Abstract
Study Design:
Case series/systematic review.
Objectives:
To report on patients undergoing posterior cervical fusion for symptomatic pseudarthrosis following anterior cervical discectomy and fusion (ACDF), and to assess outcomes reporting in the literature.
Methods:
Patients undergoing posterior instrumented fusion for pseudarthrosis after primary ACDF from 2013 to 2018 by a single surgeon were reviewed consecutively. Neck Disability Index (NDI) and visual analogue scale (VAS) arm/neck were recorded at preoperative, 6-month, and 1-year time points. A systematic review of the literature was performed, and outcomes reporting was recorded.
Results:
NDI scores were 54.4 (SD 19.1), 36.6 (SD 18.1), and 41.2 (SD 19.2) at preoperative, 6-month, and 1-year time points, respectively, with improvement from preoperatively to 6 months (P = .004). VAS neck scores were 8.1 (SD 1.3), 5.0 (SD 2.9), and 5.8 (SD 2.2) at preoperative, 6-month, and 1-year time points, respectively, with improvement from preoperatively to 6 months (P = .038). VAS arm scores were 5.1 (SD 4.1), 3.5 (SD 3.2), and 3.6 (SD 2.7) at preoperative, 6-month, and 1-year time points, respectively, with improvement although these did not reach statistical significance (P = .145). The most common subjective outcomes reported in the literature were general symptoms assessments (43%), ordinal scales (43%), and VAS neck (19%) scales, with the majority of studies (67%) documenting one measure.
Conclusions:
Patient-reported outcomes demonstrate clinically meaningful improvement within the first 6 months after posterior fusion for pseudarthrosis. Studies demonstrate substantial variability and no standardization in outcomes reporting, limiting the ability to compare results across interventions and pathologies. Standardized reporting will enable comparisons to inform patients and physicians on the optimal approach to treat this difficult problem.
Keywords: cervical, anterior cervical discectomy and fusion (ACDF), pseudarthrosis, instrumentation, nonunion, revision, fusion, clinical outcomes, patient-reported outcomes, systematic review
Introduction
One of the most common spinal procedures, anterior cervical discectomy and fusion (ACDF) accounts for approximately 80% of cervical surgeries.1 While its success in improving pain, quality of life, and disability scores has been well documented,2-6 one complication that remains a significant challenge is pseudarthrosis, which has been shown to occur at a rate of up to 20% in single level cases and at even higher rates in multilevel surgery.7-11 A failure of postoperative fusion, pseudarthrosis should be suspected based on a clinical history of persistent neck pain or radicular symptoms.3,12-16 Radiographically, signs of pseudarthrosis include lack of bridging trabeculae between the endplates, graft resorption, and/or motion exceeding 1 to 2 mm between spinous processes on flexion-extension radiographs.17,18 This phenomenon is clinically relevant, as pseudarthrosis has been shown to compromise improvements in neck pain and Neck Disability Index (NDI) scores,19 and is one of the most common indications for additional intervention after ACDF, implicated in up to 56% of revision cases.17,20,21
Multiple strategies have been employed to treat symptomatic pseudarthrosis, including anterior, posterior, or circumferential approaches, and attempts have been made to identify those at highest risk before surgery.22 There has been some debate in the literature regarding which of these treatments is most effective,11,23 yet there is a paucity of data to support any given approach, as the majority of studies available do not report standardized outcomes and often lack the power necessary to draw generalizable conclusions about the superiority of one approach over another.
The objective of this study is to report on a consecutive series of single-surgeon patients who underwent posterior cervical fusion for symptomatic pseudarthrosis following ACDF. Additionally, we provide a systematic review of the currently available literature regarding management of these cases to report on the data and to assess the variability in outcomes reporting following revision for pseudarthrosis after ACDF.
Materials and Methods
Data Collection
Institutional review board approval was obtained prior to initiation of the study (ID, 2018-1580). Fourteen consecutive patients undergoing revision surgery for symptomatic pseudarthrosis from 2013 to 2018 by a single surgeon were reviewed. Exclusion criteria included patients who did not undergo revision surgery or those undergoing revision without pseudarthrosis. All 14 patients had previously undergone attempted cervical fusion and underwent revision surgery for pseudarthrosis via posterior instrumented fusion. The majority (n = 11) of patients had their index operations performed at outside facilities. Patients were indicated for surgery based on findings of radiographic and clinical pseudarthrosis, using criteria by Song et al24 whereby fusion was defined as <1-mm motion between flexion/extension radiographs as well as computed tomography.24 Demographic characteristics and surgical details were obtained and recorded. Patients underwent a posterior approach to the cervical spine via a midline approach to expose the levels of interest. 3.5 mm and/or 4.0 mm lateral mass screws and 3.5 mm and/or 4.0 mm titanium or cobalt-chromium rods were placed and the fusion bed was prepared using a high-speed burr, decorticating the facet joints. Morselized local autograft in all cases, and biologic augmentation (recombinant human bone morphogenetic protein–2 [INFUSE Bone Graft, Medtronic Spinal and Biologics, Memphis, TN], demineralized bone matrix [Grafton, Medtronic Spinal and Biologics, Memphis, TN], and/or allograft) was used to enhance the fusion bed. Selection of fusion levels was based on the levels of pseudarthrosis as well as any additional sites of pathology at the time of revision, based on history, physical exam, imaging, and intraoperative findings. Vancomycin powder (1 g) was placed in the wound prior to layered closure. All patients were placed into a brace postoperatively. One patient with failed prior posterior fusion underwent removal of hardware at the pseudarthrosis levels with wire fixation and anterior fusion. Patient-reported outcomes including NDI and visual analogue scale (VAS) Neck and Arm pain were collected preoperatively, and at 6 months and 1 year postoperatively. All patients were assessed for fusion based on the aforementioned criteria on dynamic radiographs at 6 months and 1 year postoperatively.
Systematic Review
A systematic review of the available literature was performed, adhering to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.25 The search was performed in the Medline, PubMed, Embase, and Cochrane Library databases, using Medical Subject Headings and keyword search terms and their respective combinations, including “pseudarthrosis,” “anterior cervical discectomy,” and “treatment outcome” (full search is given in the appendix). The final search was performed on October 23, 2018.
Selection Criteria
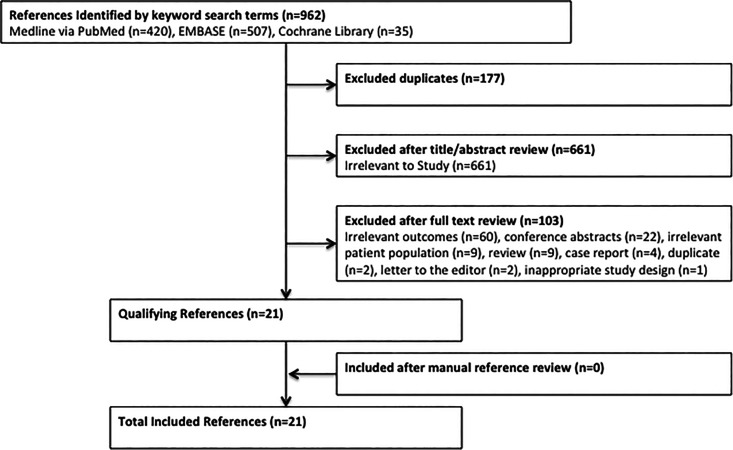
Clinical studies were evaluated and included if they were in English and reported patient outcomes after revision for pseudarthrosis following ACDF. Nonclinical studies, literature reviews, expert opinions, case reports, conference abstracts, and those not reporting on outcomes were excluded. Studies were reviewed by 2 of the study authors (M.E.S. and P.J.Y.), who performed title and abstract reviews separately. The full texts of articles meeting inclusion criteria based on title and abstract were then reviewed for final inclusion in the study, with authors coming to a consensus in the case of disagreement. A total of 21 studies were included in the systematic review, with selection process summarized in Figure 1.
