Abstract
Background:
Patients with Peutz–Jeghers syndrome develop hamartomatous polyps in the small bowel, possibly causing anemia, intussusception, and obstruction. We aimed to evaluate the impact of an enteroscopy-based approach, including both device-assisted and intraoperative enteroscopy, on the reduction of the polyp burden in a cohort of adult Peutz–Jeghers syndrome patients.
Materials and methods:
A retrospective study was conducted at Azienda Ospedaliero-Universitaria Città della Salute e della Scienza in Turin, Italy. Consecutive Peutz–Jeghers syndrome patients eligible for device-assisted or intraoperative enteroscopy, between January 2003 and November 2019, were included. Enteroscopy technical issues and complications were recorded. At the time of index enteroscopy, the patients’ clinical records were retrospectively reviewed, and clinical data were recorded until November 2019.
Results:
Overall, 24 patients were included. Before inclusion, 16/24 patients (66.7%) underwent small bowel surgery for polyp-related complications, 13 of which (81.2%) in an emergent setting. Two patients had a history of small bowel neoplasms. During the timeframe, 47 device-assisted enteroscopies and 9 intraoperative enteroscopies were performed, and 247 small bowel polyps were endoscopically removed. The overall complication rate was 12.8% (8.5% for device-assisted enteroscopy, 22.2% for intraoperative enteroscopy). The median observation time was 108 months: in this timeframe, two patients developed small bowel polyp-related complications requiring emergent surgery. No patients developed small bowel cancer, but nine extra-gastrointestinal neoplasms were recorded.
Conclusion:
An enteroscopy-based approach appears to be well tolerated and effective in decreasing polyp-related complications in Peutz–Jeghers syndrome patients, thus reducing the need for emergent surgery. Although the prevention of small bowel polyp-related complications remains the main goal in these patients, the high incidence of extra-gastrointestinal neoplasms appears to be a rising issue.
Keywords: device-assisted enteroscopy, enteroscopy, Peutz–Jeghers syndrome, small bowel
Background
Peutz–Jeghers syndrome (PJS) is a hereditary polyposis syndrome due to mutations of STK11 (LKB1) gene, with an autosomal dominant transmission and incomplete penetrance. The incidence of PJS is estimated to be 0.5–2 out of 100,000 births per year.1 Its main features are a typical mucocutaneous pigmentation and hamartomatous polyps in the gastrointestinal (GI) tract.2–4 Patients affected by PJS also have an increased risk of developing GI and extra-GI cancers.5 In these patients, GI cancers have the highest incidence with a cumulative risk of 33% at the age of 60, mainly occurring in the colon and in the small bowel (SB).5 Breast and pancreatic cancers are the most frequent extra-GI neoplasms in PJS patients.
PJS hamartomatous polyps can occur anywhere in the GI tract, but they are most commonly located in the small intestine; up to 96% of PJS patients develop nondysplastic hamartomatous polyps in the SB, while about one-third of them have gastric or colonic polyps.6 SB PJS polyps usually arise during childhood and the first complications, such as intussusception, intestinal obstruction, or bleeding, typically occur within the second decade.7,8 Moreover, as demonstrated in historical cohorts, more than two-thirds of the patients underwent surgery because of SB polyp-related complications by the end of the second decade.9,10
Screening strategies aimed at reducing SB polyp-related complications have evolved during the years. The latest European guidelines suggest to start the screening at the age of 8 years and to repeat diagnostic procedures every 1–3 years according to the clinical phenotype: video capsule endoscopy (VCE) and magnetic resonance enterography (MRE) are the recommended imaging techniques for the study of the SB.7,11,12 Computed tomography enterography (CTE) can also be used to detect SB polyps, especially when ⩾1 cm.13 The effectiveness of the dedicated SB diagnostic tools, along with the widespread adoption of the recommended screening policy, has resulted in an increase in PJS patients needing polyp removal. Until recently, the management of polyps of the SB was mainly surgical, often requiring several interventions over time with multiple resections, potentially resulting in short bowel syndrome. However, the development in recent years of device-assisted enteroscopy (DAE) systems, coupling an extensive evaluation of the SB with endoscopic polyp removal, has offered the opportunity for a nonsurgical management of SB polyps. In fact, recent guidelines agree that polypectomy or endoscopic mucosal resection (EMR) by means of DAE, or intraoperative enteroscopy (IOE) in selected cases, is the most effective treatment for the reduction of SB polyp burden in PJS patients (Figure 1).11,14 However, although recent studies on the endoscopic management of PJS patients seem to provide consistent results, they include a limited number of patients, are often focused on the pediatric patients, and mostly have a short follow-up (Table 1).8,15–31
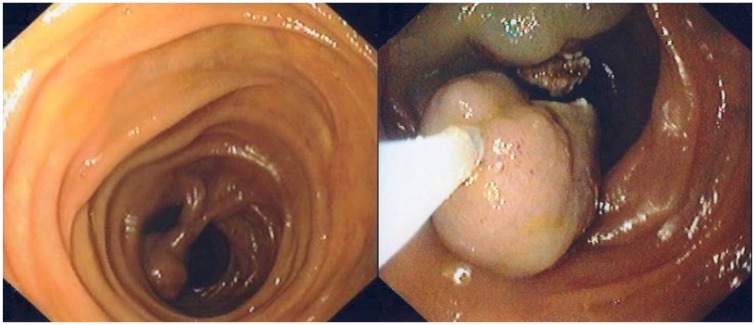
Figure 1.
