micronutrient that regulates the inflammatory response.
Zinc supplementation has been shown to reduce mortality in acute gastroenteritis, but in pneumonia its role is controversial.
What this study adds?
This systematic review and meta-analysis showed no benefit from adjunctive zinc in treatment of pneumonia in children aged 2 to 60 months.
Background
About 80% of the world’s under-five deaths occur in low and middleincome countries (LMICs).1–4 The majority are due to either acute respiratory infection or acute gastroenteritis. To a large extent, these deaths are preventable through strategies generic to both including exclusive breast feeding, safe complementary feeding and universal vaccination.5–7
Child pneumonia (specifically between the ages of 2 and 60 months) contributes approximately 30% of the total global pneumonia mortality but, despite a gradual recent fall in both incidence and case fatality rates over the Millennium Development Goals era (1990–2015), it remains, by some distance, the single largest contributor to global post-neonatal mortality.8 9 A recent review for the Child Health Epidemiology Action Group estimated an incidence of community-acquired childhood pneumonia in LMICs of 0.22 (IQR 0.11–0.51) episodes per child per year. Of these, 11.5% progress to severe episodes.2 Recent Global Burden of Disease data estimate 68 million incident cases of pneumonia and 652 572 deaths per year (uncertainty interval 586 000 to 720 000). Pneumonia, therefore, contributes of the order of 15% of the total 5.6 million annual under-five year child deaths, the vast majority in LMICs and in primary care settings where resources are more stretched and populations more susceptible.10
Standard treatment includes fluids, antibiotics appropriate to local microbiological epidemiology and oxygen.11 Pulse oximeters have enhanced detection of hypoxia, but this has little influence on pneumonia-related mortality in the absence of supplemental oxygen.12
Micronutrients and their relation to pneumonia have been the subject of research interest for many years. The consensus is that mass infant vitamin A preventative supplementation reduces subsequent all-cause mortality,13 but that adjunctive treatment in acute pneumonia does not alter disease trajectory.14
The role of zinc, another key micronutrient, remains controversial. It has immune-enhancing properties and deficiency is associated with increased susceptibility to infection as well as impaired growth and development.15 Zinc supplementation as an adjunct to standard rehydration enhances recovery from acute gastroenteritis in LMICs and in meta-analysis reduces mortality by an estimated 23%.16 Population preventative supplementation reduces all-cause mortality17 and reduces pneumonia incidence in young children if administered regularly.18 19 Given this immune protective effect and prevalence of dietary deficiencies in LMICs, it seems plausible to assume that the effect in the most common infective cause of mortality is greater than it would be in highincome countries.
However, the role of adjunctive zinc at presentation in pneumonia is still controversial: meta-analyses published in 2011, 2012 and 201620–22 found no effect, while another published in 2018 estimated a significant reduction in mortality (risk ratio 0.43, 95% CI 0.22 to 0.83), but no difference in composite treatment failure rates.23 The last study was published in 2018 and included studies up to October 2015, 4 years before our search. In addition, this meta-analysis included only studies of severe pneumonia at randomisation. These represent a minority of all pneumonia cases and the public health implications of even a small gain in non-severe pneumonia would be considerable.
As this area of research remains active with new findings published since the last meta-analysis, we aimed to update the evidence for the efficacy of adjunctive zinc treatment in all pneumonia in children in LMICs.
Methods
Aim, registration, eligibility and search strategy
The aim of this study was to investigate the efficacy of adjunctive zinc supplementation in children aged 2 to 60 months with pneumonia in LMICs. The study was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis statement) guidance.24 The review protocol was registered in advance on the PROSPERO database (CRD42019141602)
Papers and conference abstracts of placebo-controlled randomised controlled trials (RCTs), where zinc was given as an adjunctive treatment to standard pneumonia therapy including antibiotics, were considered eligible for inclusion.
In terms of simplicity, ease of interpretation and power, we chose not to include factorial designs or those comparing zinc with another potentially active adjunctive treatment.
