Abstract
Varicocele is a common cause of sperm damage. Some studies showed higher concentration of seminal leukocytes in patients with varicocele. The aim of the study was to evaluate seminal leukocyte subpopulations in patients with varicocele. We enrolled 20 patients with varicocele and 20 age-matched healthy men. Sperm analysis was conducted according to the World Health Organization (WHO) 2010 criteria. We evaluated seminal leukocyte subpopulations and bio-functional sperm parameters by flow cytometry. Patients with varicocele had significantly lower sperm concentration and total number than controls. Regarding seminal leukocyte subpopulations, patients with varicocele had a significantly lower percentage of CD8+ and CD16+ leukocytes and a significantly higher percentage of CD4+ leukocytes than controls. As for bio-functional sperm parameters, we found that patients with varicocele had a significantly lower percentage of alive spermatozoa compared to the control group. These results may explain the increased level of cytokines in the seminal plasma of patients with varicocele.
Keywords: bio-functional sperm parameters, conventional sperm parameters, seminal leukocytes, varicocele
Background
Varicocele is a common cause of male infertility. The relationship between varicocele and impaired conventional and bio-functional sperm parameters is well known, although there is no agreement on pathogenic mechanisms of sperm damage.1–4
In addition, there is evidence that patients with varicocele have higher concentration of seminal leukocytes compared to fertile men.5
Some authors found a negative correlation between seminal leukocyte concentrations and sperm morphology,6 whereas other studies have not reported any sperm damage in patients with leukocytospermia.7 Although the role of leukocytes in male infertility is still debated, the prevalence of leukocytospermia among infertile patients ranges from 15% to 28% versus 10% in fertile men.8 These conflicting data may be due to the difficulty to recognize seminal leukocytes, since the World Health Organization (WHO) manual suggested staining technique (peroxidase method) allows only quantification of polymorphonuclear granulocytes, while peroxidase-negative cells remained colorless.9 Thus, the inability to identify other subpopulations of leukocytes may cause underestimation of the inflammatory process in the semen of patients with varicocele. Flow cytometry makes possible to identify the different seminal leukocyte populations using monoclonal antibodies. Seminal leukocyte subpopulations may not influence or slightly alter conventional sperm parameters, but they may compromise the bio-functional sperm parameters, thus explaining the infertility found in different pathological models, including in patients with varicocele.
Therefore, the aim of this study was to evaluate seminal leukocyte subpopulations in men with and without varicocele and to evaluate a possible relationship between these subpopulations and conventional and bio-functional sperm parameters.
Patients and methods
Patients
In this prospective case–control study, we enrolled 20 patients (mean age: 29.5 ± 3 years) with varicocele (III–IV degree according to the Sarteschi classification) and 20 age-matched healthy men (mean age: 34.7 ± 1.9 years) as control group. For sample size calculation for continued outcomes, the PASS Software (PASS 13, Hintze, J. (2014); PASS 13, NCSS, LLC, Kaysville, Utah, USA; www.ncss.com) was adopted considering a power of 80% and accepting an alpha error of 0.05. All patients attended the Division of Andrology and Endocrinology, University Teaching Hospital “Policlinico-Vittorio Emanuele,” University of Catania, from December 2017 to April 2018. The Intra-Divisional Ethic Committee of the Endocrinology Section approved the research protocol.
We excluded from the study patients under 18 years, with azoospermia, follicle-stimulating hormone (FSH) serum levels >8 mIU/mL, primary testicular diseases other than varicocele, male accessory gland infections, central hypogonadism, systemic diseases, chronic exposition to environmental and/or occupational toxicants, intake of spermiotoxic drugs, smoking, alcohol, or drug abuse.
Before enrollment, patients underwent testicular ultrasound (US) and echo color Doppler to establish testicular volume and to estimate the presence and the degree of varicocele. Varicocele was classified according to the Sarteschi classification.10 We excluded from the study patients with varicocele degree ⩽II. Each patient who participated in this study was asked to sign a written informed consent.
Semen analysis, seminal leukocyte subpopulations and bio-functional sperm parameter evaluation
Each patient was asked to collect semen samples by masturbation after 3–4 days of sexual abstinence. Sperm analysis was conducted according to the WHO 2010 criteria.9 We evaluated the following bio-functional sperm parameters by flow cytometry: percentage of alive and apoptotic spermatozoa, mitochondrial membrane potential (MMP), degree of chromatin compactness and percentage of DNA fragmentation. We also evaluated seminal leukocyte subpopulations by flow cytometry.
