Abstract
Study Design:
Narrative literature review.
Objective:
To review and present details on the occipitocervical fixation (OCF) technique as well as considerations for planning the procedure.
Methods:
We present the surgical technique of OCF in a step-by-step didactic and practical manner with surgical tips and tricks, including C1 and C2 screw fixation techniques. Additionally, we discuss complications, the extension of fusion, types of OCF, and how to avoid common side effects associated with OCF.
Results:
The complex and mobile anatomy of the craniocervical junction, when requiring fixation and fusion, warrants rigid instrumentation that can be achieve using a modern screw-plate-rod construct. Indications for OCF are craniocervical instability, and atlantoaxial instability when selective atlantoaxial fusion is not feasible. OCF generally involves occiput-C2 fusion. C1 fixation is generally unnecessary, since it increases the surgical time and is associated with the risk of vascular complications. Selective occiput-C2 fusion is recommended when there is no need for including the cervical subaxial region (eg, when stenosis or fractures coexist in the subaxial spine), and good fixation is achieved at C2. Most instrumentation systems now have occipital plates that are not pre-integrated to rods, making fixation much simpler. Surgical steps, from position to wound closure, are presented in detail, with pearls for practice and discussion of cervical alignment.
Conclusions:
OCF is a challenging procedure, with potential risk of severe adverse effects. Understanding the surgical indications, as well as the nuances of the surgical technique, is required to improve patient outcomes and avoid complications.
Keywords: occipitocervical, craniocervical, fusion, surgical technique
Introduction
Occipitocervical fixation (OCF), also known as craniocervical fixation, is a procedure used for treating instability between the skull and the cervical spine.1-5 This instability may be secondary to conditions such as spinal trauma (eg, atlanto-occipital dislocation and occipital condyle fractures), congenital diseases (eg, basilar invagination [BI], os odontoideum, Down’s and Morquio’s syndrome), tumor destruction leading to instability (eg, clival chordomas or spinal metastasis), iatrogenic injuries (eg, after a far lateral neurosurgical approach), infectious diseases (eg, tuberculosis), and inflammatory arthritides (eg. rheumatoid or psoriatic arthritis).1-5
The highly mobile relationship between the occipital condyles and the atlanto-axial joints is associated with a complex set of force vectors between the skull and the spine that makes stabilization particularly challenging (there are 4 synovial joints in the occipito-atlantoaxial complex that are considered when undergoing stabilization).6,7 Nearly half of the cervical flexion/extension, and cervical rotation, are generated from the craniocervical junction.8-10 These highly mobile segments are due to the rounded architecture of the occipital condyle joints (allowing movements of flexion-extension in the sagittal plane); additionally, the rotational capacity of this region results from the architecture of the dens of the atlanto-axial complex.8-10 All of these joints are highly stabilized by a strong ligamentous complex that preserves stability and, importantly, protects the spine cord integrity; these ligaments include the transverse, alar, and apical ligaments, among others.8-10
Before the development of modern instrumentation specifically designed for craniocervical stabilization, OCF was limited to in situ fusions, wiring, and cable techniques. Autologous bone graft was generally harvested from the iliac crest or the ribs, and it was placed between the squamous part of the occipital bone and the posterior cervical spine for an in situ fusion, with or without supplemental wiring or cables. These surgeries required a prolonged postoperative period of immobilization with a cervical collar or a halo vest and had high rate of pseudoarthrosis.1,11 Today, OCF are based on screws, plates, and rods, with rigid constructs that offer immediate stability. Additionally, recent designs allow for intraoperative repositioning of the craniocervical junction that aid in realignment, which may be useful in cases where the joints are dislocated or indirect ventral decompression is necessary; this can occur in basilar invagination cases with atlanto-axial dislocation.4,12 Distraction techniques can be used to anteriorly translate the cervical spine relative to the foramen magnum; this can be achieved by distracting the rod (using a rod-holder) and the plate away from each other. This operative technique can similarly be used in a vertical direction to translate the spine when the indication calls for pulling the dens out of the foramen magnum (pushing the occipital plate away from the cervical spine).4,12,13
Basic Surgical Anatomy of the Occipitocervical Junction
Before inserting occipital screws, proper knowledge of the patient-specific occipital bone anatomy and the location of the internal dural venous sinus is necessary. The external occipital protuberance (EOP) is a midline boney prominence on the outer surface of the occipital bone; this is the point of attachment of the nuchal ligament.14 The superior nuchal line is the ridge that extends laterally from the EOP, in both directions, to the lateral angle of the occipital bone.14 The trapezius, splenius capitis and sternocleidomastoid muscles all attach at the superior nuchal line.14 Of note, the inion is the most prominent projection of the EOP. It is within the inner portion of the skull, at the level of the EOP, that the torcula lies.14 The torcula is also known as the confluence of the dural venous sinuses: the superior sagittal sinus, the straight sinus, and the occipital sinus.14 Inappropriate screw insertion causing injury or occlusion to the torcula, or any of these sinuses (in the setting of sinus dominance), has the potential to cause a rare but catastrophic vascular injury, and even death. This can be avoided by placing the plate just below the EOP (to avoid a prominent plate) and using unicortical screws.
The squamous portion of occipital bone is often thickest at the midline (especially at the EOP), and it decreases in thickness from medial to lateral; it also decreases in thickness inferiorly toward the foramen magnum.15 As a general rule, occipital screw fixation is performed just below the EOP to keep it from being prominent. In cases where a large suboccipital decompression has been performed, and there is not sufficient occipital bone below the transverse sinus for plate fixation, instrumentation at the EOP may be required. In such cases, it is preferable to shave down part of the inion to partially recess the plate under the bone to prevent it from being too prominent under the skin. It is always preferred to place occipital screws near the midline given that the bone is thickest in this region. It is critical to understand the patient specific anatomy during the planning stages of the operation, given that this anatomy could be highly variable. In patients with occipital dysplasia, such as those with BI and condylar hypoplasia, a vestigial occipital bone may preclude screw insertion below the superior nuchal line. Figure 1 demonstrates key features of the occipital bone anatomy that are often referenced when discussing occipital fixation.
