Abstract
Objective: To review the clinical effects of nebulized heparin and N-acetylcysteine (NAC) in patients with smoke inhalation injury (IHI) and provide recommendations for use. Data Sources: A search of PubMed, MEDLINE, and Scopus databases was completed from database inception through April 15, 2020, using terms: heparin, acetylcysteine, smoke inhalation injury, and burn injury. Study Selection and Data Extraction: All studies pertaining to efficacy and safety of nebulized heparin and/or NAC for IHI in adult patients were evaluated. Reference lists were reviewed for additional publications. Nonhuman studies, non-English, and case report publications were excluded. Data Synthesis: Eight studies were included. Four demonstrated positive outcomes, 3 demonstrated no benefit or possible harm, and 1 assessed safety. Supporting trials treated patients within 48 hours of injury with 10 000 units of nebulized heparin with NAC for 7 days or until extubation. Two trials with negative findings treated patients within 72 hours, or unspecified, with 5000 units of nebulized heparin with NAC for 7 days, while the third used 25 000 units within 36 hours but was grossly underpowered for analysis. Clinical findings include reduced duration of mechanical ventilation and improved lung function with possible increase risk of pneumonia and no evidence of increased bleeding risk. Conclusions: Nebulized heparin may improve oxygenation and reduce duration of mechanical ventilation in IHI. If nebulized heparin is used, 10 000 units every 4 hours alternating with NAC and albuterol at 4-hour intervals is recommended. Sterile technique should be emphasized. Monitoring for bronchospasm or new-onset pneumonia should be considered.
Keywords: heparin, acetylcysteine, smoke inhalation injury, burn injury
Introduction
Burn and fire-related inhalation injury (IHI) carry a significant burden of morbidity and mortality. Over one million burns occur annually in the United States and up to 20% present with IHI.1 Smoke IHI independently increases mortality by 25% to 65% and has been shown to substantially increase complications such as pneumonia, prolonged mechanical ventilation, hyperinflation, and acute respiratory distress syndrome (ARDS).2-5 Although thermal injury can occur through steam inhalation or prolonged heat exposure, the majority of IHI is attributed to chemical injury through inhalation of carbonaceous compounds and combustion by-products including nitrogen and sulfur aldehydes and oxides.4,6-8 Inhalation of carbon monoxide and cyanide can also worsen outcomes, but this occurs by a systemic mechanism rather than localized injury to the lungs.7
Smoke IHI may be suspected in patients with facial burns, singed nasal hairs, carbonaceous sputum, or a presenting clinical scenario describing smoke exposure in a closed space.6,8,9 Rales, rhonchi, wheezes, and hypoxia are infrequently observed on physical examination.6 Exclusion of an IHI diagnosis by chest radiographs is discouraged as changes on imaging are seldom present until secondary complications occur (ie, pneumonia or ARDS).5,6,9 Airway mucosa can be assessed with chest computed topography, but some prefer fiber-optic bronchoscopy for diagnosis. If, however, fiber-optic bronchoscopy is performed within the first 48 hours, it may result in a false negative or underestimation of disease severity as damage to the parenchymal tissue has not fully developed.4,7,10 Xenon scanning has been used to evaluate parenchymal damage in research, though is cost prohibitive and not common in practice.4,6
IHI can be classified based on anatomical location and severity. Classification based on anatomy include upper airway (supraglottic), lower airway (subglottic), or lung parenchyma.4 Upper airway injury is usually due to direct thermal injury, whereas lower airway and parenchymal injuries are associated with chemical irritation or steam injury.4,6 Severity of IHI is influenced by the smoke composition and duration of smoke and heat exposure.11 The Abbreviated Injury Score (AIS) for IHI is a severity grading scale classified by bronchoscopic examination scores ranging from grade 0 (absence of injury) to grade 4 (massive injury with evidence of mucosal sloughing, necrosis, or endoluminal obliteration).8,9,11 Higher grades of severity (grades 2, 3, and 4) have been correlated with increased mortality risk.8,9,11 Although frequently used in the literature for severity classification, this severity scale is not standard of care in clinical practice.
Smoke IHI occurs in 4 physiologic phases: exudation, degeneration, proliferation, and reparation.1,12 The first 3 phases involve obstructive cast formation, airway edema, and airway narrowing.1,12 The exudative phase is characterized by an increase in microvascular permeability caused by neuropeptide release, which leads to inflammation, plasma extravasation, and pulmonary edema.4,12 The degenerative phase is defined by neutrophil-mediated epithelial damage, migration of exudate into the airways, and formation of airway casts and fibrin clots.1,13 Accumulation of these casts may increase the risk of pneumonia and induce atelectasis or barotrauma.4,6 The proliferative phase is marked by further parenchymal damage as impaired mucociliary function and surfactant inactivation produces an aggregation of mucus, which contributes to airway narrowing and further ventilation perfusion mismatching.3,6-8 Narrowing of the airways leads to air trapping and alveolar hyperinflation, causing direct alveolar damage.3 Finally, the reparative phase describes patient recovery. Tissue that has sustained mild injury can return to baseline, but severe tissue injury can be complicated by prolonged or permanent intra-alveolar fibrosis.14
Nonpharmacologic Treatment
