Abstract
A 36-year-old woman presented at 16 weeks’ gestation with severe hypertension. In comparison to the non-pregnant reference normal ranges, potassium was 3.1-3.9 mmol/L, aldosterone 2570-3000 pmol/L (N 250-2885) renin was unsuppressed (24-76.4 ng/L (N1.7–23.9)), with aldosterone to renin ratios in the reference range. An adrenal MRI scan demonstrated a 1.8 × 1.4 cm left adrenal adenoma. Primary aldosteronism was strongly suspected and surgery considered. However, she was managed conservatively with labetalol and modified-release nifedipine with no obstetric complications. Post-partum blood pressures remained elevated with normal aldosterone (539 pmol/L), unsuppressed renin (5.2 ng/L) and normal aldosterone-to-renin ratio (104 (N < 144)). Suspected primary hyperaldosteronism is challenging to investigate and manage in pregnancy. The accepted screening and confirmatory tests are either contraindicated or not validated in pregnancy. Pregnancy has significant effects on the renin-angiotensin-aldosterone pathway leading to physiologic elevations in both aldosterone and renin. While primary hyperaldosteronism has been associated with poor pregnancy outcomes, optimal management in pregnancy is not clearly established.
Keywords: Primary hyperaldosteronism, adrenal adenoma
Case
A 36-year-old multiparous Caucasian woman Gravida 3, Para 2 presented to hospital at 16 weeks’ gestation with severe hypertension (BP 180-190/130 mmHg). She had no personal history of hypertension. She was initially managed with a an infusion of hydralazine with improvement in her blood pressure to 120–140/80–90 mmHg. Potassium was intermittently low, with values down to 3.1 mmol/L (Table 1). Her body mass index (BMI) was 22.75 kg/m2, she had no symptoms or risk factors for obstructive sleep apnoea, she was not taking non-steroidal anti-inflammatory drugs (NSAIDs) or eating licorice. Her serum magnesium was 0.80 mmol/L. Given her young age and presentation with severe hypertension prior to 20 weeks’ gestation, workup for secondary causes of hypertension was conducted, with high suspicion for primary hyperaldosteronism given her hypokalaemia. Serum levels were obtained for aldosterone and renin as a first screening test, while the patient was on modified-release nifedipine 30 mg twice daily. The aldosterone levels were significantly elevated, while the renin remained unsuppressed, but the resulting aldosterone to renin ratio was within the non-pregnant reference range (Table 1). The progesterone to aldosterone ratio obtained at 25 weeks’ gestational age was consistent with physiologic progesterone-induced hyperaldosteronism (Table 1).
Table 1.
Endocrine investigations.
| Gestational age: | K + mmol/L(N 3.5–5.0)a(N 3.3–5.0)b | Aldosteronepmol/L(N < 1118)a(N 250–2885)b | Reninng/L(N 1.7–23.9)a,c | Progesteronenmol/L(N 0.3–86.0)a(N 315–1088)d | ARR(N < 144)a | Aldosterone to progesterone ratio(N 18--80)5 |
|---|---|---|---|---|---|---|
| 17 Weeks | 3.5 | 2570 | 30.4 | 85 | ||
| 18 Weeks | 3.1 | >2770 | 76.4 | >36 | ||
| 19 Weeks | 3.9 | 3000 | 40.7 | 74 | ||
| 25 Weeks | 3.8 | 2690 | 24 | 310.0 | 36.2 | |
| 3 Weeks PP | 4.2 | 539 | 5.2 | 104 |
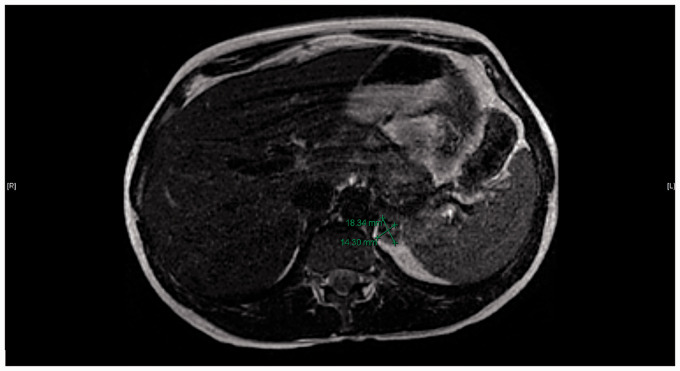
Despite the negative plasma-aldosterone renin ratio screening test, the significant elevation in aldosterone raised concerns about the reliability of this screening test in pregnancy, prompting imaging of the adrenal glands. The Magnetic Resonance Imaging (MRI) scan demonstrated a 1.8 × 1.4 cm left adrenal adenoma (Figure 1). The 24-h urine collection for metanephrines and catecholamines was normal, excluding pheochromocytoma. The clinical suspicion for Cushing’s syndrome was low so testing for Cushing's was not undertaken during pregnancy.