Polypectomy of a jejunal PJS polyp.
PJS, Peutz–Jeghers syndrome.
Table 1.
Literature.
| References | No. of patients | No. of procedures | Observation time (months) | SBE (oral/anal) | DBE (oral/anal) | IOE | No. of polypectomies | Size of resected polyps | Maximum polyp size | No. of complications (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ohmiya and colleagues15 | 2 | 5 | NA | – | Oral (2); anal (3) | – | 18 | >10 mm | 60 mm | 1 fever, 1 abdominal pain (40%) |
| Plum and colleagues16 | 16 | 47 | NA | – | Oral (39); anal (8) | – | 47 | >15 mm | 50 mm | 2 bleeding, 1 perforation, 1 propofol desaturation (8.5%) |
| Ohmiya and colleagues17 | 18 | 80 | 54.0 (median) | – | Oral (42); anal (38) | – | 387 | >5 mm | 50 mm | 1 perforation, 1 pancreatitis, 1 postpolypectomy syndrome (3.8%) |
| Gao and colleagues18 | 13 | 29 | 19.0 (median) | – | Oral (26); anal (3) | – | 79 | >10 mm | 50 mm | 0 (0%) |
| Kopácová and colleagues19 | 10 | 21 | NA | – | Oral (14); anal (0) | 7 | 205 | All polyps | 60 mm | 1 bleeding (4.8%) |
| Sakamoto and colleagues20 | 15 | 88 | 29.9 (mean) | – | 88 (NA) | – | 341 | NA | NA | 2 pancreatitis, 2 late bleeding, 1 perforation (5.7%) |
| Chen and colleagues21 | 6 | 17 | 32.0 (mean) | 5 (NA) | 12 (NA) | – | NA | >10 mm | 60 mm | 0 (0%) |
| Akarsu and colleagues8 | 7 | 31 | NA | – | Oral (21); anal (9) | 1 | 110 | >10 mm | 100 mm | 1 bleeding (3.2%) |
| Gorospe and colleagues22 | 22a | 34 | 27.0 (mean) | – | Oral (20); anal (14) | – | >10 mm | 50 mm | 2 abdominal pain, 1 intraprocedural bleeding, 1 self-limiting postprocedural bleeding (11.8%) | |
| Serrano and colleagues23 | 25 | 46 | 56.5 (median) | – | Oral (44); anal (2) | – | 214 | >10 mm | 40 mm | 2 postprocedural bleeding, 1 perforation (6.5%) |
| Bizzarri and colleagues24 | 10 | 23 | NA | Oral (16); anal (7) | – | – | 53 | >15 mm | NA | 3 abdominal pain, 1 perforation (17.4%) |
| Torroni25 | 7 | 7 | NA | Oral (7); anal (0) | – | – | 24 | >15 mm | 50 mm | 0 (0%) |
| Kröner and colleagues26 | 2 | 2 | NA | – | Oral (2); anal (0) | – | 2 | >20 mm | 25 mm | 0 (0%) |
| Belsha and colleagues27 | 16 | 22 | 26.0 (median) | – | Oral (11); anal (8) | 3 | 45 | NA | NA | 1 pelvic abscess (4.5%) |
| Blanco-Velasco and colleagues28 | 4 | 12 | NA | Oral (5); anal (2) | Oral (3); anal (2) | – | 35 | >10 mm | 40 mm | 1 pancreatitis (8.3%) |
| Wang and colleagues29 | 97 | 320 | 46.7 (median) | – | Oral (185); anal (135) | – | 1661 | NA | NA | 8 postprocedural bleeding, 4 perforation, 1 intussusception, 1 postpolypectomy syndrome (4.4%) |
| Perrod and colleagues30 | 25 | 54b | 60.0 (median) | – | Oral (25); anal (8) | 4 | 274 | >10 mm | 60 mm | 2 postprocedural bleeding, 1 pancreatitis (5.5%) |
| Kirakosyan and Lokhmatov31 | 30 | 30 | NA | 30 (NA) | – | – | 68 | >10 mm | NA | 1 postprocedural perforation (3.3%) |
DBE, double-balloon enteroscopy; IOE, intraoperative enteroscopy; NA, not applicable; PE, push enteroscopy; SBE, single-balloon enteroscopy; –, not performed.
Patients with all hamartomatous polyposis syndromes.
Includes 2 PE and 15 spiral enteroscopies.
In this study, we aimed to assess the impact of an enteroscopy-based approach (including both DAE and IOE) on the reduction of the polyp burden in a cohort of PJS patients.
