Abstract
Study Design:
Cross-sectional, international survey.
Objectives:
This study addressed the global perspectives concerning perioperative use of pharmacologic thromboprophylaxis during spine surgery along with its risks and benefits.
Methods:
A questionnaire was designed and implemented by expert members in the AO Spine community. The survey was distributed to AO Spine’s spine surgeon members (N = 3805). Data included surgeon demographic information, type and region of practice, anticoagulation principles, different patient scenarios, and comorbidities.
Results:
A total of 316 (8.3% response rate) spine surgeons completed the survey, representing 64 different countries. Completed surveys were primarily from Europe (31.7%), South/Latin America (19.9%), and Asia (18.4%). Surgeons tended to be 35 to 44 years old (42.1%), fellowship-trained (74.7%), and orthopedic surgeons (65.5%) from academic institutions (39.6%). Most surgeons (70.3%) used routine anticoagulation risk stratification, irrespective of geographic location. However, significant differences were seen between continents with anticoagulation initiation and cessation methodology. Specifically, the length of a procedure (P = .036) and patient body mass index (P = .008) were perceived differently when deciding to begin anticoagulation, while the importance of medical clearance (P < .001) and reference to literature (P = .035) differed during cessation. For specific techniques, most providers noted use of mobilization, low-molecular-weight heparin, and mechanical prophylaxis beginning on postoperative 0 to 1 days. Conversely, bridging regimens were bimodal in distribution, with providers electing anticoagulant initiation on postoperative 0 to 1 days or days 5-6.
Conclusion:
This survey highlights the heterogeneity of spine care and accentuates geographical variations. Furthermore, it identifies the difficulty in providing consistent perioperative anticoagulation recommendations to patients, as there remains no widely accepted, definitive literature of evidence or guidelines.
Keywords: anticoagulation, bridging, guidelines, spine surgery, survey, thromboprophylaxis
Introduction
Venous thromboembolism (VTE) is a preventable cause of perioperative morbidity and mortality. The incidence of VTE after spine surgery varies widely, ranging between 0.3% and 31%.1-11 Multiple factors contribute to this heterogeneity. These studies have consisted of patients undergoing surgery for elective, trauma, or oncologic indications (as some present with higher baseline rates of VTE), and also lack consistency in the method and timing of diagnosis. Given the heterogeneity of medical comorbidities, spinal pathology, and surgical techniques, a need for patient-specific anticoagulation guidelines is mounting.
Early patient mobilization, sequential compression devices (SCDs), and compression stockings are common nonpharmacologic approaches to VTE prophylaxis.12-14 Several pharmacologic agents exist that are prescribed for VTE prophylaxis, such as heparin, low-molecular-weight heparin (LMWH), and warfarin. These medications directly target factors involved in the coagulation cascade.15-20 Other medications indirectly target similar factors, such as factor Xa.21-23 A combination of these modalities are often parts of the multimodal VTE prevention strategy. Unfortunately, pharmacologic VTE prophylaxis can also cause a postoperative epidural hematoma or persistent wound drainage leading to higher rates of infection.
Recent practice guidelines have been generated related to the type and timing of VTE prophylaxis for patients with acute spinal cord injury, although uncertainty remains regarding the optimal timing for the initiation of therapy.24 Moreover, there is no consensus about perioperative VTE prophylaxis for patients undergoing spine surgery in patients with disorders other than an acute SCI. The risk of developing a clinically significant VTE must be balanced with the risk of early postoperative bleeding and epidural hematoma formation.25-27 Several attempts to create surveys have been made to elicit perioperative VTE prophylaxis patterns. However, several surveys have noted that the use of thromboprophylactic medications in the perioperative setting differed greatly28,29 and that there is no clear consensus on its use.30 Previous studies are limited by small sample sizes that prevent extensive assessment of risk factors and heterogeneity among anticoagulation methods. They also do not account for the different perspectives of spine surgeons globally. In addition, in 2009, the North American Spine Society attempted to create clinical guidelines on antithrombotic therapies in spine surgery.18 Although comprehensive, these guidelines have yet to be widely adopted worldwide.
Given the lack of consensus surrounding perioperative anticoagulation management in spine surgery, we conducted a global survey of spine surgeons to gauge their knowledge, attitudes, and practices on this topic. These results will shed light on how spine surgeons manage thromboprophylaxis over multiple countries, specialties, time in practice, type of practice, and many other specific variables. We hypothesize that the survey responses will show heterogeneity in anticoagulation practices with few instances of general consensus surrounding perioperative thromboprophylaxis in spine surgery.
Methods
Survey Design
A survey questionnaire was developed, including demographic information regarding surgeon and their practice, general anticoagulation principles, and scenarios based on patient factors, comorbidities, and region of spine pathology necessitating surgical intervention. The questions were developed by the Global Spine Journal Editorial Board and the Regional Research Chairs of AO Spine. Question selection was based on a Delphi-esque style for consensus, following several rounds of review before finalization.
Demographics were obtained on geography, specialty training, time in practice, practice type, and surgical volume. The general anticoagulation questions focused on current rationale for anticoagulation following spine surgery, risk stratification applications, the use of published/unpublished guidelines to guide treatment, and the use of multidisciplinary teams. The specific anticoagulation section was further subdivided into cervical, lumbar, and thoracolumbar surgery. Questions sought to assess perioperative factors that affect the timing of anticoagulation prophylaxis, how the diagnosis of a spinal cord injury affects thromboprophylaxis, period of bridging (the use of short-acting anticoagulants during interruption of warfarin therapy), and individual anticoagulation treatments based on medical comorbidities and previous episodes of thrombosis and/or embolus.
The survey was subsequently designed on a SurveyMonkey platform (San Mateo, CA) and distributed to the AO Spine membership through emails that classified themselves as independently performing spine surgery and who agreed to receiving such surveys via email (n = 3805 members out of approximately 6000 members). The survey recipients were provided a total of 4 weeks to complete the survey. The responses were anonymized and stored separately from the list of respondents.
Statistical Analyses
All statistical analyses were performed with Stata version 13.1 (StataCorp LC, College Station, TX). Graphical representation of survey responses was performed using RStudio v1.2.1335 (RStudio Inc, Boston, MA). Interpretation and categorization of all free-response survey answers were made by one independent reviewer to group similar categories for analyses. Calculation of percentages and means was made for count data and rank-order questions, respectively. Depiction of data was performed using a combination of RStudio’s ggplot2, rworldmap, PieDonut, and other required packages. Basic statistical analyses were performed to assess significant differences in count data using a combination of Fisher’s exact and χ2 tests. Differences in continuous variables between groups was assessed using analysis of variance (ANOVA). The threshold for statistical significance for all tests was established at P < .05.