Figure 1.
Within the left adrenal gland there is a 1.8 × 1.4 cm mass which demonstrates signal drop out on the opposed phased imaging consistent with intracellular lipid content characteristic of adrenal adenoma. The contralateral right adrenal gland is unremarkable.
Despite the lack of biochemical confirmation there was considerable debate over whether this presentation represented an aldosterone-producing adenoma causing hypertension and hypokalaemia. Surgical adrenalectomy was even considered given that she was nearing the end of her second trimester, traditionally thought to be the safest time for surgery in pregnancy in terms of both maternal and fetal complications.1 Ultimately however, given the uncertainty of the diagnosis and potential surgical risks, a conservative management strategy was instituted, with close surveillance, removal from work and regular fetal growth assessment. Traditional pregnancy antihypertensive drugs labetalol and modified-release nifedipine were initiated and she achieved reasonable blood pressure control and normal potassium levels throughout the remainder of her pregnancy, although induction of labour was initiated at 38 weeks’ gestation for rising blood pressures. A healthy baby girl (3.23 kg) was delivered at term without complications.
Post-partum the mother’s blood pressure remained elevated, but aldosterone levels returned to normal (539 pmol/L), with a non-suppressed renin (5.2 ng/L) and a normal aldosterone-to-renin ratio 104 (N < 144)) (Table 1), measured at three weeks post-partum. A recumbent saline suppression test conducted 19 weeks post-partum showed borderline suppressibility of aldosterone (830 pmol/L at baseline to 196 pmol/L post-suppression; normal <140 pmol/L, confirmation >280 pmol/L2). Despite the borderline test, the overall impression was that primary hyperaldosteronism was unlikely given that she did not meet the screening test cut-off prior to proceeding with the saline suppression test. A 1 mg dexamethasone suppression test conducted post-partum, showed normal suppressibility of cortisol to 26 mmol/L, making Cushing’s syndrome highly unlikely. A vascular CT was performed post-partum and showed no evidence of renal artery stenosis. She continues on nifedipine 120mg and is being managed as essential hypertension.
Discussion
In this patient, the presentation with hypertension and hypokalaemia during pregnancy and the simultaneous presence of high aldosterone levels and an adrenal adenoma raised concern for primary hyperhyperaldosteronism. In women of childbearing age with hypertension, 7.4% have primary hyperaldosteronism3 and it should be considered in select patients, especially those with severe or resistant hypertension, spontaneous hypokalaemia, or those who present at a young age.2 Aldosterone acts on the mineralocorticoid receptors in the kidneys to retain sodium and water and secrete potassium. The main clinical features associated with primary hyperaldosteronism are moderate to severe hypertension and possible (present in 9-37% of cases).2 Adrenal adenomas are the underlying cause of primary hyperaldosteronism in approximately 35–40% of cases, with the remaining 60% caused by hyperplasia, which is generally bilateral.4 Presentation in pregnancy is rare with less than 50 cases reported in the literature, but this causal distribution would be expected to be similar.5,6
The accepted screening test for primary hyperaldosteronism is the aldosterone to renin ratio (ARR), with confirmatory tests including a saline suppression test, captopril challenge or fludrocortisone suppression test, or salt load with 24-h urine aldosterone collection.2 Saline suppression, salt and fludrocortisone tests are rarely performed in pregnancy due to concerns of exacerbating the physiologic volume expansion that occurs during gestation, although normal values exist for the saline suppression test in healthy pregnancy.7 The majority of antihypertensive medications can also impact the ARR, including nifedipine, which can falsely lower the ARR, and could potentially have led to a false-negative test in this patient.8 Medications that have minimal impact on the ARR include verapamil, hydralazine and alpha blockers such as prazosin.2 Potassium levels should also ideally be corrected to 4.0 mmol/L or higher prior to testing to avoid false-negative results.2
Furthermore, pregnancy has significant effects on all components of the renin-angiotensin-aldosterone pathway. Progesterone, increases 1000-fold during pregnancy and competitively binds to aldosterone receptors, which leading to a physiological elevation in aldosterone in healthy pregnancies.5,9–12 Additionally, the ovaries and placental tissues secrete renin, further raising renin and aldosterone levels.5,10,12 Estrogen stimulates hepatic angiotensinogen production, again leading to down-stream up-regulation of aldosterone.10 The net effect of the physiologic changes in the renin-angiotensin-aldosterone system in pregnancy is a significant elevation in both aldosterone and renin.
