Abstract
Objectives
We examined bidirectional, time-ordered associations between age-related changes in depressive symptoms and memory.
Method
Data came from 107,599 community-dwelling adults, aged 49–90 years, who participated in the Survey of Health, Ageing, and Retirement in Europe (SHARE). Depressive symptoms were measured with the EURO-D inventory, and memory was evaluated as delayed recall of a 10-word list. Participants were assessed up to five times at 2-year intervals. Dynamic structural equation models were used to estimate longitudinal and time-ordered (lead-lag) relations between depressive symptoms and memory performance.
Results
Depressive symptoms increased and memory scores decreased across the observed age range, with worsening mostly evident after age 62 years. These long-term changes were moderately negatively correlated (r = −.53, p < .001). A time-ordered effect emerged such that age-specific memory deficits preceded shorter-term increases in depression symptoms. This effect can be translated such that each 1-point decrement on a 10-point memory scale at a given age predicted a 14.5% increased risk for depression two years later. Statistical adjustment for covariates (sex, education, re-test, smoking, and body mass index) had little influence on these associations.
Conclusion
In later adulthood, lower memory performance at a given age predicts subsequent 2-year increases in depressive symptoms.
Keywords: Bi-directional, Depression, Longitudinal change, Memory
Depression and cognitive impairment are salient mental health problems associated with aging. These conditions have adverse personal and social consequences, including reduced quality of life, heightened mortality risk, and increased caregiver burden (Vinkers, Gussekloo, Stek, Westendorp, & van der Mast, 2004). Numerous cross-sectional studies have shown that depression and cognitive impairment frequently co-occur and appear to be closely related (Christensen, Griffiths, Mackinnon, & Jacomb, 1997; Perrino, Mason, Brown, Spokane, & Szapocznik, 2008). However, associations between age-related changes in depressive symptoms and cognitive abilities are complex, both temporally and causally, and they remain poorly understood (Bennett & Thomas, 2014).
Comorbidity of depression and cognitive impairment in later life may reflect a shared etiology in cerebrovascular disease or neurochemical imbalance (Alexopoulos et al., 2002; Schweitzer, Tuckwell, O’Brien, & Ames, 2002). Depression may also act as an independent risk factor for cognitive impairment, and it may lower the threshold for manifesting dementia (Butters et al., 2008; Fernández Martínez et al., 2008). Cognitive decline may influence behaviors that put one at increased risk for depression. Depression may also occur in reaction to the awareness of one’s declining mental capacities (Huang, Wang, Li, Xie, & Liu, 2011; van den Kommer et al., 2013). These hypotheses are not mutually exclusive, and each implies a different causal-temporal ordering of influence: i.e., cognitive impairment and depression arise concurrently, or elevated depressive symptoms precede cognitive impairment, or cognitive impairment puts one at increased risk for depression.
Longitudinal research on time-ordered, bi-directional relations between depression and cognition is essential for evaluating the plausibility of these hypotheses, but to date there have been few such studies. Of the 10 such studies that we could identify, five suggested that cognitive deficits precede increases in depressive symptoms (Chen, Ganguli, Mulsant, & DeKosky, 1999; Chiao & Weng, 2016; Jajodia & Borders, 2011; Perrino et al., 2008; Vinkers et al., 2004), three suggested that elevated levels of depressive symptoms precede cognitive declines (Bunce, Batterham, Christensen, & Mackinnon, 2014; Panza et al., 2009; van den Kommer et al., 2013), and the remaining studies either showed reciprocal associations or no dynamic associations between cognitive performance and depressive symptoms (Gale, Allerhand, & Deary, 2012; Petersen, McGue, Tan, Christensen, & Christiansen, 2016).
Statistical analyses for most of these studies used latent growth curve (LGC) models, from which directional influences were deduced based on bivariate symmetric associations between levels and slopes of cognitive performance and depressive symptoms. However, LGC levels and slopes summarize static information taken concurrently from all time points (across an entire observation period) and hence are referred to as “constant change” parameters. Thus, although bivariate LGC models are effective for elucidating static associations of stability (level) and change (slope) between variables, they do not establish temporal precedence between variables in the way that “lead-lag” or “dynamic effects” models are designed to do (Grimm, Ram, & Estabrook, 2017; McArdle & Hamagami, 2001). This is an important distinction because temporal precedence is necessary for inferring causal influence—especially in an observational study.
Four of the identified bidirectional studies did examine lead-lag relations between depression and cognition. Two of these studies used cross-lag panel (CLP) models with data obtained at two occasions (Bunce et al., 2014; Petersen et al., 2016). In general, two-occasion studies are very susceptible to bias (e.g., due to regression to the mean), and confidence in CLP models is minimal when data are limited to two time points (Hamaker, Kuiper, & Grasman, 2015; Rogosa, 1980). This leaves only two studies wherein lead-lag relations between depression and cognition were examined at three or more occasions. In a sample of 273 community-dwelling older adults, Perrino et al. (2008) found that lower cognitive ability (tracking and memory) predicted subsequent increases in depressive symptoms across 1-year intervals, whereas depressive symptoms were not predictive of changes in cognition. In a sample of 14,000 middle-aged and older adults, Jajodia and Borders (2011) found that lower levels of delayed recall memory predicted increased depressive symptoms across 2-year intervals, whereas depression levels did not predict changes in memory. Thus, of existing studies investigating bi-directional depression-cognition relations, those using more rigorous data analytical tools show that memory impairment precedes increases in depressive symptoms, while the opposite does not hold.
Scope and Aims of the Current Study
Data for the current analyses come from the Survey of Health, Ageing, and Retirement in Europe (SHARE; Börsch-Supan et al., 2008, 2013), a longitudinal study of health and socioeconomic conditions in individuals aged 50+ years in Europe and Israel. Here, in a sample of 107,599 adults, we use bivariate latent change score models (BLCSM; McArdle, 2001; McArdle & Hamagami, 2001) to examine associations between longitudinal changes in memory and depressive symptoms. BLCSM combine aspects of latent growth models (Meredith and Tisak, 1990) and cross-lag regression models (Jöreskog, 1970) to allow for concurrent estimation of constant and time-ordered (dynamic) change relations within a single framework.
We modeled relations between changes in depressive symptoms and cognition as a function of chronological age. This is advantageous compared to the more common approach of modeling change as a function of measurement occasion with age as a covariate (i.e., statistical control variable) because age can attenuate associations between other variables of interest, because age provides little explanatory value in itself (Spiro & Brady, 2011)—and also because it allowed us to statistically adjust for retest effects (i.e., independently of age). We hypothesized that depressive symptoms and memory performance would worsen over the long-term (i.e., from age 49–90 years). Given outcomes from previous lead-lag depression-cognition studies (summarized above), we also expected that time-ordered effects would show that lower memory scores precede increases in depressive symptoms, while depressive symptoms would not influence changes in cognitive performance.