Results
Overall, 316 spine surgeons from 64 countries completed the survey (Figure 1). The largest number of responses were from the United States (12.3%), India (10.1%), and Germany (6.0%). When stratified by continent, Europe had the largest survey representation (31.7%), followed by South America/Latin America (19.9%) and Asia (18.4%).
Figure 1.
Distribution of survey responses by country.
Respondents were between the ages of 35 to 44 (42.1%) and 45 to 54 (27.2%) years, and were typically fellowship-trained (74.7%) and orthopedic surgeons (65.5%). Most were within 5 years (26.4%) or 5 to 10 years (23.1%) of completing their training, and they practiced at academic (39.6%) or combined private/academic institutions (46.2%). The vast majority of respondents performed an estimated 101 to 200 cases per year (35.4%). Nearly all surgeons answered that they would likely adopt anticoagulation guidelines, if established (91.8%; Table 1).
Table 1.
Survey Respondent Demographics and Practice Characteristics.
| n | % | |
|---|---|---|
| Total | 316 | 100 |
| Age | ||
| 25-34 | 43 | 13.61 |
| 35-44 | 133 | 42.09 |
| 45-54 | 86 | 27.22 |
| 55-64 | 44 | 13.92 |
| Over 65 | 10 | 3.16 |
| Specialty | ||
| Orthopedics | 207 | 65.51 |
| Neurosurgery | 102 | 32.28 |
| Trauma | 7 | 2.22 |
| Spine fellowship | 236 | 74.68 |
| Years post-training | ||
| <5 | 81 | 26.38 |
| 5-10 | 71 | 23.13 |
| 10-15 | 57 | 18.57 |
| 15-20 | 46 | 14.98 |
| >20 | 52 | 16.94 |
| Practice type | ||
| Academic | 125 | 39.56 |
| Private | 45 | 14.24 |
| Both | 146 | 46.2 |
| Practice volume (cases/year) | ||
| <100 | 79 | 25 |
| 101-200 | 112 | 35.44 |
| 201-300 | 61 | 19.3 |
| 301-400 | 34 | 10.76 |
| 401-500 | 12 | 3.8 |
| >500 | 18 | 5.7 |
| Willing to adopt guidelines | ||
| Yes | 151 | 47.78 |
| Probably yes | 139 | 43.99 |
| Unsure | 17 | 5.38 |
| Probably no | 7 | 2.22 |
| No | 2 | 0.63 |
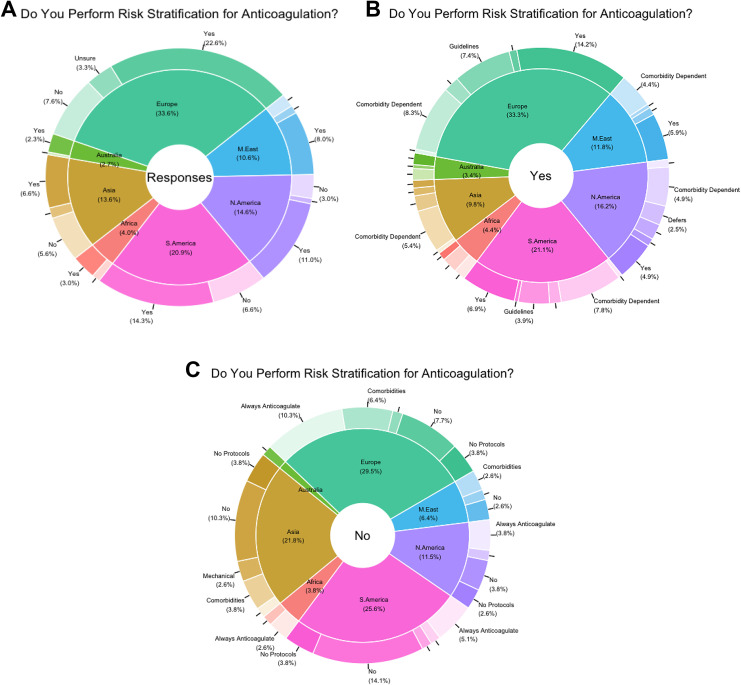
Regarding current practices, most surgeons (70.3%) admitted to routine anticoagulation risk-stratification techniques, irrespective of geographic location. Of these respondents, the most common risk-stratification method cited use of a comorbidity-based evaluation (31.5%) followed by the use of either hospital, national, or other unspecified guidelines (14.4%). Overall, reported methods were roughly similar between continents, though significant differences were observed in the number of recipients who reported use of multiple risk-stratification techniques (P = .01). Among recipients who reported no use of routine risk-stratification, when a reason was specified, most reported indiscriminate anticoagulation use (23.1%). No significant differences in reporting for risk-stratification abstinence was noted between continents (Tables 2 -4; Figures 2A, 2B, and 2C).
Table 2.
Do You Perform Risk Stratification for Anticoagulation?a
| Yes | No | Unsure | Total | |
|---|---|---|---|---|
| All continents | 222 | 78 | 18 | 319 |
| Africa | 9 | 3 | 0 | 12 |
| Asia | 38 | 16 | 3 | 57 |
| Australia | 7 | 1 | 0 | 8 |
| Europe | 68 | 22 | 11 | 101 |
| Middle East | 24 | 5 | 3 | 32 |
| North America | 33 | 8 | 2 | 43 |
| South America | 43 | 20 | 0 | 63 |
| P valueb | .748 | .559 | .899 | — |
a Vote totals exceed 316 since 2 respondents reported “Yes” and “No.” One respondent reported “No” and “Unsure.”
b Calculation of P values was performed using a combination of χ2 and Fisher exact tests.
Table 3.
Reasons for Risk Stratification.
| Yes (Unspecified) | Comorbidity Dependent | Guidelines | Defer to Other Specialty | Combination of Others | Miscellaneous | Total | |
|---|---|---|---|---|---|---|---|
| All continents | 88 | 70 | 32 | 17 | 8 | 7 | 222 |
| Africa | 2 | 4 | 0 | 0 | 3 | 0 | 9 |
| Asia | 18 | 11 | 2 | 4 | 1 | 2 | 38 |
| Australia | 3 | 3 | 1 | 0 | 0 | 0 | 7 |
| Europe | 29 | 17 | 15 | 4 | 1 | 2 | 68 |
| Middle East | 12 | 9 | 2 | 1 | 0 | 0 | 24 |
| North America | 10 | 10 | 4 | 5 | 2 | 2 | 33 |
| South America | 14 | 16 | 8 | 3 | 1 | 1 | 43 |
| P valuea | .626 | .476 | .240 | .646 | .010 | .840 | — |
a Calculation of P values was performed using Fisher exact test. Bolded value indicates statistical significance at P < .05.