Figure 1.
Flow diagram of systematic review: A total of 21 studies were included.
Quality Evaluation
Included studies were evaluated using the MINORS (Methodological Index for Nonrandomized Studies) checklist.26 Studies were evaluated on 8 to 12 items, with each scored as 0 (not reported), 1 (reported but poorly or inadequately done), or 2 (reported, well done and adequate), with a maximum score of 16 and 24 for noncomparative and comparative studies, respectively. Articles were each scored by 2 of the study authors (M.E.S. and P.J.Y.) with the authors coming to consensus in the case of disagreement.
Statistical Analysis
Descriptive statistics of the study cohort were summarized as mean (±SD) for normally distributed continuous variables, median (interquartile range [IQR]) for nonnormally distributed continuous variables and count (frequency) for categorical variables. All analyses were conducted using Stata SE 14.0 (Stata Corp, College Station, TX).
Results
A total of 14 patients were included in the study (Table 1). Mean age at revision surgery was 53.6 years (range 40-68 years) with mean follow-up of 12.3 months (range 6.0-22.9 months). The mean time between index and revision surgery was 86.8 months (range 23.5-323.8 months). Four (29%) patients had pseudarthrosis at a single level, while 10 (71%) had pseudarthrosis at 2 levels. Revision surgery involved 2, 3, and 4 levels in 7 (50%), 6 (43%), and 1 (7%) patients, respectively. Mean intraoperative blood loss (n = 10) was 130 mL (range 50-200 mL) and mean procedure duration was 131 minutes (range 55-280 minutes). Of note, 3 additional cases had “minimal” blood loss and were not included in the calculation. The mean length of stay was 2.9 days (range 2-5 days). Thirteen patients demonstrated radiographic fusion at 6 months postoperatively. One patient did not follow-up at 6 months but presented at 8 months postoperatively with evidence of fusion on computed tomography scan. Individual patient characteristics are shown in Table 2. Patients presented with axial neck pain, while a subset also had radicular symptoms and headaches. Recombinant human bone morphogenetic protein-2 (rhBMP-2) was used in 10 (71%) of the revisions to enhance the fusion, whereas 7 (50%) had demineralized bone matrix (DBM) and 6 (43%) had allograft. Morselized local autograft was used in all cases.
Table 1.
Demographics and Operative Details.
| Variable | |
|---|---|
| Age, y, mean (range) | 53.6 (40-68) |
| Follow-up, mo, mean (range) | 12.3 (6.0-22.9) |
| Gender, n | 7 male; 7 female |
| BMI, kg/m2, mean (range) | 29.9 (range 23.6-39.9) |
| ASA class (n = 11), n (%) | |
| II | 11 (100) |
| Smokers (n = 13), n (%) | |
| Current | 2 (15) |
| Former | 5 (38) |
| Never | 6 (46) |
| Index no. of levels, n (%) | |
| 1 | 1 (7) |
| 2 | 9 (64) |
| 3 | 1 (7) |
| 4+ | 3 (21) |
| Time from index to revision, mo, mean (range) | 86.8 (23.5-323.8) |
| No. of pseudarthrosis levels, n (%) | |
| 1 | 4 (29) |
| 2 | 10 (71) |
| Revision levels, n (%) | |
| 2 | 7 (50) |
| 3 | 6 (43) |
| 4 | 1 (7) |
| Intraoperative blood loss (n = 10),a mL, mean (range) | 130 (50-200) |
| Procedure duration, min, mean (range) | 131 (55-280) |
| Hospital length of stay, days, mean (range) | 2.9 (2-5) |
| Fusion at 6 months, n (%) | 14/14 (100)b |
Abbreviations: BMI, body mass index; ASA, American Society of Anesthesiologists.
a Three cases had “minimal” blood loss and were not calculated in the total.
b One patient did not have 6-month follow-up, but had computed tomography demonstrating fusion at 8 months postoperatively.
Table 2.
Individual Patient Characteristics.
| Patient | Age, y | Index Procedure | Clinical Presentation | Diagnosis | Revision Procedure | Biologic Augmentationa |
|---|---|---|---|---|---|---|
| 1 | 44 | ACDF C5-7 | NA | Pseudo C5-7, radiculopathy C4-7 | PCF C4-7, Foraminotomy C4-7 | DBM |
| 2 | 60 | ACDF C4-5, C6-7 | NA | Pseudo C6-7 | PCF C4-7 | Allograft, DBM |
| 3 | 40 | ACDF C4-7 | Neck and arm pain, LE weakness, loss of dexterity | Pseudo C6-7, radiculopathy C6-7 | PCF C4-7, Foraminotomy C6-7 | DBM |
| 4 | 56 | ACDF C3-5 | NA | Pseudo C3-5, central stenosis C3-5 | PCF C3-6, laminectomy C3-5 | DBM |
| 5 | 60 | Posterior C2-T1 fusion | Headache, neck and arm pain/numbness/tingling | Pseudo C2-3 and C4-5 | PCF C2-3, 4-5, wiring, ACDF C2-3 | Allograft, rhBMP-2 |
| 6 | 54 | ACDF C3-7 | Neck and arm pain, dysphagia | Pseudo C5-7 | PCF C4-7 | rhBMP-2, DBM |
| 7 | 47 | ACDF C4-7 | Neck and arm pain | Pseudo C6-7 | PCF C5-7 | Allograft, rhBMP-2, DBM |
| 8 | 53 | ACDF C5-7 | Neck and shoulder pain | Pseudo c5-7 | PCF C5-7 | Allograft, rhBMP-2 |
| 9 | 53 | ACDF C5-7 | Neck and arm pain | Pseudo C5-7, radiculopathy C6-T1 | PCF C5-T1, foraminotomy C6-T1 | rhBMP-2, DBM |
| 10 | 51 | ACDF C4-6 | Neck and arm pain, headaches | Pseudo C4-6 | PCF C4-6 | Allograft, rhBMP-2 |
| 11 | 47 | ACDF C3-7 | Neck pain, UE numbness/tingling | Pseudo C5-7 | PCF C5-7 | rhBMP-2 |
| 12 | 60 | ACDF C5-7 | Neck stiffness, imbalance | Pseudo C5-7 | PCF C5-7 | rhBMP-2 |
| 13 | 68 | ACDF C5-7 | Neck pain, headaches | Pseudo C5-7, radiculopathy C5-7 | PCF C5-7, foraminotomy C5-7 | Allograft, rhBMP-2 |
| 14 | 57 | ACDF C5-7 | Neck pain | Pseudo C5-7, central stenosis C4-5 | PCF C5-7, laminectomy C4-5 | rhBMP-2 |
Abbreviations: ACDF, anterior cervical discectomy and fusion; NA, not available; Pseudo, pseudarthrosis; PCF, posterior cervical fusion; DBM, demineralized bone matrix; rhBMP-2, recombinant human bone morphogenetic protein–2; LE, lower extremity; UE, upper extremity.
aLocal autograft was used in all cases.