Method
Study design and data collection for SHARE have previously been described in detail (Börsch-Supan et al., 2008, 2013). In brief, SHARE is a consortium study of health and aging in 20 European countries and Israel, and for which national survey organizations are responsible for selecting and interviewing representative samples of people aged approximately 50 years and older. Survey materials were administered as a Computer Assisted Personal Interview (CAPI), supplemented by paper and pencil questionnaire. Questions covered health-related, psychological, and socioeconomic variables. Interviews were conducted in the respondents’ homes and took about 90 min.
Participants entered the study in six waves (from 2004 to 2015) spaced at approximately 2-year intervals such that participants were repeatedly assessed at each wave following wave of entry. Participants selected for the current analysis provided memory performance and depressive symptoms data at one or more occasions and were between the ages of 49 and 90 years at time of measurement. Table 1 shows summary statistics for participants who met these criteria (N = 107,599). Data collection was approved by the internal review board of the University of Mannheim, Germany (until 2011) and by the Ethics Council of the Max-Planck-Society for the Advancement of Science (2011 onward).
Table 1.
Characteristics of the Sample (N = 107,599)
| Variable | Value |
|---|---|
| Age in years at entry | M = 62.0, IQR = 55–70 |
| Age in years when last observed | M = 66.0, IQR = 59–74 |
| Women | 58,417 (54.3%) |
| Education level (ISCED-1997) | |
| Primary | 20,508 (19.1%) |
| Lower secondary | 20,071 (18.6%) |
| Upper secondary | 34,133 (31.7%) |
| Postsecondary, nontertiary | 4,421 (4.1%) |
| First stage tertiary | 21,138 (19.7%) |
| Second stage tertiary | 810 (0.7%) |
| Missing observations | 6,518 (6.1%) |
| Smoking | |
| Never | 41,110 (38.2%) |
| Not currently | 20,749 (19.3%) |
| Yes, currently | 15,369 (14.3%) |
| Missing observations | 30,371 (28.2%) |
| Body Mass Index | M = 26.3, IQR = 23.9–29.2 |
| Longitudinal Summaries by Study Wave | |||||
|---|---|---|---|---|---|
| Wave 1 | Wave 2 | Wave 4 | Wave 5 | Wave 6 | |
| N (all cohorts) | 27,826 | 34,050 | 53,178 | 58,328 | 53,880 |
| Words recalled (mean) | 3.37 | 3.58 | 3.80 | 3.96 | 4.02 |
| EURO-D score (mean) | 2.31 | 2.29 | 2.57 | 2.37 | 2.37 |
Note: IQR = 25%–75% interquartile range; M = Median.
Measures
Delayed recall memory
At each measurement occasion, participants were asked to recall a list of 10 words either immediately or following completion of two additional cognitive tasks (numeracy ability, verbal fluency). Delayed recall memory has previously been shown to be especially sensitive for early detection of mild cognitive impairment (Sano et al., 2011). Therefore, and consistent with a similar study byJojodia and Borders (2011), we examined only delayed recall memory (number of words recalled following the additional cognitive tasks). For participants who entered in wave 1, the words list was the same at first and second assessment. To control for this statistically, we created a dummy variable “re-test effect” to flag repeated exposure to the same words.
Depressive symptoms
The EURO-D scale was initially developed to compare depressive symptoms in older persons in 11 European countries (Prince et al., 1999). The scale consists of 12 dichotomous items corresponding to the following depressive symptoms: sadness, pessimism, suicidality, guilt, sleep problems, interest in things, irritability, poor appetite, fatigue, difficulty concentrating, enjoyment, and tearfulness. Items are scored 0 or 1 such that 1 always indicates negative valence (i.e., to indicate the presence of a negative symptom such as sadness, or the absence of a positive symptom such as enjoyment). These item scores are then summed to give a final score between 0 and 12, where a summary score ≥ 4 indicates the presence of a depressive disorder for which clinical intervention may be warranted. The psychometric properties of the EURO-D scale have been extensively investigated and criterion validity established cross-culturally in European, Indian, and Latin-American populations (Castro-Costa et al., 2007; Prince et al., 2004).
Other covariates
Sex, education level, smoking status, and body mass index (BMI) were included as covariates (summarized in Table 1). Education level was standardized across countries according to the International Standard Classification of Education (UNESCO, 2006). The ISCED 1997 is a seven-point scale in ascending order of highest education level completed: We treated this as an ordinal (rather than categorical) covariate, centered at level 1. Smoking status was assigned as one of three categories: never smoked, smoked in the past but not currently, and currently smoke. BMI was calculated as body weight in kilograms divided by height in meters squared.
Statistical Analyses
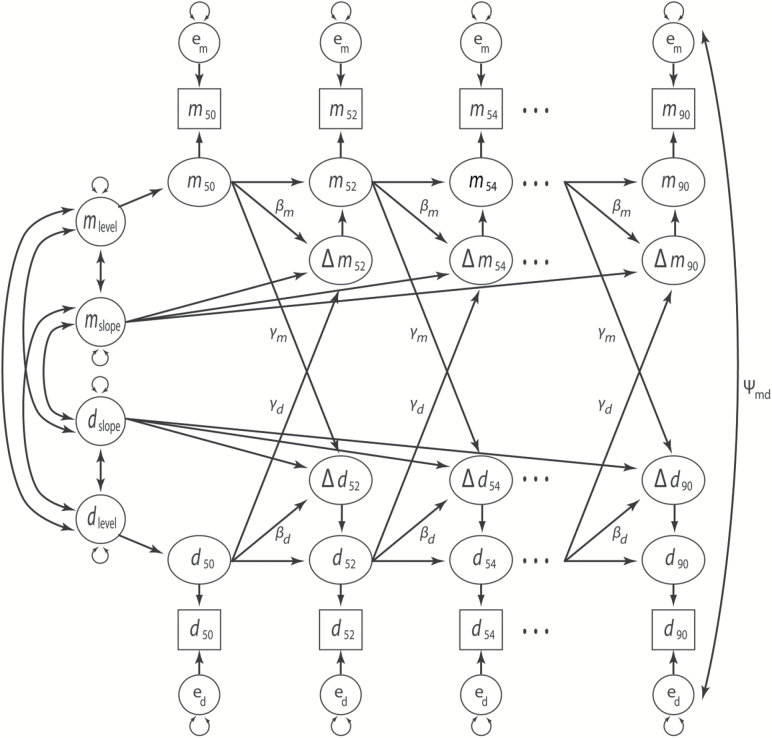
Analyses were based on a structural equation modeling (SEM) approach using bivariate latent change score models (BLCSM; McArdle, 2001; McArdle & Hamagami, 2001). BLCSM combine latent growth models (Meredith and Tisak, 1990) and autoregressive cross-lag models (Jöreskog, 1970). The former provides a way to examine relations between variables’ longitudinal change trajectories (i.e., levels and slopes calculated across all time points). The latter provides a way to examine time-sequential lead-lag relations between variables X and Y, or “couplings”, such that variable X at time t predicts change in variable Y across times t and t +1, and vice versa. Thus, BLCSM allows for concurrent estimation of overall (constant) change and time-ordered (dynamic) change relations between variables.