Figure 1.
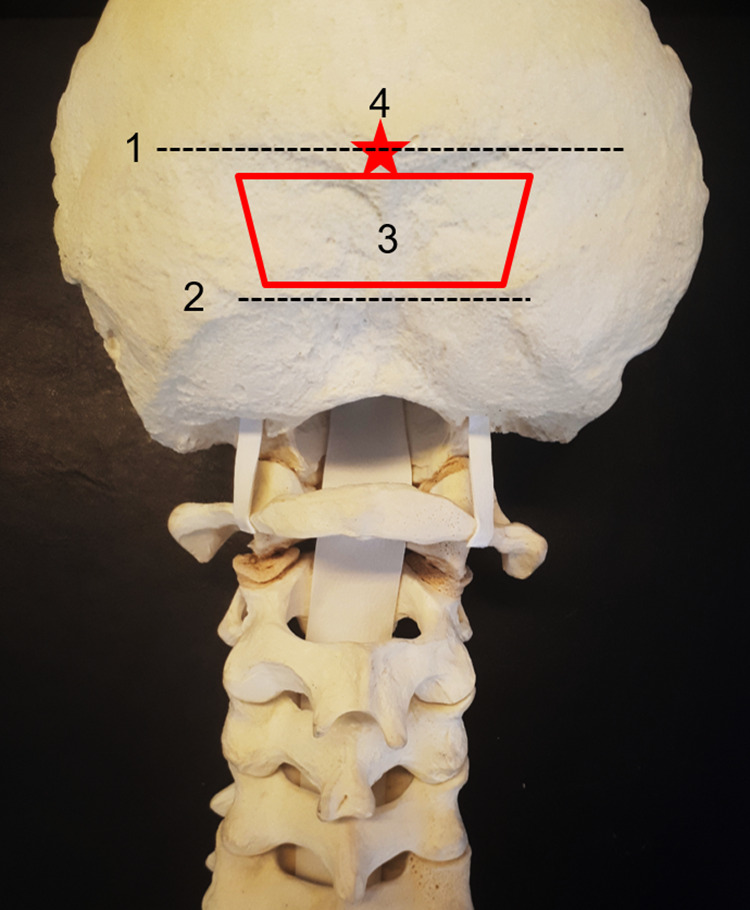
(1) Superior nuchal line—insertion of the trapezius, splenius capitis, and sternocleidomastoid muscle. The transverse sinus and the torcula are internal to this line. (2) Inferior nuchal line—insertion of the obliquus capitis superior, rectus capitis posterior major, and rectus capitis posterior minor muscle. We generally perform, when necessary, decompression below this area. (3) The “red trapezium” corresponds to the area of preference to insert the occipital screws—just below the superior nuchal line to avoid a prominent plate, in the midline, where the occipital squama is thicker. (4) The “red star” is the external occipital protuberance, which corresponds to the confluence of the dural sinuses (torcula).
Batista et al15 performed a study in 100 asymptomatic adult patients reporting on the occipital bone thickness variability. They reported that the EOP thickness ranged from 7.4 to 22.3 mm (mean of 14 mm); 1 cm below the EOP, the thickness ranged from 2.9 to 13.4 mm.15 Given this wide range of measurements in occipital bone thickness, it is critical for surgeons to be aware of the patient-specific anatomy in order to plan appropriate occipital screw fixation.
Preoperative imaging for the OC fusion patient should include a magnetic resonance imaging (MRI) to evaluate the underlying suboccipital neuroanatomy (vascular and neural elements), and a computed tomography (CT) scan of the occipital region, in addition to routine imaging of the cervical region. The occipital bone imaging with CT will provide insight on the thickness and morphology of the region for planned fixation. Additionally, an MR or CT angiogram may also be necessary in cases with potential vertebral artery anomalies, such as those with congenital craniocervical malformations or syndromic diseases, in order to avoid iatrogenic injuries.
Cervical Alignment in Occipito-Cervical Fusion
It is important to have a clear understanding of the sagittal parameters involved in cervical alignment prior to performing an OCF. The vast majority of cervical lordosis (about 77%) is located in the occiptal-C2 region.16 The assessment of this is made with the O-C2 angle—an angle measured between the McGregor’s line and the inferior end plate of C2.16,17 Additionally, the T1 slope correlates with the O-C7 angle (the angle created between the McGregor line and the inferior end plate of C7); thus, patients with an increased T1 slope will require higher O-C7 angles (increased cervical lordosis) to maintain horizontal gaze.16,17 After occipitocervical fusions (with the lower instrumented vertebrae being either at C2 or C3), Matsubayashi et al17 reported that there was an inverse correlation between the O-C2 angle, and the C2-7 angle after an OCF (O-C2 or O-C3); therefore, the high O-C2 angle, had a reciprocal decrease in the C2-7 angle, and vice versa. In other words, patients with O-C2 kyphosis would require a large amount of C2-7 lordosis as a compensatory alignment change to maintain horizontal gaze. Additionally, excessive lordosis at the craniovertebral junction can lead to reciprocal kyphotic changes in the subaxial cervical spine. On the other hand, the O-C7 angle is generally stable after OCF fusion since it is dependent on the T1 slope. Understanding these compensatory changes are important in order to avoid unnecessary complications. A description of these angles is shown in Figure 2.
Figure 2.