The cornerstones of nonpharmacologic treatment for IHI are pulmonary clearance techniques and mechanical ventilation optimization. Therapeutic coughing, either reflexive or voluntary, promotes airway clearance of mucus and fibrin casts and should be encouraged every 1 to 2 hours.6 This technique can be limited by pain and medications that suppress the cough reflex such as opioids, H1 antagonists, and GABAa agonists.15 Chest physiotherapy, including percussive therapy and vibrations on exhalation, increases bronchial drainage.6 Care should be taken to avoid disturbance of skin graft sites with either method. Nasotracheal airway suctioning is largely effective at removing secretions but can induce bradycardia and hypoxia.6 Therapeutic bronchoscopy is also effective at removing secretions and foreign particles, thereby reducing atelectasis, and has been shown to reduce duration of mechanical ventilation in patients with IHI who subsequently developed pneumonia.8,9,16 Ventilator strategies such as high-frequency percussive ventilation, high-frequency oscillatory ventilation, and prone positioning have been shown to improve pulmonary clearance and oxygenation, though the optimal approach is currently unknown.4,8,9,17
Pharmacologic Treatment
The negative sequelae attributed to IHI originate from a multitude of pathophysiologic pathways, which present several possible targets for pharmacologic intervention. However, numerous interventions including corticosteroids, prophylactic antibiotics, exogenous surfactant replacement, nebulized nitric oxide, parenteral heparin, and allopurinol have not shown definitive improvement in clinical outcomes after IHI.18-22 Two promising treatment options are nebulized heparin and N-acetylcysteine (NAC), which may improve ventilation-perfusion matching and prevent atelectasis and barotrauma through the inhibition of obstructive cast formation.4
Nebulized NAC and β-Agonist Mechanism of Action
NAC acts as a mucolytic and free radical scavenger.12,23,24 Free sulfhydryl groups hydrolyze disulfide bonds between mucin monomers causing depolymerization of larger glycoprotein oligomers.23 The resulting mucus breakdown can slow or prevent the propagation of obstructive casts as they are primarily composed of mucus and extravascular plasma during the initial phase of formation.12 NAC has additionally been shown to decrease nitric oxide production in animal models.24 This diminishes the inflammatory response thereby preventing endothelial damage and vascular permeability.3 The reduction in nitric oxide also improves the mismatch in ventilation and perfusion by minimizing inappropriate perfusion of poorly ventilated lung tissue.3 One concern with NAC administration is airway irritation resulting in bronchospasm, especially in patients with asthma.4,25 This is of special consideration in patients with IHI as they are prone to bronchospasm secondary to airway debris and cytokines.5,18 Bronchodilators are, therefore, commonly coadministered.1,12,26-28 In addition to alleviation of bronchospasm, nebulized β2 adrenergic agonists such as epinephrine, albuterol, and levalbuterol may improve mucociliary clearance, pulmonary compliance, and ventilation-perfusion matching.4,29
Nebulized Heparin Mechanism of Action
Heparin potentiates antithrombin III activity, preventing activated factor X from converting prothrombin to thrombin and inhibiting the conversion of fibrinogen to fibrin.10,30 These mechanisms inhibit the cross-linking of fibrin, which is critical in the formation of obstructive casts. An additional benefit of reduced fibrin formation is the preservation of surfactant activities that are critical for preventing reduced lung compliance, atelectasis, and ultimately decreased functional residual capacity.1,31 Increased risk of bleeding is a primary concern with the administration of nebulized heparin, though limited safety data have been published. A study in healthy subjects sought to assess the systemic coagulopathy effects of inhaled heparin, escalating doses up to 32 000 units.32 Authors identified a dose-dependent effect on anti-factor Xa and activated partial thromboplastin time (aPTT), but changes were relatively small and deemed clinically insignificant.
These theoretical alterations in IHI pathophysiology have sparked numerous investigations to determine the utility of nebulized heparin and NAC in this vulnerable population. Therefore, the aim of this review is to evaluate existing literature regarding the efficacy and safety of nebulized heparin and NAC therapy in patients with IHI and provide recommendations for their use in clinical practice.
Methods
A search of the PubMed, MEDLINE, and Scopus databases from inception through April 15, 2020, was completed utilizing the following terms: heparin, acetylcysteine, smoke inhalation injury, and burn injury. All abstracts and titles were screened to identify studies pertaining to the efficacy, safety, or pharmacology of nebulized heparin and/or NAC in patients with IHI. Case reports and studies including an exclusively pediatric population were excluded. Nonhuman studies and non-English language publications were excluded. Reference lists were reviewed to identify additional relevant publications. All identified articles in which nebulized heparin and/or NAC was used for IHI were reviewed by the authors (MJT, CVM, MKP) for possible inclusion. Data abstracted included patient population included, intervention(s) provided (including dose, frequency, timing, and duration), mortality, hospital and intensive care unit (ICU) length of stay (or ICU-free days), duration of mechanical ventilation (or ventilator-free days), pulmonary function markers, and any safety endpoints reported (eg, bleeding, coagulation laboratory values, pneumonia).
Results
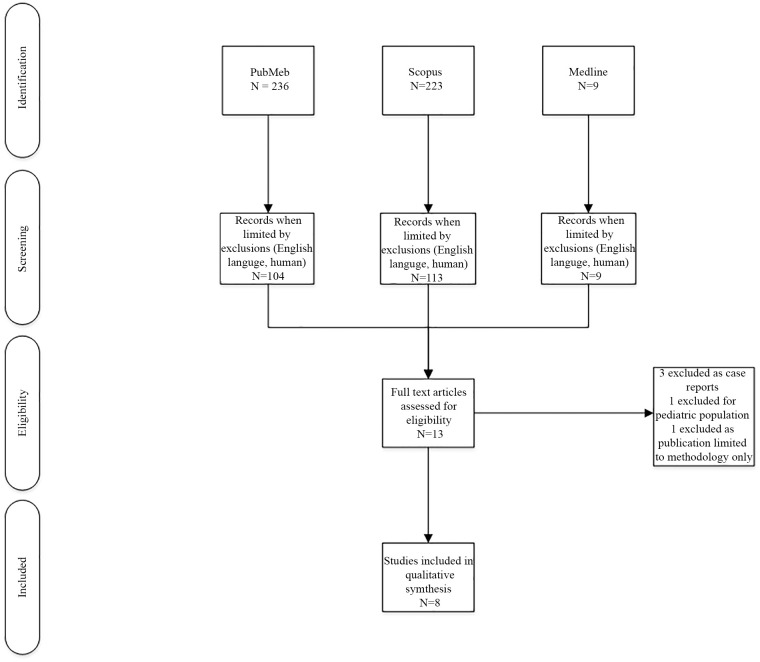
After application of exclusion criteria, 13 articles were reviewed by the authors for inclusion. Three articles were excluded as they were case reports, 1 was excluded based on a pediatric population, and 1 was excluded as the publication was limited to study protocol description, and no results were reported (Figure 1). One study included both pediatric and adult patients in their retrospective evaluation; however, the decision was made to include the article as pediatric patients comprised only 16% of the study population.12 A total of 8 studies remained for inclusion in the qualitative review (Figure 1). The majority of these studies were retrospective and observational (6 studies),1,12,26-28,33 with only 2 prospective, randomized controlled trials.34,35 One of the retrospective, observational studies reported only safety data.33 No additional studies were identified through the review of reference lists. Study design and interventions are summarized in Table 1. Efficacy and safety outcomes reported are summarized in Table 2.