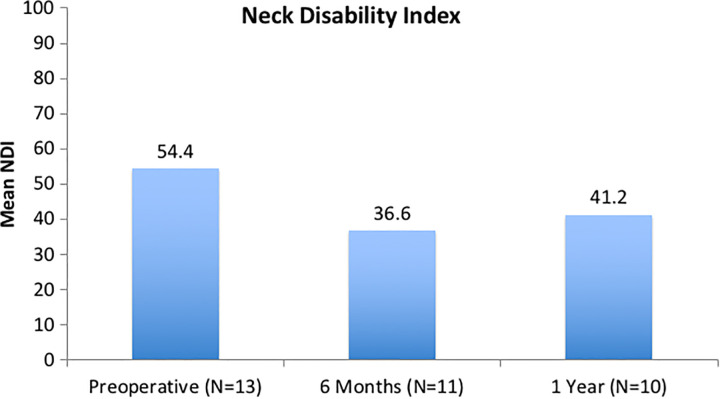
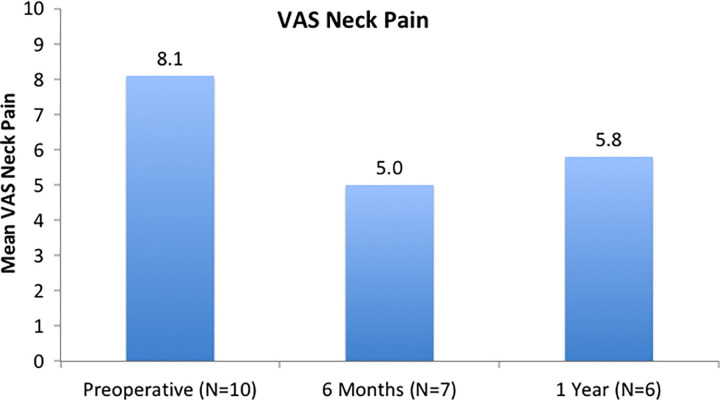
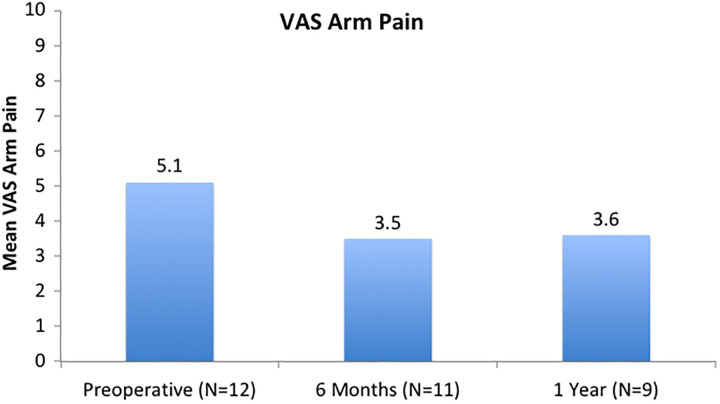
Patient-reported outcomes are shown in Figures 2, 3, and 4. NDI scores were 54.4 (SD 19.1), 36.6 (SD 18.1), and 41.2 (SD 19.2) at the preoperative, 6-month, and 1-year time points, respectively. There was a significant improvement noted in NDI from preoperatively to 6 months postoperatively (P = .004). VAS neck scores were 8.1 (SD 1.3), 5.0 (SD 2.9), and 5.8 (SD 2.2) at the preoperative, 6-month, and 1-year time points, respectively. There was significant improvement noted from preoperatively to 6 months postoperatively (P = .038). While mean VAS Arm pain scores improved over time, these results were not statistically significant (P = .145 from preoperative to 6 months).
Figure 2.
Neck Disability Index (NDI): There was a significant, clinically important improvement noted from preoperatively to 6 months postoperatively (P = .004).
Figure 3.
Visual analogue scale (VAS) Neck: There was a significant, clinically important improvement noted from preoperatively to 6 months postoperatively (P = .038).
Figure 4.
Visual analogue scale (VAS) Arm: While mean VAS arm pain scores improved over time, these results were not statistically significant (P = .145).
Systematic Review
Twenty-one studies, published between 1990 and 2017, were included in the systematic review (Table 3).10,13,23,27-44 The majority of studies were retrospective (n = 19) with level IV evidence (n = 16). Of the 21 studies, 19 were specifically assessing the outcomes after revision for pseudarthrosis, whereas in 2 studies this data was reported as a subgroup of the overall population. A total of 530 patients were included in the review. The mean number of patients in each study was 25.2 (range 3-120) with a frequency-weighted mean age of 48.3 years (n = 16 studies; range 18-79 years) and follow-up of 39.6 months (n = 17 studies; range 2-216 months). The frequency-weighted mean number of revision levels was 1.43 (n = 12 studies; range 1-4 levels) with a mean interval from index to revision procedure of 23.5 months (n = 17 studies; range 2-230 months). The posterior approach was most commonly used (n = 18 studies), followed by anterior (n = 9 studies), and circumferential (n = 2 studies) approaches. Five studies13,23,40-42 reported on subgroups undergoing anterior and posterior revision; one study27 noted subgroups undergoing posterior and circumferential fusion; and another study39 reported on subgroups undergoing anterior, posterior, and circumferential fusion. On quality evaluation, 16 noncomparative studies were found to have a mean MINORS score of 8.9 (range 5-12) of 16, and 5 comparative studies had a mean MINORS score of 9.2 (range 6-13) of 24.
Table 3.
Details on Included Studies.