Methods
All consecutive adult patients (⩾18 years old) with clinical or genetic diagnosis of PJS who underwent DAE or IOE between January 2003 and November 2019, in a tertiary care center (Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino, Turin, Italy), were included. This center has a well-established dedicated pathway for diagnosis and therapy of PJS patients and, since the introduction of DAE in 2003, taking into account the limitation of diagnostic procedures in locating the SB polyps, all the endoscopic procedures were performed by DAE or IOE, whereas push enteroscopies were no longer performed. In this center, patients were considered eligible for DAE or IOE if ⩾1 SB polyp ⩾1.5 cm in size was detected by diagnostic procedures (e.g. VCE, MRE, CTE, and SB series/enteroclysis). The route of insertion was decided depending on the imaging findings: when necessary, the patients underwent a combined approach (i.e. antegrade procedure followed by retrograde procedure some days after) in order to remove all the detected polyps. The choice between DAE and IOE was taken on a case-by-case basis, taking into account not only diagnostic test results (namely, number, size, and location of polyps) but also the characteristics of the patient, ongoing comorbidities, the previous history of surgical resection, the risk of short bowel, and the will of the patient. In case of possible IOE, we discussed the case during multidisciplinary meeting with radiologists, anesthesiologists, and surgeons.
For each included patient, the following data were collected through a dedicated database: patient’s characteristics at time of index enteroscopy (e.g. demographics, diagnostic SB procedures performed before enteroscopy, previous oncological history, history of GI previous surgical interventions, etc.), enteroscopic procedures performed during the study timeframe (e.g. type of procedure, procedural issues, complications, etc.), as well as any other clinically relevant event occurring during the study timeframe (e.g. surgical interventions, results of screening tests, etc.). These data were retrieved by checking hospital medical records and procedures’ reports or, when unavailable, by phone contact with the patients at time of data collection (i.e. November 2019). In our study, the observation time was defined as the time interval between the index enteroscopy (either DAE or IOE) and the data collection time.
All enteroscopic procedures were performed under deep sedation or under general anesthesia with tracheal intubation on a case-by-case basis, by a gastroenterologist experienced in enteroscopy (M.P., P.C.V.) after obtaining a written medical informed consent. As far as DAE is concerned, the insertion route was based on the location of the largest polyp at previous imaging.
DAE was performed with either single- or double-balloon system: the choice between single-balloon enteroscopy (SBE) and double-balloon enteroscopy (DBE) was related to the availability of the equipment at the time of the examination. SBE was performed with either a standard Olympus SIF-Q180 (Olympus Optical Co., Tokyo, Japan) or a prototype, equipped with a wider operative channel (3.2 mm). DBE was performed with Fujinon EN-450P5 or Fujinon EN-580T (Fujifilm Inc., Tokyo, Japan). Both systems are described in detail elsewhere.32 IOE was performed with Olympus SIF-100, SIF-Q140, or SIF-Q180 (Olympus Optical Co.); after surgical enterotomy, the progression in the SB loops was performed directly by the surgeon.
All polyps were resected endoscopically, with the en bloc or piecemeal technique according to the polyp size, morphology, and location. Submucosal injection prior to the polyp removal was performed with saline solution or diluted epinephrine (epinephrine ratio was at the discretion of the endoscopist). Prophylactic clipping after the polyp removal was done at the discretion of the endoscopist.
All resected polyps were sent to the Pathology Department of our hospital for histopathological analysis. Incidental polyps <5 mm were not included in the total count of removed polyps.
Enteroscopy-related complications (e.g. bleeding, perforation, and acute pancreatitis) were systematically recorded: these were defined as intraprocedural when occurring during the procedure, early, or delayed when occurring within or over 24 h after the procedure, respectively. Bleeding was classified as minor when self-limiting or when hemostasis was achieved with endotherapy during the same procedure with a hemoglobin drop <2 g/dL, and as major when a hemoglobin drop ⩾2 g/dL was observed or when transfusions were required or when further procedures were planned (i.e. hembolization or a new DAE) to perform hemostasis or when it increased the length of the hospital stay. The diagnosis of acute pancreatitis was made according to the established diagnostic criteria.33 For IOE, surgically related complications were also collected and systematically recorded.
Results
Patients’ characteristics at time of index enteroscopy
Between January 2003 and November 2019, 24 patients who met the inclusion criteria were included; 15 of them were male (62.5%). At time of inclusion in this study, the median age was 33.5 years [interquartile range (IQR): 25.0–43.5]. The index enteroscopy was DAE in 18 patients and IOE in 6 patients.
The patients were referred for index enteroscopy because of the detection of an SB polyp requiring removal, in at least one dedicated diagnostic procedure: as reported in Table 2, overall 19 VCE, 4 MRE, 3 CTE, and 3 SB series were performed. In detail, 5 patients underwent more than one diagnostic procedure, whereas, out of the remaining 19 patients, 14 underwent only VCE, 1 only MRE, 1 only CTE, and 3 only SB series.
Table 2.
Summary of cases.