Figure 1.
Flow diagram for study inclusion.
Table 1.
Study Design and Treatment Interventions of Nebulized Heparin Studies.
| Holt et al12 | Miller et al1 | Yip et al33 | Elsharnouby et al34 | Kashefi et al28 | McIntire et al26 | McGinn et al27 | Glas et al35 | |
|---|---|---|---|---|---|---|---|---|
| Design | Single-center, retrospective cohort | Single-center, retrospective, matched cohort based on APACHE-III, LIS | Single-center, retrospective cohort | Single-center, randomized, double-blind | Single-center, retrospective, matched cohort based on gender, burn severity, age | Single-center, retrospective, matched cohort based on %TBSA burned, age | Single-center, retrospective, cohort | Multicenter, randomized, double-blind, placebo-controlled |
| Population | • Adult and pediatric patients • IHI based on ABA/TRACS • Admitted 1999-2005 |
• Adults • Bronchoscopy-confirmed IHI • Mechanically ventilated • Admitted over a 5-year period |
• Adults • Bronchoscopy-confirmed IHI • Mechanically ventilated • Admitted 2006-2009 (treatment) or 2001-2005 (control) |
• Adults • Bronchoscopy-confirmed IHI • Mechanically ventilated |
• Adults • Suspected or bronchoscopy confirmed IHI • Admitted January 2011 to July 2012 (treatment) or 2006-2010 (control) |
• Adults • Bronchoscopy-confirmed IHI • Mechanically ventilated >24 hours • Admitted June 2014 to February 2016 (treatment) or January 2000 to February 2016 (control) |
• Adults • Suspected IHI • Mechanically ventilated >24 hours • Admitted between August 2014 and March 2016 |
• Adults • Bronchoscopy-confirmed IHI • Mechanically ventilated for <24 hours prior to randomization |
| Treatment group | N = 62 | N = 16 | N = 52 | N = 15 | N = 20 | N = 36 | N = 22 | N = 7 |
| Treatment timing and duration | Within 72 hours of injury; 7 days | Within 48 hours of injury; 7 days | No time limit; 7 days or at discretion of physician | Within 24 hours of injury; duration not reported | No time limit; 7 days | Within 48 of injury; 7 days or until extubation | On admission; 5 days or until extubation | Within 36 of injury; 14 days or until extubation |
| Heparin dosing | 5000 units every 4 hours | 10 000 units every 4 hours | 5000 units every 4 hours | 10 000 units every 4 hours | 5000 units every 4 hours | 10 000 units every 4 hours | 5000 units every 4 hours | 25 000 units every 4 hours |
| Mucolytic and β-agonist therapy | NAC 20% 3 mL + albuterol every 4 hours | NAC 20% 3 mL + albuterol every 4 hours | NAC 20% 3 mL + salbutamol every 4 hours | NAC 20% 3 mL + salbutamol every 4 hours | NAC 20% 3 mL + albuterol every 4 hours | NAC 20% 3 mL or 4% sodium bicarbonate 3 mL + albuterol every 4 hours | NAC (dose not reported) + albuterol; frequency not reported | No routine use of mucolytics; allowed only if problematic viscous mucus |
| Comparator group | N = 88 | N = 14 | N = 11 | N = 14 | N = 20 | N = 36 | N = 26 | N = 6 |
| Comparator interventions | No intervention β-agonists allowed |
No intervention Albuterol every 4 hours as needed |
No intervention Supportive care |
Heparin 5000 units every 4 hours + NAC 20% 3 mL + salbutamol every 4 hours | No intervention Albuterol or NAC as needed at the discretion of physician |
No intervention | No intervention Albuterol ± ipratropium at the discretion of the physician |
Placebo (0.9% sodium chloride) every 4 hours |
Abbreviations: ABA/TRACS, American Burn Association/Trauma Registry for the American College of Surgeons; APACHE, Acute Physiology, Age, Chronic Health Evaluation; IHI, smoke inhalation injury; LIS, lung injury score; NAC, N-acetylcysteine, TBSA, total body surface area.
Table 2.
Efficacy and Safety Outcomes of Nebulized Heparin for Smoke Inhalation Injury.
| Holt et al12 | Miller et al1 | Yip et al33 | Elsharnouby et al34 | Kashefi et al28 | McIntire et al26 | McGinn et al27 | Glas et al35 | |
|---|---|---|---|---|---|---|---|---|
| Efficacy outcomes | ||||||||
| Mortality | No difference, 24% vs 20% | Lower mortality in treatment group, 6% vs 43%, RR = 0.0055 (95% CI = 0.0314-0.02004) | No difference, 36.5% vs 54.5%, P = .32 | No difference, 4% vs 3%, P = .6 | No difference, 30% vs 25%, P = .72 | No difference in 28-day mortality, 2.8% vs 2.8%, P = 1 | No difference, 22.7% vs 23.1%, P = .99 | — |
| Hospital LOS (days) | No difference, 31 vs 31.9 | — | — | — | No difference, 15.3 vs 16.3, P = .8 | No difference, 17 vs 22, P = .195 | No difference, 9.0 vs 18.5, P = .093 | — |
| ICU LOS (days) | — | — | No difference, 6 vs 7, P = .86 | No difference, 19 vs 25, P = .17 | — | — | Shorter in treatment group, 5.5 vs 13, P = .033 | No difference in ICU-free days and alive at day 90, 71 vs 49, P = .73 |
| Duration of mechanical ventilation (days) | No difference, 18.2 vs 17.2, P = .76 | — | No difference, 5 vs 5, P = .63 | Shorter for treatment group, 11 vs 19, P = .037 | No difference, 8.5 vs 8.8, P = .9 | No difference, 7.0 vs 14.5, P = .60 Shorter duration in treatment group when patients who died or discharged on the ventilator excluded, 7 vs 14.5, P = .044 |
Shorter for treatment group, 3.0 vs 6.5, P = .022 Heparin associated with duration of mechanical ventilation in multivariable linear regression model (P = .039) |
No difference in ventilator-free days and alive at day 28, 16 vs 20, P = .62 |
| Pulmonary function | No difference in markers of oxygenation | Lower LIS, days 2-7 Lower lung resistance, days 2 and 4 Lower average hypoxemia score in first 7 days |
— | Lower LIS on days 5-7 | — | — | — | — |
| Safety outcomes | ||||||||
| Pneumonia | No difference, 63% vs 50%, P = .12 | — | No difference, 17.3% vs 18.2% | No difference, 27% vs 43%, P = .4 | Increased in treatment group, 45% vs 11%, P = .03 | No difference in VAP, 63.9% vs 72.2% | Increased in treatment group 18% vs 0%, P = .0376 | 28.5% vs 16.7%, No statistical analysis performed |
| Bleeding | — | — | No difference in PT or aPTT No difference in platelet trends |
No significant effect on coagulation parameters or platelet count No significant blood staining of bronchial secretions |