We modeled changes in memory scores and depressive symptoms across the range of participant ages (49–90 years). As testing occurred at approximately 2-year intervals, we correspondingly scaled the model age range in nonoverlapping 2-year intervals (e.g., participant ages 49–50, 51–52, …, 89–90 years). Therefore, a given participant provided data, at most, across six 2-year time intervals, but the time metric for the statistical models spanned twenty-one 2-year time intervals.
Model development proceeded in stages. We first estimated univariate LCSM (i.e., separate models for depressive symptoms and memory performance). We then combined these models to create a BLCSM (using estimates from the univariate LCSM as starting values). The BLCSM also included paths between the constant change parameters (i.e., correlations between levels and slopes for memory and depressive symptoms). This served as the baseline, or “no coupling” BLCSM, to which we then added cross-lagged paths between memory and depression variables to create three more models: (a) a model with a uni-directional coupling for memory scores predicting changes in depressive symptoms, memory → ∆depression, (b) a model with a uni-directional coupling for depressive symptoms predicting changes in memory scores, depression → ∆memory, and (c) a model with full bi-directional coupling between memory scores and depressive symptoms. To determine whether the couplings were significant, we performed likelihood ratio tests of change in model fit, as outlined by Grimm et al. (2017).
A general path diagram for all BLCSM is provided in Figure 1. In the initial BLCSM specification, we constrained proportional change parameters (βm, βd) and bivariate cross-lag “coupling” parameters (γm, γd) to be equal across the entire age range (i.e., 49–90 years). In a follow-up series of analyses, we respecified these equality constraints as tripartite (rather than singular) effects by the following age ranges: 49–62, 63–76, and 77–90 years. Theoretically, this models the expectation that lead-lag relations between memory performance and depressive symptoms differ across these age windows and where, for example, the middle window corresponds to the transition from active to progressive, and eventually full, retirement.
Figure 1.
Bivariate latent change score model. Memory (m) and depressive symptoms (d) are modeled across 2-year age intervals (i.e., the first age interval, 49–50 years, is noted by subscript 50; the last age interval, 89–90 years, is noted by subscript 90). Change scores are noted by delta (triangle symbol). Unlabeled paths with single-headed arrows show regression effects fixed at 1. Unlabeled paths with doubleheaded arrows show freely estimated variances and covariances. Labels on paths show model parameter constraints. Paths from levels and slopes onto covariates and a constant term are not shown.
Finally, we reran these analyses with covariates added to the models (predictors of intercepts and slopes for memory and depressive symptoms). Covariates were sex, highest education level, retest effect, smoking status, and BMI. Participants who entered in later waves generally provided data at fewer time points than those who entered earlier, so we included test cohort (i.e., study entry wave) as an auxiliary variable (Enders, 2010; Graham, 2003) to account for missing data and to reduce the estimation bias without directly influencing associations of substantive interest. We used MPLUS statistical software (version 6; Muthén & Muthén, 2012) with full information maximum likelihood for model estimation. Given the very large sample size, we chose a significance cutoff value of p < .00001 for model fit comparison tests.
Results
Model fit statistics and model comparison results are summarized in Table 2. Models with couplings (γm, γd) only converged when couplings were estimated with tripartite (rather than singular) constraints across the modeled age range (more specific technical notes on these constraints are provided as Supplementary Materials). Additionally, all models with a uni-directional coupling of depression → ∆memory failed to converge. This left two sets of three models that converged and for which we conducted likelihood ratio tests.
Table 2.
Latent Change Score Model Comparisons
| Description | Parameters | Deviance | CFI | BIC | RMSEA | Likelihood ratio tests | ||
|---|---|---|---|---|---|---|---|---|
| Model | Comparison | ∆χ2 (df) | ||||||
| No Covariates | ||||||||
| M0a | No Coupling | 19 | 2,321,187 | 0.958 | 2,321,917 | 0.009 | ||
| M0b | Memory → ∆Depression | 22 | 2,320,070 | 0.971 | 2,320,835 | 0.007 | M0a vs M0b | 1117 (3) |
| M0d | Bi-directional Coupling | 25 | 2,319,870 | 0.974 | 2,320,669 | 0.007 | M0b vs M0d | 200 (3) |
| Covariates = Sex, Education, Re-test, Smoking, BMI | ||||||||
| M1a | No Coupling | 45 | 3,456,192 | 0.972 | 3,457,596 | 0.006 | ||
| M1b | Memory → ∆Depression | 48 | 3,455,942 | 0.974 | 3,457,381 | 0.006 | M1a vs M1b | 250 (3) |
| M1d | Bi-directional Coupling | 51 | 3,455,892 | 0.974 | 3,457,366 | 0.006 | M1b vs M1d | 50 (3) |
Note: CFI = Comparative fit index; BIC = Bayesian information criterion; RMSEA = Root mean square error of approximation: 90% RMSEA confidence intervals were all within ±.0005 of reported estimates. ∆χ2 (df) = change in deviance per degrees freedom: Higher values indicate the model with more parameters fit the data better. ∆ = “change in”. Models with a uni-directional coupling for Depression → ∆Memory failed to converge and so are not included here.
For each set of models, we first compared the no-coupling model against the model with a uni-directional coupling for memory → ∆depression. There was significant improvement in fit with the addition of the memory → ∆depression coupling parameter for models without covariates (M0a vs M0b) and for models with covariates (M1a vs M1b). We next compared the uni-directional memory → ∆depression model against the bi-directional coupling model. Here, the bi-directional model significantly outperformed the uni-directional model, with and without covariates present. This provides some indication that inclusion of the depression → ∆memory coupling was influential (despite nonconvergence of the uni-directional depression → ∆memory models). However, this effect was comparatively small.
Expected scores for memory and depressive symptoms generated by the BLCSM are a function of constant change parameters (levels, slopes), auto-regressive parameters (memory → ∆memory; depression → ∆depression), and cross-lag parameters (memory → ∆depression; depression → ∆memory). Therefore, to facilitate interpretation, we first plot and describe the trajectories of estimated memory scores within subsamples of participants stratified by baseline levels of depressive symptoms (Figure 2a) and the trajectories of estimated depressive symptoms within subsamples of participants stratified by baseline levels of memory scores (Figure 2b). We then summarize the model parameters statistically (Table 3). We report standard errors rather than repeating the large sample size value (N = 107,599) and correspondingly small p values for significant effects (i.e., < .00001).