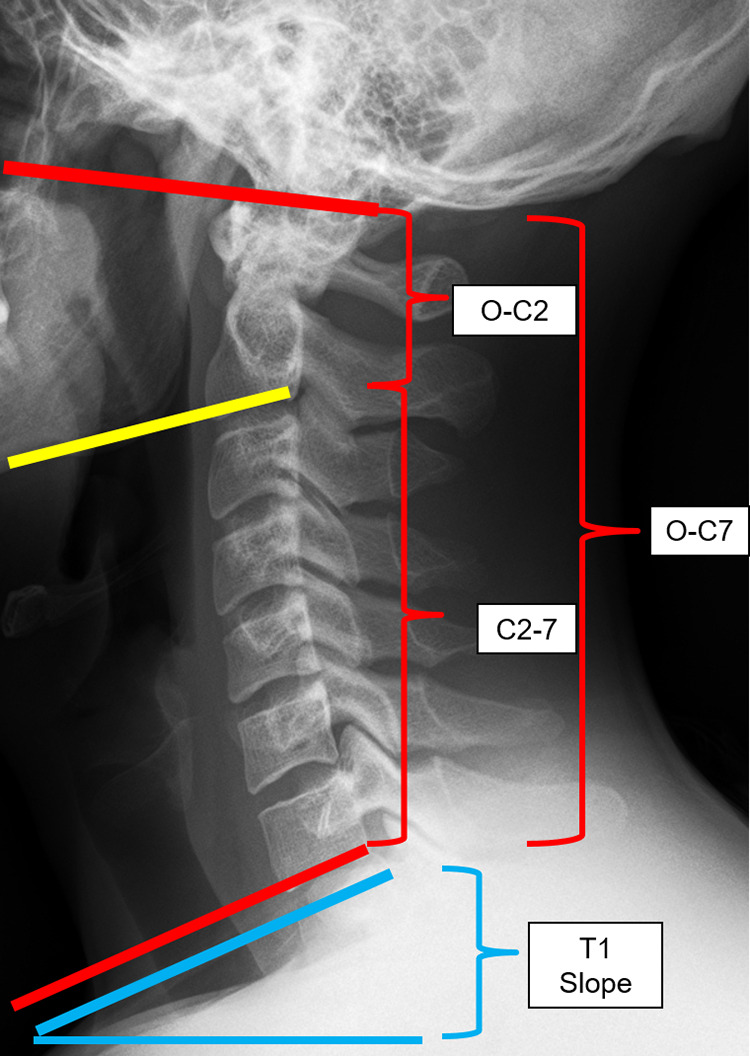
O-C2 angle—angle between the McGregor line and the inferior end plate of C2. O-C7 angle—angle between the McGregor line and the inferior end plate of C7 (O-C7 angle = O-C2 + C2-7 angle). C2-7 angle—angle between the inferior endplate of C2 and the inferior endplate of C7. T1 slope—the angle between the horizontal line and the superior endplate of C7. T1 slope has correlation with O-C7 angle (a high T1 slope requires high O-C7 angle) and O-C2 and C2-7 angles had inverse correlation to each other to maintain horizontal gaze.
Atlas Fixation in Occipitocervical Fusion
Hankinson et al18 performed a multi-institutional study evaluating the need for C1 fixation when performing pediatric OC fusions. They analyzed 77 cases of O-C2 instrumented fusions for atlantooccipital instability that were performed in 9 different hospitals, and they excluded cases with subaxial instability. They divided patients into 3 groups: Group 1—16 patients (20.8%) with C2 instrumentation, group 2—22 patients (28.6%) with C1 and C2 instrumentation but without transarticular screw placement, and group 3—39 patients (50.6%) with at least 1 transarticular screw. Groups were evaluated for rates of fusion and perioperative complications. There were no differences in the groups for fusion rates and complication rates were low. They concluded that there was not a distinct advantage of including C1 in occipitocervical fusions in children.
We believe that when good C2 fixation is obtained (C2 pedicle screws), C1 lateral mass fixation for OC arthrodesis may be unnecessary in routine cases. C1 lateral mass screws are relatively more demanding with an increased risk of vertebral artery injury, venous bleeding, and C2 neuropathy. However, if fixation at C1 is necessary or preferred, we recommend leaving the Atlas screws more proud than for C1-2 cases, as that will make it easier to engage the rod to the C1 screws.
Axis Fixation Versus Extended Fixation to the Subaxial Cervical Spine
The extension of the cervical spine fixation in OCF depends on factors that include associated subaxial cervical spine pathology (subaxial stenosis or highly unstable fractures with multilevel involvement), poor screw fixation at C2 (bone anomalies, bone fractures, etc), and poor bone quality requiring additional fixation. As a general rule, choosing C2 as the lower instrumented vertebrae is reasonable and is associated with high rates of fusion in the majority of the cases.3,18,19
Pan et al20 compared OCF in 2 different groups: those who had O-C2 fusion (short segment fusion: SSF), and occiput to subaxial spine fusion (multisegment fusion: MSF). The mean follow-up was 33.9 months; fusion rates and neurological improvement was not statistically different in both groups. The authors concluded that including subaxial fusion was unnecessary with adequate C2 fixation when treating occipitocervical instability. Biomechanical studies have also suggested no difference in stiffness of an OC construct that ends with good fixation at C2, versus one with additional subaxial instrumentation.20
Current Options of Occipital Fixation Systems: Condylar Screws, Plates Integrated to the Rods and Nonintegrated Occipital Plates
Although there are many different commercial systems for OCF, most modern systems have a skull plate that attaches to the rod. This is preferable to older systems where the plate was integrated into the distal end of the rod. Such integrated rod-plate systems required complex bends to fit perfectly and the skull sometimes was reduced to the rod, instead of placing the rod onto the reduced skull. Additionally, some of the manufactured integrated rod-plate systems have large plates that preclude the insertion of C1 lateral mass screws, and at times even make placement of C2 screws challenging; in some, the plate system extends down into the cervical spine making the connection to the heads of screws at C1 and C2 challenging). Finally, integrated rod-plate systems require paramedian occipital screws, where the bone is thinner than midline; in comparison to nonintegrated systems that can take advantage of fixation onto the midline occipital bone, allowing for improved occipital bone fixation.