Table 4.
Reasons for No Risk Stratification.
| No (Unspecified) | No Established Protocols | Always Anticoagulate | Comorbidity Deponent | Uses Mechanical Methods | Defer to Other Specialty | Bleeding Risk | Total | |
|---|---|---|---|---|---|---|---|---|
| All continents | 31 | 11 | 18 | 12 | 2 | 3 | 1 | 78 |
| Africa | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 3 |
| Asia | 8 | 3 | 1 | 3 | 2 | 0 | 0 | 17 |
| Australia | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Europe | 6 | 3 | 8 | 5 | 0 | 1 | 0 | 23 |
| Middle East | 2 | 0 | 0 | 2 | 0 | 1 | 0 | 5 |
| North America | 3 | 2 | 3 | 1 | 0 | 0 | 0 | 9 |
| South America | 11 | 3 | 4 | 0 | 0 | 1 | 1 | 20 |
| P valuea | .260 | .949 | .094 | .102 | .276 | .456 | .705 | — |
a Calculation of P values was performed using Fisher exact test.
Figure 2.
(A) Overall responses by continent. (B) Yes responses. (C) No responses.
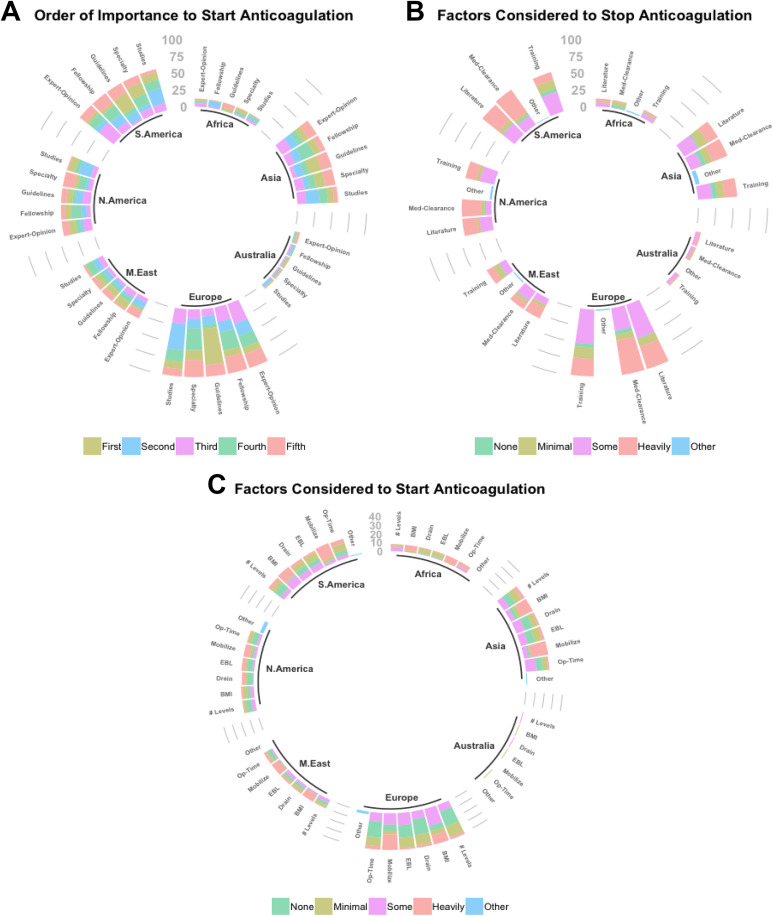
When querying recipients on factors considered during anticoagulation initiation and cessation, significant differences were seen in reporting between continents. Specifically, significant differences in perceived importance of fellowship training (P = .016), unspecified guidelines (P = .022), and choice of specialty (P = .025) were observed between continents in anticoagulation initiation practices, while usage of medical clearance (P < .001) and reference to the literature (P = .035) differed during anticoagulant cessation. Moreover, within continents, differences were seen between mean rankings for various categories for initiation and cessation as well (Tables 5 and 6). Similarly, for specific patient-related factors employed in initiation of anticoagulation, significant differences in importance of length of operation (P = .036) and body mass index (P = .008) were also observed by geographic location (Table 7; Figures 3A, 3B, and 3C). Interestingly, no significant differences were seen between continents regarding anticoagulation methodology employed in the setting of spinal cord injury (Tables 8 and 9).
Table 5.
Mean Importance for Factors Influencing Anticoagulation Initiationa.
| Expert Opinion | Fellowship Training | Guidelines | Specialty | Studies | P Valueb | |
|---|---|---|---|---|---|---|
| Mean Rank | Mean Rank | Mean Rank | Mean Rank | Mean Rank | ||
| Africa | 2.670 | 2.580 | 3.080 | 3.080 | 3.580 | .458 |
| Asia | 3.120 | 3.070 | 3.140 | 2.780 | 2.880 | .459 |
| Australia | 4.130 | 3.130 | 3.380 | 2.250 | 2.130 | .021 |
| Europe | 3.380 | 3.410 | 3.230 | 2.780 | 2.200 | <.001 |
| Middle East | 2.910 | 2.590 | 3.440 | 3.150 | 2.910 | .175 |
| North America | 3.720 | 3.050 | 3.070 | 2.350 | 2.810 | <.001 |
| South America | 2.970 | 3.480 | 3.300 | 2.650 | 2.600 | <.001 |
| P valueb | .923 | .016 | .022 | .025 | .080 | — |
a Categories were ranked by respondents on a scale from 1 (highest influence) to 5 (lowest influence).
b Calculation of P values was performed using ANOVA. Bolded values indicate statistical significance at P < .05.
Table 6.
Mean Importance for Factors Influencing Cessation of Anticoagulationa.
| Other | Med-Clearance | Previous Training | Literature | P Valueb | |
|---|---|---|---|---|---|
| # | Mean Rank | Mean Rank | Mean Rank | ||
| Africa | 2 | 1.080 | 1.920 | 2.170 | .017 |
| Asia | 6 | 1.980 | 1.910 | 1.980 | .905 |
| Australia | 0 | 2.000 | 2.130 | 2.500 | .298 |
| Europe | 2 | 2.320 | 1.990 | 2.210 | .012 |
| Middle East | 2 | 2.130 | 2.000 | 2.380 | .165 |
| North America | 3 | 2.440 | 2.260 | 2.400 | .481 |
| South America | 1 | 2.480 | 1.820 | 2.380 | <.001 |
| P valueb | <.001 | <.001 | .279 | .035 | — |
a Categories were ranked by respondents on a scale from 0 (no influence) to 3 (high influence).
b Calculation of P values was performed using a combination of Fisher’s exact test and ANOVA. Bolded values indicate statistical significance at P < .05.