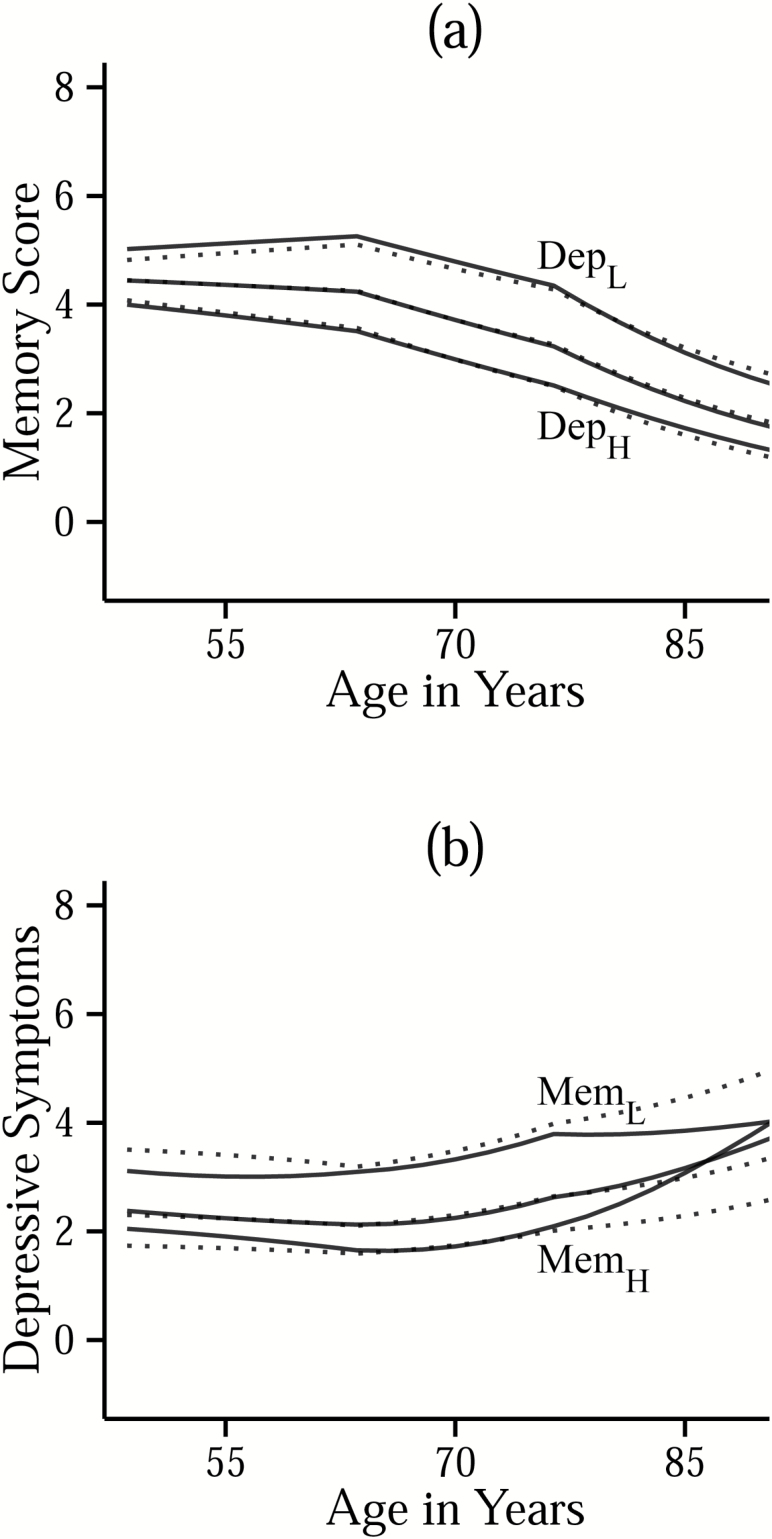
Figure 2.
Panel (a) shows trajectories of estimated memory scores within sub-samples of participants with different baseline levels of depressive symptoms: low (≤ -1.5SD; DepL), mid (> -1.5SD and < 1.5SD; unlabeled), and high (≥ 1.5SD; DepH). Panel (b) shows trajectories of estimated depressive symptoms within sub-samples of participants with different baseline levels of memory scores: low (≤ -1.5SD; MemL), mid (> -1.5SD and < 1.5SD; unlabeled), and high (≥ 1.5SD; MemH). Solid lines indicate trajectories estimated from the full coupling model (M1d), and dotted lines are trajectories estimated from the nocoupling model (M1a).
Table 3.
BLCSM Parameter Estimates (from Model M1d)
| Parameter | Estimate (SE) | |
|---|---|---|
| Means | ||
| Level of Memory | 3.416 | (0.058) |
| Slope of Memory | −0.302 | (0.052) |
| Level of Depression | 1.825 | (0.078) |
| Slope of Depression | 0.573 | (0.068) |
| Correlations | ||
| Level of Memory w/Slope of Memory | −0.788 | (0.051) |
| Level of Depression w/Slope of Depression | −0.472 | (0.078) |
| Level of Memory w/Level of Depression | −0.128 | (0.015) |
| Level of Memory w/Slope of Depression | 0.859 | (0.032) |
| Level of Depression w/Slope of Memory | −0.151 | (0.127)ns |
| Slope of Memory w/Slope of Depression | −0.531 | (0.090) |
| Proportional changes | ||
| Memory → ∆ Memory (βm) | ||
| Age window 49–62 years | 0.064 | (0.012) |
| Age window 63–76 years | 0.037 | (0.012)ns |
| Age window 77–90 years | −0.017 | (0.015)ns |
| Depression → ∆ Depression (βd) | ||
| Age window 49–62 years | 0.029 | (0.018)ns |
| Age window 63–76 years | 0.014 | (0.022)ns |
| Age window 77–90 years | −0.062 | (0.017)ns |
| Coupling effects | ||
| Memory → ∆ Depression (γm) | ||
| Age window 49–62 years | −0.210 | (0.016) |
| Age window 63–76 years | −0.201 | (0.015) |
| Age window 77–90 years | −0.178 | (0.020) |
| Depression → ∆ Memory (γd) | ||
| Age window 49–62 years | 0.018 | (0.014)ns |
| Age window 63–76 years | 0.023 | (0.016)ns |
| Age window 77–90 years | 0.051 | (0.014)ns |
Note: Slope = constant change per 2-year interval (i.e., between ages 49–90 years). Proportional changes and coupling effects reflect score at time t as predictive of change in score between time t to t + 2 years.
ns = Nonsignificant at p = .00001.