Modern systems have also improved the rods used for OCF. Several systems have articulated rods that allow for minimal bends and adjustment of the occipitocervical angle after fixing the rod to the occipital plate and cervical screws. Other systems have prebent rods that make it easier to attach the skull to C2. Because titanium rods are notch sensitive, they should never be bent in more than one direction. If the bend has been too extreme, it should be discarded, since bending it back will likely make it fail in the early postoperative periods.
More recently, some authors have proposed the use of condylar screws. Uribe et al21 performed a biomechanical study comparing occipital plate, C1 lateral mass screws and C2 pars screws versus occipital condylar screws, C1 lateral mass screws and C2 pars screws. They reported that both configurations reduced the range of motion by 80%, compared with normal anatomy, and there was no statistical differences between the fixation techniques. Ahamadian et al22 reported the results of 12 patients with occipital condyle screws that were used as the primary cranial fixation point. After 6 months, all had achieved occipitocervical fusion without neural or vascular complications. Electromyographic monitoring of the hypoglossal nerve is recommended when performing occipital condyle screw fixation. Although this technique has been shown to provide adequate fixation, we believe it should be reserved for selected cases when the occipital bone has largely been removed or destroyed by tumor, and there is an inability to achieve fixation to the occiput.
Finally, for young children (generally younger than 6 years), the vertebral dimensions are too small and therefore preclude the use of occipital plates and cervical screws. In these cases, we recommend wiring and cables, use of rib allograft, and postoperative rigid cervical orthosis fixation.23 Figures 3 and 4 are illustrative cases of condylar screw fixation, integrated rod-plate systems, and occipital plate constructs.
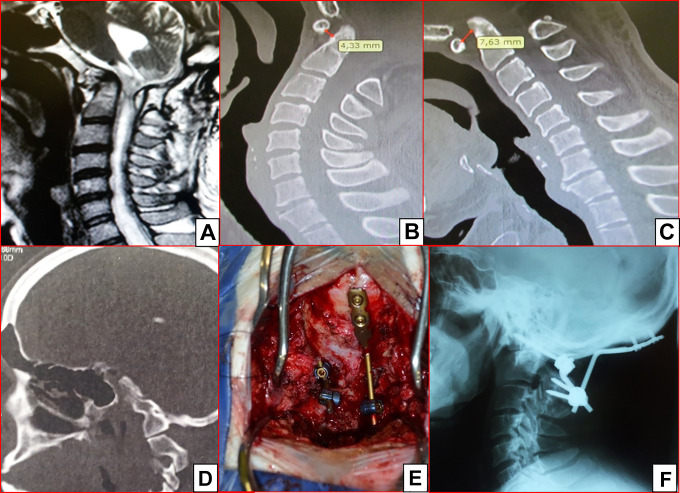
Figure 3.
This patient underwent a posterior fossa decompression some years ago. He developed new neurological worsening with progressive weakness in the 4 limbs and occipito-cervical pain. In (A), a sagittal T2 MRI with a syringomyelia and posterior fossa compression (a fibrous tissue was compressing the spinal cord at the level of C1). Sagittal CT scan in extension (B) and flexion (C), with the atlanto-dens interval increasing from 4.33 mm in extension to 7.63 mm in flexion, suggesting atlanto-axial instability. Also, there is occipital assimilation of the atlas at the condyle (D). Intraoperative picture of a condyle screw on the left side and a plate integrated to the rod with C2 laminar screws (E). Final lateral plain radiograph (F).
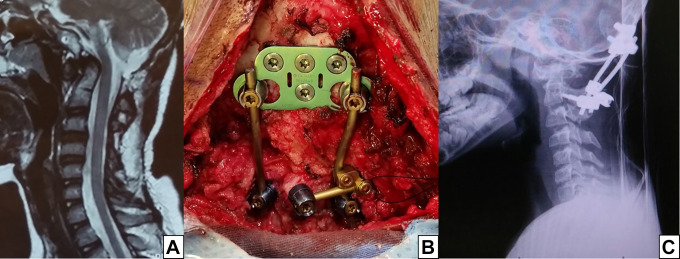
Figure 4.
This patient underwent a posterior fossa decompression and C1-2 wiring 3 years before being referred to our center. He developed severe pain when flexing the head, along with dysphagia. In (A), a sagittal T2 MRI showing the dens compressing the spinal cord and posterior fossa decompression. In (B), an occipital plate was affixed with 4 screws just below the superior nuchal line. On the axis, there were 3 screws: bilateral pars screws and a unilateral laminar screw. (C) Postoperative lateral plain radiograph (C).
Surgical Steps
Many patients who go on to need an OCF, often come into surgery using a rigid cervical brace. For patients with instability, intubation is commonly performed using fiberoptic guidance, in order to minimize neck extension or flexion. For those with high-risk instability, we prefer to obtain motor and somatosensory evoked potentials while they are in a neutral supine position, prior to positioning, to allow for baseline data prior to prone positioning.
Patients are positioned prone using either a fixed head holder or with tongs for continuous traction with the neck in the neutral position. Traction is contra-indicated when the etiology is a traumatic atlanto-occipital dislocation. When using traction, pay attention to minimize rotation, which if unrecognized, can go on to affect the final head position; the result of a misaligned neck positioned during surgery could result in a fusion with the head laterally rotated. Thus, checking the position is of paramount importance; the patient should be positioned so that they are looking directly at the floor. Excessive extension or flexion may result in swallowing problems; excessive flexion may result in a spinal deformity where patients compensate by hyperextending the spine or flexing at the knees in order to walk and look straight. We obtain a preoperative standing lateral radiograph with the head and neck in a comfortable position for swallowing and use this as a template to compare with an intraoperative lateral radiograph, in order to prevent misalignment.