Flow cytometric analysis was performed using flow cytometer CytoFLEX (Beckman Coulter Life Science, Milan, Italy). CytoFLEX is equipped with two solid-state lasers at 488 and 638 nm and with seven fluorescence channels: 525/40 BP, 585/42 BP, 610/20 BP, 690/50 BP, 780/60 BP for excitation at 488 nm and 660/10 BP, 712/25 BP, 780/60 BP for excitation at 638 nm. Data were analyzed by the software CytExpert1.2.
Seminal leukocyte subpopulation evaluation
Leukocyte subpopulations were identified by flow cytometry using a threefold established by labeled antibodies to membrane-specific antigens. In particular, after liquefaction, an aliquot of 100 μL of seminal fluid underwent three cycles of washes with PBS (phosphate-buffered saline), the supernatant was removed and the pellet re-suspended in 1 mL of PBS. Subsequently, spermatozoa of each sample were labeled with anti-CD45 antibody(Ab) (ECD emission), anti-CD14 Ab, and anti-CD16 Ab (APC emission) to identify neutrophils (co-expression of CD45 and CD16) and monocyte-macrophages (co-expression of CD45 and CD14; these cell subpopulations were divided up, in the gate, according to cell dimension), anti-CD3 Ab (PC 5.5 emission), anti CD4-Ab (FITC emission), and anti-CD8 Ab (PE emission) were used to identify T-helper and T-suppressor lymphocytes, respectively.
The other leukocyte subpopulations are not described in literature since they seem to be poorly represented in the seminal fluid.
Evaluation of sperm apoptosis/vitality
The externalization of phosphatidylserine (PS) on the outer cell membrane is used as an indicator of early apoptosis. The assessment of PS externalization was performed using annexin V (FITC-labeled), a protein that binds selectively the PS in presence of calcium ions, and propidium iodide (PI). The simultaneous staining marked spermatozoa. Therefore, marking simultaneously the cells with annexin V and/or PI allows us to distinguish alive (with intact cytoplasmic membrane), apoptotic, or necrotic spermatozoa. Staining with annexin V and PI was obtained using a commercially available kit (Annexin V-FITC Apoptosis; Beckman Coulter, IL, Milan, Italy). Briefly, an aliquot containing 0.5 × 106/mL was suspended in 0.5 mL of buffer containing 10 µL of annexin V-FITC and 20 µL of PI and incubated for 10 min in the dark. After incubation, the sample was analyzed by the fluorescence channels 525/40 BP (FITC) and 585/42 BP, 610/20 BP, 690/50 BP (PI). The different patterns of staining allowed to identify the different cell populations: FITC negative and PI negative indicate alive sperm cells, FITC positive and PI negative indicate spermatozoa in early apoptosis, and FITC positive and PI positive indicate sperm cells in late apoptosis.
Evaluation of the MMP
The percentage of spermatozoa with low MMP was evaluated using the lipophilic probe 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethyl-benzimidazolylcarbocyanine iodide (JC-1) able to selectively penetrate into mitochondria where it is in monomeric form, emitting at 527 nm. Therefore, JC-1 excited at 490 nm is able to form aggregates emitting at 590 nm in relation to the membrane potential. When the mitochondrial membrane becomes more polarized, the fluorescence changes reversibly from green to orange. In cells with normal membrane potential, JC-1 is in the mitochondrial membrane in form of aggregates, emitting an orange fluorescence, while in the cells with low membrane potential, it remains in the cytoplasm in a monomeric form, emitting a green fluorescence. As regards the sample preparation, we incubated an aliquot containing 1 × 106/mL spermatozoa with JC-1 (JC-1 Dye, Mitochondrial Membrane Potential Probe; DBA s.r.l, Milan, Italy) for 10 min, at a temperature of 37°C and in the dark; after 10 min of incubation, the cells were washed in PBS and analyzed by the fluorescence channels 525/40 BP (FITC) and 585/42 BP (PE).