On average, memory scores and depressive symptoms changed little between ages 49–62 years but then worsened with increasing age. Linear slope estimates from univariate models (generated during development of the BLCSM) provide simplified approximations of these longitudinal changes, per decade, for memory scores (M = −0.73, SE = 0.01) and depressive symptoms (M = 0.26, SE = 0.01). Figure 2a and b shows that individuals with better memory scores generally had fewer depressive symptoms, and vice versa, across most of the observed age range—as would be expected from the significant negative correlations between level-level (r = −.13, SE = .02) and slope-slope (r = −.53, SE = .09) memory-depression parameters of the BLCSM.
Additionally, level of depressive symptoms was not significantly correlated with slope of memory (r = −.15, SE = .13). Thus, depression-related differences in memory performance at age 50 years were preserved across later adulthood, as shown by nonoverlap of the solid line trajectories in Figure 2a. Depression → ∆memory couplings were nonsignificant (see Table 3), as shown in Figure 2a by virtually nonexistent differences between the memory score trajectories estimated from the no-coupling model (dotted lines) versus those estimated from the bi-directional coupling model (solid lines).
In contrast, in Figure 2b, there are noticeable differences in the trajectories of depressive symptoms estimated from the no-coupling model (dotted lines) versus trajectories estimated from the bidirectional coupling model (solid lines). In the no-coupling model, level of memory scores and slope of depressive symptoms were only weakly correlated (r = 0.13, SE = .01), whereas in the coupling model, there was a strong positive correlation between level of memory and slope of depressive symptoms (r = .86, SE = .03), accompanied by significant negative memory → ∆depression couplings (Table 3). Thus, addition of the memory → ∆depression couplings had a twofold influence on the estimated trajectories of depressive symptoms: First, by strengthening the positive correlation, or “baseline effect,” between levels of memory performance and slopes of depressive symptoms, and second, by either counter-acting or augmenting longer-term changes in depressive symptoms conditional on differences in baseline levels of memory performance.
This means that for individuals who were memory-impaired at baseline (MemL), the model predicted a higher overall level of depressive symptoms, a negative slope for depressive symptoms, and compounding 2-year increases in depressive symptoms (the effect of the memory → ∆depression coupling). The latter two of these effects counter-balanced one another, with the net result being an elevated but relatively stable trajectory of depressive symptoms (as shown by the solid line trajectory for MemL in Figure 2b). For individuals with relatively good baseline memory performance (MemH), the model predicted a lower baseline level of depressive symptoms, a shallow long-term increase in depressive symptoms, and compounding 2-year increases in depressive symptoms. Combined, these resulted in a depressive symptoms trajectory that started out at low values but accelerated upward in later life (as shown by the solid line trajectory for MemH in Figure 2b).
It is important to bear in mind that whereas BLCSM level and slope parameters varied (and co-varied) across individuals, the couplings were fixed effects and therefore constant in their influences, irrespective of individual differences in levels and slopes. This means that the memory → ∆depression couplings can be simply interpreted as regression coefficients in a multivariate equation (e.g., the effect of X on Y, adjusted for Z)—with a time-ordered influence. In brief, every one-point drop in memory performance at a given age predicted subsequent 2-year increases in depressive symptoms of .21 (between ages 49–62 years), .20 (between ages 63–76 years), and .18 (between ages 77–90 years). We note that although increases in depressive symptoms were most sensitive to memory deficits in the earlier age windows, falling memory scores with age meant that the effect of the memory → ∆depression coupling compounded over time (i.e., across successive 2-year intervals), such that acceleration in depressive symptoms trajectories became more pronounced in later life.
To express the memory → ∆depression coupling in terms of depression risk, we conducted a follow-up multilevel logistic analysis wherein we evaluated memory performance at age t as predictive of depression status at age t + 2 years, controlling for depression status at age t. We used the R statistical software package lme4 (Bates, Maechler, Bolker, & Walker, 2014; R Development Core Team, 2016). The log-odds coefficient for the effect of memory performance on depression status was −0.1357 (p < .001). Thus, adjusted statistically for current depression status at any given age, every one-point lower score on the 10-item memory inventory predicted a 14.5% increase in the odds of being depressed 2 years later. We thank an anonymous reviewer for motivating our thinking in terms of translating the memory → ∆depression coupling effect into a statement of risk for depression at age t + 2 years given a lower memory score at age t.
Covariate Associations
Tabled results of the effects of covariates are provided as Supplementary Materials. Women on average had higher levels than men both for memory performance (b = 0.552, SE = .017) and for depressive symptoms (b = 0.819, SE = .022), but there were no significant sex-related differences in slopes for memory or depressive symptoms. Higher education level was associated with a higher level of memory performance (b = 0.391, SE = .007), a lower level of depressive symptoms (b = −0.157, SE = .009), and larger long-term increases in depressive symptoms (b = 0.088, SE = .005). Retest effect was significantly positively associated with change in memory performance (b = 0.020, SE = .003). Current smokers’ memory and depressive symptoms estimates did not differ significantly from nonsmokers; however, past smokers who quit had lower levels of memory performance (b = −0.151, SE = .024), less age-related decline in memory performance (b = 0.016, SE = .003), and relatively lower levels of depressive symptoms (b = −0.375, SE = .026) compared to nonsmokers. Finally, BMI was negatively related to level of memory performance (b = −0.015, SE = .002) and positively related to level of depression (b = 0.028, SE = .002), but not to slopes for either.
Discussion
In a large-sample study of community-dwelling middle aged and older adults, we found that average memory performance declined and depressive symptoms increased between ages 49 and 90 years, with worsening most evident following age 62 years. Longitudinal increases in depressive symptoms were most pronounced for individuals who, at baseline, were not already memory impaired, whereas for individuals with low baseline levels of memory performance, depressive symptoms tended to remain elevated across the observed age range. Adjusting statistically for differences in baseline memory performance and depressive symptoms, we also observed a time-ordered effect such that memory decrements at a given age were predictive of worsening depressive symptoms across subsequent 2-year intervals. This lead-lag effect appeared to gradually weaken with advancing age but remained significant and was not reciprocated (i.e., elevated depressive symptoms did not predict declines in memory scores). Statistical adjustment for sex, education level, smoking behavior, and body mass index had little influence on these outcomes.
At present, we know of only two other studies that examined time-ordered, bi-directional associations between depressive symptoms and cognitive performance in older adults and using data collected from more than two time points. The current study design and analysis is most consistent with research by Jajodia and Borders (2011). These researchers used data from the Health and Retirement Study (a large population-representative and independent research effort cross-linked to SHARE) and also used BLCSM to examine associations between delayed recall memory and depressive symptoms across 2-year intervals. In a smaller study of older Hispanic Americans, Perrino et al. (2008) used cross-lagged path models to estimate lead-lag relations between depressive symptoms and cognitive performance. Results from both of these studies are consistent with the current finding that lower memory performance precedes and predicts worsening depressive symptoms in older adults.