Table 7.
Mean Importance for Patient-Specific Factors Influencing Initiation of Anticoagulationa.
| Other | Length of Operation | Number of Levels | EBL | Drain Output | Mobility | BMI | P Valueb | |
|---|---|---|---|---|---|---|---|---|
| # | Mean Rank | Mean Rank | Mean Rank | Mean Rank | Mean Rank | Mean Rank | ||
| Africa | 0 | 2.375 | 1.875 | 1.250 | 1.250 | 2.750 | 2.500 | <.001 |
| Asia | 1 | 1.346 | 1.259 | 1.222 | 1.185 | 2.519 | 2.074 | <.001 |
| Australia | 0 | 1.000 | 2.000 | 1.000 | 2.000 | 0.000 | 1.000 | — |
| Europe | 3 | 1.000 | 0.905 | 1.095 | 0.929 | 2.000 | 1.857 | <.001 |
| Middle East | 0 | 1.364 | 1.273 | 1.455 | 1.364 | 2.727 | 2.636 | .015 |
| North America | 4 | 0.929 | 1.000 | 1.286 | 1.231 | 2.071 | 1.357 | .093 |
| South America | 1 | 1.409 | 1.227 | 1.273 | 1.318 | 2.478 | 2.348 | <.001 |
| P valueb | <.001 | .036 | .239 | .966 | .710 | .076 | .008 | — |
Abbreviations: EBL, estimated blood loss; BMI, body mass index.
a Categories were ranked by respondents on a scale from 0 (no influence) to 3 (high influence).
b Calculation of P values was performed using a combination of Fisher’s exact test and ANOVA. Bolded values indicate statistical significance at P < .05.
Figure 3.
(A) Ranking of importance for factors influencing anticoagulation initiation. (B) Ranking of factors influencing anticoagulation cessation. (C) Ranking of patient-specific factors influencing anticoagulation initiation.
Table 8.
Spinal Cord Injury.
| Yes | No | Total | |
|---|---|---|---|
| All continents | 75 | 47 | 122 |
| Africa | 4 | 3 | 7 |
| Asia | 15 | 11 | 26 |
| Australia | 1 | 0 | 1 |
| Europe | 23 | 18 | 41 |
| Middle East | 8 | 3 | 11 |
| North America | 9 | 4 | 13 |
| South America | 15 | 8 | 23 |
| P valuea | .48 | .48 | — |
a Calculation of P values was performed using Fisher’s exact tests.
Table 9.
Anticoagulation Methods when Considering Spinal Cord Injury.
| Start Agent Earlier | Increase Dose | Change Agent | Combination of Others | Miscellaneous | Total | |
|---|---|---|---|---|---|---|
| All continents | 54 | 9 | 3 | 6 | 3 | 75 |
| Africa | 4 | 0 | 0 | 0 | 0 | 4 |
| Asia | 12 | 2 | 1 | 0 | 0 | 15 |
| Australia | 1 | 0 | 0 | 0 | 0 | 1 |
| Europe | 14 | 5 | 0 | 2 | 2 | 23 |
| Middle East | 4 | 1 | 1 | 2 | 0 | 8 |
| North America | 7 | 0 | 1 | 0 | 1 | 9 |
| South America | 12 | 1 | 0 | 2 | 0 | 15 |
| P valuea | .646 | .776 | .298 | .426 | .715 | — |
a Calculation of P values was performed using Fisher’s exact tests.
Regarding techniques employed during various hypothetical scenarios, the vast majority of providers noted use of mobilization techniques (range: 71-82 votes), LMWH (range: 62-77 votes), and mechanical prophylaxis (SCD range: 39-46 votes; compression socks range: 35-44 votes) irrespective of the given patient history (Table 10). Similar trends were observed even where patients were utilizing prescribed anticoagulation preoperatively (Table 11).
Table 10.
Anticoagulation (AC) Methods for Hypothetical Scenarios Without Baseline AC Use.
| Scenarios | No Intervention | Mobilize | SCD | Compression Socks | LMWH | SCH | Warfarin | ASA 81 mg | ASA 81 mg bid | ASA 325 mg | ASA 325 mg bid | IVC Filter | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | |
| No Hx of thromboembolic event | 9 | 82 | 45 | 35 | 50 | 5 | 2 | 2 | 2 | 2 | 0 | 1 | 1 |
| Hx of deep vein thrombosis | 4 | 77 | 46 | 49 | 67 | 13 | 7 | 7 | 0 | 2 | 0 | 4 | 3 |
| Hx of superficial thrombophlebitis | 5 | 78 | 47 | 46 | 63 | 19 | 6 | 7 | 0 | 2 | 0 | 1 | 3 |
| Hx of pulmonary embolism | 4 | 75 | 52 | 40 | 70 | 20 | 8 | 5 | 0 | 4 | 0 | 11 | 5 |
| Hx of atrial fibrillation | 8 | 71 | 43 | 37 | 63 | 13 | 8 | 6 | 1 | 4 | 2 | 2 | 3 |
| Hx of coronary artery disease | 10 | 72 | 44 | 37 | 62 | 14 | 8 | 17 | 2 | 3 | 3 | 2 | 5 |
Abbreviations: SCD, sequential-compression device; LMWH, low-molecular-weight heparin; SCH, subcutaneous heparin; ASA, aspirin; bid, twice daily; IVC, inferior vena cava; Hx, history.
Table 11.