— | No difference in major bleeding: 5.6% vs 11.1%, P = .394 No difference in minor bleeding: 58.3% vs 52.8%, P = .635 |
— | No bleeds in either group One patient per group required blood transfusion for severe hemorrhage 7 and 13 days after last nebulization One patient in heparin group with aPTT >150 seconds aPTTs similar between groups; during nebulization: 36 vs 31 seconds After nebulization period: 33 vs 40 seconds |
| Other | No difference in unplanned reintubation, 14% vs 8%, |P = .20 | — | No difference in incidence of sepsis, 38.5% vs 63.6%, P = .18 No difference in incidence of ARDS, 23.1% vs 36.4%, P = .45 |
— | No difference in incidence of sepsis, 40% vs 33%, P = .7 No difference in incidence of ARDS, 15% vs 10%, P = 1 No documented complications directly attributed to administration of the protocol |
— | — | No HIT in either group One patient in the placebo group with ARDS Two patients in heparin group with ventilatory problems (high airway pressures) Feasibility: 127 of 429 heparin nebulizations withheld |
Abbreviations: aPTT, activated partial thromboplastin time; ARDS, acute respiratory distress syndrome; CI, confidence interval; HIT, heparin-induced thrombocytopenia; ICU, intensive care unit; LIS, lung injury score; LOS, length of stay; PT, prothrombin time; RR, relative risk; VAP, ventilator-associated pneumonia.
Efficacy of Nebulized Heparin/NAC
The first of 5 retrospective studies evaluating the efficacy of nebulized heparin, NAC, and albuterol in humans was performed by Holt and colleagues in 2008.12 This was a large retrospective study of both pediatric and adult patients with IHI between 1999 and 2005 using the American Burn Association and Trauma Registry for the American College of Surgeons database.12 The diagnosis of IHI was made by bronchoscopy, elevated carboxyhemoglobin, or clinical suspicion based on mechanism of injury; grade of injury was not reported. Initiation of an institutional protocol was based on physician discretion. The protocol utilized nebulized heparin 5000 units/mL, 3 mL of 20% NAC solution, and 3 mL of 0.083% albuterol every 4 hours for the first 7 days post-admission or until extubation. Patients who received the protocol (n = 62) were compared with a control group who did not receive NAC or nebulized heparin (n = 88). Mean percent total body surface area (TBSA) burned was similar between protocol and control groups (27% vs 31.6%, P = .29), although no other severity of illness scores were reported. No significant differences were detected for length of stay, mortality, ventilator days, unplanned reintubation, or PaO2:FiO2 ratios. Twenty-five pediatric patients were included in this study, and although the authors did not specify the group these patients were assigned to, they mentioned there was no difference in the pediatric subgroup analysis.
In contrast, Miller and colleagues found positive outcomes associated with a higher dose of nebulized heparin (10 000 units) in a retrospective study.1 The primary objective of this study was to determine if the combination therapy (nebulized heparin, NAC, and albuterol) reduced 28-day mortality or lung injury scores (LIS). The authors did not indicate a primary outcome for the analysis. Patients with bronchoscopy-confirmed IHI admitted within 48 hours of injury were treated with a nebulized heparin protocol (n = 16) and compared with historical controls (n = 14) over a 5-year period. The protocol arm received 10 000 units of nebulized heparin sulfate in 3 mL of normal saline every 4 hours alternating with 3 mL of 20% nebulized NAC and 0.5 mL of albuterol every 4 hours for 7 days, beginning the day of admission. The historical control was allowed to receive 0.5 mL nebulized albuterol every 4 hours as needed. Groups were matched based on Acute Physiology, Age, Chronic Health Evaluation (APACHE)-III score, and similar mechanical ventilation strategies (tidal volume 5-8 mL/kg, plateau pressure ≤20 cm H2O) were utilized in both arms. Baseline severity of illness and LIS were similar between groups; however, the control group had a greater percent TBSA burned compared with the protocol group (44% vs 23%).36 The protocol group experienced significantly lower mean LIS scores on days 2 to 7, with improved lung compliance and reduced hypoxemia as prominent contributors to this finding. The protocol arm was associated with an absolute risk reduction of 0.366 for 28 day mortality and a number needed to treat of 2.73. This study was limited, however, by a lack of statistical analysis regarding the possible impact of differing TBSA between groups on clinical outcomes.
A study by Kashefi and colleagues also failed to detect a significant clinical benefit from the administration of nebulized heparin and NAC in a pre-/post-protocol implementation study.28 Patients treated with IHI with an institutional protocol (n = 20) were matched 1:1 to historical controls based on sex, percent TBSA burned, and age (n = 20). Mechanical ventilation was not required for inclusion, and the number of individuals requiring mechanical ventilation at any given time was not reported. The protocol group received nebulized heparin 5000 units/3 mL every 4 hours alternating with 3 mL of 20% NAC and 2.5 mg of albuterol every 4 hours for the first 7 days after admission. Time from injury to initiation of the protocol was not reported. The historical control group received as needed NAC and albuterol at the discretion of the physician. The primary outcome was duration of mechanical ventilation; however, the authors did not describe how they accounted for patients who never required mechanical ventilation. The groups were well matched for age, sex, and burn size, and the majority of patients had an LIS of 0 to 1. However, more patients in the protocol group presented with grade 1 IHI (79% vs 47%, P = .01). No difference in mean duration of mechanical ventilation was detected between protocol and historical controls (8.5 vs 8.9 days, P = .9). No significant differences in mortality, length of stay, or incidence of ARDS were found.