Pneumonia diagnosis was based on the WHO definition and grades of severity in use at the time of the study.11 The age group of interest was children from 2 to 60 months. Only trials conducted in LMICs, as defined by World Bank definitions25 at the time recruitment, were included. Studies, where all patients had simultaneously another debilitating condition such as measles, AIDS or severe malnutrition, were excluded. In accordance with International Committee of Medical Journal Editors guidance,26 advance registration was required for trial in which recruitment started 1 January 2007 or later.
Two authors (NB and AJK) independently searched the following databases from their inception to 1 August 2019: MEDLINE, the Cochrane Library, EMBASE, LILACS, SciELO, the WHO trial database, Scopus, Google Scholar and ClinicalTrials.gov. Key search terms were child AND pneumonia OR acute respiratory infection AND zinc (online supplementary appendix 1). Reference lists of the retrieved studies and relevant reviews were additionally searched for completeness. The search was repeated during the revision of the manuscript on 17 April 2020.
bmjpo-2020-000662supp001.pdf (15.9KB, pdf)
Data extraction, outcome measures and bias evaluation
The main outcome of the meta-analysis was treatment failure. We considered a change to second-line antibiotics (as other studies have done) as a treatment failure as this invariably means that the first-line drugs have not worked.
Secondary outcomes were time to recovery from pneumonia and mortality. Recovery was defined as normalisation of age-adjusted respiratory rate and oxygen saturation in air.
Data extraction was done independently by two authors (NB and AJK) onto two separate spreadsheets. The spreadsheets were compared on completion and any disagreements solved by discussion. In keeping with PRISMA guidance, we extracted data on the following: study title; first author; publication year; country; age group and any stratifications; pneumonia definition; vital parameters reported; nutritional status; HIV status; comparability of baseline characteristics; exclusion of chronic illness; trial registration data; ethical permission; consent; conflict of interest; reported outcomes and their definitions; number of participants in each group (post-randomisation and with completed follow-up); dosage and duration of zinc treatment; placebo used.
We only used outcome data available, did not impute data and did not obtain individual outcome data
For the screened studies, risk of bias was evaluated using the Cochrane tool for systemic reviews27 for the following domains: sequence generation, allocation concealment, blinding, outcome, selective reporting and other. The risk for each domain was graded independently by the two authors as low, high or unclear and disagreements after completion were resolved by discussion. The results of the bias evaluation were used for sensitivity analysis, where studies with high risk of bias in any of the fields or an unclear risk of bias in more than one field were removed from the meta-analysis.
Statistical analyses
ORs (with 95% CI) were used for calculating the pooled estimate of dichotomous outcomes. Time to recovery was treated as time-to-event data and expressed as HRs. Estimations were all made using a random-effects analysis. Though I2 was low to moderate in all analyses, the differential direction of effect in studies with low risk of bias led us to concur that for the sake of consistency, a random-effects model would be more appropriate for all analyses.
Subgroup analyses were planned for age, pneumonia severity and nutritional status. We assessed publication bias using funnel plots.
RevMan (V.5.3, The Cochrane Collaboration, Software Update) was used for all statistical analyses and risk of bias. A value of p <0.05 was considered statistically significant and p <0.1 for the significance level of the Q test.
Patient and public involvement
As this was a systematic review and meta-analysis of already conducted RCTs, there was no patient and public involvement in the manuscript.