Bridging Methods for Hypothetical Scenarios with Baseline Anticoagulation Use.
| Scenarios | No Intervention | Mobilize | SCD | Compression Socks | LMWH | SCH | Warfarin | ASA 81 mg | ASA 81 mg bid | ASA 325 mg | ASA 325 mg bid | IVC Filter | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | |
| Hx of deep vein thrombosis | 2 | 76 | 42 | 44 | 77 | 20 | 9 | 6 | 0 | 2 | 1 | 6 | 7 |
| Hx of pulmonary embolism | 3 | 74 | 46 | 40 | 75 | 20 | 10 | 5 | 0 | 1 | 0 | 11 | 7 |
| Hx of atrial fibrillation | 3 | 72 | 42 | 35 | 71 | 17 | 16 | 7 | 0 | 4 | 1 | 3 | 7 |
| Hx of coronary artery disease | 6 | 67 | 39 | 35 | 71 | 15 | 5 | 12 | 2 | 4 | 1 | 1 | 7 |
| Artificial heart valve | 3 | 71 | 41 | 41 | 67 | 18 | 20 | 12 | 1 | 3 | 2 | 0 | 13 |
| Stent | 6 | 70 | 39 | 36 | 62 | 15 | 9 | 18 | 1 | 6 | 3 | 0 | 12 |
Abbreviations: SCD, sequential-compression device; LMWH, low-molecular-weight heparin; SCH, subcutaneous heparin; ASA, aspirin; bid, twice daily; IVC, inferior vena cava; Hx, history.
Last, when queried on timing of anticoagulant initiation (for patients not previously on anticoagulation), the vast majority of respondents noted initiation on postoperative day 1 (range: 22-25 votes), followed by postoperative day 0 (range: 9-22 votes; Table 12; Figure 4). Conversely, bridging regimens appeared bimodal in distribution, with most providers electing initiation of aspirin, warfarin, or another agent on postoperative day 1 (range: 13-26 votes) or day 0 (range: 11-28 votes), as well as on day 5 (range: 8-20 votes) or day 6 and beyond (range: 7-15 votes; Table 13; Figures 5A and 5F).
Table 12.
Start Time (Days) for New Anticoagulation Regimen.
| Day 0 | Day 1 | Day 2 | Day 3 | Day 5 | Day 7 | Day 8+ | No AC | NA | |
|---|---|---|---|---|---|---|---|---|---|
| Scenarios | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes |
| No thromboembolic history | 9 | 23 | 1 | 2 | 0 | 1 | 2 | 24 | 2 |
| Hx of deep vein thrombosis | 22 | 25 | 1 | 3 | 1 | 0 | 3 | 8 | 1 |
| Hx of superficial thrombophlebitis | 16 | 25 | 1 | 2 | 0 | 0 | 3 | 17 | 0 |
| Hx of atrial fibrillation | 18 | 23 | 3 | 2 | 2 | 1 | 3 | 11 | 1 |
| Hx of coronary artery disease | 18 | 22 | 1 | 3 | 2 | 0 | 5 | 12 | 1 |
Abbreviations: NA = not applicable, no response given; Hx, history.
Figure 4.
Start date for new anticoagulation regimen.
Table 13.
Bridge Time (Days) for Previous Anticoagulation Regimen.
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6+ | No AC | NA | |
|---|---|---|---|---|---|---|---|---|---|
| Scenarios | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes | # Votes |
| Hx of deep vein thrombosis | |||||||||
| ASA | 22 | 26 | 8 | 4 | 2 | 8 | 9 | 3 | 0 |
| Warfarin | 17 | 17 | 6 | 13 | 2 | 12 | 12 | 2 | 1 |
| Other | 18 | 21 | 7 | 7 | 2 | 11 | 8 | 6 | 2 |
| Hx of pulmonary embolism | |||||||||
| ASA | 28 | 18 | 6 | 5 | 2 | 9 | 9 | 4 | 1 |
| Warfarin | 22 | 13 | 9 | 9 | 1 | 13 | 11 | 2 | 2 |
| Other | 23 | 14 | 8 | 8 | 2 | 11 | 7 | 5 | 4 |
| Hx of coronary artery disease | |||||||||
| ASA | 23 | 22 | 4 | 3 | 2 | 9 | 13 | 2 | 4 |
| Warfarin | 14 | 16 | 7 | 8 | 2 | 17 | 11 | 4 | 3 |
| Plavix | 11 | 19 | 4 | 7 | 0 | 20 | 15 | 3 | 3 |
| Other | 12 | 20 | 7 | 7 | 1 | 13 | 8 | 9 | 5 |
| Hx of atrial fibrillation | |||||||||
| ASA | 24 | 19 | 3 | 6 | 1 | 8 | 12 | 5 | 4 |
| Warfarin | 15 | 18 | 7 | 10 | 1 | 13 | 12 | 2 | 4 |
| Other | 17 | 18 | 5 | 7 | 1 | 14 | 8 | 7 | 5 |
| Hx of artificial heart valve | |||||||||
| ASA | 21 | 13 | 8 | 7 | 2 | 12 | 8 | 6 | 5 |
| Warfarin | 18 | 15 | 10 | 11 | 1 | 10 | 9 | 2 | 6 |
| Plavix | 19 | 13 | 5 | 9 | 1 | 15 | 13 | 2 | 5 |
| Other | 21 | 13 | 8 | 7 | 2 | 12 | 8 | 6 | 5 |
| Hx of stent | |||||||||
| ASA | 24 | 21 | 4 | 2 | 2 | 10 | 11 | 3 | 5 |
| Warfarin | 17 | 14 | 8 | 12 | 1 | 13 | 9 | 3 | 5 |
| Plavix | 14 | 16 | 3 | 10 | 1 | 16 | 15 | 1 | 6 |
| Other | 16 | 16 | 7 | 6 | 1 | 14 | 9 | 6 | 7 |
Abbreviations: NA, not applicable, no response given; Hx, history; ASA, aspirin.
Figure 5.
(A) History of DVT: Start date (postoperative days) for bridging regimen. (B) History of A-Fib: Start date (postoperative days) for bridging regimen. (C) History of CAD: Start date (postoperative days) for bridging regimen. (D) History of PE: Start date (postoperative days) for bridging regimen. (E) History of stent: Start date (postoperative days) for bridging regimen. (F) Artificial heart valve: Start date (postoperative days) for bridging regimen.
Discussion
Timing and use of perioperative thromboprophylaxis is controversial given the lack of consensus on best strategies. This topic remains challenging given the heterogeneity in patient presentation and difficulties in balancing the risks of developing clinically significant VTE versus early postoperative bleeding. As the largest survey to directly focus on perioperative anticoagulation practices in spine surgery to date, we brought to light the heterogeneity in practices worldwide. Most important, nearly all surgeons answered that they would likely adopt anticoagulation guidelines, if established (91.8%). Most surgeons (70.3%) admitted to routine anticoagulation risk-stratification techniques, irrespective of geographic location. The most common risk-stratification method used a comorbidity-based evaluation (31.5%) followed by the use of guidelines (14.4%). Among recipients who did not use any routine risk-stratification, when a reason was specified, most reported indiscriminate anticoagulation use (23.1%). Significant differences were observed in perceived importance of fellowship training, unspecified guidelines, and choice of specialty between continents in anticoagulation initiation practices. While usage of medical clearance and reference to the literature differed when considering timing of anticoagulant cessation. For specific patient-related factors employed in initiation of anticoagulation, significant differences in importance of length of operation and body mass index were also observed by geographic location. Interestingly, no significant differences were seen between continents regarding anticoagulation methodology employed in the setting of spinal cord injury.