A retrospective, case-control study (HIHI study) evaluated a similar treatment strategy utilizing a higher dose of nebulized heparin (10 000 units every 4 hours) in a pre-/post-protocol implementation study. Protocol patients (n = 36) were matched to historical controls (n = 36) based on percent TBSA burned and age.26 The protocol consisted of nebulized heparin 10 000 units every 4 hours alternating with a mucolytic (NAC or 4% sodium bicarbonate) and albuterol therapy every 4 hours for 7 days or until extubation. Use of the protocol was at the discretion of the physician. Patients were similar at baseline, with the exception of higher smoking incidence in the protocol group (63.9% vs 38.9%, P = .034). The primary outcome of duration of mechanical ventilation was not statistically different between the protocol and control groups (median 7 vs 14.5 days, P = .06). However, after excluding 5 patients who died or were discharged on the ventilator, 2 in the protocol group (1 death, 1 discharged on ventilator) and 3 in the control group (1 death, 2 discharged on ventilator), the protocol group had a shorter duration of mechanical ventilation (median 7 vs 14.5 days, P = .044). In addition, the protocol group had more ventilator-free days than the control group (median 21 vs 13.5 days, P = .031). The authors did not report how ventilator-free days were determined for patients who died before day 28 or for patients who were discharged on the ventilator. The small sample size may have contributed to the lack of a statistically significant difference in the primary outcome, as they lacked power to detect a difference. In addition, the exclusion of patients who either died or were discharged on mechanical ventilation appears to be a post hoc analysis, which was not indicated by the authors. These findings were potentially confounded further by an increased incidence of repeat bronchoscopy in the protocol group, which may have included therapeutic bronchoscopy (72.2% vs 50%, P = .053). As the use of nebulized heparin protocol was at the discretion of the treating physician, 42 patients did not receive treatment after protocol implementation, indicating a risk for treatment bias in the protocol group.
McGinn and colleagues performed a retrospective, observational, cohort study comparing a nebulized heparin, NAC, and albuterol protocol (n = 22) to albuterol ± ipratropium only (n = 26).27 The protocol was initiated on admission at the discretion of the provider and was continued for 5 days or until extubation and consisted of nebulized heparin 5000 units every 4 hours with NAC and albuterol, though administration details were not provided for these agents. Protocol patients had a significantly shorter median duration of mechanical ventilation (3 vs 6.5 days, P = .022) and ICU length of stay (5.5 vs 13 days, P = .033), though individuals treated with the protocol had smaller burns (median percent TBSA burned 5.25% vs 29%, P = .009). Multivariable linear regression analysis controlling for age, percent TBSA burned, and AIS inhalation grade suggested that the protocol was independently associated with reduced duration of mechanical ventilation. No significant differences in hospital length of stay or mortality were observed.
Two prospective studies examining the efficacy of nebulized heparin have been published. Elsharnouby and colleagues performed a prospective, double-blind, randomized controlled trial comparing 2 nebulized heparin dosing strategies: 5000 (n = 14) and 10 000 units/mL (n = 15) administered every 4 hours in patients requiring mechanical ventilation for bronchoscopy-confirmed IHI.34 Patients with percent TBSA burn greater than 50% were excluded. Patients in both groups received 3 mL of 20% NAC and salbutamol every 4 hours, alternating with nebulized heparin. All patients received fiber-optic bronchoscopy on day 1 and were resuscitated using the Parkland formula. Patient demographics, percent TBSA burned, APACHE II score, and bronchoscopy scores were similar at baseline. The LIS was not different between groups on days 1 to 4 but was significantly improved in the high-dose heparin group on days 5 to 7. The mean duration of mechanical ventilation was reduced in the high-dose heparin group (11 vs 19 days, P = .037). No differences in ICU length of stay or mortality were observed between groups.
The final prospective evaluation of nebulized heparin was published by Glas and colleagues in 2020.35 However, this multicenter, randomized, double-blind, placebo-controlled trial was stopped early due to slow recruitment and high cost of blinded placebo medication. Patients were randomized to receive either 25 000 units nebulized heparin every 4 hours or placebo within 36 hours of IHI, and therapy was continued for 14 days or until extubation or death. Routine use of mucolytics use discouraged, and use was only allowed at the discretion of the attending provider when viscous mucus was problematic. A total of 13 patients (n = 7 heparin vs n = 6 placebo) were enrolled prior to termination. No differences in ICU-free days and alive at day 28 or ventilator-free days and alive at day 28 were observed, although this study was grossly underpowered.
Safety of Nebulized Heparin/NAC
Yip and colleagues retrospectively compared patients who received nebulized heparin 5000 units, 3 mL of 20% NAC, and salbutamol 5 mg every 4 hours (n = 52) with historical controls (n = 11).33 Patients in the control group had higher percent TBSA burned compared with the protocol group (51% vs 20%, P = .09), although this difference was not statistically significant. Prothrombin time, aPTT, and platelet values were similar between both the groups over 7 days. No differences in bloody secretions from the endotracheal tube, wound bleeding, hemoserous exudates, or bleeding from other sites were observed. Combined rates of bleeding from all sites were high in both the groups; however, the protocol arm had a lower numerical rate (71% vs 81%, P = .87). Limitations include the lack of a standardized definition for bleeding, reliance on documentation of bleeding within the medical record, and that blood transfusion requirements and changes in hemoglobin were not reported.