Results
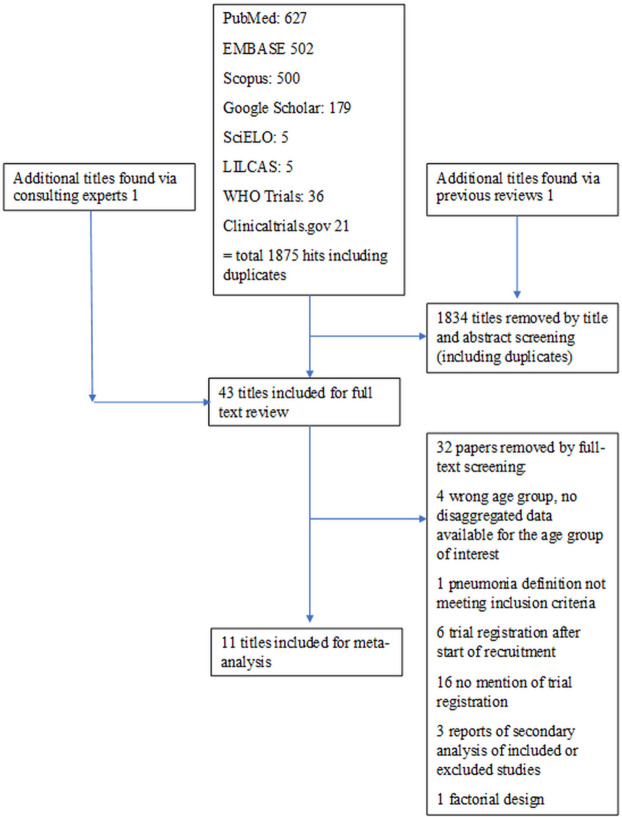
We retrieved a total of 1877 records up to 17 April 2020. After screening of titles and abstracts, 43 studies were included in a full-text screening, where further 32 studies were removed. References to and reasons for excluding studies at the full-text evaluation are presented in online supplementary table 1, appendix 2. Eleven trials were included in the meta-analysis (references 28–38 and figure 1).
Figure 1.
Flow chart of study selection.
bmjpo-2020-000662supp002.pdf (60.3KB, pdf)
The characteristics of the included studies are presented in table 1. Seven studies were from South Asia and three from Africa and one from South America. Total number of children included was 6497 and sample sizes varied between 95 and 2628 cases. All but one study28 enrolled only children with severe pneumonia. For children above 12 months of age, dosage of zinc was 20 mg per day in all but one study where all children were given 25 mg of zinc daily.29 In four studies,30–33 children younger than that received 10 mg daily and in five papers they had the same 20 mg daily dose.28 34–37 In one study, the cut-off for 20 mg daily dose was 7 months.38
Table 1.
Main characteristics of the included studies
| Lead author, year, reference | Country | Age group (months) | N zinc/N control | Zinc dosage |
| Baruah, 201838 | India | 2–60 | 280/280 | 10 mg ×1 for <7 mo, 20 mg ×1 for ≥7 mo for 2 wk |
| Basnet, 201230 | Nepal | 2–35 | 305/305 | 10 mg ×1 for <12 mo, 20 mg ×1 for ≥12 mo until discharge (maximum 14 d) |
| Bose, 200634 | India | 2–23 | 150/150 | 10 mg ×2 until discharge (maximum 14 d) |
| Brooks, 200435 | Bangladesh | 2–23 | 135/135 | 20 mg/d until discharge |
| Fataki, 201429 | Tanzania | 6–36 | 47/45 | 12.5 mg ×2 until discharge |
| Howie, 201931 | The Gambia | 2–59 | 303/301 | 10 mg ×1 for <12 mo, 20 mg ×1 for ≥12 mo for 7 d |
| Sempértegui, 201436 | Ecuador | 2–59 | 225/225 | 10 mg ×2 until discharge |
| Shah, 201237 | Nepal | 2–60 | 64/53 | 20 mg ×1 first day, then 10 mg ×2 for 7 d |
| Srinivasan, 201232 | Uganda | 6–59 | 176/176 | 10 mg ×1 for <12 mo, 20 mg ×1 for ≥12 mo for 7 d |
| Valentiner-Branth, 201028 | Nepal | 2–35 | 1314/1314 | 10 mg ×1 for <12 mo, 20 mg ×1 for ≥12 mo for 14 d |
| Wadhwa, 201333 | India | 2–24 | 274/276 | 10 mg ×2 until recovery (maximum 14 d) |
Estimation on risk of bias for the included studies is presented in table 2. Four included studies had high risk of bias in one of the evaluated fields29 32 37 38 and two other unclear risk of bias in one field each.30 36
Table 2.