The vast majority of providers used mobilization techniques (range: 71-82 votes), LMWH (range: 62-77 votes), and mechanical prophylaxis (SCD range: 39-46 votes; compression socks range: 35-44 votes) irrespective of the given patient history. Similar trends were observed even where patients were utilizing prescribed anticoagulation preoperatively. The vast majority of respondents who used chemoprophylaxis initiated it on postoperative day 1 (range: 22-25 votes), followed by postoperative day 0 (range: 9-22 votes). Conversely, bridging regimens appeared bimodal in distribution, with most providers electing initiation of aspirin, warfarin, or another agent on postoperative day 1 (range: 13-26 votes) or day 0 (range: 11-28 votes), as well as on day 5 (range: 8-20 votes) or day 6 and beyond (range: 7-15 votes).
Only a limited number of surveys have been published that describe perioperative VTE prophylaxis patterns. In a survey created for spine and trauma-trained surgeons, Ploumis et al28 concluded that spine trauma surgeons do not routinely use chemical prophylaxis after cervical spine surgery, and that the use of thromboprophylaxis was tailored to the risk factors involved with each patient’s presentation. However, this survey consisted of only 47 surgeons focusing primarily on patients presenting following spine trauma. Based on a survey of 40 spine surgeons practicing in Switzerland, Baschera et al29 concluded that the administration and discontinuation of thromboprophylactic medications in the perioperative setting differed vastly between different units and surgeons. This survey focused on the decision to start several anticoagulation medications based on various surgeries. However, the survey was distributed in only one country, which may limit generalizability. Similarly, 8 different clinical scenarios were presented to 50 spine surgeons at a British Association of Spine Surgeon by Bryson et al.30 This group concluded that there was no clear consensus in thromboprophylaxis in any of the surgeries. These findings highlight the discrepancies and uncertainties, likely attributable to the paucity of literature examining VTE in spinal surgery. Last, in 2009, the North American Spine Society attempted to create clinical guidelines on antithrombotic therapies in spine surgery.18 Albeit comprehensive, these guidelines have yet to be widely adopted; as only 14% of our respondents described following hospital, national, or other unspecified guidelines.
The results of this survey highlights the lack of consensus on various aspects of thromboprophylaxis in spine surgery. Although a large percentage of respondents subscribed to routine anticoagulation risk-stratification techniques, reported methods differed largely irrespective of geographic location. Additionally, when querying respondents on factors considered during anticoagulation initiation and cessation, significant differences were seen in reporting between geographic locations of practice. Specifically, the utilization of medical clearance and adherence to the practices described in literature also widely varied geographically. Among recipients who reported no use of routine risk-stratification, when a reason was specified, most reported indiscriminate anticoagulation use. One area in which there was relative agreement was the approach to thromboprophylaxis in the setting of spinal cord injury (SCI). Patients with acute SCI have been shown to present with the highest risk of VTE among hospitalized patients, ranging from 50% to 100% in untreated patients, and pulmonary embolism is the third most common cause of mortality in these patients.31-33 The global adherence to thromboprophylactic principles in SCI patients are likely a result of the various well-studied and widely established/distributed recommendations and protocols for this presentation.24,34-37
Despite the large amount of data published on specific thromboprophylactic therapies for various spine surgery indications, well-established guidelines and algorithms have had difficulty gaining widespread acceptance and adherence.2,15-18,20-23,38-43 Although spine surgery VTE prophylaxis recommendations from North American Spine Society and American College of Chest Physicians have been published, these groups describe “spine surgery” as a broad category, not taking into account the differing VTE and bleeding complication risks associated with specific surgical procedures and the location in the spine.18,44 To this point, Eskildsen et al45 worked to develop an algorithm for thromboprophylaxis in spine surgery. This group created a score based on patient-related and surgery-specific risk factors for elective spine surgeries. This algorithm has difficulty addressing scenarios in which patients are unable to receive standard prophylaxis and fails to provide a score for some spine surgeries. Nonetheless, this algorithm may help guide spine surgeons when deciding on thromboprophylaxis in the majority of clinical situations. Despite these attempts at creating widely accepted recommendations and algorithms, this survey shows that reference to these published items is not commonly practiced globally. The question remains how we can fundamentally address thromboprophylaxis as a spine community and develop a widely accepted algorithm or guideline to reference worldwide. Although difficult, future prospective studies are necessary to determine whether certain recommendations and algorithms can improve outcomes in VTE and bleeding complication rates following common spine surgeries. We plan to use the information from this survey to better understand global-, training-, and practice-specific indications for perioperative thromboprophylaxis to design a prospective randomized trial that can develop future guidelines and algorithms.
As with any survey, there are limitations to our current survey and its findings. The survey distribution was limited to current spine surgeon members of the AO Spine network that opted-in to receive email notifications, survey requests falling into this category. As such, there is still questionable generalizability, especially in regions in which there were very few or no respondents, and potential selection bias that may represent a unique make-up of those spine surgeons opting to receive the survey as opposed to those that did not. The survey was sent out to 3805 spine surgeons worldwide; however, only 316 surgeons responded (8.3%). Although the response rate may appear low, perhaps we have captured respondents who take special interest in this topic and have placed greater thought to their anticoagulation practices. Previous studies have described that a low response rate does not necessarily mean the study results have low validity, they simply indicate a greater risk of this. Therefore, response rates can be informative, but independently should not be considered a good proxy for study validity.46-48 Furthermore, such a response rate affiliated with AO Spine surveys distributed to the membership have been consistent. Given the length limit of surveys in general, we were not able to capture all of the possible patient comorbidities, specific spine surgeries, nor pharmaceutical options. However, given the variety of unique presentations and treatment practices worldwide, our goal was to capture the majority of spine surgeons. Although these limitations exist, this remains the largest, international survey to date focused on perioperative anticoagulation practices during spine surgery.