The cranio-cervical area is shaved and cleaned with antiseptic agents, followed by proper draping. The incision is performed in the midline, and it is carried down along an avascular and amuscular plane, in the standard fashion. A subperiostal exposure of the occipital squama is performed with monopolar cautery, and minimal midline monopolar electrocautery is used on the axis. We avoid exposure overlying the levels not included in the fusion to preserve the posterior tension band.
In cases where decompression is necessary, we generally performed bone removal below the inferior nuchal line. The inferior nuchal line is located about 2 to 3 cm below the EOP, and it is the insertion of the obliquus capitis superior muscle, rectus capitis posterior major muscle and rectus capitis posterior minor muscle. The region between the superior and inferior nuchal line is sufficient to occipital plating in the vast majority of the cases. The occipital bone is carefully drilled to remove surface imperfections and allow for the plate to lie flat. Minor plate bending is also required in the majority of cases (slight curvature added to the plate). We then position the plate just below the inion. A matchstick burr is then used to create a pilot hole for the top midline screw. Then, we use a handheld drill to achieve unicortical screws into the EOP and bicortical screws lower, using a drill guide set initially to a depth of 4 to 6 mm, depending on the bone thickness on the preoperative CT and progressively increase the drill length by 2 mm to avoid drilling too deep. The depth achieved is measured according to the length of the hand drill. Because the EOP has the second strongest bone in the body (after one’s teeth), we rarely use bicortical screws into the inion and typically stop once the midline top screw hole is >16 mm. With at least three 10-mm screws, it is extremely rare for the screws to fail.
For patients with cranial settling or basilar invagination, distraction on the rods when connecting them from the occipital plate to the cervical screws could be used, but for atlanto-occipital dislocation, distraction is not recommended. Instead, when treating atlanto-occipital dislocation, vertex compression onto the rods may be required.
The rods should be placed without any tension; sometimes in situ benders are necessary to achieve proper occipitocervical adjustment. Proper decompression of the neural elements should be performed when necessary, based on preoperative MRI findings. After proper connection of the rods, bone graft is placed from the occipital squama to the cervical spine. Wound closure is performed in multiple layers.
C1 Lateral Mass Screw Fixation
The exposure of C1 involves a posterior arch subperiosteal dissection. The superior aspect of the C1 posterior arch is preserved and not exposed in order to avoid injury to the vertebral artery.24 The lamina and the spinous process of C2 are also exposed, as well as the inferior portion of the occipital squama, which will allow for lateral retraction of the soft tissues for a working corridor.24 The large venous plexus covering the region can easily be disrupted, resulting in a significant amount of bleeding that can obscure the working corridor.24 One strategy that we utilize is to expose the atlanto-axial ligament at the midline, and from this ligament expose from medial to lateral; when exposing, you are using the bipolar to coagulate the veins above the dura mater until you have reached the lateral mass of C1 bilaterally. Bipolar cauterization with loupes, or a microscope, allow for improved visualization of these delicate structures. As the exposure moves laterally, the C2 nerve root is encountered and visualized, and the surrounding soft tissues adjacent to the C2 nerve root is removed or retracted to allow exposure of the lateral mass. After dissection of the surrounding soft tissues, the C2 nerve root is mobilized inferiorly, and the medial aspect of the lateral mass is palpated. In some cases, such as in congenital craniovertebral anomalies with severe atlantoaxial subluxation, the lateral mass is anteriorly located and access may be difficult. In these cases, proximal coagulation and cutting the C2 nerve root may improve the visualization of the lateral mass. Huang et al25 reported that sacrifice of C2 nerve root carries a high risk of postoperative numbness, but preservation of the nerve can also result in neuralgia. Yeom et al26 performed a post hoc analysis of prospectively collected patient-derived outcome data to compare 24 consecutive patients who had bilateral C2 nerves transected versus 41 consecutive patients without transection. Seventeen percent of patients in the transected group had persistent neuralgic pain requiring medications at final follow-up (44-80 months) versus none in the patients without transection. Despite this article, the topic remains controversial with strong proponents for and against transection. Therefore, there is no consensus about preserving or cutting the C2 nerve root for C1 lateral mass fixation, and each case should be thoughtfully planned while also discussing the risks associated with the planned procedure with the patient.
Once the lateral mass exposure is complete, the entry point can be identified. The entry point is in the midportion of the lateral mass (often 4-6 mm lateral to the medial border of the lateral mass), and the trajectory is generally of about 15° medially oriented (the preoperative CT can allow for visualization of atlas and a planned trajectory). We generally use the junction of the lateral mass and the inferior aspect of the C1 arch to be sure we are not violating the C1-2 joint. Lateral fluoroscopic guidance can be utilized at this point to align the screw trajectory in the direction of the anterior tubercle of C1. Matching the angulation of the C1-2 joints can also provide an accurate trajectory. Short bicortical purchase is usually safe at this level but a longer screw can violate the internal carotid artery.27 For bicortical purchase, a hand drill is used until some resistance is achieved. Commonly, the screw length is chosen about 1 to 2 mm longer than the measured length of the depth achieved with the hand drill to allow for bicortical purchase. To avoid excess bleeding, hemostatic agents are used as well as bipolar cauterization. Additionally, raising the head position, in a reverse Trendelenburg position, will allow for decreased venous pressure. Finally, we use a screw size of about 10 to 12 mm longer than the measured depth in the lateral mass, so that the head of the C1 screw is in line with C2 screw, which will facilitate rod placement (otherwise the head of the C1 lateral mass screw will be deep adjacent to C1-2 joint).