| Author/Year | Design; LOE | No. of Patients | Mean Follow-up, mo (Range) | Mean Age, y (Range) | No. of Levels Revised | Interval From Index to Revision, mo (Range) | Intervention |
|---|---|---|---|---|---|---|---|
| Smith/2017 | R; IV | 25 | 18 (13-45) | 56 (36-75) | NR | 27 (2-230) | Tissue-sparing post fusion with facet cages (all); Ant plating with cage or allograft (9/25) |
| Elder/2015 | R; IV | 22 | 12 (2-146) | 51 (33-67) | 1 level: 17 Multilevel: 5 |
11 (3-151) | Post fusion: lateral mass screws (20), decompression (19), wiring (1), noninstrumented fusion (1) |
| Kasliwal/2015 | R; IV | 19 | 20 (12-56) | 54 (31-65) | 1 level: 13 2 level: 6 |
20 (6-33) | Post fusion with cervical interfacet spacers and lateral mass screw fixation |
| Liu/2012 | R; IV | 38 | 28 (24-60) | 45 (24-60) | NR | 18 (12-55) | Post fusion with lateral mass screw fixation (multiaxial screw-rod or plate) |
| Carreon/2006 | R; III | 120 | Ant: 42 (24-132) Post: 52 (24-120) |
NR | Ant: 1.48 (1-2) Post: 1.58 (1-4) |
Ant: 28.7 (5.2-115.3) Post: 26.5 (6.3-135.9) |
Ant: autograft + plate Post: wires (34), lateral mass plates (32), screw/rod (27) |
| Toohey/2006 | R; IV | 18 | NR | 53 | 1 level: 15 2 levels: 3 |
NR | Post fusion with Halifax clamp fixation |
| Kuhns/2005 | R; IV | 33 | 46 (20-86) | 47 (28-63) | 1 level: 26 2 levels: 7 |
16 (3-63) | Selective nerve root decompression (18) + post fusion with wiring (31) and/or lateral mass plating (21) |
| Gore/2003 | R; IV | 25 | 60 (12-168) | NR | 1 level: 16 2 levels: 8 3 levels: 1 |
36 (12-132) | Post interspinous wiring with autograft (20) or allograft (5) |
| Bolesta/2002 | P; III | 9 | NR | NR | 1 level: 4 2 levels: 5 |
NR | Post instrumentation/fusion |
| Bolesta/2000 | P; IV | 3 | 40.7 (24-50) | NR | NR | 15.3 (4-24) | Post fusion |
| Epstein/1998 | R; IV | 3 | 24 (12-36) | 53 (35-68) | 1 level: 1 2 levels: 1 4 levels: 1 |
9.7 (3-22) | Post wiring/fusion |
| Siambanes/1998 | R; IV | 14 | 43.2 (6-126) | 43 (33-52) | All 1 level | NR | Post interspinous wiring with autograft |
| Coric/1997 | R; IV | 19 | 22.4 (12-42) | 49.1 (25-72) | 1 level: 11 2 levels: 6 3 levels: 2 |
20 (3-48) | Ant, iliac crest allograft with plating |
| Phillips/1997 | R; III | 22 | 32 (min 12) | NR | NR | 28 (6-110) | Ant revision with autograft (16), post wire or lateral mass screw-plate with autograft (6) |
| Tribus/1997 | R; IV | 16 | 43 (19-61) | 42.1 (33-62) | 1 level: 14 2 levels: 2 |
51 (5-58) | Ant revision with autograft and plate (14), with corpectomy/strut grafting and plate (2) |
| Zdeblick/1997 | R; IV | 23 | 44 (24-216) | 50.3 (31-60) | NR | 32 (4-168) | Ant with autograft (20), corpectomy + strut grafting (3) |
| Lowery/1995 | R; III | 37 | 28 (12-60) | 47 (18-79) | NR | 19 (6-81) | Ant grafting and plating (20); post fusion and articular pillar plating (17); circumferential (7) |
| Mutoh/1993 | R; IV | 15 | NR | 55.6 (36-74) | NR | 27.2 (16 to 86) | Ant with graft/plating (1), post interspinous wiring and graft/facet fusion (12), post fusion with graft (2) |
| Newman/1993 | R; IV | 16 | NR | 40 | NR | NR | Ant fusion (14), post fusion (2) |
| Brodsky/1992 | R; III | 34 | 60 (24-165) | 47 (31-72) | 1 level: 18 2 levels: 14 3 levels: 2 |
20 (3-121) | Ant revision with grafting (17), post modified Rogers wiring with grafts/laminal fusion (17) |
| Farey/1990 | R; IV | 19 | 44 (24-54) | 44.9 (23-57) | 1 level: 11 2 levels: 8 |
25 (9-69) | Post decompression/fusion with triple wire technique + autograft |
| Totals: | R: 19, P: 2 III (5), IV (16) | 25.2 (3-120) | 39.6 (2-216) | 48.3 (18-79) | 1.43 (1-4) | 23.5 (2-230) | Post: 18; Ant: 9; circumferential: 2 |
Abbreviations: LOE, level of evidence; R, retrospective; P, prospective; NR, not reported; Ant, anterior; Post, posterior.
Outcomes Reporting
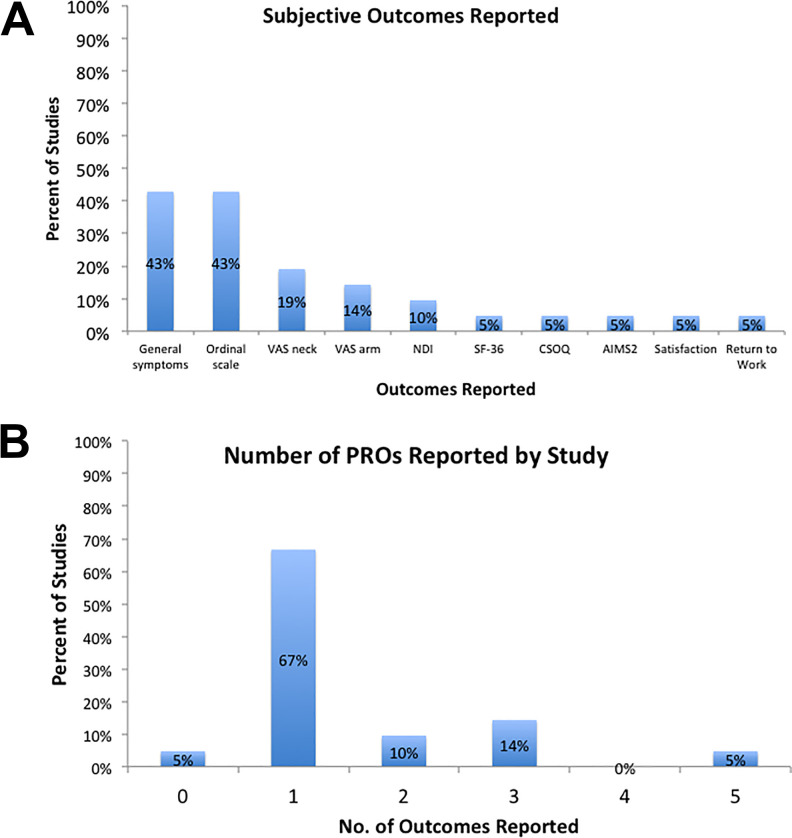
Outcomes following revision for pseudarthrosis are shown in Table 4. Objective outcomes were reported sparsely in the reviewed studies. Length of hospital stay was most frequently reported, noted in 24% (n = 5) of studies, whereas estimated blood loss and duration of operation were reported by 19% (n = 4) and 14% (n = 3) of studies, respectively. Each study was assessed for inclusion of patient-reported outcomes with respect to frequency of reporting. The 10 most commonly cited outcomes are shown in Figure 5a. The most common subjective outcomes reported were general symptoms assessments (n = 9 studies) and ordinal scales (n = 9 studies), frequently with Odom’s criteria.45 The next most commonly noted subjective instruments were VAS Neck and Arm pain, reported in 4 (19%) and 3 (14%) studies, respectively. The majority of subjective outcomes were noted in one study each. The number of subjective outcomes reported per study is depicted in Figure 5b. The majority of studies (67%) document one subjective outcome measure. One study10 was found to report 5 subjective measures, whereas one study23 supplied none.