The HIHI study discussed above also evaluated bleeding as a secondary endpoint and categorized bleeding events as major and minor.26 Major bleeds either prompted discontinuation of heparin or were documented as clinically significant, while minor bleeds required both documented hemoptysis and a positive gastroccult or hemoccult. No difference was detected between protocol (n = 36) and control groups (n = 36) in the incidence of major (5.6% vs 11.1%, P = .394) or minor bleeding (58.3% vs 52.8%, P = .635). One patient receiving nebulized heparin had documented alveolar hemorrhage, which prompted discontinuation. Of note, the HIHI study is the first to report the incidence of bleeding in patients receiving 10 000 units of nebulized heparin per dose.26 Kashefi and colleagues attributed no episodes of bleeding to heparin, though bleeding was not defined.28 In the study by Glas and colleagues, no severe bleeding was reported, and there was no difference in rates of transfusions between the groups. There was 1 patient in the treatment arm of this study who had an aPTT greater than 150 seconds; however, this normalized after discontinuation of the heparin nebulization.35 Most studies evaluating coagulopathy associated with nebulized heparin excluded patients with hypersensitivity to heparin and those with bleeding disorders or platelet counts less than 50 000/µL.1,26,33 Nebulized heparin has not been shown to increase the rates of clinically significant bleeding at doses of up to 25 000 units in patients not meeting the aforementioned exclusion criteria. Furthermore, no studies in patients with IHI have documented any cases of heparin-induced thrombocytopenia.
Increased risk of pneumonia has also been reported with nebulized heparin for IHI. Six of the 7 included studies comparing nebulized heparin with a control group reported rates of pneumonia, with 2 reporting statistically higher rates of pneumonia with nebulized heparin.12,26-28,33,35 Holt and colleagues were the first to report a higher incidence of pneumonia in patients receiving heparin, although this finding was not statistically significant (63% vs 50%, P = .12).12 Yip and colleagues reported no difference in the incidence of pneumonia (17.3% vs 18.2%).33 Kashefi and colleagues were the first to find a significantly higher incidence of pneumonia among patients treated with nebulized heparin (45% vs 11%, P = .03) when compared with historical controls.28 In contrast, the HIHI study did not observe any differences in rates of ventilator-associated pneumonia between protocol and control groups (63.9% vs 72.2%, P = .448).26 McGinn and colleagues also found a higher incidence of pneumonia among those who received the heparin protocol (18% vs 0%, P = .04).27 Glas and colleagues found the incidence of pneumonia to be similar between heparin and placebo groups (2 vs 1 patient); however, these findings are limited by the premature cessation of the trial due to low enrollment and were not statistically analyzed.35
Discussion
The use of nebulized heparin and NAC for IHI remains controversial. To date, of the 7 trials reporting efficacy endpoints, there are 4 trials favoring and 3 trials opposing routine use. Trials supporting the use of nebulized heparin and NAC for IHI started therapy early within 24 to 48 hours of injury with 10 000 units of nebulized heparin every 4 hours for 5 to 7 days or until extubation. In contrast, 2 of the 3 trials opposing routine use initiated therapy later, within 72 hours of injury (or did not specify timeframe), with 5000 units of nebulized heparin every 4 hours for 7 days. Early initiation (within 48 hours) with higher dosing (10 000 units) may therefore be most effective.12,28,34 One study utilizing a higher dose of heparin (25 000 units), within 36 hours of IHI, was terminated early and was, therefore, not powered to detect a difference in the primary efficacy endpoint.35 Clinical outcomes vary, and only reduction in mechanical ventilation duration and improvements in LIS have been replicated in 2 retrospective studies.1,27 Elsharnouby and colleagues saw improvement in LIS over days 5 through 7, while Miller and colleagues reported an improvement over days 2 through 7.1,34 No study reported changes in LIS beyond 7 days, making it difficult to determine clinical benefit beyond this time; however, nebulized heparin and NAC were continued until extubation in the studies that reported benefit in other clinical outcomes. These promising outcomes are tempered, however, by the potential for an increased risk of pneumonia associated with nebulized heparin and NAC therapy. It had been historically hypothesized that reduced rates of pneumonia could be anticipated in those treated with nebulized heparin due to the reduction of cast formation and improved lung surfactant activity; however, this correlation has not been demonstrated, and in fact, a higher risk of pneumonia has been seen.32 It is possible that these findings are due to frequent disruptions in the ventilator circuit for the administration of medications, which may compromise sterility. In addition, inconsistency may exist in the preparation of nebulized medications under sterile conditions.28
Many limitations such as small sample size, retrospective design, provider bias, and uneven distribution of confounding variables exist in the majority of the discussed studies making definitive conclusions elusive. There is also limited information reported on ventilator management strategies, which could affect clinical outcomes such as ventilator days and markers of pulmonary function (eg, oxygenation, LIS). In addition, it is difficult to distinguish the benefit of NAC versus nebulized heparin due to the comparator group lacking both interventions in multiple of the studies discussed.1,12,27
Consideration should be given to the acquisition or creation of these inhaled products and potential barriers to their administration. Heparin for nebulization does not currently exist as a manufactured product, but it is available as 5000 unit/mL and 10 000 unit/mL products intended for intravenous injection.37 Most studies that provided information on the concentration of heparin utilized, compounded either 5000 units/3 mL or 10 000 units/3 mL products diluted in normal saline, with one study using a 25 000 unit/5 mL dose.1,28,34,35 The authors suggest that nebulized heparin be compounded in polypropylene containers, stored for up to 10 days if kept refrigerated (2-8°C) or for 4 days at room (20-25°C) temperature.38,39 NAC for nebulization exists as a manufactured product in a 20% solution, and all studies that give a detailed description of their inhaled NAC product utilize 3 mL of this solution (600 mg) per dose.1,12,28,33,34,40
Barriers specific to nebulized heparin therapy include training of respiratory personnel, the creation of a protocol for administration, and development of an order within the electronic medical record system. In one study, 24% of scheduled doses were omitted for all patients enrolled, which highlights the difficulty in feasibility of every 4-hour nebulization in patients with IHI.35 Safety concerns exist regarding this preparation as it could be incorrectly administered (intravenous or subcutaneous rather than inhaled) or compounded. Strict adherence to administration protocols and aseptic technique should be employed to mitigate these risks. Safety concerns have also been raised regarding the risk of localized and systemic bleeding. Only one study, to date, has reported bleed incidence as a primary outcome; however, a standard definition of major and minor bleeding was not employed to assess these outcomes.33 This makes it difficult to compare with other studies where bleeding is reported without the use of nebulized heparin.