Assessment of DNA fragmentation
The evaluation of DNA fragmentation was performed by the TUNEL assay. This method uses terminal deoxynucleotidyl transferase (TdT), an enzyme that polymerizes at the level of DNA breaks, modifying nucleotides conjugated to a fluorochrome. The TUNEL assay was performed using a commercially available kit (Apoptosis Mebstain Kit; DBA s.r.l). To obtain a negative control, TdT was omitted from the reaction mixture; the positive control was obtained pre-treating spermatozoa (about 0.5 × 106) with 1 mg/mL of deoxyribonuclease I, not containing RNAse, at 37°C for 60 min prior to staining. The reading was performed by flow cytometry using the 525/40 BP fluorescence channels.
Assessment of the degree of chromatin compactness
Chromatin compactness assessment was evaluated after a process of cell membrane permeabilization to allow fluorophore to penetrate into the nucleus. In brief, an aliquot of 1 × 106/mL spermatozoa was incubated with LPR DNA-Prep Reagent containing 0.1% potassium cyanate, 0.1% NaN3, non-ionic detergents, saline, and stabilizers (Beckman Coulter, IL), in the dark, at room temperature, for 10 min, and incubated with Stain DNA-Prep reagent containing 50 µg/mL of PI (<0.5%), RNase A (4 KUnitz/mL), <0.1% NaN3, saline, and stabilizers (Beckman Coulter, IL) in the dark at room temperature for 30 min. The samples were analyzed by cytometer using 585/42 BP and 610/20 BP fluorescence channels. PI enters the cells, after adequate permeabilization of the cell membrane, and the more the chromatin is compact, the less it can bind to it.
Statistical analysis
The results are reported as mean ± SEM throughout the study. Statistical analysis of the data was performed using the Student t test. Spearman’s rank correlation coefficient was used to evaluate correlations between seminal leukocyte subpopulations and conventional or bio-functional sperm parameters. SPSS 22.0 for Windows was used for statistical analysis (SPSS Inc., Chicago, IL, USA). A P value lower than 0.05 was considered statistically significant.
Results
Patients with varicocele had significantly lower sperm concentration (P ⩽ 0.05) and total sperm number (P ⩽ 0.05) than controls (Table 1). Seminal fluid volume, progressive and total sperm motility and the percentage of spermatozoa with normal forms did not differ significantly between the two groups. Moreover, we did not find significant differences in seminal leukocyte concentration, evaluated as suggested by the WHO criteria,11 between the two groups.
Table 1.
Conventional sperm parameters in patients with varicocele and in normal controls.
| Sperm parameters | Controls | Varicocele |
|---|---|---|
| Volume (mL) | 3.6 ± 0.4 | 3.6 ± 0.3 |
| Concentration (mil/mL) | 49.5 ± 8.0 | 28.6 ± 3.9* |
| Total sperm count (mil/ejaculate) | 165.1 ± 32.2 | 94.0 ± 12.1* |
| Progressive motility (%) | 28 ± 0.9 | 22.7 ± 2.2 |
| Total motility (%) | 66.0 ± 2.0 | 56.9 ± 13.3 |
| Normal forms (%) | 7.6 ± 0.8 | 8.3 ± 1.1 |
| Leukocytes (mil/mL) | 0.4 ± 0.1 | 0.7 ± 0.3 |
P ⩽ 0.05 (Student’s t test).
The evaluation of seminal leukocyte subpopulations by flow cytometry showed that patients with varicocele had a significantly lower percentage of CD8+ cytotoxic T lymphocytes and CD16+ leukocytes (macrophages) (P < 0.05) and a significantly higher percentage of CD4+ helper T-lymphocytes (P < 0.05) than controls (Figure 1). The number of CD14+ leukocytes did not differ significantly between the two groups. As a secondary finding, no change was found in the proportion between CD4 and CD8 lymphocytes in peripheral blood. Table 2 reports the percentage of different seminal leukocyte subpopulations in patients with varicocele and in the control group.
Figure 1.