Table 4.
Outcomes of Included Studies.
| Author/Year | No. of Patients | Approach | Clinical Outcomes | Radiographic Outcomes | Complications |
|---|---|---|---|---|---|
| Smith/2017 | 25 | Post, Circum | VAS neck: 7.9 ± 1.5 to 3.8 ± 2.3 VAS arm: 7.24 ± 2.2 to 3.12 ± 2.5 NDI: 65.1 ± 20.3 to 29.1 ± 17.9 Hospital stay 1.4 d OR time 104 min EBL 88 mL |
All fused by 1 y | Radicular pain requiring surgery (1); recurrent laryngeal nerve palsy (1); superficial infection (1) |
| Elder/2015 | 22 | Post | VAS neck: 8 to 4 (P = .012); symptoms resolved/improved (18), stable (3), worse (1) EBL 388 mL (50-1200) Hospital stay 4 d (1-8) |
Ant/Post fusion (18), Ant only (2), Post only (2), median 10 mo (2-14) | ASD (4), wound infection (1), PNA (1), transient C5 palsy (1) |
| Kasliwal/2015 | 18 | Post | Improvement in VAS neck in 83% (P < .004), arm in 72% (P < .007), and NDI in 67% (P < .06) | All levels; Ant/ post 84%, post alone 16% | Asymptomatic pseudarthrosis at level unrelated to index pseudarthrosis (1), wound revision for seroma (2) |
| Liu/2012 | 38 | Post | Excellent (10), good (22), fair (6), poor (0) | All fused | Superficial infection (3), graft site drainage (1) |
| Carreon/2006 | 120 | Ant, Post | OR time: 134.9 min (Ant), 138.9 min (Post) EBL: 102.7 mL (Ant), 282.1 mL (Post) Hospital stay: 2.3 d (Ant), 4.4 d (Post) |
Persistent nonunion: 12/27 (Ant), 2/93 (Post) | Ant: graft site infection (1), surgery for nonunion (12) Post: wound infection (4), graft site infection (3), surgery for nonunion (2) |
| Toohey/2006 | 18 | Post | Continued pain (5/18) | All had interbody fusion, not all with lateral fusion mass | Hardware removal (5), PNA (1) |
| Kuhns/2005 | 33 | Post | All had improvement in symptoms Mild/no pain (13), “discomforting” pain (5), moderate/severe pain (7) SF-36 PC 35.9, MC 46.2 CSOQ: neck pain 46.0, shoulder-arm pain 35.2, physical symptoms 56.0, functional disability 28.2 AIMS2: hand and finger 1.86, arm 1.42 Satisfied (18), “not sure” (4), not satisfied (3) |
All fused | Hardware removal (2), revision foraminotomy (2), ASD (1), retained drain (1) |
| Gore/2003 | 25 | Post | Pain relief (17), little/no pain relief (8) | All fused | ASD requiring surgery (4) |
| Bolesta/2002 | 9 | Post | 1 level unplated: excellent (2), satisfactory (1); plated: good (1) 2 level unplated: poor (1); plated: excellent (3), satisfactory (1) |
All fused | NR |
| Bolesta/2000 | 3 | Post | Odom excellent (2), good (1) | All fused | NR |
| Epstein/1998 | 3 | Post | All “clinically stable” | NR | NR |
| Siambanes/1998 | 14 | Post | Mean axial pain 7 (3-9), appendicular pain 6 (0-10) Axial: improved (7), unchanged/worse (2) Appendicular: improved (4), unchanged/worse (5) Good (1), fair (1), poor (7) |
All fused; Ant (12), interlaminal bridge but no ant fusion (2) | Wound infection (2) |
| Coric/1997 | 19 | Ant | Excellent (6), good (9), fair (2), poor (1) Hospital stay 3.3 d (2-6) |
All fused | MI/death (1), transient hoarseness (2) |
| Phillips/1997 | 22 | Ant, Post | Odom’s excellent (19), good (1), symptomatic nonunion (2) Motor weakness resolved in 5 (ant) and 1 (post) No significant difference in pain, medication use, work status, or daily activity detected between ant/post |
Ant: 14/16 fused Post: 6/6 fused |
Symptomatic nonunion (2) |
| Tribus/1997 | 16 | Ant | Symptom improvement (11), same/worse (3), initial improvement before ASD (2) Weakness resolution (5), persistent weakness (2) Return to work 69%, hospital stay 2.2 days OR time 152 min, EBL 125 mL |
Disc space obliteration (10), endplates partially obliterated/no lucent lines (3), lucent lines <1 mm (2), lucent lines >1 mm (1) | Persistent dysphagia (1), ASD (2) |
| Zdeblick/1997 | 23 | Ant | Excellent (20), fair (3) | All fused | Recurrent laryngeal n palsy (1), graft site drainage (2) |
| Lowery/1995 | 37 | Ant, Post, Circum | Ant: 43% reduction in axial pain, 56% appendicular; 40% felt better than before surgery, 25% same, 35% worse Post: 77% reduction in axial pain, 83% appendicular; 82% better, 12% same, 6% worse Circumferential: 68% reduction in axial pain, 65% appendicular; 71% felt better, 29% same, 0% worse |
Circum: 100% fusion Post: 16/17 (94%) Ant: 9/20 (45%) |
Failed revision (6), hardware failure (9/20 Ant, 2/7 circum, 2/17 Post), durotomy (1 Ant, 1 circum), C5 palsy (2 Post) |
| Mutoh/1993 | 15 | Ant, Post | 4/5 symptomatic resolved | All fused | NR |
| Newman/1993 | 16 | Ant, Post | Excellent/good (9), persistent pain/unsatisfactory (4) | 13/16 fused | NR |
| Brodsky/1992 | 34 | Ant, Post | Ant: excellent/good in 59% (excellent 5, good 10, fair 2, 0 poor) Post: excellent/good in 88% (excellent 7, good 3, fair 7, 0 poor) |
Ant: 13 (76%) fusion Post: 16 (94%) fusion |
NR |
| Farey/1990 | 19 | Post | Radiculopathy resolved (18/19), motor weakness resolved (4/4) Cervical pain: complete relief (14), pain not interfering w/ADLs (5) |
All fused | Graft donor site pain (2), donor site numbness (1), superior gluteal artery laceration (1) |
Abbreviations: VAS, visual analogue scale; NDI, Neck Disability Index; NR, not reported; OR, operating room; EBL, estimated blood loss; Ant, anterior; Post, posterior; circum, circumferential; ASD, adjacent segment disease; PNA, pneumonia; MI, myocardial infarction; SF, Short Form; PC, physical component; MC, mental component; CSOQ, Cervical Spine Outcomes Questionnaire; AIMS2, Arthritis Impact Measurement Scales 2; ADL, activities of daily living.
Figure 5.