Risk of bias of the included studies
| Author, year, reference | Sequence generation | Allocation concealment | Blinding | Outcome data | Selective reporting | Other bias |
| Baruah, 201838 | Low | Low | Low | Low | Low | High |
| Basnet, 201230 | Unclear | Low | Low | Low | Low | Low |
| Bose, 200634 | Low | Low | Low | Low | Low | Low |
| Brooks, 200435 | Low | Low | Low | Low | Low | Low |
| Fataki, 201429 | Low | Low | Low | Low | Low | High |
| Howie, 201831 | Low | Low | Low | Low | Low | Low |
| Sempértegui, 201436 | Low | Low | Low | Unclear | Low | Low |
| Shah, 201237 | High | Low | Low | Low | Low | Unclear |
| Srinivasan, 201232 | Unclear | Low | Low | High | Low | Low |
| Valentiner-Branth, 201028 | Low | Low | Low | Low | Low | Low |
| Wadhwa, 201333 | Low | Low | Low | Low | Low | Low |
The study by Baruah and Saikia38 seemed to be well designed, but outcomes of recovery and duration of hospitalisation were reported only dichotomously using a random cut-off of 3 and 5 days, respectively, and, therefore, risk of bias towards the null and questionable clinical applicability. The study by Fataki et al 29 was terminated prematurely due to difficulties in recruitment and only one-sixth of the planned sample was analysed. The process of randomisation in the study by Shah et al 37 was unclear. In the papers by Shah et al 37 and Srinivasan et al,32 randomisation was unclear. In the latter, the focus of discussion was based on a protective effect in HIV-infected children limiting generalisability.
The paper by Basnet et al 30 was deemed to have unclear risk of sequence generation bias as the description of sequence generation. The study by Sempértegui et al 36 had an unclear risk of biased outcome data due to high and uneven rate of fall-out (19% in zinc arm and 12% in placebo arm).
Treatment failure
A pre-specified outcome of treatment failure was reported in five studies28 30 31 36 37 while in four studies change to second-line antibiotics was reported and, therefore, used as a marker of failure.29 34 35 37 In the study by Baruah and Saikia, we used failure to recover from severe pneumonia within 3 days to define treatment failure.38
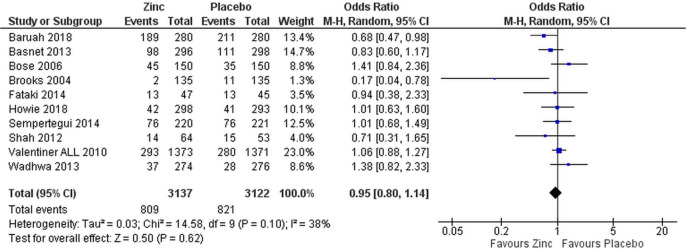
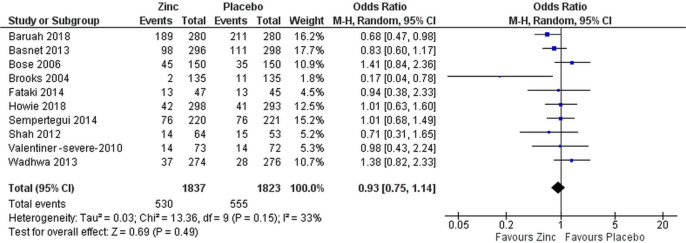
Proportional treatment failure was comparable in both zinc and placebo groups when analysed for all patients (OR 0.95 (95% CI 0.80 to 1.14), figure 2) and only for patients with severe pneumonia (OR 0.93 (95% CI 0.75 to 1.14), figure 3).
Figure 2.
Efficacy of supplemental zinc in preventing treatment failure in comparison with placebo in all patients.
Figure 3.
Subgroup analysis of efficacy of supplemental zinc in preventing treatment failure in comparison with placebo in children with severe pneumonia.