C1 Posterior Arch Screws
In some patients, the posterior arch of C1 is large enough that one can start the screw on the arch of C1 instead of the lateral mass.28 This has the advantage of avoiding lateral mass dissection, with the attendant blood loss and risk of C2 nerve irritation. In addition, it allows for a longer screw with better bony purchase. The risk of this screw is cranial violation of the arch, resulting in vertebral artery injury. To help avoid this, we routinely obtain CT images with 3-dimensional reconstructed views to determine if the arch is thick enough to accept a 3.5- or 4-mm screw. If the arch appears to be thick enough to accept a screw, intraoperatively, we carefully dissect the artery off of the cranial surface of the C1 posterior arch and place a small paddy between the arch and the artery to protect it. Then a starting hole is made on the arch with a burr until it is deeper than the artery, followed by a drill to complete the hole. Care must be taken to identify a ponticulus posticus, which gives the false impression of a thick posterior arch, but in fact is a thin bridge of bone covering the vertebral artery (see Figure 5).29 Even when the arch is not very thick, it is possible to place screws starting from the dorsal arch.28 However, if the arch is split, it may compromise the C1-2 fusion, unless an intra-articular fusion is performed.
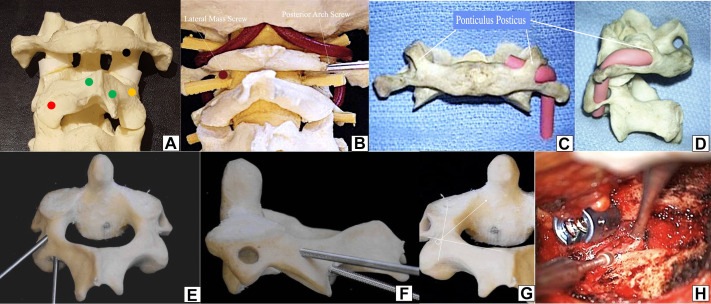
Figure 5.
(A) Sawbone model with a posterior view of C1-2-3. Black dot—entry point of C1 lateral mass screw—midportion of the lateral mass just below the inferior posterior C1 arch. Red dot—entry point of C2 pars screws and C2 transarticular screw—about 3 to 5 mm above the medial junction of C2-C3 facet joint, the medial position should not violate the medial portion of the spinal canal. Orange dot—entry point of C2 pedicle screw—transition between the lateral portion of the lateral mass of C2, a little inferior (2 mm) to the transition of the lateral mass-pars. Green dots—upper and inferior junction of the lamina-spinous process, which are entry points for laminar screws (crossing screws). (B) Sawbone model comparing the lateral mass screw entry point (left) with the entry point of posterior arch screw (right). (C) and (D) Posterior and lateral view of the ponticulis posticus—a thin thick bone covering the vertebral artery. (E) Posterior and (F) lateral comparative perspective of a C2 pedicle screw with a C2 pars screw—the entry point of the pedicle is above and lateral to the pars screw. (G) Illustrative direction of the C2 pedicle screw, with an intraoperative (H) picture of the entry point.
C2 Screw Fixation Techniques
The most commonly used fixation techniques for C2 screws are pars, transarticular C1-2, pedicle, and laminar screws. In Table 1, we described the main techniques as well as specific considerations for each one, as it follows:
Table 1.
Surgical Techniques and Special Considerations for C2 Screw Fixation.
| Type of Axis Fixation | Surgical Techniques30 | Special Considerations—Pearls for Practice30 |
|---|---|---|
| Pars screws | Entry point: 3-5 mm above the C2-3 junction, medial position without violating the spinal canal. Trajectory: Parallel trajectory to the pars, may be guided by lateral fluoroscopy |
Average screw length is 12-18 mm. Evaluate the foramen transversarium: Generally, just anterior to the posterior vertebral line—more vertical trajectory increases the risk of vertebral artery injury |
| Transarticular C1-2 screws | Same entry point as a pars screw with greater craniocaudal direction. Palpating the medial and superior border of C2 pedicle to avoid breaking the cortical bone. Removal of the C1-2 articular cartilage—improves fusion rates. Trajectory: Guided by lateral fluoroscopy, through the pars into the lateral mass of C1 |
Average screw length is 20-30 mm. Computed tomography (CT) scan: The entire pars should be visualized on a single image slice of a parasagittal CT scan (average CT scan cut is 3 mm)—failure to identify a medial located vertebral artery may result in vascular injury C1-2 joints must be aligned on fluoroscopy Axial support in the vertex may be useful to avoid C1-2 joints distraction Excessive angulation may cause: (1) cranial—may violate the condyle-C1 joint, (2) caudal—inadequate fixation of C1, (3) medial—spinal cord injury, (4) lateral—vertebral artery injury |
| Pedicle screws | Entry point: The entry point in the cranio-caudal direction is an imaginary line extending the rostral border of the C2 lamina. Medial-laterally, it is 2 mm lateral to the midpoint of the pars Trajectory: Palpating the medial and rostral portion of the pedicle for guidance |
True pedicle screws—cross obliquely into the pedicle toward the body of the axis CT scan: The entire pars should be visualized on a single image slice of a parasagittal CT scan (average CT scan cut is 3 mm)—failure to identify a medial located vertebral artery may result in vascular injury Up to 20% of the patients do not have pedicles large enough to allow for pedicle screw cannulation |
| Laminar | Entry point: Junction of the spinous process and lamina. The trajectory is directly into the lamina but avoiding a ventral breech (dorsal perforation is possible and may also improve purchase with a bicortical screw) | Free hand technique. May require of head screw extension to capture the rod Contraindicated when there is a hemilaminectomy of C2 Alternative to pedicle and transarticular screws given 20% of the patients cannot have safely placed pedicle screws With a dissector it is possible to palpate the inner portion of the lamina, which may help in selecting trajectory |
Figure 5 illustrates the entry points of the most common techniques utilized for C1 and C2 screw fixation techniques.