(A) Subjective outcomes reported: The most common subjective outcomes reported were general symptoms assessments and ordinal scales (each in 43%), followed by VAS neck (19%), VAS arm (14%), and NDI (10%) scales. Other outcomes were found in 1 study each. VAS, visual analog scale; NDI, Neck Disability Index; SF-36, Short Form 36; CSOQ, Cervical Spine Outcomes Questionnaire; AIMS2, Arthritis Impact Measurement Scales 2. (B) Patient-reported outcomes reported by study: The majority of studies (67%) document 1 subjective outcome measure, with 5 studies reporting 2 (n = 2 studies) and 3 (n = 3 studies) outcomes. One study reported 5 subjective measures and one reported none.
Discussion
Based on the results of this study, patients undergoing posterior fusion for symptomatic pseudarthrosis after ACDF can expect to have improvements in NDI and VAS Neck pain at 6 months and 1 year postoperatively. At a mean follow-up of 12.3 months, NDI scores were 54.4 and 36.6 at preoperative and 6-month time points (P = .004), respectively, and VAS Neck scores were 8.1 and 5.0 at preoperative and 6-month time points, respectively (P = .038). These outcomes are statistically significant as well as clinically important, with both exceeding minimally clinically important differences.46-49 Furthermore, these outcomes were attained with acceptable perioperative risk, with mean intraoperative blood loss of 130 mL (not including 3 patients with “minimal” blood loss), procedure duration of 131 minutes, and length of stay of 2.9 days. All patients went on to fusion postoperatively based on radiographic criteria.
To our knowledge, this is the first report on clinical outcomes in patients undergoing posterior instrumented fusion with a screw/rod construct alone for pseudarthrosis after ACDF. The results of our cohort are important, suggesting that these patients exhibit clinically meaningful improvements in NDI and neck pain within the first 6 months postoperatively. While many have noted improvements after revision for pseudarthrosis, few studies have reported on validated instruments in cervical pathology. Kuhns et al10 studied 33 patients undergoing lateral mass plating and/or wiring, reporting postoperative Short Form–36 (SF-36), Cervical Spine Outcomes Questionnaire (CSOQ), and Arthritis Impact Measurement Scales 2 (AIMS2) but no baseline preoperative comparisons to assess change after surgery. Two more recent studies assessed posterior fusion focusing on preparation of the facet joints. Kasliwal et al44 evaluated posterior fusion using interfacet spacers and lateral mass screws, reporting significant improvements in VAS Neck and Arm pain for 83% and 72% of patients, respectively.44 They further noted improvement in NDI scores in 67% of patients although this did not reach statistical significance (P < .06). A more recent study by Smith et al27 assessed tissue-sparing posterior fusion using bilateral facet cages in 25 patients, noting significant improvements in VAS Neck and Arm scores from 7.9 ± 1.5 to 3.8 ± 2.3 and from 7.24 ± 2.2 to 3.12 ± 2.5, respectively, and significant improvements in NDI from 65.1 ± 20.3 to 29.1 ± 17.9 at a mean 18 months postoperatively. These findings are consistent with what was found in our study.
There remains some debate as to whether symptomatic pseudarthrosis after ACDF is best managed with an anterior or posterior approach. In their study of 120 patients with symptomatic pseudarthrosis after ACDF, Carreon et al23 found similar operative times for anterior (134.9 minutes) and posterior (138.9 minutes) groups, although noted greater estimated blood loss (282.1 vs 102.7 mL) and longer hospital stay (4.4 vs 2.3 days) in those undergoing posterior revision. Similarly, Elder et al28 note estimated blood loss of 388 mL and mean hospital stay of 4 days in their cohort undergoing posterior revision, whereas in a group of patients undergoing anterior revision, Tribus et al37 report estimated blood loss of 125 mL and mean hospital stay of 2.2 days. More recent literature has noted a more favorable risk profile for the posterior approach. Smith et al. noted a short length of stay (1.4 days), operative time (104 minutes), and estimated blood loss (88 mL),27 while in our study we found length of stay of 2.9 days, procedure duration of 131 minutes with 130 mL of blood loss, demonstrating the possibility of achieving a better risk profile than what has been previously reported.
Reviewing the literature, we identified 21 studies, the majority of which included a posterior approach to revise pseudarthrosis after ACDF. Of note, there was substantial heterogeneity in outcomes reporting. The most commonly reported objective variable (length of stay) was noted in 24% of studies, whereas blood loss and operative duration were reported in 19% and 14% of studies, respectively. Similar shortcomings were found in the reporting of patient-reported outcomes. The most commonly noted outcomes were assessments of general symptoms (43%) and ordinal scales (43%), followed by VAS Neck (19%) and VAS Arm (14%) scales. Overall, the quality of the studies was low, with 16 noncomparative studies having a mean MINORS score of 8.9 (range 5-12) of 16, and 5 comparative studies having a mean MINORS score of 9.2 (range 6-13) of 24.
A prior meta-analysis assessed clinical and fusion outcomes to evaluate the optimal approach for revision in the setting of pseudarthrosis after ACDF.11 Sixteen studies were included in the meta-analysis, with 10 of the studies each reporting on the results after anterior and posterior revision. The authors found a significant difference in fusion success, noting 86.4% success for anterior and 97.1% for the posterior approach (P = .028), but found no difference in terms of clinical outcomes. Nevertheless, the authors note significant heterogeneity and poor overall quality of studies as key factors limiting the accuracy of their conclusions.
Given the overall low prevalence of this condition, it is unlikely that a randomized trial or even a well-powered cohort study would be feasible. Rather, comparing different strategies to treat symptomatic pseudarthrosis may lie in our ability to amalgamate the outcomes of contemporary case series such as this one. However, this systematic review highlights the imposing limitation of this strategy—inadequate use of standardized outcome instruments. The most common patient-reported outcomes noted are general symptoms assessments and ordinal scales—not validated instruments in cervical spine disease—and many of the studies do not distinguish between neck and arm pain, which could represent different underlying pathologies.11 This lack of standardized outcome measures make results difficult to interpret and compare across interventions.
Our study has several limitations. This series contains a small number of patients treated by a single surgeon without long-term follow-up, limiting the study’s power and ability to draw conclusions about the generalizability and long-term outcomes associated with posterior fusion. In this vein and given the number of patients, a power analysis was not performed and all consecutive patients meeting the inclusion criteria were included in the study. Similarly, as patients underwent revision with a posterior approach and only one with an anterior approach, we are unable to compare across different treatment strategies (anterior vs posterior vs combined). Regarding the systematic review, our inclusion of studies relied on our previously described search strategy and the inclusion of works in the aforementioned databases. To limit the possibility of excluding studies, we used broad search terms as well as multiple databases. Additionally, the results of our study were dependent on data reported in the included studies and were therefore limited by the clarity of reporting in the primary works. Finally, analysis of outcomes reporting is limited by the small number of studies. Yet this is reflective of the current literature on revision for symptomatic pseudarthrosis after ACDF, and this shortcoming is an important reality to highlight as we consider future research in this field.
In conclusion, patient-reported outcomes improve after posterior fusion in the setting of pseudarthrosis after ACDF. These gains are made in the first 6 months postoperatively with an acceptable perioperative risk profile. The current literature for this condition contains substantial variability of outcomes reporting, with few studies documenting validated instruments, limiting our ability to compare results across studies. Randomized trials comparing surgical approaches for symptomatic pseudarthrosis would prove useful but are likely not feasible. In the future, broader adoption of standardized patient-reported outcomes will enable more accurate comparison across studies, informing patients and surgeons about the optimal approach to treat this difficult problem.