| Patient | Evaluation at time of index DAE/IOE |
Enteroscopic procedures during the study timeframe |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at enteroscopy | SB diagnostic procedure |
Observation time (months) | SBE (oral/anal) | DBE (oral/anal) | IOE | Resected polyps: location (number) [dimension range] | Complications (no.) |
|||||
| VCE | MRE | CTE | SB series | DAE related | IOE related | |||||||
| P.F. | 45 | y | – | – | – | 46 | Oral (1); anal (0) | – | – | Jejunum (2) [15–20 m] | Minor intraprocedural bleeding (1) | |
| P.P. | 25 | y | – | – | – | 124 | Oral (3); anal (2) | – | 1 | Duodenum (2); jejunum (38); ileum (12) [5–60 mm] | Late perforation after IOE (1) | |
| P.R. | 21 | y | – | – | – | 170 | Oral (1); anal (0) | Oral (1); anal (1) | 1 | Jejunum (9) [10–40 mm] | ||
| P.N. | 19 | y | – | – | – | 162 | Oral (0); anal (1) | – | 1 | Jejunum (4); ileum (2) [15–50 mm] | ||
| T.L. | 39 | y | – | – | – | 77 | – | – | 1 | Duodenum (0)a | ||
| M.N. | 52 | y | – | – | – | 121 | – | Oral (1); anal (1) | – | Duodenum (2); jejunum (3) [30–40 mm] | ||
| G.L. | 33 | y | – | – | – | 157 | Oral (3); anal (1) | Oral (1); anal (1) | – | Duodenum (1); jejunum (9); ileum (4) [5–30 mm] | ||
| F.S. | 32 | y | – | – | – | 167 | Oral (1); anal (1) | Oral (1); anal (0) | – | Duodenum (3); jejunum (3) [10–40 mm] | ||
| A.A. | 25 | y | y | – | – | b | Oral (1); anal (0) | – | – | Jejunum (2) [5–10 mm] | ||
| S.R. | 43 | – | – | – | y | 73 | Oral (2); anal (0) | – | – | Duodenum (1); jejunum (1) [10–50 mm] | ||
| M.A. | 31 | – | y | – | – | 21 | Oral (1); anal (0) | – | – | Jejunum (3) [25–40 mm] | Minor intraprocedural bleeding (1) | |
| C.M. | 29 | y | – | – | – | 34 | Oral (2); anal (0) | – | – | Jejunum (3); ileum (1) [5–40 mm] | ||
| P.G. | 21 | y | y | – | – | 16 | Oral (1); anal (0) | – | – | Duodenum (1); jejunum (3) [40–50 mm] | ||
| G.V. | 20 | y | y | – | – | 18 | Oral (1); anal (0) | – | – | Jejunum (3) [10–60 mm] | ||
| S.G. | 54 | y | – | – | – | 197 | – | – | 1 | Duodenum (1); jejunum (2); ileum (3) [5–10 mm] | ||
| S.A. | 34 | – | – | – | y | b | – | – | 1 | Jejunum (6); ileum (1) [10–45 mm] | ||
| D.D. | 30 | y | – | – | – | 163 | Oral (2); anal (0) | Oral (2); anal (0) | 1 | Duodenum (32); jejunum (17); ileum (15) [5–60 mm] | Minor intraprocedural bleeding (1) | |
| P.R. | 42 | – | – | – | y | 173 | Oral (3); anal (2) | Oral (1); anal (1) | 1 | Jejunum (10); ileum (26) [10–50 mm] | Intraprocedural pneumothorax (1) | |
| S.A. | 51 | y | – | – | – | 164 | – | Oral (1); anal (0) | – | Jejunum (1) [10 mm] | ||
| S.A. | 46 | y | – | – | – | 195 | – | – | 1 | Jejunum (4); ileum (2) [10–50 mm] | ||
| R.A. | 25 | y | – | y | – | 7 | – | Oral (1); anal (0) | – | Ileum (1) [15 mm] | ||
| M.A. | 41 | y | – | – | – | 95 | – | Oral (2); anal (1) | – | Jejunum (4); ileum (2) [10–35 mm] | ||
| P.G. | 37 | y | – | y | – | 40 | – | Oral (1); anal (0) | – | Jejunum (1) [20 mm] | ||
| C.L. | 65 | – | – | y | – | 31 | – | Oral (1); anal (0) | – | Duodenum (3); jejunum (4) [10–45 mm] | Minor intraprocedural bleeding (1) | |
CTE, computed tomography enterography; DAE, device-assisted enteroscopy; DBE, double-balloon enteroscopy; IOE, intraoperative enteroscopy; MRE, magnetic resonance enterography; SB, small bowel; SBE, single-balloon enteroscopy; VCE, video capsule endoscopy.
Polyp not removable by enterotomy (descending duodenum, medial wall, size) and the patient underwent elective duodenopancreatectomy.
Not possible to retrieve data.
At time of inclusion, 16 patients had already received SB surgery (66.7%) for SB polyp-related complications, such as intussusception or intestinal obstruction, even if 13 of them (81.2%) were not diagnosed yet with PJS at the time of surgery. Surgical intervention was performed in an emergent setting in these 13 patients. Two patients (2/24; 8.3%) had a history of SB adenocarcinoma, which was treated surgically in an elective setting, whereas no patients had a history of extra-GI neoplasms. The patient’s characteristics are summarized in Table 3.
Table 3.
Patients’ characteristics at time of inclusion.
| Demographics | |
| No. of patients | 24 |
| Males, n (%) | 15 (62.5) |
| Median age (IQR) | 33.5 (25.0–43.5) |
| History of SB surgery | |
| n (%) | 16 (66.7) |
| In emergency, n (%) | 13/16 (81.2) |
| History of SB neoplasms, n (%) | 2 (8.3) |
| History of extra-GI neoplasms, n (%) | 0 (0) |
GI, gastrointestinal; IQR, interquartile range; SB, small bowel.