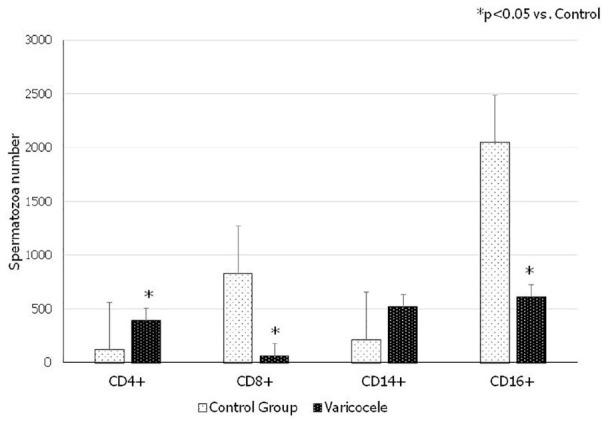
Seminal leukocyte subpopulations in patients with varicocele and in normal controls.
Table 2.
Percentage and absolute number of different seminal leukocyte subpopulations.
| Leukocyte subpopulations | Controls (%) (n = 20) | Varicocele (%) (n = 20) | Controls (n = 20) | Varicocele (n = 20) |
|---|---|---|---|---|
| CD45+—CD3+—CD4+ | 2.9* | 24.5 | 113.2 ± 30.3* | 385.6 ± 82.7 |
| CD45+—CD3+—CD8+ | 23.7 | 4* | 925.7 ± 354.5 | 63.2 ± 18.4* |
| CD45+—CD14+ | 5.8 | 32.6 | 227.2 ± 67.2 | 513.4 ± 183 |
| CD45+—CD16+ | 67.6 | 38.8* | 2646.9 ± 790.4 | 611.5 ± 197.5* |
P < 0.05 (Student’s t test).
The evaluation of the bio-functional sperm parameters showed that patients with varicocele had a significantly lower percentage of alive spermatozoa (P < 0.05) compared to controls (Figure 2). The other parameters did not differ significantly, although patients with varicocele had lower percentage of spermatozoa with chromatin compactness, higher DNA fragmentation, and higher percentage of spermatozoa with low MMP.
Figure 2.

Bio-functional sperm parameters in patients with varicocele and in normal controls.
Finally, we did not find any correlation between seminal leukocyte subpopulations and conventional or bio-functional sperm parameters.
Discussion
The relationship between varicocele and impaired sperm parameters is well known and it is the frequent cause of infertility.4 According to literature, we found that patients with varicocele had significantly lower sperm concentration and total sperm number than controls.1–4
Some studies have shown that patients with varicocele have worse bio-functional sperm parameters.1,11 We found that patients with varicocele had a significantly lower percentage of alive spermatozoa compared to the control group, whereas the other parameters did not differ significantly, although the group of patients with varicocele showed lower chromatin compactness, higher DNA fragmentation, and lower percentage of spermatozoa with low MMP.
The exact mechanisms of sperm damage in patients with varicocele are not clear, but a number of potential harmful conditions have been called into play.4 Experimental and epidemiological studies have shown that inflammation has an essential role in the pathogenesis of varicocele-induced sperm alteration.5 Accordingly, the role of some pro-inflammatory and anti-inflammatory cytokines has been investigated.5 Cytokines increase also the oxidative stress, because of which spermatozoa are highly sensitive.
Little evidences in literature have shown a higher concentration of seminal leukocytes in patients with varicocele compared to fertile men.5 In this study, we found that leukocyte concentration did not differ significantly between patients with varicocele and normal controls. However, their subpopulations differ significantly.
It is not possible to reliably identify seminal leukocytes. Indeed, the peroxidase method identifies only granulocytes. Although granulocytes are the most prevalent leukocyte subpopulation in semen (50%–60%), macrophages (20%–30%) and T lymphocytes (2%–5%) are also present.12
Very few studies have investigated seminal leukocyte subpopulations, and to our knowledge, no study has evaluated the seminal leukocyte formula in patients with varicocele. We found that these patients had a significantly lower percentage of CD8+ cytotoxic T lymphocytes and CD16+ leukocytes (macrophages) and a significantly higher number of CD4+ helper T lymphocytes than the control group. This is an interesting discovery that may explain why patients with varicocele have increased cytokine levels in the seminal fluid, even if they have a normal of leukocyte concentration.
This study may have some limitations. The first one could be related to the relatively small number of the samples. Moreover, seminal leukocyte subpopulation evaluation by flow cytometry is not a standardized method. Further studies will be needed.