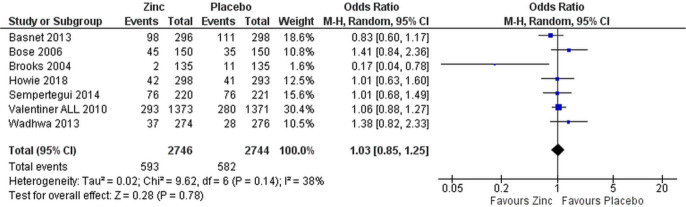
Removing the three studies with high risk of bias did not change the overall results (OR 1.03 (95% CI 0.85 to 1.25), figure 4 for all patients and OR 1.01 (95% CI 0.79 to 1.30) for those with severe pneumonia only, data not shown). Only two studies, Baruah and Saikia38 and Brooks et al,35 showed lower treatment failure rate in the zinc-supplemented group (figures 2 and 3).
Figure 4.
Sensitivity analysis of efficacy of supplemental zinc in preventing treatment failure in comparison with placebo in children with pneumonia in studies with low risk of bias.
Case fatality
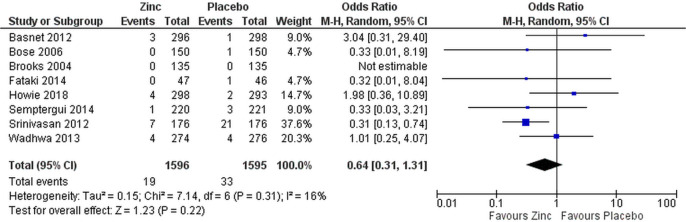
Data on case fatality were available from eight studies all recruiting only cases with severe pneumonia.29–36 No difference was seen in mortality between zinc and placebo groups when including all studies (OR 0.64 (95% CI 0.31 to 1.31), figure 5) or only those without high risk of bias (OR 1.10 (95% CI 0.47 to 2.62), data not shown).
Figure 5.
Efficacy of supplemental zinc in preventing deaths from severe pneumonia.
Time to recovery
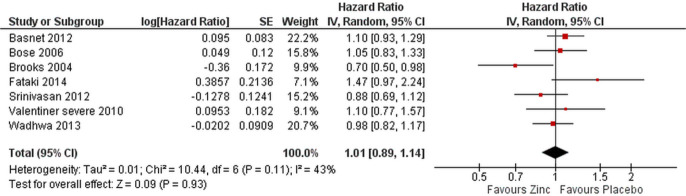
Time to recovery from severe pneumonia did not differ between the treatment and control groups for patients with severe pneumonia in the seven studies28–30 32–35 reporting this outcome using time to event data (HR 1.01 (95% CI 0.89 to 1.14), figure 6). The study by Brooks was the only one to show an effect (of marginal significance) of zinc on recovery time (figure 6).
Figure 6.
Time to recovery from severe pneumonia in zinc vs placebo groups.
It should be noted that in the study by Fataki et al,29 the authors state that they will use Cox proportional hazards to evaluate this outcome, but in their table they present the results as incidence rate ratio. We presumed that HR was used for analysis and also converted the reported outcome to its inverse to match the usual way of reporting HRs. Removing this study and the one by Srinivasan et al 32 also considered to have high risk of bias did not change the overall results (OR 1.00 (95% CI 0.88 to 1.14), data not shown).
Subgroup analyses
We had originally intended to examine subgroup effects by age and nutrition status but were unable to do so because of lack of data reported. We assumed a priori that the trials would include children with both non-severe (since 2014 WHO change in categorisation ‘fast breathing’) and severe (since 2014, fast breathing with danger signs) pneumonia.11 However, only one study28 included children with non-severe pneumonia and, though we ran a sensitivity analysis, this did not alter our outcome.
Publication bias
Funnel plots were not suggestive of publications bias (online supplementary figures 1–3, appendix 3).