Barriers specific to inhaled NAC include bronchospasm and medication interactions. Inhaled NAC should be administered with β-agonists, and patients should be closely monitored for wheezing with lung auscultation after administration to detect bronchospasm.25 The 2 β-agonists described in previous studies, albuterol and salbutamol, are commercially available and typically administered with NAC at doses of 2.5 mg and 5 mg, respectively.12,28,33 NAC 20% solution has undergone in vitro compatibility testing with a myriad of other potentially nebulized medications and is notably compatible with nitrous oxide and colistimethate sodium (must be used immediately after mixing), but not amphotericin.40 A summary of other known interacting medications can be found in the NAC package insert.40
Avoidance of concomitant administration is considered best practice for scenarios when compatibility data are unavailable. With continuously nebulized medications such as epoprostenol, this practice may not be feasible. Although no in vitro compatibility data exist for inhaled heparin, NAC, and epoprostenol, a single case utilizing these therapies reported no apparent drug incompatibilities with concomitant administration.41
Though the combination of nebulized heparin and NAC has been almost exclusively evaluated in patients requiring invasive mechanical ventilation, it may be reasonable to administer these medications to patients only requiring noninvasive mechanical ventilation support. This can be accomplished by connecting a nebulizer to a single-limb circuit close to the patient and, when possible, utilizing low inspiratory pressures and prolonged inspiratory time to aid in aerosol deposition.42 Such an administration method could also be employed for most situations when interruption of the ventilator circuit is not feasible.
Conclusion
Current evidence suggests that nebulized heparin with NAC may have benefits for patients with IHI requiring intubation. Based on the available literature, if nebulized heparin is used for IHI, we recommend nebulized heparin 10 000 units every 4 hours alternating with nebulized NAC and albuterol at opposite 4-hour intervals (patient would receive treatment with either regimen every 2 hours). Initiation should occur within 48 hours of injury to maximize benefit. It is reasonable to continue treatment for 7 days or until liberation from mechanical ventilation. Administration to patients not requiring mechanical ventilation may also be feasible to prevent escalation of respiratory support. Standardized compounding and sterile preparation and administration processes should be developed to safeguard against contamination and inappropriate administration. Routine monitoring of coagulation tests is unlikely to be valuable, but clinicians should be observant to signs of bronchospasm or new-onset pneumonia.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Megan K. Phelps  https://orcid.org/0000-0002-4673-2392
https://orcid.org/0000-0002-4673-2392
Logan M. Olson  https://orcid.org/0000-0002-1727-6244
https://orcid.org/0000-0002-1727-6244
Megan A. Van Berkel Patel  https://orcid.org/0000-0003-2282-3043
https://orcid.org/0000-0003-2282-3043
Claire V. Murphy  https://orcid.org/0000-0001-5339-8735
https://orcid.org/0000-0001-5339-8735
References
- 1. Miller AC, Rivero A, Ziad S, Smith DJ, Elamin EM. Influence of nebulized unfractionated heparin and N-acetylcysteine in acute lung injury after smoke inhalation injury. J Burn Care Res. 2009;30:249-256. doi: 10.1097/BCR.0b013e318198a268 [DOI] [PubMed] [Google Scholar]
- 2. Shirani KZ, Pruitt BA, Mason AD. The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205:82-87. doi: 10.1097/00000658-198701000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller AC, Elamin EM, Suffredini AF. Inhaled anticoagulation regimens for the treatment of smoke inhalation–associated acute lung injury. Crit Care Med. 2014;42:413-419. doi: 10.1097/CCM.0b013e3182a645e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Enkhbaatar P, Pruitt BA, Jr, Suman O, et al. Pathophysiology, research challenges, and clinical management of smoke inhalation injury. Lancet. 2016;388:1437-1446. doi: 10.1016/S0140-6736(16)31458-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark WR. Smoke inhalation: diagnosis and treatment. World J Surg. 1992;16:24-29. doi: 10.1007/bf02067110 [DOI] [PubMed] [Google Scholar]
- 6. Mlcak RP, Suman OE, Herndon DN. Respiratory management of inhalation injury. Burns. 2007;33:2-13. doi: 10.1016/j.burns.2006.07.007 [DOI] [PubMed] [Google Scholar]
- 7. Bittner EA, Shank E, Woodson L, Martyn JAJ. Acute and perioperative care of the burn-injured patient. Anesthesiology. 2015;122:448-464. doi: 10.1097/ALN.0000000000000559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dries DJ, Endorf FW. Inhalation injury: epidemiology, pathology, treatment strategies. Scand J Trauma Resusc Emerg Med. 2013;21:31. doi: 10.1186/1757-7241-21-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Deutsch CJ, Tan A, Smailes S, Dziewulski P. The diagnosis and management of inhalation injury: An evidence based approach. Burns. 2018;44:1040-1051. doi: 10.1016/j.burns.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 10. Olson ST, Björk I. Predominant contribution of surface approximation to the mechanism of heparin acceleration of the antithrombin-thrombin reaction. Elucidation from salt concentration effects. J Biol Chem. 1991;266:6353-6364. [PubMed] [Google Scholar]
- 11. Endorf FW, Gamelli RL. Inhalation injury, pulmonary perturbations, and fluid resuscitation. J Burn Care Res. 2007;28:80-83. doi: 10.1097/BCR.0B013E31802C889F [DOI] [PubMed] [Google Scholar]
- 12. Holt J, Saffle JR, Morris SE, Cochran A. Use of inhaled heparin/N-acetylcystine in inhalation injury: does it help? J Burn Care Res. 2008;29:192-195. doi: 10.1097/BCR.0b013e31815f596b [DOI] [PubMed] [Google Scholar]