Enteroscopic procedures
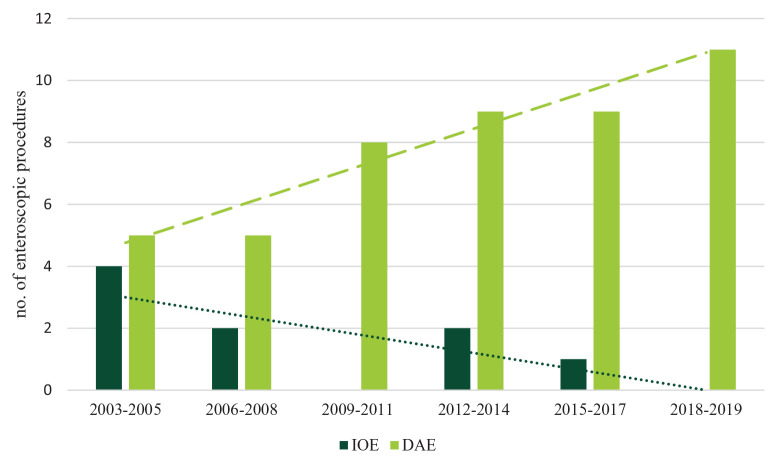
During the study timeframe, 56 enteroscopic procedures were performed overall: 47 DAE (47/56; 83.9%) and 9 IOE (9/56; 16.1%). Out of 47 DAE procedures, 29 were SBE (22 with an antegrade approach) and 18 were DBE (13 with an antegrade approach). On six occasions, a combined (oral and anal) approach was performed (DBE in five cases, SBE in one case) because the polyp/s identified in previous diagnostic tests (target polyp/s) was not identified during the first examination; in these patients, a tattoo was placed and a second enteroscopy examination was planned, via the alternate route. During the second examination, the target polyp/s was always reached without the need of a complete SB exploration. The time trend of IOE and DAE performed in the study timeframe is presented in Figure 2.
Figure 2.
Time trend of IOE and DAE.
DAE, device-assisted enteroscopy; IOE, intraoperative enteroscopy.
The dashed line shows the linear trend of DAE procedures over time, whereas the dotted line refers to the linear time trend of IOE.
Endoscopic resection of 247 SB polyps ⩾5 mm (size range: 5.0–60.0 mm) was carried out: 181 of these polyps (73.3%) measured ⩾15 mm. 158/247 polyps (64.0%) were resected during DAE (median number of polyps per DAE: 2.0, IQR: 1.0–4.0) and 89 (36.0%) during 8 IOE (median number of polyps per IOE: 7.5, IQR: 6.0–17.0). During one IOE, no polyps were resected: the previously detected lesion was considered unresectable due to its localization (descending duodenum, medial wall) and its extremely large size, and the patient has recently undergone duodenopancreatectomy. Out of 247 polyps, 242 were histologically confirmed as hamartomas, 1 was a tubulovillous adenoma with high-grade dysplasia, and 1 was a serrated polyp with low-grade dysplasia; 3 polyps were not retrieved after resection.
Complications occurred in 6/47 procedures (12.8%): the per-procedure complication rate was 8.5% (4/47) and 22.2% (2/9) in patients undergoing DAE and IOE, respectively. Regarding DAE-related complications, one was defined as major and three as minor. The only major complication was a pneumothorax at the end of a per-oral DAE: the patient was treated conservatively and recovered uneventfully. In three cases, a minor intraprocedural bleeding was observed. Two IOE-related complications were reported: one case of minor intraprocedural bleeding and one case of delayed perforation, the latter being related to enterotomy. Neither perforation nor acute pancreatitis was reported in our series. Procedural and per-patient details are reported in Tables 4 and 2, respectively.
Table 4.
Enteroscopic procedures.
| Type of enteroscopy | No. of procedures, n (%) | Antegrade procedures | No. of resected polyps, n (%) | Complications (%) |
|---|---|---|---|---|
| SBE | 29 (51.8) | 22 | 113 (45.8) | 3 minor intraprocedural bleeding, 1 pneumothorax (4/47 (8.5%)) |
| DBE | 18 (32.1) | 13 | 45 (18.2) | |
| IOE | 9 (16.1) | NA | 89 (36.0) | 1 minor intraprocedural bleeding, 1 late perforation (2/9 (22.2%)) |
| Total | 56 (100) | 35 | 247 (100) | 6 (12.8%) |
DBE, double-balloon enteroscopy; IOE, intraoperative enteroscopy; NA, not applicable; SBE, single-balloon enteroscopy.
Clinical data during the study timeframe
After index enteroscopy, no further data were retrieved in two patients (medical data were unavailable and we could not contact them by phone): therefore, they were excluded from further analysis. Data collected in 22 patients are summarized in Table 5.
Table 5.
Patients’ characteristics during the study timeframe.
| No. of patients | 22 |
| Observation time (months), median (IQR) | 108.0 (35.5–163.7) |
| SB surgery, n (%) | 2/22 (9.1) |
| SB neoplasms, n (%) | 0/22 (0) |
| Extra-GI neoplasms, n (%) | 8/22 (36.4) |
| Deaths, n (%) | 3/22 (13.6) |
GI, gastrointestinal; IQR, interquartile range; SB, small bowel.