Supplemental Material
Supplemental Material, Appendix_-_Search_Strategy for Outcomes of Revision Surgery for Pseudarthrosis After Anterior Cervical Fusion: Case Series and Systematic Review by Michael E. Steinhaus, Philip J. York, Rachel S. Bronheim, Jingyan Yang, Francis Lovecchio and Han Jo Kim in Global Spine Journal
Footnotes
Authors’ Note: Investigation performed at the Department of Orthopaedic Surgery, Hospital for Special Surgery, New York, NY.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michael E. Steinhaus, MD  https://orcid.org/0000-0002-0348-0754
https://orcid.org/0000-0002-0348-0754
Francis Lovecchio, MD  https://orcid.org/0000-0001-5236-1420
https://orcid.org/0000-0001-5236-1420
Han Jo Kim, MD  https://orcid.org/0000-0003-2170-3592
https://orcid.org/0000-0003-2170-3592
References
- 1. Oglesby M, Fineberg SJ, Patel AA, Pelton MA, Singh K. Epidemiological trends in cervical spine surgery for degenerative diseases between 2002 and 2009. Spine (Phila Pa 1976). 2013;38:1226–1232. doi:10.1097/BRS.0b013e31828be75d [DOI] [PubMed] [Google Scholar]
- 2. Bohlman HH, Emery SE, Goodfellow DB, Jones PK. Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am. 1993;75:1298–1307. doi:10.2106/00004623-199309000-00005 [DOI] [PubMed] [Google Scholar]
- 3. Smith GW, Robinson RA. The treatment of certain cervical-spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40:607–624. [PubMed] [Google Scholar]
- 4. Carette S, Fehlings MG. Clinical practice. Cervical radiculopathy. N Engl J Med. 2005;353:392–399. doi:10.1056/NEJMcp043887 [DOI] [PubMed] [Google Scholar]
- 5. Emery SE, Bolesta MJ, Banks MA, Jones PK. Robinson anterior cervical fusion comparison of the standard and modified techniques. Spine (Phila Pa 1976). 1994;19:660–663. [PubMed] [Google Scholar]
- 6. Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602–617. [DOI] [PubMed] [Google Scholar]
- 7. Wright IP, Eisenstein SM. Anterior cervical discectomy and fusion without instrumentation. Spine (Phila Pa 1976). 2007;32:772–774. doi:10.1097/01.brs.0000258846.86537.ad [DOI] [PubMed] [Google Scholar]
- 8. Wang JC, McDonough PW, Kanim LEA, Endow KK, Delamarter RB. Increased fusion rates with cervical plating for three-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2001;26:643–646. doi:10.1097/00007632-200103150-00015 [DOI] [PubMed] [Google Scholar]
- 9. Emery SE, Fisher JR, Bohlman HH. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine (Phila Pa 1976). 1997;22:2622–2625. [DOI] [PubMed] [Google Scholar]
- 10. Kuhns CA, Geek MJ, Wang JC, Delamarter RB. An outcomes analysis of the treatment of cervical pseudarthrosis with posterior fusion. Spine (Phila Pa 1976). 2005;30:2424–2429. doi:10.1097/01.brs.0000184314.26543.7d [DOI] [PubMed] [Google Scholar]
- 11. McAnany SJ, Baird EO, Overley SC, Kim JS, Qureshi SA, Anderson PA. A meta-analysis of the clinical and fusion results following treatment of symptomatic cervical pseudarthrosis. Global Spine J. 2014;5:148–155. doi:10.1055/s-0035-1544176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simmons EH, Bhalla SK. . Anterior cervical discectomy and fusion. A clinical and biomechanical study with eight-year follow-up. J Bone Joint Surg Br. 1969;51:225–237. [PubMed] [Google Scholar]
- 13. Phillips FM, Carlson G, Emery SE, Bohlman HH. Anterior cervical pseudarthrosis: natural history and treatment. Spine (Phila Pa 1976). 1997;22:1585–1589. doi:10.1097/00007632-199707150-00012 [DOI] [PubMed] [Google Scholar]
- 14. Lindsey RW, Newhouse KE, Leach J, Murphy MJ. Nonunion following two-level anterior cervical discectomy and fusion. Clin Orthop Relat Res. 1987;(223):155–163. [PubMed] [Google Scholar]
- 15. Riley LH, Jr, Robinson RA, Johnson KA, Walker AE. The results of anterior interbody fusion of the cervical spine. Review of ninety-three consecutive cases. Spine (Phila Pa 1976). 1969;30:127–133. [DOI] [PubMed] [Google Scholar]
- 16. Martin GJ, Jr, Haid RW, Jr, Macmillan M, Rodts GE, Jr, Berkman R. Anterior cervical discectomy with freeze-dried fibula allograft: overview of 317 cases and literature review. Spine (Phila Pa 1976). 1999;24:852–859. doi:10.1097/00007632-199905010-00004 [DOI] [PubMed] [Google Scholar]
- 17. Leven D, Cho SK. Pseudarthrosis of the cervical spine: risk factors, diagnosis and management. Asian Spine J. 2016;10:776–786. doi:10.4184/asj.2016.10.4.776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cannada LK, Scherping SC, Yoo JU, Jones PK, Emery SE. Pseudoarthrosis of the cervical spine: a comparison of radiographic diagnostic measures. Spine (Phila Pa 1976). 2003;28:46–51. doi:10.1097/00007632-200301010-00012 [DOI] [PubMed] [Google Scholar]
- 19. Lee DH, Cho JH, Hwang CJ, et al. What is the fate of pseudarthrosis detected 1 year after anterior cervical discectomy and fusion? Spine (Phila Pa 1976). 2018;43:E23–E28. doi:10.1097/BRS.0000000000002077 [DOI] [PubMed] [Google Scholar]
- 20. Van Eck CF, Regan C, Donaldson WF, Kang JD, Lee JY. The revision rate and occurrence of adjacent segment disease after anterior cervical discectomy and fusion: a study of 672 consecutive patients. Spine (Phila Pa 1976). 2014;39:2143–2147. doi:10.1097/BRS.0000000000000636 [DOI] [PubMed] [Google Scholar]
- 21. Whitecloud TS. 3rd Anterior surgery for cervical spondylotic myelopathy. Smith-Robinson, Cloward, and vertebrectomy. Spine (Phila Pa 1976). 1988;13:861–863. doi:10.1097/00007632-198807000-00030 [DOI] [PubMed] [Google Scholar]
- 22. Steinhaus ME, Hill PS, Yang J, et al. Urinary N-telopeptide can predict pseudarthrosis after anterior cervical decompression and fusion (ACDF): a prospective study. Spine (Phila Pa 1976). 2019;44:770–776. doi:10.1097/BRS.0000000000002935 [DOI] [PubMed] [Google Scholar]
- 23. Carreon L, Glassman SD, Campbell MJ. Treatment of anterior cervical pseudoarthrosis: posterior fusion versus anterior revision. Spine J. 2006;6:154–156. doi:10.1016/j.spinee.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 24. Song KS, Piyaskulkaew C, Chuntarapas T, et al. Dynamic radiographic criteria for detecting pseudarthrosis following anterior cervical arthrodesis. J Bone Joint Surg Am. 2014;96:557–563. doi:10.2106/JBJS.M.00167 [DOI] [PubMed] [Google Scholar]