Evaluation of suspected primary hyperaldosteronism in pregnancy can be challenging. However, some clinical features may be useful. Despite the physiologic elevation of renin in pregnancy, it is usually still suppressed in pregnant women with primary hyperaldosteronism, although may be higher than in non-pregnant women with primary hyperaldosteronism.5,6 Calculating an ARR may still be useful, but there is an increased risk of false negatives due to the physiologic elevation of renin in pregnancy.5 Elevated ARR with suppressed Plasma Renin Activity (PRA) occurs in only 61% of women with known primary hyperaldosteronism in pregnancy.13
Hypertension and hypokalaemia are unreliable markers of primary hyperaldosteronism in pregnancy. Blood pressure may worsen or improve in pregnancy. Improvement is secondary to antagonism of aldosterone by progesterone at the level of the mineralocorticoid receptor.5,14 Blood pressure may worsen in certain individuals, who may have higher baseline aldosterone levels that overcome the competitive inhibition by progesterone.5 Genetic variation in the mineralocorticoid receptor may also contribute to the variability in blood pressure response in pregnancy.6,9,11 Potassium levels down to 3.3 mmol/L are within reference range in healthy pregnancy.15 Hypokalaemia may also improve in pregnancy due to the antikaliuretic effects of progesterone.5 Proteinuria has been seen in 43% of women with primary hyperaldosteronism in pregnancy, which can lead to difficulties in distinguishing it from preeclampsia, which also presents with hypertension and proteinuria in pregnancy.13
Rare mutations in the mineralocorticoid receptor gene can allow for potent receptor activation by progesterone as well as aldosterone and can lead to a presentation of abrupt onset severe hypertension in pregnancy, and warrant special consideration in this population.16,17 While similar in presentation, these cases can be readily distinguished from primary hyperaldosteronism as they are characterized by hyporeninemic hypohyperaldosteronism.
Riester et al. suggested that calculating a progesterone to aldosterone ratio may be useful as a measure of mineralocorticoid excess in pregnancy. In normal pregnancy, this ratio increases to between 18 and 80 pmol/L/pmol/L.5 A ratio below 20 was associated with worse outcomes in one case report and may be more consistent with primary hyperaldosteronism.5,14 Therefore, a ratio above this number may portend a favourable pregnancy outcome, and suggest a benign, physiologic process of progesterone-mediated-hyperhyperaldosteronism.5 However, the clinical data for using this ratio are limited and establishment of a validated reference range, both during and outside of pregnancy, as well as more data for its link to outcomes would be required before it should be used to guide clinical decisions.
Poorly-controlled hypertension in primary hyperaldosteronism has been associated with poor pregnancy outcomes, including placental abruption, preterm labour, intrauterine death, fetal distress, end-organ damage and superimposed preeclampsia.1,18,19 It may also present as severe post-partum hypertension due to the loss of competitive progesterone binding to the mineralocorticoid receptor. Optimal management in pregnancy is not clearly established, although most experts favour a trial of conservative management of hypertension and/or hypokalaemia during pregnancy and proceeding to targeted treatment only if blood pressure remains uncontrolled despite first-line treatments.5,6 Some authors, however, advocate surgical adrenalectomy early in the second trimester in cases of identified adrenal adenoma due to the risks of significant obstetric complications associated with uncontrolled hypertension in later pregnancy.1,18
The use of mineralocorticoid receptor antagonists (MRA), including spironolactone and eplerenone, can be considered as these will directly oppose the action of aldosterone at its renal site of action. Spironolactone has been associated with feminization in male rats, and in one reported case of ambiguous genitalia in a male born to a mother taking spironolactone until 5 weeks’ gestation for polycystic ovarian syndrome.5 Spironolactone is considered safe for breastfeeding. Eplerenone lacks the antiandrogenic effects of spironolactone, but has not been widely used in pregnancy, although no adverse events have been reported to date with accidental or intentional exposure in pregnancy.5,20 Amiloride blocks the renal luminal sodium channels, the principal site of aldosterone-mediated sodium reabsorption. It has not been studied in pregnancy, but its use has been described in one case series, with reasonable blood pressure control, improvement in hypokalaemia and no safety concerns.21