Other researchers have examined bi-directional cognition-depression associations and concluded the converse to be true; i.e., that elevated depressive symptoms predict cognitive declines. However, outcomes from those studies that we could identify were either based on data collected at only two time points or were based on statistical models that did not impose a lead-lag framework necessary for inferring temporal precedence (see Introduction). In the current results, uni-directional models with a depression → ∆memory coupling (i.e., that did not estimate a memory → ∆depression effect) failed to converge. And, although models with bi-directional couplings fit the data better than uni-directional memory → ∆depression models, parameter estimates for the depression → ∆memory couplings in the bidirectional models were nonsignificant. From these results, we conclude that age-specific decrements in memory performance temporally precede 2-year increases in depressive symptoms, and not vice versa.
This outcome is consistent with several previously conveyed hypotheses. First, the association between memory deficits and elevated depressive symptoms may stem from a shared etiology in one or more underlying brain disorders—such as cerebral or limbic atrophy, or neuro-chemical imbalance (Alexopoulos et al., 2002; Schweitzer et al., 2002)—and memory decrements may simply be detectable behaviorally prior to changes in depressive symptoms. A second explanation is that older adults may grow increasingly depressed when becoming aware of their own cognitive decline (Vinkers et al., 2004). A third possibility is that age-related cognitive decrements adversely affect behaviors important for self-care and day-to-day functioning (e.g., social engagement, management of personal finances, exercise), which in turn creates conditions for worsening emotional health (e.g., Ritchie, Gillham, Ledesert, Touchoun, & Kotzki, 1999).
The current work also extends earlier findings of Jajodia and Borders (2011) by showing that the lead-lag relation between memory decrements and increasing depressive symptoms loses strength with advancing age. We were able to demonstrate this effect by modeling change as a function of chronological age rather than measurement occasion and by imposing different constraints on the coupling parameters across three age ranges (i.e., 49–62, 63–76, and 77–90 years). Although the leading effect of memory on biennial changes in depressive symptoms was significant within each of these age ranges, the strength of this association lessened following age 77 years. This provides some incentive for monitoring changes in memory prior to the onset of advanced age to ensure that necessary support is provided when it is most effective for improving emotional well-being. We also note that although this lead-lag effect was itself strongest in earlier age ranges, its influence accrued over time (much like compounding interest paid on an investment) and therefore became most pronounced visually as later-life acceleration in the depressive symptoms trajectories of individuals who were not chronically memory impaired (or depressed) at baseline (Figure 2b, solid trajectory for MemH).
Although our primary aim for the study was to examine time-ordered associations between memory and depressive symptoms, we would be remiss to not briefly comment on the estimated long-term worsening in memory performance and depressive symptoms. The observed declines in memory are consistent with previous reports (e.g., McArdle, Fisher, & Kadlec, 2007). Age-related differences in recall memory have been linked to changes in resting state functional connectivity across cortical networks, but the specific mechanisms behind these age-varying relations are not yet well understood (Fjell et al., 2015). In contrast with the current depressive symptoms outcome, accumulating evidence from other studies shows that for most adults, depressive symptoms are relatively stable following middle age (Montagnier et al., 2014; Musliner, Munk-Olsen, Eaton, & Zandi, 2016). We note that individual differences in age-related trajectories of depressive symptoms are largely attributed to the presence/absence of chronic disease and physical impairment (Chen et al., 2011; Musliner et al., 2016). SHARE data includes a single measure to track the number of chronic diseases and multiple measures of functional capacity (e.g., grip strength, difficulties in daily activities). We did not include these variables in the current analyses (due to nonspecificity of the former and added complexity of the latter): Had we done so, the observed increases in depressive symptoms may have been attenuated thereby.
With respect to sociodemographic covariates, the finding that women, on average, had better levels of memory performance and also higher levels of depressive symptoms has been observed in similar large sample studies (e.g., Gale et al., 2012; Jajodia & Borders, 2011). And others have similarly found no link between education level and longitudinal changes in cognitive ability (e.g., Christensen et al., 2001). However, there is mixed evidence as to whether education may protect against increasing depressive symptoms in later life: Some studies similarly report a favorable influence of education on depression trajectories (e.g., Chen et al., 2011; Jajodia & Borders, 2011; Musliner et al., 2016), whereas others find no significant relation between these variables (e.g., Gale et al., 2012). Here, we find that better-educated individuals have lower average levels of depressive symptoms but show larger changes in depressive symptoms across age. This suggests a “baseline effect” for depressive symptoms, such that those with more room to worsen indeed appear to worsen more.
An important limitation of the study concerns the vascular hypothesis of cognitive aging, which implicates cardiovascular (and by extension cerebrovascular) disease both in cognitive declines and depression risk. Regrettably, we were unable to include biological measures of cardiovascular illness or diabetes in the analyses because blood samples for SHARE were first collected in 2012 and are still being processed (and hence not available for analyses). That said, we did include two established cardiovascular risk factors (BMI and smoking). BMI was linked to relatively worse levels of depressive symptoms and memory performance, consistent with vascular theories of mental aging. As for smoking, participants who previously smoked but then quit had lower levels of memory performance but also lower levels of depressive symptoms than nonsmokers. Perhaps the ability to quit smoking reflected a broader motivation for health-related change that benefitted emotional well-being.
Indeed, lower motivational capacity has been implicated both in depression and impaired cognitive performance, especially on effortful tasks (Scheurich et al., 2008). Regrettably, we did not consider motivational differences in associations between depressive symptoms and memory performance. It could be argued that the Euro-D, as a subjective measure of depressive symptoms, was itself to some degree a proxy measure for motivational differences, whereas the more objective measure of recall memory lacked such information. Inclusion of measures of subjective cognitive effort in future studies may help to further disambiguate potential mediating roles of motivation and effort in longitudinal cognition-depression associations.
Additionally, modeling change as a function of chronological age rather than measurement occasion meant that maximum longitudinal data coverage for a given individual was capped at approximately 23.5% (12 years of the modeled 51-year age range). We sought to account for missingness by including study cohort, sociodemographic, and health-related variables in the statistical models, but models with a uni-directional coupling for depression → ∆memory may have failed to converge due to insufficient within-person variability. As a check, we ran a follow-up analysis on participants with data at four or more occasions (n = 12,483). All models converged: The uni-directional effect of depression → ∆memory remained negligible, whereas the effect of memory → ∆depression was again substantial. This indicates that dynamic associations observed in the full data set were robust to data selection.
Strengths of this study are (a) its use of a very large and representative sample of European older adults, (b) repeated assessments at five occasions (with individual-level data spanning up to 12 years and group-level data spanning an age range of 49–90 years), and (c) the application of sophisticated statistical models to infer both temporal precedence and long-term associations between changes in memory and depression. At present, we believe this to be the only depression-cognition study of middle-aged and older adults to examine these bi-directional associations as a function of chronological age rather than measurement occasion—and to show that the dynamic effect of memory as predictive of changes in depressive symptoms itself becomes less pronounced with advancing age.