Adverse Events
Numerous complications can occur during and after an OCF. Before surgery, especially in patients with additional craniofacial abnormalities, intubation, and airway-related complications can occur. Preoperative evaluation of the airway and fiberoptic assistance may be necessary.2,3 In select cases, a tracheostomy may be necessary, such as in some children with mucopolysaccharidosis or other syndromic diseases. Neurological injury is avoided using proper stabilization before position, with rigid craniofixation during surgery and neurophysiological monitoring (before positioned and then, just after head fixation before starting the procedure itself).2,3 In our experience, some severe neurologically compromised patients with craniovertebral junction instability may require head position adjustment prior to fixation before beginning the procedure due to decreased neuromonitoring data (motor and sensory evoked potentials). Given the likelihood of this occurring, we strongly advise obtaining baseline neurophysiological data before positioning and prior to starting the procedure.
Venous bleeding can be minimized by elevating the patient body (torso and head) to about 30° of reverse Trendelenburg, and additionally having careful hemostasis in the venous plexus when C1 lateral mass fixation is required. Arterial injury can be avoided with proper preoperative radiologic evaluation, careful evaluation for arterial anomalies, and good surgical strategy. Preoperative evaluation using an MRI and/or a CT scan is necessary. In cases where an MRI does not allow sufficient resolution or detail to evaluate the vertebral artery, a CT angiogram should be obtained at that time. Examples where this could be encountered are in complex congenital craniovertebral junction anomalies or in patients with anomalous position of the foramen transversarium. If a vertebral artery injury is encountered, direct repair is the preferred treatment modality, although it may be not possible in the majority of the cases.31 If the injury is addressed using an endovascular approach, the vertebral artery can be sacrificed if it is nondominant and sufficient collateral circulation is observed (generally the smaller diameter artery in preoperative exams). Immediately following a vertebral artery injury, the surgeon should remain calm and control the hemorrhage with direct pressure.31 Additionally, a vascular surgeon should be consulted (an endovascular procedure may avoid ligation and reestablish arterial flow). If hemostasis is difficult to achieve, applying direct pressure with a small cottonoid in the foramen transversarium of C2 may help decreasing bleeding.
In some cases, dural injury may occur when placing the occipital screws. Generally, the leak will stop immediately after screw insertion. If a persistent leak continues after occipital screw placement, fibrin glue and a subarachnoid lumbar drain may be considered.
Implant failure generally occurs when the surgeon cannot achieve good screw purchase, or when there is an insufficient amount of bone graft. To avoid this, a careful preoperative bone anatomy evaluation is necessary, followed by a meticulous surgical technique, and placement of large screws (maximizing screw length). A large amount of bone graft is generally required for fusion, preferentially with autologous iliac or rib graft. Fixation of the patient with the neck hyperextended, will impact walking because it limits the patients ability to view the ground while walking; excessive flexion will result in compensatory torso bending backward in order to perform activities of daily living.3 Difficulty with swallowing will occur with both hyperextension and hyperflexion.
Finally, general complications such as posterior fossa hematoma, infection, thromboembolism, among others, may also occur. In the setting of any of these being suspected, close monitoring in an intensive care unit during the immediate post-operative period may be preferred.
Postoperative Use of a Cervical Orthosis
With higher fusion rates, modern hardware for OCF does not require routine use of a cervical orthosis. However, in complex cases, such as in patients with bone abnormalities, poor bone quality, or suboptimal screw purchase, a hard cervical collar or a halo may be advisable.32
Conclusions
OCF can sometimes be a challenging procedure, with the potential risk of severe complications. Understanding the surgical indications, as well as the details of the surgical techniques, is necessary to improve outcomes and avoid complications.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrei Fernandes Joaquim, MD, PhD  https://orcid.org/0000-0003-2645-0483
https://orcid.org/0000-0003-2645-0483
References
- 1. Newman P, Sweetnam R. Occipito-cervical fusion. An operative technique and its indications. J Bone Joint Surg Br. 1969;51:423–431. [PubMed] [Google Scholar]
- 2. Sonntag VK, Dickman CA. Craniocervical stabilization. Clin Neurosurg. 1993;40:243–272. [PubMed] [Google Scholar]
- 3. Lu DC, Roeser AC, Mummaneni VP, Mummaneni PV. Nuances of occipitocervical fixation. Neurosurgery. 2010;66(3 suppl):141–146. [DOI] [PubMed] [Google Scholar]
- 4. Joaquim AF, Tedeschi H, Chandra PS. Controversies in the surgical management of congenital craniocervical junction disorders—a critical review. Neurol India. 2018;66:1003–1015. [DOI] [PubMed] [Google Scholar]