The median time of observation was 108.0 months (IQR: 35.5–163.7). During this timeframe, 2/22 patients (9.1%) developed polyp-related complications (intussusception in both cases) which led to emergent surgery. No SB neoplasms were reported, whereas in 8/22 patients (36.4%) 9 extra-GI neoplasms were recorded: pancreatic cancer (n = 1), lung cancer (n = 2), breast cancer (n = 2), ovarian cancer (n = 1), papillary thyroid cancer (n = 1), basal cell carcinoma (n = 1), and hepatocellular carcinoma (n = 1). Out of 22 patients, 3 died during the observation period (13.6%): all deaths were directly related to extra-GI neoplasms (lung, pancreatic, and ovarian cancers).
Discussion
In our series, during a 17-year timeframe, we included 24 adult patients who underwent 56 enteroscopic procedures overall (46 DAE and 9 IOE). For each included patient, we systematically collected the technical data of all the procedures, thus confirming the high efficacy and safety profile of the enteroscopy-based approach. For the majority of the patients, it was possible to collect clinical data during the study timeframe, which highlighted a reduction in SB polyp-related complications and an increase in extra-GI neoplasms.
As our study timeframe is long (about 17 years), we could observe the distribution of enteroscopic procedures over time. In detail, we observed a stable increase in device-assisted procedures throughout the years, whereas intraoperative procedures decreased progressively. In our study, the choice between DAE and IOE was not standardized: upfront IOE was considered after multidisciplinary discussion on a case-by-case basis, given its high rate of complications, when large or multiple polyps were detected during baseline imaging workup; no predefined cutoff for the polyp size or number was set to take IOE into consideration, but indeed an evaluation combining clinical and technical parameters, as well as the will of the patient, was performed. IOE was also indicated when polyps were considered too difficult to remove endoscopically. Nevertheless, all our data show that DAE has almost totally replaced IOE and that it is, by now, the reference standard technique for the removal of SB polyps in PJS patients.11
Although the patients were included in the study with different timings, at the time of index enteroscopy, in our case series the median time of observation was long (about 9 years). Interestingly, during this timeframe, there were only 2 cases of SB polyp-related complications with consequent emergent surgery: conversely, about two-thirds of these patients had a positive history for SB surgery due to polyp-related complications prior to joining the study. These data indirectly highlight the efficacy of enteroscopy in the management of SB polyps and appear to confirm the results of other studies in which, in patients undergoing multiple DAE sessions, the median number and dimension of resected SB polyps progressively decreased.20,29
Of note, during the observation period, no SB neoplasms were detected even though two patients had a positive history for SB cancer: although our cohort is small, it is intriguing to hypothesize a direct effect in SB cancer prevention in these patients, as suggested by Latchford and colleagues.34
In PJS patients, the endoscopic management of polyps is technically challenging due to their localization in the SB: the SB wall is thin, the working space is limited, and, sometimes, it is difficult to maintain a stable position with the scope. The complication rate of DAE in our study is relatively high (8.5%), especially when compared with studies with a large population of patients undergoing operative DAE.35 Nevertheless, it should be noted that these series take into account all operative enteroscopies, including hemostatic procedures which are generally less risky. Conversely, our data are exclusively related to polypectomy, which is considered to be the most dangerous procedure due to the thin SB wall, and they appear to be in line with the complication rate reported in similar case series of PJS patients undergoing DAE. Of note, most of the complications in our study were minor and were easily managed by the endoscopist during the same procedure.
On the contrary, the safety profile of IOE is different: although in line with the literature data, in our study IOE’s complication rate is higher than that of DAE. Possible explanations may be related to the higher number of polypectomies performed during IOE (median number of polyps removed during DAE and IOE being 2 and 7, respectively), to the localization of polyps and to their size (patients undergoing IOE were considered having more ‘difficult’ polyps), and to the surgical setting per se.
Interesting data came out of the oncological analysis: during the 9-year median observation period, eight patients developed nine extra-GI neoplasms, whereas at the time of inclusion in our network none of them had such a diagnosis. Moreover, extra-GI neoplasms are the cause of all deaths in our cohort. Although the median age at index enteroscopy was 35 years and both the study timeframe and the median observation time (17 and 9 years, respectively) were relatively long, in our opinion the trend observed in our cohort might not be due to aging only, but it is likely to reflect a true increase in extra-GI neoplasm incidence. Recent guidelines propose a dedicated multiorgan screening protocol for oncological prevention, which is also applied in our center.13 The evidence of surveillance recommendations is low, due to the rarity of the syndrome: the variety of the pathways of genetic mutations makes the understanding of cancer development in PJS patients extremely problematic.7,36,37 Currently, the rising incidence of extra-GI neoplasms appears to be the most significant burden for patients affected by PJS. Due to these data, it seems that ongoing cancer surveillance protocols are not sufficient yet.
Our study has several limitations. First of all, it is a descriptive study with a retrospective design. Second, although the study timeframe is wide, the overall number of included patients is relatively small; however, our sample size is in line with that reported in previous paper and the rarity of the syndrome, as well as the relative novelty of the DAE approach, compared with previous management strategies, makes the patient collection difficult. Third, as the main goal of enteroscopy examinations was to remove the polyp/s identified during previous diagnostic procedures (either VCE or SB dedicated cross-sectional imaging techniques), we did not attempt to perform complete SB explorations. Moreover, we are not able to estimate the impact of enteroscopy techniques taking into account the whole SB detection rate. However, we can hypothesize that the impact of a complete evaluation of the SB could be even higher than that observed in our study. Last but not least, some patients were referred to our tertiary care center by other small hospitals in Italy, and therefore we cannot be sure that the oncological extra-GI screening had been carried out properly (some patients could already harbor extra-GI lesions when they came to our attention) or that some other relevant data had been missed or not reported. Despite these limitations, we believe that these data might be one of the resources for establishing management strategies in patients with PJS in the future.