Conclusions
This AO Spine Anticoagulation Global Survey is the largest to date focusing on perioperative anticoagulation attitudes, practices, and beliefs among spine surgeons worldwide. Through the distribution of this large, global survey directly focused on perioperative anticoagulation practices in spine surgery, our study highlights the heterogeneous practices across geography, specialties, time in practice, type of practice, and several other variables. The one area of agreement across various backgrounds is the thromboprophylaxis treatment plan in patients presenting with spinal cord injury. This survey raises awareness and quantitatively highlights the difficulty in providing consistent perioperative anticoagulation recommendations to patients as there have been no widely accepted guidelines to date. Future studies will utilize this data to better understand global-, training-, and practice-specific indications for perioperative thromboprophylaxis to design robust future study designs, such as prospective randomized trials, that can develop future guidelines and algorithms or the need for expert group consensus recommendations for anticoagulation management.
Acknowledgements
The authors wish to thank Jayr Bass, Regula Bleuler, Niccole Germscheid, and Kaija Kurki-Suonio from AO Spine for providing administrative support, and with assistance in survey building and circulation to members.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Garrett Harada  https://orcid.org/0000-0002-7024-3783
https://orcid.org/0000-0002-7024-3783
Michael Fehlings  https://orcid.org/0000-0002-5722-6364
https://orcid.org/0000-0002-5722-6364
Venugopal Menon  https://orcid.org/0000-0003-0108-6451
https://orcid.org/0000-0003-0108-6451
References
- 1. Cox JB, Weaver KJ, Neal DW, Jacob RP, Hoh DJ. Decreased incidence of venous thromboembolism after spine surgery with early multimodal prophylaxis: clinical article. J Neurosurg Spine. 2014;21:677–684. [DOI] [PubMed] [Google Scholar]
- 2. Lee HM, Suk KS, Moon SH, Kim DJ, Wang JM, Kim NH. Deep vein thrombosis after major spinal surgery: incidence in an East Asian population. Spine (Phila Pa 1976). 2000;25:1827–1830. [DOI] [PubMed] [Google Scholar]
- 3. Oda T, Fuji T, Kato Y, Fujita S, Kanemitsu N. Deep venous thrombosis after posterior spinal surgery. Spine (Phila Pa 1976). 2000;25:2962–2967. [DOI] [PubMed] [Google Scholar]
- 4. Akeda K, Matsunaga H, Imanishi T, et al. Prevalence and countermeasures for venous thromboembolic diseases associated with spinal surgery: a follow-up study of an institutional protocol in 209 patients. Spine (Phila Pa 1976). 2014;39:791–797. [DOI] [PubMed] [Google Scholar]
- 5. Yoshioka K, Murakami H, Demura S, et al. Comparative study of the prevalence of venous thromboembolism after elective spinal surgery. Orthopedics. 2013;36:e223–e228. [DOI] [PubMed] [Google Scholar]
- 6. Goz V, McCarthy I, Weinreb JH, et al. Venous thromboembolic events after spinal fusion: which patients are at high risk? J Bone Joint Surg Am. 2014;96:936–942. [DOI] [PubMed] [Google Scholar]
- 7. Piasecki DP, Poynton AR, Mintz DN, et al. Thromboembolic disease after combined anterior/posterior reconstruction for adult spinal deformity: a prospective cohort study using magnetic resonance venography. Spine (Phila Pa 1976). 2008;33:668–672. [DOI] [PubMed] [Google Scholar]
- 8. Hohl JB, Lee JY, Rayappa SP, et al. Prevalence of venous thromboembolic events after elective major thoracolumbar degenerative spine surgery. J Spinal Disord Tech. 2015;28:E310–E315. [DOI] [PubMed] [Google Scholar]
- 9. Fineberg SJ, Oglesby M, Patel AA, Pelton MA, Singh K. The incidence and mortality of thromboembolic events in lumbar spine surgery. Spine (Phila Pa 1976). 2013;38:1154–1159. [DOI] [PubMed] [Google Scholar]
- 10. Sansone JM, del Rio AM, Anderson PA. The prevalence of and specific risk factors for venous thromboembolic disease following elective spine surgery. J Bone Joint Surg Am. 2010;92:304–313. [DOI] [PubMed] [Google Scholar]
- 11. Schairer WW, Pedtke AC, Hu SS. Venous thromboembolism after spine surgery. Spine (Phila Pa 1976). 2014;39:911–918. [DOI] [PubMed] [Google Scholar]
- 12. Kepler CK, McKenzie J, Kreitz T, Vaccaro A. Venous thromboembolism prophylaxis in spine surgery. J Am Acad Orthop Surg. 2018;26:489–500. [DOI] [PubMed] [Google Scholar]
- 13. Epstein NE. Efficacy of pneumatic compression stocking prophylaxis in the prevention of deep venous thrombosis and pulmonary embolism following 139 lumbar laminectomies with instrumented fusions. J Spinal Disord Tech. 2006;19:28–31. [DOI] [PubMed] [Google Scholar]
- 14. Epstein NE. Intermittent pneumatic compression stocking prophylaxis against deep venous thrombosis in anterior cervical spinal surgery: a prospective efficacy study in 200 patients and literature review. Spine (Phila Pa 1976). 2005;30:2538–2543. [DOI] [PubMed] [Google Scholar]
- 15. Strom RG, Frempong-Boadu AK. Low-molecular-weight heparin prophylaxis 24 to 36 hours after degenerative spine surgery: risk of hemorrhage and venous thromboembolism. Spine (Phila Pa 1976). 2013;38:E1498–E1502. [DOI] [PubMed] [Google Scholar]
- 16. Schizas C, Neumayer F, Kosmopoulos V. Incidence and management of pulmonary embolism following spinal surgery occurring while under chemical thromboprophylaxis. Eur Spine J. 2008;17:970–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moayer A, Mohebali N, Razmkon A. Incidence of deep vein thrombosis in patients undergoing degenerative spine surgery on prophylactic dalteparin; a single center report. Bull Emerg Trauma. 2016;4:38–42. [PMC free article] [PubMed] [Google Scholar]
- 18. Bono CM, Watters WC, 3rd, Heggeness MH, et al. An evidence-based clinical guideline for the use of antithrombotic therapies in spine surgery. Spine J. 2009;9:1046–1051. [DOI] [PubMed] [Google Scholar]
- 19. Rokito SE, Schwartz MC, Neuwirth MG. Deep vein thrombosis after major reconstructive spinal surgery. Spine (Phila Pa 1976). 1996;21:853–859. [DOI] [PubMed] [Google Scholar]
- 20. Young EY, Ahmadinia K, Bajwa N, Ahn NU. Does chronic warfarin cause increased blood loss and transfusion during lumbar spinal surgery? Spine J. 2013;13:1253–1258. [DOI] [PubMed] [Google Scholar]