Data Availability
Data from SHARE and SHARELIFE, release version 6.0.0 (March 31, 2017), can be downloaded free of charge from the SHARE Research Data Center by researchers who sign a statement confirming their use only for scientific purposes, at: http://www.share-project.org/data-access.html.
Funding
This work was supported by the Swiss National Centre of Competence in Research LIVES – Overcoming vulnerability: Life course perspectives, which are financed by the Swiss National Science Foundation (grant 51NF40-160590). SHARE data collection was primarily funded by the European Commission through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006–062193, COMPARE: CIT5-CT-2005–028857, SHARELIFE: CIT4-CT-2006–028812) and FP7 (SHARE-PREP: N°211909, SHARE-LEAP: N°227822, SHARE M4: N°261982)—with additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C) and others.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
The authors are thankful to an anonymous reviewer for recommending simplified summary statements for the principal results and for providing useful examples thereof.
References
- Alexopoulos G. S., Buckwalter K., Olin J., Martinez R., Wainscott C., & Krishnan K. R (2002). Comorbidity of late life depression: An opportunity for research on mechanisms and treatment. Biological Psychiatry, 52, 543–558. doi:10.1016/S0006-3223(02)01468-3 [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., & Walker S (2014). lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–7 Retrieved from http://CRAN.R-project.org/package=lme4.
- Bennett S., & Thomas A. J (2014). Depression and dementia: Cause, consequence or coincidence?Maturitas, 79, 184–190. doi:10.1016/j.maturitas.2014.05.009 [DOI] [PubMed] [Google Scholar]
- Börsch-Supan A., Brandt M., Hunkler C., Kneip T., Korbmacher J., Malter F., Schaan B ... Zuber S., on behalf of the SHARE Central Coordination Team. (2013). Data Resource Profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). International Journal of Epidemiology, 42, 992–1001. doi:10.1093/ije/dyt088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börsch-Supan A., Brugiavini A., Jürges H., Kapteyn A., Mackenbach J., Siegrist, & Weber G (Eds.). (2008). First results from the Survey of Health, Ageing and Retirement in Europe (2004–2007). Starting the longitudinal dimension. Mannheim: Mannheim Research Institute for the Economics of Aging (MEA). [Google Scholar]
- Bunce D., Batterham P. J., Christensen H., & Mackinnon A. J (2014). Causal associations between depression symptoms and cognition in a community-based cohort of older adults. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 22, 1583–1591. doi:10.1016/j.jagp.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Butters M. A., Young J. B., Lopez O., Aizenstein H. J., Mulsant B. H., & Reynolds C. F 3rd (2008). Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience, 10, 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Costa E., Dewey M., Stewart R., Banerjee S., Huppert F., Mendonca-Lima C., Bula >C...Prince M (2007). Prevalence of depressive symptoms and syndromes in later life in ten European countries: The SHARE study. The British Journal of Psychiatry: The Journal of Mental Science, 191, 393–401. doi:10.1192/bjp.bp.107.036772 [DOI] [PubMed] [Google Scholar]
- Chen P., Ganguli M., Mulsant B. H., & DeKosky S. T (1999). The temporal relationship between depressive symptoms and dementia: A community-based prospective study. Archives of General Psychiatry, 56, 261–266. doi:10.1001/archpsyc.56.3.261 [DOI] [PubMed] [Google Scholar]
- Chen C. M., Mullan J., Griffiths D., Kreis I. A., Lan T. Y., & Chiu H. C (2011). Trajectories of depression and their relationship with health status and social service use. Archives of Gerontology and Geriatrics, 53, e118–e124. doi:10.1016/j.archger.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Chiao C., & Weng L. J (2016). Mid-life socioeconomic status, depressive symptomatology and general cognitive status among older adults: Inter-relationships and temporal effects. BMC Geriatrics, 16, 88. doi:10.1186/s12877-016-0257-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H., Griffiths K., Mackinnon A., & Jacomb P (1997). A quantitative review of cognitive deficits in depression and Alzheimer-type dementia. Journal of the International Neuropsychological Society: JINS, 3, 631–651. [PubMed] [Google Scholar]
- Christensen H., Hofer S. M., Mackinnon A. J., Korten A. E., Jorm A. F., & Henderson A. S (2001). Age is no kinder to the better educated: Absence of an association investigated using latent growth techniques in a community sample. Psychological Medicine, 31, 15–28. doi:10.1017/S0033291799002834 [DOI] [PubMed] [Google Scholar]
- Enders C. K. (2010). Applied missing data analysis: Methodology in the social sciences. New York: The Guilford Press. [Google Scholar]
- Fernández Martínez M., Castro Flores J., Pérez de Las Heras S., Mandaluniz Lekumberri A., Gordejuela Menocal M., & Zarranz Imirizaldu J. J (2008). Risk factors for dementia in the epidemiological study of Munguialde County (Basque Country-Spain). BMC Neurology, 8, 39. doi:10.1186/1471-2377-8-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A. M., Sneve M. H., Grydeland H., Storsve A. B., Glasø de Lange A-M., Amlien I. K., Røgeberg O. J., &Walhovd K. B (2015). Functional connectivity change across multiple cortical networks relates to episodic memory changes in aging. Neurobiology of Aging, 36, 3255–3268. doi:10.1016/j.neurobiolaging.2015.08.020 [DOI] [PubMed] [Google Scholar]
- Gale C. R., Allerhand M., & Deary I. J (2012). Is there a bidirectional relationship between depressive symptoms and cognitive ability in older people? A prospective study using the English Longitudinal Study of Ageing. Psychological Medicine, 42, 2057–2069. doi:10.1017/S0033291712000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. W. (2003). Adding missing-data relevant variables to FIML-based structural equation models. Structural Equation Modeling: A Multidisciplinary Journal, 10, 80–100. doi:10.1207/S15328007SEM1001_4 [Google Scholar]
- Grimm K. J., Ram N., & Estabrook R (2017). Growth modeling: Structural equation and multilevel modeling approaches. New York: The Guilford Press. [Google Scholar]
- Hamaker E. L., Kuiper R. M., & Grasman R. P (2015). A critique of the cross-lagged panel model. Psychological Methods, 20, 102–116. doi:10.1037/a0038889 [DOI] [PubMed] [Google Scholar]
- Huang C. Q., Wang Z. R., Li Y. H., Xie Y. Z., & Liu Q. X (2011). Cognitive function and risk for depression in old age: A meta-analysis of published literature. International Psychogeriatrics, 23, 516–525. doi:10.1017/S1041610210000049 [DOI] [PubMed] [Google Scholar]