- 25. McInnes MDF, Moher D, Thombs BD, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy studies: the PRISMA-DTA statement. J Am Med Assoc. 2018;319:388–396. doi:10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 26. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi:10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 27. Smith W, Gillespy M, Huffman J, Vong V, McCormack BM. Anterior cervical pseudarthrosis treated with bilateral posterior cervical cages. Oper Neurosurg (Hagerstown). 2018;14:236–242. [DOI] [PubMed] [Google Scholar]
- 28. Elder BD, Sankey EW, Theodros D, et al. Successful anterior fusion following posterior cervical fusion for revision of anterior cervical discectomy and fusion pseudarthrosis. J Clin Neurosci. 2016;24:57–62. [DOI] [PubMed] [Google Scholar]
- 29. Liu H, Ploumis A, Schwender JD, Garvey TA. Posterior cervical lateral mass screw fixation and fusion to treat pseudarthrosis of anterior cervical fusion. J Spinal Disord Tech. 2012;25:138–141. [DOI] [PubMed] [Google Scholar]
- 30. Toohey JS, Stromberg L, Neidre A, Ramsey M, Fogel GR. Treatment of cervical pseudarthrosis after Smith-Robinson procedure with Halifax clamp fixation. J Surg Orthop Adv. 2006;15:201–202. [PubMed] [Google Scholar]
- 31. Gore DR, Brechbuler M. Treatment of nonunions following anterior cervical discectomy and fusion with interspinous wiring and bone grafting. J Surg Orthop Adv. 2003;12:214–217. doi:10.1007/s12206-014-0831-x [PubMed] [Google Scholar]
- 32. Bolesta MJ, Rechtine GR, 2nd, Chrin AM. Three- and four-level anterior cervical discectomy and fusion with plate fixation. Spine (Phila Pa 1976). 2000;25:2040–2046. [DOI] [PubMed] [Google Scholar]
- 33. Bolesta MJ, Rechtine GR, Chrin AM. One- and two-level anterior cervical discectomy and fusion: the effect of plate fixation. Spine J. 2002;2:197–203. doi:10.1016/S1529-9430(02)00186-9 [DOI] [PubMed] [Google Scholar]
- 34. Epstein NE. Evaluation and treatment of clinical instability associated with pseudarthrosis after anterior cervical surgery for ossification of the posterior longitudinal ligament. Surg Neurol. 1998;49:246–252. [DOI] [PubMed] [Google Scholar]
- 35. Siambanes D, Miz GS. Treatment of symptomatic anterior cervical nonunion using the Rogers interspinous wiring technique. Am J Orthop (Belle Mead NJ). 1998;27:792–796. [PubMed] [Google Scholar]
- 36. Coric D, Branch CL, Jr, Jenkins JD. Revision of anterior cervical pseudarthrosis with anterior allograft fusion and plating. J Neurosurg. 1997;86:969–974. [DOI] [PubMed] [Google Scholar]
- 37. Tribus CB, Corteen DP, Zdeblick TA. The efficacy of anterior cervical plating in the management of symptomatic pseudoarthrosis of the cervical spine. Spine (Phila Pa 1976).1999;24:860–864. [DOI] [PubMed] [Google Scholar]
- 38. Zdeblick TA, Hughes SS, Riew KD, Bohlman HH. . Failed anterior cervical discectomy and arthrodesis. Analysis and treatment of thirty-five patients. J Bone Joint Surg Am. 1997;79:523–532. [PubMed] [Google Scholar]
- 39. Lowery GL, Swank ML, McDonough RF. . Surgical revision for failed anterior cervical fusions. Articular pillar plating or anterior revision? Spine (Phila Pa 1976). 1995;20:2436–2441. [DOI] [PubMed] [Google Scholar]
- 40. Mutoh N, Shinomiya K, Furuya K, Yamaura I, Satoh H. Pseudarthrosis and delayed union after anterior cervical fusion. Int Orthop. 1993;17:286–289. [DOI] [PubMed] [Google Scholar]
- 41. Newman M. The outcome of pseduarthrosis after cervical anterior fusion. Spine (Phila Pa 1976). 1993;18:2380–2382. [DOI] [PubMed] [Google Scholar]
- 42. Brodsky AE, Khalil MA, Sassard WR, Newman BP. Repair of symptomatic pseudarthrosis of anterior cervical fusion: posterior versus anterior repair. Spine (Phila Pa 1976). 1992;17:1137–1143. [DOI] [PubMed] [Google Scholar]
- 43. Farey ID, McAfee PC, Davis RF, Long DM. Pseudarthrosis of the cervical spine after anterior arthrodesis. J Bone Joint Surg Am. 1990;72:1171–1177. [PubMed] [Google Scholar]
- 44. Kasliwal MK, Corley JA, Traynelis VC. Posterior cervical fusion using cervical interfacet spacers in patients with symptomatic cervical pseudarthrosis. Neurosurgery. 2016;78:661–668. [DOI] [PubMed] [Google Scholar]
- 45. Odom GL, Finney W, Woodhall B. Cervical disk lesions. J Am Med Assoc. 1958;166:23–28. doi:10.1001/jama.1958.02990010025006 [DOI] [PubMed] [Google Scholar]
- 46. Young IA, Dunning J, Butts R, Mourad F, Cleland JA. Reliability, construct validity, and responsiveness of the neck disability index and numeric pain rating scale in patients with mechanical neck pain without upper extremity symptoms [published online June 1, 2018]. Physiother Theory Pract. doi:10.1080/09593985.2018.1471763 [DOI] [PubMed] [Google Scholar]
- 47. Pool JJM, Ostelo RWJG, Hoving JL, Bouter LM, de Vet HC. Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine (Phila Pa 1976). 2007;32:3047–3051. doi:10.1097/BRS.0b013e31815cf75b [DOI] [PubMed] [Google Scholar]
- 48. Parker SL, Godil SS, Shau DN, Mendenhall SK, McGirt MJ. Assessment of the minimum clinically important difference in pain, disability, and quality of life after anterior cervical discectomy and fusion. J Neurosurg Spine. 2013;18:154–160. doi:10.3171/2012.10.SPINE12312 [DOI] [PubMed] [Google Scholar]
- 49. Carreon LY, Glassman SD, Campbell MJ, Anderson PA. Neck Disability Index, Short Form-36 physical component summary, and pain scales for neck and arm pain: the minimum clinically important difference and substantial clinical benefit after cervical spine fusion. Spine J. 2010;10:469–474. doi:10.1016/j.spinee.2010.02.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Appendix_-_Search_Strategy for Outcomes of Revision Surgery for Pseudarthrosis After Anterior Cervical Fusion: Case Series and Systematic Review by Michael E. Steinhaus, Philip J. York, Rachel S. Bronheim, Jingyan Yang, Francis Lovecchio and Han Jo Kim in Global Spine Journal