In the case of an aldosterone-secreting adrenal adenoma, surgical adrenalectomy can be curative and could be considered in pregnancy for cases of severe refractory hypertension. Successful adrenalectomy for primary hyperaldosteronism in pregnancy has been reported in a handful of case reports,4,18 including a series of five patients who had improvement in hypertension and no significant adverse outcomes related to the surgery.1 This is perhaps most safely performed in the second trimester, which carries the lowest risk for fetal and maternal complications.1 However, in a pooled analysis of nine women with primary hyperaldosteronism who had laparoscopic adrenalectomy at 14–24 weeks’ gestation, there was considerable morbidity despite biochemical cure of primary hyperaldosteronism, including one intrauterine fetal demise at 26 weeks’ gestational age (GA); and three pre-term deliveries (two at 26 weeks GA and one at 34 weeks GA).13
The adrenal finding described here is likely to represent a non-functional adenoma, which is present in approximately 1% of the adult population in this patient’s age range,22 but resulted in considerable uncertainty and possibly unnecessary investigations in this case. This case highlights the importance of following the correct sequence of diagnostic evaluation. Securing a biochemical diagnosis is an essential first step in the diagnosis of primary hyperaldosteronism and should be confirmed prior to proceeding with imaging to avoid the potential for introducing confounding information. Based on biochemical testing alone there was no indication to proceed with adrenal imaging in this case. Furthermore, other diagnostic considerations for secondary hypertension were not fully explored prior to proceeding with unwarranted imaging, including renal artery imaging or testing for Cushing’s syndrome with a 24 hour urinary free cortisol level, which were not completed until after pregnancy. This case illustrates the potential danger of bypassing steps in diagnostic reasoning, where imaging prior to biochemical confirmation nearly led to an inappropriate adrenalectomy in a pregnant women.
Conclusion
Significant challenges still exist when managing a patient with suspected primary hyperaldosteronism in pregnancy. Firstly, a definitive diagnosis of primary hyperaldosteronism is difficult to make in pregnancy for a number of reasons including the fact that traditional tests may not be valid, there is significant physiological disruption of the RAAS during pregnancy and there is heterogeneity of individual response to pregnancy in patients with primary hyperaldosteronism that is only partially understood. It should also be noted that adrenal adenomas are not uncommon (1–5% of the general population), and that incidental findings of adrenal adenomas are much more likely to be non-functional than secretory. Given this, following correct diagnostic procedures and securing a biochemical confirmation of diagnosis prior to imaging should be a high priority. While best management strategies are not yet fully established, even if biochemical diagnosis is reached, most experts suggest starting with more conservative treatments, proceeding to imaging only if considering surgery and to give consideration to the use of MRAs before surgery with the awareness of the limited safety data available for their use in pregnancy. Areas of potential further study include establishment of valid pregnancy reference ranges for renin concentration, validation of the progesterone to aldosterone ratio as a clinical prediction tool and a more systematic evaluation of the outcomes for available management options for primary hyperaldosteronism in pregnancy.
Supplemental Material
Supplemental Material for High aldosterone, hypertension and adrenal adenoma in a 36-year-old pregnant patient: Is this primary aldosteronism? by Amanda J Berberich, Deborah Penava, Dongmei Sun, Arlene MacDougall, Andrea Lum and Stan Van Uum in Obstetric Medicine
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Guarantor
AB
Contributorship
AJB, DP, DS, AL, SVU were involved in the clinical care of the patient described. AJB drafted the first draft manuscript. AJB, DP, AM, DS, AL, SVU reviewed and revised the first drafts.