Conclusion
Results of our study show that the enteroscopy-based approach appears to be effective in decreasing polyp-related complications, thus reducing the need for emergent surgery, with an acceptable rate of procedural adverse events, particularly as far as DAE is concerned. Although the prevention of SB polyp-related complications remains the main goal of screening programs in PJS patients, the high incidence of extra-GI neoplasms is a rising issue in PJS patients.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Pablo Cortegoso Valdivia  https://orcid.org/0000-0002-8793-5890
https://orcid.org/0000-0002-8793-5890
Marco Pennazio  https://orcid.org/0000-0001-6151-0395
https://orcid.org/0000-0001-6151-0395
Contributor Information
Pablo Cortegoso Valdivia, University Division of Gastroenterology, Department of Medical Sciences, City of Health and Science University Hospital, Turin, Italy.
Emanuele Rondonotti, Gastroenterology Unit, Valduce Hospital, Como, Italy.
Marco Pennazio, University Division of Gastroenterology, Department of Medical Sciences, City of Health and Science University Hospital, Via Cavour 31, 10123 Turin, Italy.
References
- 1. Giardiello FM, Trimbath JD. Peutz-Jeghers syndrome and management recommendations. Clin Gastroenterol Hepatol 2006; 4: 408–415. [DOI] [PubMed] [Google Scholar]
- 2. Peutz J. Very remarkable case of familial polyposis of mucous membrane of intestinal tract and nasopharynx accompanied by peculiar pigmentation of skin and mucous membrane. Nederl Maandschr Geneesk 1921; 10: 134–146. [Google Scholar]
- 3. Jeghers H, McKusick VA, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits: a syndrome of diagnostic significance. N Engl J Med 1949; 241: 993, illust; passim. [DOI] [PubMed] [Google Scholar]
- 4. Jeghers H, McKusick VA, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits: a syndrome of diagnostic significance. N Engl J Med 1949; 241: 1031–1036. [DOI] [PubMed] [Google Scholar]
- 5. Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006; 12:3209–3215. [DOI] [PubMed] [Google Scholar]
- 6. Schreibman IR, Baker M, Amos C, et al. The hamartomatous polyposis syndromes: a clinical and molecular review. Am J Gastroenterol 2005; 100: 476–490. [DOI] [PubMed] [Google Scholar]
- 7. Beggs AD, Latchford AR, Vasen HFA, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010; 59: 975–986. [DOI] [PubMed] [Google Scholar]
- 8. Akarsu M, Ugur Kantar F, Akpinar H. Double-balloon endoscopy in patients with Peutz-Jeghers syndrome. Turk J Gastroenterol 2012; 23: 496–502. [DOI] [PubMed] [Google Scholar]
- 9. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol 2011; 106: 940–945. [DOI] [PubMed] [Google Scholar]
- 10. Hinds R, Philp C, Hyer W, et al. Complications of childhood Peutz-Jeghers syndrome: implications for pediatric screening. J Pediatr Gastroenterol Nutr 2004; 39: 219–220. [DOI] [PubMed] [Google Scholar]
- 11. van Leerdam ME, Roos VH, van Hooft JE, et al. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2019; 51: 877–895. [DOI] [PubMed] [Google Scholar]
- 12. Gupta A, Postgate AJ, Burling D, et al. A prospective study of MR enterography versus capsule endoscopy for the surveillance of adult patients with Peutz-Jeghers syndrome. Am J Roentgenol 2010; 195: 108–116. [DOI] [PubMed] [Google Scholar]
- 13. Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015; 110: 223–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Latchford AR, Phillips RK. Gastrointestinal polyps and cancer in Peutz-Jeghers syndrome: clinical aspects. Fam Cancer 2011; 10: 455–461. [DOI] [PubMed] [Google Scholar]
- 15. Ohmiya N, Taguchi A, Shirai K, et al. Endoscopic resection of Peutz-Jeghers polyps throughout the small intestine at double-balloon enteroscopy without laparotomy. Gastrointest Endosc 2005; 61: 140–147. [DOI] [PubMed] [Google Scholar]
- 16. Plum N, May AD, Manner H, et al. Peutz-Jeghers syndrome: endoscopic detection and treatment of small bowel polyps by double-balloon enteroscopy. Z Gastroenterol 2007; 45: 1049–1055. [DOI] [PubMed] [Google Scholar]
- 17. Ohmiya N, Nakamura M, Takenaka H, et al. Management of small-bowel polyps in Peutz-Jeghers syndrome by using enteroclysis, double-balloon enteroscopy, and videocapsule endoscopy. Gastrointest Endosc 2010; 72: 1209–1216. [DOI] [PubMed] [Google Scholar]
- 18. Gao H, van Lier MG, Poley JW, et al. Endoscopic therapy of small-bowel polyps by double-balloon enteroscopy in patients with Peutz-Jeghers syndrome. Gastrointest Endosc 2010; 71: 768–773. [DOI] [PubMed] [Google Scholar]