bmjpo-2020-000662supp003.pdf (34KB, pdf)
Discussion
This systematic review and meta-analysis identified 11 eligible trials testing the efficacy of zinc as an adjunctive treatment for pneumonia in children aged 2–60 months in LMICs.28–38 Seven of these studies were deemed to have low risk of overall bias.28 30 31 33–36 We identified two additional large RCTs with a total number of 1151 children31 38 published since the search for the last meta-analysis was undertaken in October 2015.23
We found no evidence of effect on any outcome parameter. Our findings are largely concordant with previous meta-analyses.20–23 In difference to Wang and Song,23 we did not find decreased mortality in the zinc supplementation group. The study by Srinivasan et al 32 with high risk of bias in outcome reporting was driving the results presented by Wang and Song and neither of the two new large studies published since then showed any increased risk of mortality. A sensitivity analysis presented by Wang and Song, where the Srinivasan study was removed, led to null effect.23
Zinc has many hypothetically attractive properties which may alter host response to respiratory pathogens including the regulation of pro-inflammatory cytokines, lymphocyte proliferation and T lymphocyte function.16 21 23 Whether modulation of these factors in vivo during an acute infection is beneficial is, however, unknown.22 Despite the preventative role in reducing incidence of acute respiratory infection and improving recovery time from acute gastroenteritis,16 there appears to be no benefit of effect when used adjunctively in the acute phase. In this respect, it appears to behave like vitamin A.14
Three studies identified some benefit from zinc in some outcome. The study by Srinivasan et al 32 discussed earlier had lower mortality overall (relative risk 0.33 (95% CI 0.15 to 0.76)) and in HIV-positive patients in particular (relative risk 0.1 (95% CI 0.0 to 1.0)). The study by Baruah and Saikia38 showed slightly lower odds of pneumonia duration more than 3 days (unadjusted OR 0.68 (95% CI 0.47 to 0.98)) and hospital stay more than 5 days (unadjusted OR 0.70 (95% CI 0.50 to 0.97)). Also, Brooks et al 35 showed improvement in recovery time (HR 0.70 (95% CI 0.51 to 0.98)) and treatment failure (HR 0.14 (95% CI 0.04 to 0.78)).
In addition, three studies identified marginal subgroup benefits: Sempértegui et al 36 showed faster median recovery times in children with higher baseline zinc levels and better nutritional status with height and weight for age z scores predicting time to resolution of indrawing and tachypnoea, respectively. Wadhwa et al 33 showed marginally faster recovery (HR 1.52 (95% CI 1.07 to 2.23)) in very severe pneumonia, but this was not robust to adjustment for nutritional status. Though we were interested in deriving effect sizes for children with both severe and non-severe pneumonia, only one study included children in the latter group and we were unable to draw any conclusions. Non-severe (fast breathing without danger signs) pneumonia is by far the more common phenotype and therefore a potentially greater contributor to global illness burden if not mortality. Were zinc to be efficacious in this group, there would be substantial implications for public health and this is arguably an area of importance for future work.
There might be alternative explanations for the null effect. It is conceivable that absorption of zinc is impaired in the acute inflammatory state in pneumonia, but, given the increases in serum level in the studies in which it was measured, this seems unlikely.28 29 34 35 38
There are some limitations to the systematic review and meta-analysis. Though we searched standard databases, we cannot exclude the possibility that we did not identify unpublished positive studies. However, the funnel plots analysed do not support this presumption. A large number of studies could not be included because of lacking or late trial registration (online supplementary appendix 2). We did not have sufficient data to extrapolate to season or type of pathogen as the clinical definition of pneumonia used in all studies is likely to include a large number of viral infections. However, case management of pneumonia has to be pragmatic and minor variations in response by season or by variation between viral and bacterial agents are unlikely to be helpful at the individual patient level.
Finally, it is worth noting that the WHO definitions of pneumonia were modified in 2014, the three previous categories being simplified to two, fast breathing with or without chest indrawing and severe pneumonia, defined as fast breathing, chest indrawing with danger signs including hypoxia, lethargy and poor feeding.11 However, as none of the studies started recruitment after this period, it is unlikely to have biased the findings.
Conclusion
This systematic review and meta-analysis did not provide evidence that zinc used adjunctively in children aged 2–60 months with pneumonia in LMIC improves recovery.
Supplementary Material
Footnotes
Contributors: NB: original idea, primary analyses and manuscript writing. AJK: co-searcher, analysis, figures and manuscript writing. AM: critical overview of concept, content and manuscript writing. We all fulfil the ICMJE requirements for authorship.