References
- 1.Kosaka K, Onoda N, Ishikawa T, et al. Laparoscopic adrenalectomy on a patient with primary hyperaldosteronism during pregnancy. Endocr J 2006; 53: 461–466. [DOI] [PubMed] [Google Scholar]
- 2.Funder JW, Carey RM, Mantero F, et al. The management of primary hyperaldosteronism: case detection, diagnosis, and treatment: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016; 101: 1889–1916. [DOI] [PubMed] [Google Scholar]
- 3.Camelli S, Bobrie G, Postel-Vinay N, et al. Lb01.11: prevalence of secondary hypertension in young hypertensive adults. J Hypertens 2015; 33: e47. [Google Scholar]
- 4.Nursal TZ, Caliskan K, Ertorer E, et al. Laparoscopic treatment of primary hyperhyperaldosteronism in a pregnant patient. Can J Surg 2009; 52: E188–E190. [PMC free article] [PubMed] [Google Scholar]
- 5.Riester A, Reincke M. Progress in primary hyperaldosteronism: mineralocorticoid receptor antagonists and management of primary hyperaldosteronism in pregnancy. Eur J Endocrinol 2015; 172: R23–R30. [DOI] [PubMed] [Google Scholar]
- 6.Malha L, August P. Secondary hypertension in pregnancy. Curr Hypertens Rep 2015; 17: 53. [DOI] [PubMed] [Google Scholar]
- 7.Bentley-Lewis R, Graves SW, Seely EW. The renin-aldosterone response to stimulation and suppression during normal pregnancy. Hypertens Pregnancy 2005; 24: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiad TM, Cunningham SK, Hayes FJ, et al. Effects of nifedipine treatment on the renin-angiotensin-aldosterone axis. J Clin Endocrinol Metab 1997; 82: 457–460. [DOI] [PubMed] [Google Scholar]
- 9.Shigematsu K, Nishida N, Sakai H, et al. Primary hyperaldosteronism with aldosterone-producing adenoma consisting of pure zona glomerulosa-type cells in a pregnant woman. Endocr Pathol 2009; 20: 66–72. [DOI] [PubMed] [Google Scholar]
- 10.Lumbers ER, Pringle KG. Roles of the circulating renin-angiotensin-aldosterone system in human pregnancy. Am J Physiol Regul Integr Comp Physiol 2014; 306: R91–R101. [DOI] [PubMed] [Google Scholar]
- 11.Campino C, Trejo P, Carvajal CA, et al. Pregnancy normalized familial hyperhyperaldosteronism type I: a novel role for progesterone? J Hum Hypertens 2015; 29: 138–139. [DOI] [PubMed] [Google Scholar]
- 12.Escher G. Hyperhyperaldosteronism in pregnancy. Ther Adv Cardiovasc Dis 2009; 3: 123–132. [DOI] [PubMed] [Google Scholar]
- 13.Morton A. Primary hyperaldosteronism and pregnancy. Pregnancy Hypertens 2015; 5: 259–262. [DOI] [PubMed] [Google Scholar]
- 14.Ronconi V, Turchi F, Zennaro MC, et al. Progesterone increase counteracts aldosterone action in a pregnant woman with primary hyperaldosteronism. Clin Endocrinol (Oxf) 2011; 74: 278–279. [DOI] [PubMed] [Google Scholar]
- 15.Abbassi-Ghanavati M, Greer LG, Cunningham FG. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2009; 114: 1326–1331. [DOI] [PubMed] [Google Scholar]
- 16.Geller DS, Farhi A, Pinkerton N, et al. Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science 2000; 289: 119–123. [DOI] [PubMed] [Google Scholar]
- 17.Zennaro MC, Fernandes-Rosa F. 30 Years of the mineralocorticoid receptor: mineralocorticoid receptor mutations. J Endocrinol 2017; 234: T93–T106. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto J, Miyake H, Isozaki T, et al. Primary hyperaldosteronism in pregnancy. J Nippon Med Sch 2000; 67: 275–279. [DOI] [PubMed] [Google Scholar]
- 19.Kamoun M, Mnif MF, Charfi N, et al. Adrenal diseases during pregnancy: pathophysiology, diagnosis and management strategies. Am J Med Sci 2014; 347: 64–73. [DOI] [PubMed] [Google Scholar]
- 20.Cabassi A, Rocco R, Berretta R, et al. Eplerenone use in primary hyperaldosteronism during pregnancy. Hypertension 2012; 59: e18–e19. [DOI] [PubMed] [Google Scholar]
- 21.Al-Ali NA, El-Sandabesee D, Steel SA, et al. Conn's syndrome in pregnancy successfully treated with amiloride. J Obstet Gynaecol 2007; 27: 730–731. [DOI] [PubMed] [Google Scholar]
- 22.Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest 2006; 29: 298–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for High aldosterone, hypertension and adrenal adenoma in a 36-year-old pregnant patient: Is this primary aldosteronism? by Amanda J Berberich, Deborah Penava, Dongmei Sun, Arlene MacDougall, Andrea Lum and Stan Van Uum in Obstetric Medicine