- 21. Zhang K, Zhao S, Kan W, Xiao J, Pu F, Li K. Comparison of apixaban and rivaroxaban for anticoagulant effect after lumbar spine surgery: a single-center report. Future Sci OA. 2018;4:FSO297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chunhong L, Jin L, Qin Z, Guohui X. Anticoagulant effects of rivaroxaban after surgical fixation of spinal fracture. Pak J Pharm Sci. 2018;31:1131–1135. [PubMed] [Google Scholar]
- 23. Du W, Zhao C, Wang J, Liu J, Shen B, Zheng Y. Comparison of rivaroxaban and parnaparin for preventing venous thromboembolism after lumbar spine surgery. J Orthop Surg Res. 2015;10:78 doi:10.1186/s13018-015-0223-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fehlings MG, Tetreault LA, Aarabi B, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the type and timing of anticoagulant thromboprophylaxis. Global Spine J. 2017;7(3 suppl):212S–220S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976). 2002;27:1670–1673. [DOI] [PubMed] [Google Scholar]
- 26. Glotzbecker MP, Bono CM, Wood KB, Harris MB. Postoperative spinal epidural hematoma: a systematic review. Spine (Phila Pa 1976). 2010;35:E413–E420. [DOI] [PubMed] [Google Scholar]
- 27. Schroeder GD, Hilibrand AS, Arnold PM, et al. Epidural hematoma following cervical spine surgery. Global Spine J. 2017;7(1 suppl):120S–126S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ploumis A, Ponnappan RK, Sarbello J, et al. Thromboprophylaxis in traumatic and elective spinal surgery: analysis of questionnaire response and current practice of spine trauma surgeons. Spine (Phila Pa 1976). 2010;35:323–329. [DOI] [PubMed] [Google Scholar]
- 29. Baschera D, Oberle J, Grubhofer F, Schmid SL. Perioperative use of anticoagulant and platelet-inhibiting medications for elective spine surgery: results of a nationwide survey. J Neurol Surg A Cent Eur Neurosurg. 2018;79:398–407. [DOI] [PubMed] [Google Scholar]
- 30. Bryson DJ, Uzoigwe CE, Braybrooke J. Thromboprophylaxis in spinal surgery: a survey. J Orthop Surg Res. 2012;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eichinger S, Eischer L, Sinkovec H, et al. Risk of venous thromboembolism during rehabilitation of patients with spinal cord injury. PLoS One. 2018;13:e0193735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Teasell RW, Hsieh JT, Aubut JAL, Eng JJ, Krassioukov A, Tu L; Spinal Cord Injury Rehabilitation Evidence Review Research Team. Venous thromboembolism after spinal cord injury. Arch Phys Med Rehabil. 2009;90:232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsumoto S, Suda K, Iimoto S, et al. Prospective study of deep vein thrombosis in patients with spinal cord injury not receiving anticoagulant therapy. Spinal Cord. 2015;53:306–309. [DOI] [PubMed] [Google Scholar]
- 34. Piran S, Schulman S. Thromboprophylaxis in patients with acute spinal cord injury: a narrative review. Semin Thromb Hemost. 2019;45:150–156. [DOI] [PubMed] [Google Scholar]
- 35. Aito S, Pieri A, D’Andrea M, Marcelli F, Cominelli E. Primary prevention of deep venous thrombosis and pulmonary embolism in acute spinal cord injured patients. Spinal Cord. 2002;40:300–303. [DOI] [PubMed] [Google Scholar]
- 36. Christie S, Thibault-Halman G, Casha S. Acute pharmacological DVT prophylaxis after spinal cord injury. J Neurotrauma. 2011;28:1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arnold PM, Harrop JS, Merli G, et al. Efficacy, safety, and timing of anticoagulant thromboprophylaxis for the prevention of venous thromboembolism in patients with acute spinal cord injury: a systematic review. Global Spine J. 2017;7(3 suppl):138S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee JS, Son DW, Sung SK, Lee SW, Song GS. Effects of discontinuance of preoperative anti-platelet medication in multi-level thoracolumbar spine surgery. Turk Neurosurg. 2018;28:99–104. [DOI] [PubMed] [Google Scholar]
- 39. Akhavan-Sigari R, Rohde V, Abili M. Continuation of medically necessary platelet aggregation inhibitors—acetylsalicylic acid and clopidogrel—during surgery for spinal degenerative disorders: results in 100 patients. Surg Neurol Int. 2014;5(suppl 7):S376–S379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cuellar JM, Petrizzo A, Vaswani R, Goldstein JA, Bendo JA. Does aspirin administration increase perioperative morbidity in patients with cardiac stents undergoing spinal surgery? Spine (Phila Pa 1976). 2015;40:629–635. [DOI] [PubMed] [Google Scholar]
- 41. Soleman J, Baumgarten P, Perrig WN, Fandino J, Fathi AR. Non-instrumented extradural lumbar spine surgery under low-dose acetylsalicylic acid: a comparative risk analysis study. Eur Spine J. 2016;25:732–739. [DOI] [PubMed] [Google Scholar]
- 42. Park JH, Ahn Y, Choi BS, et al. Antithrombotic effects of aspirin on 1- or 2-level lumbar spinal fusion surgery: a comparison between 2 groups discontinuing aspirin use before and after 7 days prior to surgery. Spine (Phila Pa 1976). 2013;38:1561–1565. [DOI] [PubMed] [Google Scholar]
- 43. Park HJ, Kwon KY, Woo JH. Comparison of blood loss according to use of aspirin in lumbar fusion patients. Eur Spine J. 2014;23:1777–1782. [DOI] [PubMed] [Google Scholar]
- 44. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eskildsen SM, Moll S, Lim MR. An algorithmic approach to venous thromboembolism prophylaxis in spine surgery. J Spinal Disord Tech. 2015;28:275–281. [DOI] [PubMed] [Google Scholar]
- 46. Holbrook AL, Krosnick JA, Pfent A, et al. The causes and consequences of response rates in surveys by the news media and government contractor survey research firms In: Lepkowski JM, Tucker C, Brick JM, et al. , eds. Advances in Telephone Survey Methodology. New York, NY: Wiley; 2008:499–528. [Google Scholar]
- 47. Curtin R, Presser S, Singer E. The effects of response rate changes on the index of consumer sentiment. Public Opin Q. 2000;64:413–428. [DOI] [PubMed] [Google Scholar]
- 48. Keeter S, Kennedy C, Dimock M, et al. Gauging the impact of growing nonresponse on estimates from a National RDD Telephone Survey. Public Opin Q. 2006;70:759–779. [Google Scholar]