Funding: This study was funded by Uppsala Universitet.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: SR and MA on already undertaken RCTs.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information. This is a systematic review and meta-analysis based on already available data.
References
- 1. McAllister DA, Liu L, Shi T, et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: a systematic analysis. Lancet Glob Health 2019;7:e47–57. 10.1016/S2214-109X(18)30408-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudan I, O'Brien KL, Nair H, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health 2013;3:010401. 10.7189/jogh.03.010401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40. 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 4. Pneumonia. Available: https://data.unicef.org/topic/child-health/pneumonia/ [Accessed 26 May 2018].
- 5. Bhutta ZA, Das JK, Walker N, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet 2013;381:1417–29. 10.1016/S0140-6736(13)60648-0 [DOI] [PubMed] [Google Scholar]
- 6. Lassi Z, Mallick D, et al. Eessential interventions for child health. Reproductive Health 2014;11:S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chopra M, Mason E, Borrazzo J, et al. Ending of preventable deaths from pneumonia and diarrhoea: an achievable goal. Lancet 2013;381:1499–506. 10.1016/S0140-6736(13)60319-0 [DOI] [PubMed] [Google Scholar]
- 8. Leung DT, Chisti MJ, Pavia AT. Prevention and control of childhood pneumonia and diarrhea. Pediatr Clin North Am 2016;63:67–79. 10.1016/j.pcl.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nair H, Simões EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013;381:1380–90. 10.1016/S0140-6736(12)61901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. GBD 2016 Lower Respiratory Infections Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Infect Dis 2018;18:1191–210. 10.1016/S1473-3099(18)30310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO Revised WHO classification and treatment of childhood pneumonia at health facilities, 2014. ISBN: 978 92 4 150781 3. [PubMed] [Google Scholar]
- 12. Enoch AJ, English M, Shepperd S. Does pulse oximeter use impact health outcomes? A systematic review. Arch Dis Child 2016;101:1–7. 10.1136/archdischild-2015-309638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neonatal Vitamin A Supplementation Evidence group Early neonatal vitamin A supplementation and infant mortality: an individual participant data meta-analysis of randomised controlled trials. Arch Dis Child 2019;104:1–10. 10.1136/archdischild-2018-315242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brown N, Roberts C. Vitamin A for acute respiratory infection in developing countries: a meta-analysis. Acta Paediatr 2004;93:1437–42. 10.1111/j.1651-2227.2004.tb02625.x [DOI] [PubMed] [Google Scholar]
- 15. Krebs NF, Miller LV, Hambidge KM. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatr Int Child Health 2014;34:279–88. 10.1179/2046905514Y.0000000151 [DOI] [PubMed] [Google Scholar]
- 16. Walker CLF, Black RE. Zinc for the treatment of diarrhoea: effect on diarrhoea morbidity, mortality and incidence of future episodes. Int J Epidemiol 2010;39 Suppl 1:i63–9. 10.1093/ije/dyq023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yakoob MY, Theodoratou E, Jabeen A, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011;11 Suppl 3:S23. 10.1186/1471-2458-11-S3-S23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev 2016;12:CD005978. 10.1002/14651858.CD005978.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roth DE, Richard SA, Black RE. Zinc supplementation for the prevention of acute lower respiratory infection in children in developing countries: meta-analysis and meta-regression of randomized trials. Int J Epidemiol 2010;39:795–808. 10.1093/ije/dyp391 [DOI] [PubMed] [Google Scholar]
- 20. Haider BA, Lassi ZS, Ahmed A, et al. Zinc supplementation as an adjunct to antibiotics in the treatment of pneumonia in children 2 to 59 months of age. Cochrane Database Syst Rev 2011;10:CD007368. 10.1002/14651858.CD007368.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Das RR, Singh M, Shafiq N. Short-term therapeutic role of zinc in children <5 years of age hospitalised for severe acute lower respiratory tract infection. Paediatr Respir Rev 2012;13:184–91. 10.1016/j.prrv.2012.01.004 [DOI] [PubMed] [Google Scholar]
- 22. Tie H-T, Tan Q, Luo M-Z, et al. Zinc as an adjunct to antibiotics for the treatment of severe pneumonia in children <5 years: a meta-analysis of randomised-controlled trials. Br J Nutr 2016;115:807–16. 10.1017/S0007114515005449 [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: a meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Respir J 2018;12:857–64. 10.1111/crj.12646 [DOI] [PubMed] [Google Scholar]
- 24. PRISMA reporting guidelines. Available: http://www.equator-network.org/reporting-guidelines/prisma/
- 25. World Bank Country and Lending Groups [Internet]. Available: http://data.worldbank.org/about/country-and-lending-groups [Accessed 26 Oct 2015].