- 19. Kopácová M, Bures J, Ferko A, et al. Comparison of intraoperative enteroscopy and double-balloon enteroscopy for the diagnosis and treatment of Peutz-Jeghers syndrome. Surg Endosc 2010; 24: 1904–1910. [DOI] [PubMed] [Google Scholar]
- 20. Sakamoto H, Yamamoto H, Hayashi Y, et al. Nonsurgical management of small-bowel polyps in Peutz–Jeghers syndrome with extensive polypectomy by using double-balloon endoscopy. Gastrointest Endosc 2011; 74: 328–333. [DOI] [PubMed] [Google Scholar]
- 21. Chen TH, Lin WP, Su MY, et al. Balloon-assisted enteroscopy with prophylactic polypectomy for Peutz-Jeghers syndrome: experience in Taiwan. Dig Dis Sci 2011; 56: 1472–1475. [DOI] [PubMed] [Google Scholar]
- 22. Gorospe EC, Alexander JA, Bruining DH, et al. Performance of double-balloon enteroscopy for the management of small bowel polyps in hamartomatous polyposis syndromes. J Gastroenterol Hepatol 2013; 28: 268–273. [DOI] [PubMed] [Google Scholar]
- 23. Serrano M, Mão-de-Ferro S, Pinho R, et al. Double-balloon enteroscopy in the management of patients with Peutz-Jeghers syndrome: a retrospective cohort multicenter study. Rev Esp Enferm Dig 2013; 105: 594–599. [DOI] [PubMed] [Google Scholar]
- 24. Bizzarri B, Borrelli O, de’Angelis N, et al. Management of duodenal–jejunal polyps in children with Peutz-Jeghers syndrome with single-balloon enteroscopy. J Pediatr Gastroenterol Nutr 2014; 59: 49–53. [DOI] [PubMed] [Google Scholar]
- 25. Torroni F. Conservative approach in Peutz-Jeghers syndrome: single-balloon enteroscopy and small bowel polypectomy. World J Gastrointest Endosc 2014; 6: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kröner PT, Sancar A, Fry LC, et al. Endoscopic mucosal resection of jejunal polyps using double-balloon enteroscopy. GE Port J Gastroenterol 2015; 22: 137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Belsha D, Urs A, Attard T, et al. Effectiveness of double-balloon enteroscopy-facilitated polypectomy in pediatric patients with Peutz-Jeghers syndrome. J Pediatr Gastroenterol Nutr 2017; 65: 500–502. [DOI] [PubMed] [Google Scholar]
- 28. Blanco-Velasco G, Hernández-Mondragón OV, Blancas-Valencia JM, et al. Seguridad y eficacia de la polipectomía en intestino delgado utilizando enteroscopio asistido por balones en pacientes pediátricos con síndrome de Peutz-Jeghers. Rev Gastroenterol Mex 2018; 83: 234–237. [DOI] [PubMed] [Google Scholar]
- 29. Wang Y, Bian D, Zhu H, et al. The role of double-balloon enteroscopy in reducing the maximum size of polyps in patients with Peutz-Jeghers syndrome: 12-year experience. J Dig Dis 2019; 20: 415–420. [DOI] [PubMed] [Google Scholar]
- 30. Perrod G, Samaha E, Perez-Cuadrado-Robles E, et al. Small bowel polyp resection using device-assisted enteroscopy in Peutz-Jeghers syndrome: results of a specialised tertiary care centre. United European Gastroenterol J 2020; 8: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirakosyan E, Lokhmatov M. High-tech diagnostic methods and enteroscopic treatment of children with Peutz-Jeghers syndrome. Eur J Pediatr Surg. Epub ahead of print 26 November 2019. DOI: 10.1055/s-0039-3400286. [DOI] [PubMed] [Google Scholar]
- 32. Pennazio M, Venezia L, Cortegoso Valdivia P, et al. Device-assisted enteroscopy: an update on techniques, clinical indications and safety. Dig Liver Dis 2019; 51: 934–943. [DOI] [PubMed] [Google Scholar]
- 33. Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association Institute guideline on initial management of acute pancreatitis. Gastroenterology 2018; 154: 1096–1101. [DOI] [PubMed] [Google Scholar]
- 34. Latchford AR, Neale K, Phillips RKS, et al. Peutz-Jeghers syndrome: intriguing suggestion of gastrointestinal cancer prevention from surveillance. Dis Colon Rectum 2011; 54: 1547–1551. [DOI] [PubMed] [Google Scholar]
- 35. Möschler O, May A, Müller MK, et al. Complications in and performance of double-balloon enteroscopy (DBE): results from a large prospective DBE database in Germany. Endoscopy 2011; 43: 484–489. [DOI] [PubMed] [Google Scholar]
- 36. Aretz S, Stienen D, Uhlhaas S, et al. High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Hum Mutat 2005; 26: 513–519. [DOI] [PubMed] [Google Scholar]
- 37. Alhopuro P, Phichith D, Tuupanen S, et al. Unregulated smooth-muscle myosin in human intestinal neoplasia. Proc Natl Acad Sci USA 2008; 105: 5513–5518. [DOI] [PMC free article] [PubMed] [Google Scholar]