Conclusion
Varicocele is a common cause of sperm damage. This study reports, for the first time, seminal leukocyte subpopulation evaluated by flow cytometry. The inversion of the “seminal fluid leukocyte formula” compared to fertile men may be one of the pathogenic mechanisms underlying the sperm damage found in patients with varicocele. This result may also explain the increased level of cytokines in the seminal plasma of patients with varicocele.
Footnotes
Author contributions: LMM is the principal investigators. AA, AEC, MC, FG, RC, and SLV have contributed in methodological and statistical aspects. RAC is the coordinator of the study.
Availability of data and material: All data generated or analyzed during this study are included in this published article.
Consent to participate: Each patient who participated in this study was asked to sign a written informed consent.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The Intra-Divisional Ethic Committee of the Endocrinology Section approved the research protocol.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Laura Maria Mongioì  https://orcid.org/0000-0003-2341-0996
https://orcid.org/0000-0003-2341-0996
Angela Alamo  https://orcid.org/0000-0003-4391-880X
https://orcid.org/0000-0003-4391-880X
Aldo E Calogero  https://orcid.org/0000-0001-6950-335X
https://orcid.org/0000-0001-6950-335X
Sandro La Vignera  https://orcid.org/0000-0002-7113-2372
https://orcid.org/0000-0002-7113-2372
References
- 1. La Vignera S, Condorelli R, Vicari E, et al. (2012) Effects of varicocelectomy on sperm DNA fragmentation, mitochondrial function, chromatin condensation, and apoptosis. Journal of Andrology 33(3): 389–396. [DOI] [PubMed] [Google Scholar]
- 2. Condorelli RA, Calogero AE, Mongioi’ L, et al. (2016) Varicocele and concomitant dilation of the periprostatic venous plexus: Effects on semen viscosity sperm parameters. Journal of Endocrinological Investigation 39(5): 543–547. [DOI] [PubMed] [Google Scholar]
- 3. Mongioì LM, Mammino L, Compagnone M, et al. (2019) Effects of varicocele treatment on sperm conventional parameters: Surgical varicocelectomy versus sclerotherapy. Cardiovasc Intervent Radiol 42: 396–404. [DOI] [PubMed] [Google Scholar]
- 4. Agarwal A, Sharma R, Harlev A, et al. (2016) Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: A systematic review and meta-analysis. Asian Journal of Andrology 18(2): 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tortolero I, Duarte Ojeda JM, Pamplona Casamayor M, et al. (2004) The effect of seminal leukocytes on semen quality in subfertile males with and without varicocele. Archivos Espanoles De Urologia 57(9): 921–928. [PubMed] [Google Scholar]
- 6. Thomas J, Fishel SB, Hall JA, et al. (1997) Increased polymorphonuclear granulocytes in seminal plasma in relation to sperm morphology. Human Reproduction (Oxford, England) 12(11): 2418–2421. [DOI] [PubMed] [Google Scholar]
- 7. Seshadri S, Flanagan B, Vince G, et al. (2012) Detection of subpopulations of leucocytes in different subgroups of semen sample qualities. Andrologia 44(Suppl. 1): 354–361. [DOI] [PubMed] [Google Scholar]
- 8. Zorn B, Virant Klun I, Meden Vrtovec H, et al. (2000) Semen granulocyte elastase: Its relevance for the diagnosis and prognosis of silent genital tract inflammation. Hum Reprod 15(9): 1978–1984. [DOI] [PubMed] [Google Scholar]
- 9. Lu JC, Huang YF, Lu NQ. (2010) WHO laboratory manual for the examination and processing of human semen. Zhonghua Nan Ke Xue = National Journal of Andrology 16: 867–871. [PubMed] [Google Scholar]
- 10. Sarteschi LM. (1993) Lo studio del varicocele con eco-color-Doppler. Giornale Italiano di Ultrasonologia 4; 43–49. [Google Scholar]
- 11. Roque M, Esteves SC. (2018) Effect of varicocele repair on sperm DNA fragmentation: A review. International Urology and Nephrology 50(4): 583–603. [DOI] [PubMed] [Google Scholar]
- 12. La Vignera S, Condorelli RA, Morgia G, et al. (2015) Different levels of Cd45pos leukocytes in the semen of patients with low testicular volume. International Journal of Immunopathology and Pharmacology 28(1): 85–92. [DOI] [PubMed] [Google Scholar]