- 26. International Committee of Medical Journal Editors Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals. [Internet], 2019. Available: http://www.icmje.org/icmje-recommendations.pdf [Accessed 25 Apr 2020]. [PubMed]
- 27. Cochrane Handbook: risk of bias tool. Available: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
- 28. Valentiner-Branth P, Shrestha PS, Chandyo RK, et al. A randomized controlled trial of the effect of zinc as adjuvant therapy in children 2–35 mo of age with severe or nonsevere pneumonia in Bhaktapur, Nepal. Am J Clin Nutr 2010;91:1667–74. 10.3945/ajcn.2009.28907 [DOI] [PubMed] [Google Scholar]
- 29. Fataki MR, Kisenge RR, Sudfeld CR, et al. Effect of zinc supplementation on duration of hospitalization in Tanzanian children presenting with acute pneumonia. J Trop Pediatr 2014;60:104–11. 10.1093/tropej/fmt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Basnet S, Shrestha PS, Sharma A, et al. A randomized controlled trial of zinc as adjuvant therapy for severe pneumonia in young children. Pediatrics 2012;129:701–8. 10.1542/peds.2010-3091 [DOI] [PubMed] [Google Scholar]
- 31. Howie S, Bottomley C, Chimah O, et al. Zinc as an adjunct therapy in the management of severe pneumonia among Gambian children: randomized controlled trial. J Glob Health 2018;8:010418. 10.7189/jogh.08.010418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Srinivasan MG, Ndeezi G, Mboijana CK, et al. Zinc adjunct therapy reduces case fatality in severe childhood pneumonia: a randomized double blind placebo-controlled trial. BMC Med 2012;10:14. 10.1186/1741-7015-10-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wadhwa N, Chandran A, Aneja S, et al. Efficacy of zinc given as an adjunct in the treatment of severe and very severe pneumonia in hospitalized children 2–24 mo of age: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2013;97:1387–94. 10.3945/ajcn.112.052951 [DOI] [PubMed] [Google Scholar]
- 34. Bose A, Coles CL, Gunavathi JH, et al. Efficacy of zinc in the treatment of severe pneumonia in hospitalized children <2 y old. Am J Clin Nutr 2006;83:1089–96. 10.1093/ajcn/83.5.1089 [DOI] [PubMed] [Google Scholar]
- 35. Brooks WA, Yunus M, Santosham M, et al. Zinc for severe pneumonia in very young children: double-blind placebo-controlled trial. Lancet 2004;363:1683–8. 10.1016/S0140-6736(04)16252-1 [DOI] [PubMed] [Google Scholar]
- 36. Sempértegui F, Estrella B, Rodríguez O, et al. Zinc as an adjunct to the treatment of severe pneumonia in Ecuadorian children: a randomized controlled trial. Am J Clin Nutr 2014;99:497–505. 10.3945/ajcn.113.067892 [DOI] [PubMed] [Google Scholar]
- 37. Shah GS, Dutta AK, Shah D, et al. Role of zinc in severe pneumonia: a randomized double bind placebo controlled study. Ital J Pediatr 2012;38:36. 10.1186/1824-7288-38-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baruah A, Saikia H. Effect of zinc supplementation in children with severe pneumonia: a randomised controlled study. J Clin Diagn Res 2018;12. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2020-000662supp001.pdf (15.9KB, pdf)
bmjpo-2020-000662supp002.pdf (60.3KB, pdf)
bmjpo-2020-000662supp003.pdf (34KB, pdf)