- Jajodia A., & Borders A (2011). Memory predicts changes in depressive symptoms in older adults: A bidirectional longitudinal analysis. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66, 571–581. doi:10.1093/geronb/gbr035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöreskog K. G. (1970). Estimation and testing of simplex models. British Journal of Mathematical and Statistical Psychology, 23, 121–145. doi:10.1111/j.2044–8317.1970.tb00439.x [Google Scholar]
- van den Kommer T. N., Comijs H. C., Aartsen M. J., Huisman M., Deeg D. J., & Beekman A. T (2013). Depression and cognition: How do they interrelate in old age?The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 21, 398–410. doi:10.1016/j.jagp.2012.12.015 [DOI] [PubMed] [Google Scholar]
- McArdle J. J. (2001). A latent difference score approach to longitudinal dynamic structural analysis. In Cudeck R., du Toit S., & Sorbom D. (Eds.), Structural equation modeling: Present and future (pp. 342–380). Lincolnwood, IL: Scientific Software International. [Google Scholar]
- McArdle J. J., & Hamagami F (2001). Latent difference score structural models for linear dynamic analyses with incomplete longitudinal data. In Collins L. M. & Sayer A. G. (Eds.), New methods for the analysis of change (pp. 137–176). Washington, DC: American Psychological Association. doi:10.1037/10409-005 [Google Scholar]
- McArdle J. J., Fisher G. G., & Kadlec K. M (2007). Latent variable analyses of age trends of cognition in the Health and Retirement Study, 1992-2004. Psychology and Aging, 22, 525–545. doi:10.1037/0882-7974.22.3.525 [DOI] [PubMed] [Google Scholar]
- Meredith W., & Tisak J (1990). Latent curve analysis. Psychometrika, 55, 107–122. doi:10.1007/BF02294746 [Google Scholar]
- Montagnier D., Dartigues J. F., Rouillon F., Pérès K., Falissard B., & Onen F (2014). Ageing and trajectories of depressive symptoms in community-dwelling men and women. International Journal of Geriatric Psychiatry, 29, 720–729. doi:10.1002/gps.4054 [DOI] [PubMed] [Google Scholar]
- Musliner K. L., Munk-Olsen T., Eaton W. W., & Zandi P. P (2016). Heterogeneity in long-term trajectories of depressive symptoms: Patterns, predictors and outcomes. Journal of Affective Disorders, 192, 199–211. doi:10.1016/j.jad.2015.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén L. K., & Muthén B. O.(1998–2012). Mplus user’s guide (7th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Panza F., D’Introno A., Colacicco A...Solfrizzi V (2009). Temporal relationship between depressive symptoms and cognitive impairment: The Italian Longitudinal Study on Aging. Journal of Alzheimer’s disease: JAD, 17, 899–911. doi:10.3233/JAD-2009-1111 [DOI] [PubMed] [Google Scholar]
- Perrino T., Mason C. A., Brown S. C., Spokane A., & Szapocznik J (2008). Longitudinal relationships between cognitive functioning and depressive symptoms among Hispanic older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63, P309–P317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen I., McGue M., Tan Q., Christensen K., & Christiansen L (2016). Change in depression symptomatology and cognitive function in twins: a 10-year follow-up study. Twin Research and Human Genetics: The Official Journal of the International Society for Twin Studies, 19, 104–111. doi:10.1017/thg.2016.3 [DOI] [PubMed] [Google Scholar]
- Petersson S., Mathillas J., Wallin K., Olofsson B., Allard P., & Gustafson Y (2014). Risk factors for depressive disorders in very old age: A population-based cohort study with a 5-year follow-up. Social Psychiatry and Psychiatric Epidemiology, 49, 831–839. doi:10.1007/s00127-013-0771-2 [DOI] [PubMed] [Google Scholar]
- Prince M., Acosta D., Chiu H., Copeland J., Dewey M., Scazufca M., & Varghese M (2004). Effects of education and culture on the validity of the Geriatric Mental State and its AGECAT algorithm. The British Journal of Psychiatry: the Journal of Mental Science, 185, 429–436. doi:10.1192/bjp.185.5.429 [DOI] [PubMed] [Google Scholar]
- Prince, M. J., Reischies, F., Beekman, A. T., Fuhrer, R., Jonker, C., Kivela, S. L., Lawlor, B. A...Copeland, J. R (1999). Development of the EURO-D scale–a European, Union initiative to compare symptoms of depression in 14 European centres. The British Journal of Psychiatry: The Journal of Mental Science, 174, 330–338. doi:10.1192/bjp.174.4.330 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2016). R: A Language and Environment for Statistical Computing [computer software] Vienna, Austria: Available from http://www.R-project.org/. [Google Scholar]
- Ritchie K., Gilham C., Ledésert B., Touchon J., & Kotzki P. O (1999). Depressive illness, depressive symptomatology and regional cerebral blood flow in elderly people with sub-clinical cognitive impairment. Age and Ageing, 28, 385–391. doi:10.1093/ageing/28.4.385 [DOI] [PubMed] [Google Scholar]
- Rogosa D. R. (1980). A critique of cross-lagged correlation. Psychological Bulletin, 88, 245–258. doi:10.1037/0033-2909.88.2.245 [Google Scholar]
- Sano M., Raman R., Emond J., Thomas R. G., Petersen R., Schneider L. S., & Aisen P. S (2011). Adding delayed recall to the Alzheimer disease assessment scale is useful in studies of mild cognitive impairment but not Alzheimer disease. Alzheimer Disease and Associated Disorders, 25, 122–127. doi:10.1097/WAD.0b013e3181f883b7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheurich A., Fellgiebel A., Schermuly I., Bauer S., Wölfges R., & Müller M. J (2008). Experimental evidence for a motivational origin of cognitive impairment in major depression. Psychological Medicine, 38, 237–246. doi:10.1017/S0033291707002206 [DOI] [PubMed] [Google Scholar]
- Schweitzer I., Tuckwell V., O’Brien J., & Ames D (2002). Is late onset depression a prodrome to dementia?International Journal of Geriatric Psychiatry, 17, 997–1005. doi:10.1002/gps.525 [DOI] [PubMed] [Google Scholar]
- Spiro A. 3rd, & Brady C. B (2011). Integrating health into cognitive aging: Toward a preventive cognitive neuroscience of aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66, i17–i25. doi:10.1093/geronb/gbr018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNESCO (2006). International Standard Classification of Education: ISCED 1997 Retrieved from: http://www.uis.unesco.org/Library/Documents/isced97-en.pdf.
- Vinkers D. J., Gussekloo J., Stek M. L., Westendorp R. G., & van der Mast R. C (2004). Temporal relation between depression and cognitive impairment in old age: Prospective population based study. BMJ (Clinical research ed.), 329, 881. doi:10.1136/bmj.38216.604664.DE [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from SHARE and SHARELIFE, release version 6.0.0 (March 31, 2017), can be downloaded free of charge from the SHARE Research Data Center by researchers who sign a statement confirming their use only for scientific purposes, at: http://www.share-project.org/data-access.html.