- 13. Glas GJ, Muller J, Binnekade JM, et al. HEPBURN—investigating the efficacy and safety of nebulized heparin versus placebo in burn patients with inhalation trauma: study protocol for a multi-center randomized controlled trial. Trials. 2014;15:91. doi: 10.1186/1745-6215-15-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fitzpatrick JC, Cloffi WG. Inhalation injury. Trauma Q. 1994;11:114-126. [Google Scholar]
- 15. Chung KF, Widdicombe J. Pharmacology and Therapeutics of Cough. Vol 187 Springer; 2009. doi: 10.1007/978-3-540-79842-2 [DOI] [PubMed] [Google Scholar]
- 16. Carr JA, Phillips BD, Bowling WM. The utility of bronchoscopy after inhalation injury complicated by pneumonia in burn patients: results from the National Burn Repository. J Burn Care Res. 2009;30:967-974. doi: 10.1097/BCR.0b013e3181bfb77b [DOI] [PubMed] [Google Scholar]
- 17. Sheridan RL. Fire-related inhalation injury. N Engl J Med. 2016;375:464-469. doi: 10.1056/NEJMra1601128 [DOI] [PubMed] [Google Scholar]
- 18. Pruitt BA, Cioffi WG. Diagnosis and treatment of smoke inhalation. J Intensive Care Med. 1995;10:117-127. doi: 10.1177/088506669501000303 [DOI] [PubMed] [Google Scholar]
- 19. Rehberg S, Maybauer MO, Enkhbaatar P, Maybauer DM, Yamamoto Y, Traber DL. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3:283-297. doi: 10.1586/ERS.09.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murakami K, Enkhbaatar P, Shimoda K, et al. High-dose heparin fails to improve acute lung injury following smoke inhalation in sheep. Clin Sci (Lond). 2003;104:349-356. [DOI] [PubMed] [Google Scholar]
- 21. Cox CS, Jr, Zwischenberger JB, Traber DL, Traber LD, Haque AK, Herndon DN. Heparin improves oxygenation and minimizes barotrauma after severe smoke inhalation in an ovine model. Surg Gynecol Obstet. 1993;176:339-349. [PubMed] [Google Scholar]
- 22. Ahn SY, Sugi K, Talke P, et al. Effects of allopurinol on smoke inhalation in the ovine model. J Appl Physiol (1985). 1990;68:228-234. doi: 10.1152/jappl.1990.68.1.228 [DOI] [PubMed] [Google Scholar]
- 23. Sadowska AM. N-acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2012;6:127-135. doi: 10.1177/1753465812437563 [DOI] [PubMed] [Google Scholar]
- 24. Yeh STY, Guo HR, Su YS, et al. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223:181-190. doi: 10.1016/j.tox.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 25. Bernstein IL, Ausdenmoore RW. Iatrogenic bronchospasm occurring during clinical trials of a new mucolytic agent, acetylcysteine. Dis Chest. 1964;46:469-473. doi: 10.1378/chest.46.4.469 [DOI] [PubMed] [Google Scholar]
- 26. McIntire AM, Harris SA, Whitten JA, et al. Outcomes following the use of nebulized heparin for inhalation injury (HIHI study). J Burn Care Res. 2017;38:45-52. doi: 10.1097/BCR.0000000000000439 [DOI] [PubMed] [Google Scholar]
- 27. McGinn KA, Weigartz K, Lintner A, Scalese MJ, Kahn SA. Nebulized heparin with N-acetylcysteine and albuterol reduces duration of mechanical ventilation in patients with inhalation injury. J Pharm Pract. 2019;32:163-166. doi: 10.1177/0897190017747143 [DOI] [PubMed] [Google Scholar]
- 28. Kashefi NS, Nathan JI, Dissanaike S. Does a nebulized heparin/N-acetylcysteine protocol improve outcomes in adult smoke inhalation? Plast Reconstr Surg Glob Open. 2014;2:e165. doi: 10.1097/GOX.0000000000000121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Walker PF, Buehner MF, Wood LA, et al. Diagnosis and management of inhalation injury: an updated review. Crit Care. 2015;19:351. doi: 10.1186/s13054-015-1077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albers GW, Dalen JE, Laupacis A, Manning WJ, Petersen P, Singer DE. Antithrombotic therapy in atrial fibrillation. Chest. 2001;119(1 suppl):194S-206S. doi: 10.1378/chest.119.1_suppl.194S [DOI] [PubMed] [Google Scholar]
- 31. Holm BA, Matalon S. Role of pulmonary surfactant in the development and treatment of adult respiratory distress syndrome. Anesth Analg. 1989;69:805-818. [PubMed] [Google Scholar]
- 32. Bendstrup KE, Gram J, Jensen JI. Effect of inhaled heparin on lung function and coagulation in healthy volunteers. Eur Respir J. 2002;19:606-610. doi: 10.1183/09031936.02.00105202 [DOI] [PubMed] [Google Scholar]
- 33. Yip LY, Lim YF, Chan HN. Safety and potential anticoagulant effects of nebulised heparin in burns patients with inhalational injury at Singapore General Hospital Burns Centre. Burns. 2011;37:1154-1160. doi: 10.1016/j.burns.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 34. Elsharnouby NM, Eid HEA, Elezz NFA, Aboelatta YA. Heparin/N-acetylcysteine: an adjuvant in the management of burn inhalation injury: a study of different doses. J Crit Care. 2014;29:182.e1-e4. doi: 10.1016/j.jcrc.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 35. Glas GJ, Horn J, Binnekade JM, et al. Nebulized heparin in burn patients with inhalation trauma—safety and feasibility. J Clin Med. 2020;9:894. doi: 10.3390/jcm9040894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720-723. doi: 10.1164/ajrccm/138.3.720 [DOI] [PubMed] [Google Scholar]
- 37. Heparin Sodium [package insert]. New York, NY: Pfizer, Inc; 2016. [Google Scholar]
- 38. Tunbridge LJ, Lloyd JV, Penhall RK, Wise AL, Maloney T. Stability of diluted heparin sodium stored in plastic syringes. Am J Hosp Pharm. 1981;38:1001-1004. [PubMed] [Google Scholar]
- 39. United States Pharmacopeial Convention. General Chapter <797> Pharmaceutical Compounding—Sterile Preparations. Accessed July 30, 2019 https://www.usp.org/compounding/general-chapter-797
- 40. Acetylcysteine [package insert]. Shirley, NY: American Regent, Inc; 2014. [Google Scholar]
- 41. Dube KM, Ditch KL, Hills L. Use of nebulized heparin, nebulized N-acetylcysteine, and nebulized epoprostenol in a patient with smoke inhalational injury and acute respiratory distress syndrome. J Pharm Pract. 2017;30:663-667. doi: 10.1177/0897190016663071 [DOI] [PubMed] [Google Scholar]
- 42. Rzepka-Wrona P, Skoczynski S, Wrona D, Barczyk A. Inhalation techniques used in patients with respiratory failure treated with noninvasive mechanical ventilation. Can Respir J. 2018;2018:8959370. doi: 10.1155/2018/8959370 [DOI] [PMC free article] [PubMed] [Google Scholar]