- 5. Joaquim AF, Ghizoni E, Giacomini LA, Tedeschi H, Patel AA. Basilar invagination: surgical results. J Craniovertebr Junction Spine. 2014;5:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tubbs RS, Kelly DR, Humphrey ER, et al. The tectorial membrane: anatomical, biomechanical, and histological analysis. Clin Anat. 2007;20:382–386. [DOI] [PubMed] [Google Scholar]
- 7. Tubbs RS, Hallock JD, Radcliff V, et al. Ligaments of the craniocervical junction. J Neurosurg Spine. 2011;14:697–709. [DOI] [PubMed] [Google Scholar]
- 8. Dickman CA, Crawford NR, Brantley AGU, Sonntag VKH. Biomechanical effects of transoral odontoidectomy. Neurosurgery. 1992;36:1146–1153. [DOI] [PubMed] [Google Scholar]
- 9. Dickman CA, Locantro J, Fessler RG. The influence of transoral odontoid resection on stability of the craniovertebral junction. J Neurosurg. 1992;77:525–530. [DOI] [PubMed] [Google Scholar]
- 10. Dvorak J, Schneider E, Saldinger P, Rahn B. Biomechanics of the craniocervical region: the alar and transverse ligaments. J Orthop Res. 1988;6:452–461. [DOI] [PubMed] [Google Scholar]
- 11. Malcolm GP, Ransford AO, Crockard HA. Treatment of non-rheumatoid occipitocervical instability. Internal fixation with the Hartshill-Ransford loop. J Bone Joint Surg Br. 1994;76:357–366. [PubMed] [Google Scholar]
- 12. Jian FZ, Chen Z, Wrede KH, Samii M, Ling F. Direct posterior reduction and fixation for the treatment of basilar invagination with atlantoaxial dislocation. Neurosurgery. 2010;66:678–687. [DOI] [PubMed] [Google Scholar]
- 13. Joaquim AF. Treatment of craniocervical instability using a posterior-only approach [letter to the editor]. J Neurosurg Spine. 2015;22:334–335. [DOI] [PubMed] [Google Scholar]
- 14. Rhoton AL., Jr The foramen magnum. Neurosurgery. 2000;47(3 suppl):S155–S193. [DOI] [PubMed] [Google Scholar]
- 15. Batista UC, Joaquim AF, Fernandes YB, Mathias RN, Ghizoni E, Tedeschi H. Computed tomography evaluation of the normal craniocervical junction craniometry in 100 asymptomatic patients. Neurosurg Focus. 2015;38:E5. [DOI] [PubMed] [Google Scholar]
- 16. Ames CP, Blondel B, Scheer JK, et al. Cervical radiographical alignment: comprehensive assessment techniques and potential importance in cervical myelopathy. Spine (Phila Pa 1976). 2013;38(22 suppl 1):S149–S160. [DOI] [PubMed] [Google Scholar]
- 17. Matsubayashi Y, Shimizu T, Chikuda H, Takeshita K, Oshima Y, Tanaka S. Correlations of cervical sagittal alignment before and after occipitocervical fusion. Global Spine J. 2016;6:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hankinson TC, Avellino AM, Harter D, et al. Equivalence of fusion rates after rigid internal fixation of the occiput to C-2 with or without C-1 instrumentation. J Neurosurg Pediatr. 2010;5:380–384. [DOI] [PubMed] [Google Scholar]
- 19. Martin MD, Bruner HJ, Wolfla CE, Yoganandan N. Biomechanical implications of extending occipitocervical instrumentation to include the subaxial spine. Neurosurgery. 2010;66:1148–1152. [DOI] [PubMed] [Google Scholar]
- 20. Pan J, Huang D, Hao D, et al. Occipitocervical fusion: fix to C2 or C3? Clin Neurol Neurosurg. 2014;127:134–139. [DOI] [PubMed] [Google Scholar]
- 21. Uribe JS, Ramos E, Youssef AS, et al. Craniocervical fixation with occipital condyle screws: biomechanical analysis of a novel technique. Spine (Phila Pa 1976). 2010;35:931–938. [DOI] [PubMed] [Google Scholar]
- 22. Ahmadian A, Dakwar E, Vale FL, Uribe JS. Occipitocervical fusion via occipital condylar fixation: a clinical case series. J Spinal Disord Tech. 2014;27:232–236. [DOI] [PubMed] [Google Scholar]
- 23. Menezes AH. Craniocervical fusions in children. J Neurosurg Pediatr. 2012;9:573–585. [DOI] [PubMed] [Google Scholar]
- 24. Joaquim AF, Ghizoni E, Rubino PA, et al. Lateral mass screw fixation of the atlas: surgical technique and anatomy. World Neurosurg. 2010;74:359–362. [DOI] [PubMed] [Google Scholar]
- 25. Huang DG, Hao DJ, Li GL, Guo H, Zhang YC, He BR. C2 nerve dysfunction associated with C1 lateral mass screw fixation. Orthop Surg. 2014;6:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yeom JS, Buchowski JM, Kim HJ, Chang BS, Lee CK, Riew KD. Postoperative occipital neuralgia with and without C2 nerve root transection during atlantoaxial screw fixation: a post-hoc comparative outcome study of prospectively collected data. Spine J. 2013:13:786–795. [DOI] [PubMed] [Google Scholar]
- 27. Currier BL, Todd LT, Maus TP, Fisher DR, Yaszemski MJ. Anatomic relationship of the internal carotid artery to the C1 vertebra: a case report of cervical reconstruction for chordoma and pilot study to assess the risk of screw fixation of the atlas. Spine (Phila Pa 1976). 2003:28:E461–E467. [DOI] [PubMed] [Google Scholar]
- 28. Yeom JS, Kafle D, Nguyen NQ, et al. Routine insertion of the lateral mass screw via the posterior arch for C1 fixation: feasibility and related complications. Spine J. 2012;12:476–483. [DOI] [PubMed] [Google Scholar]
- 29. Young JP, Young PH, Ackermann MJ, Anderson PA, Riew KD. The ponticulus posticus: implications for C1 lateral mass screw insertion. J Bone Joint Surg Am. 2005;87:2495–2498. [DOI] [PubMed] [Google Scholar]
- 30. Joaquim AF, Riew KD. Axis screw fixation—a step-by-step review of the surgical techniques. Arq Bras Neurocir. 2017;36:101–107. [Google Scholar]
- 31. Joaquim AF, Sielatycki JA, Riew KD. Anterior surgical options for cervical spondylotic myelopathy. Indian Spine J. 2019;2:33–41. [Google Scholar]
- 32. Finn MA, Bishop FS, Dailey AT. Surgical treatment of occipitocervical instability. Neurosurgery. 2008;63:961–968. [DOI] [PubMed] [Google Scholar]