Abstract
The gut microbiota plays a fundamental role in host health and disease. Host diet is one of the most significant modulators of the gut microbial community and its metabolic activities. Evidence demonstrates that dietary patterns such as the ‘Western diet’ and perturbations in gut microbiome (dysbiosis) have strong associations with a wide range of human diseases, including obesity, metabolic syndrome, type-2 diabetes and cardiovascular diseases. However, consumption of Mediterranean-style diets is considered healthy and associated with the prevention of cardiovascular and metabolic diseases, colorectal cancers and many other diseases. Such beneficial effects of the Mediterranean diet might be attributed to high proportion of fibers, mono- and poly-unsaturated fatty acids, antioxidants and polyphenols. Concurrent literature has demonstrated beneficial modulation of the gut microbiome following a Mediterranean-style diet in humans as well as in experimental animal models such as rodents. We recently demonstrated similar positive changes in the gut microbiome of non-human primates consuming a Mediterranean-style diet for long term (30 months). Therefore, it is rational to speculate that this positive modulation of the gut microbiome diversity, composition and function is one of the main factors intermediating the health effects of Mediterranean diet on the host. The present perspective discusses the evidences that the Mediterranean diet induces gut microbiome modulation in rodents, non-human primates and human subjects, and discusses the potential role of gut microbiota and microbial metabolites as one of the fundamental catalysts intermediating various beneficial health effects of Mediterranean diet on the host.
Keywords: fiber, gut microbiota, Mediterranean diet, monkey, non-human primate, short-chain fatty acids, western diet
Introduction
Diet, gut microbiome and chronic diseases like obesity, diabetes, cancer and aging-related diseases such as Alzheimer’s disease are closely associated. Our dietary habits have a strong effect on our gut microbiome and metabolism. Unsolicited perturbations in gut microbiota diversity and composition (gut dysbiosis) are key elements underlying chronic diseases including several low-grade inflammatory disorders of human gastrointestinal tract 1. Specifically, low consumption of dietary fibers is known to induce long-term changes in the gut microbiome that are associated with low production of beneficial microbial metabolites, i.e., short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate involved in the modulation of host immune and inflammatory health status 2. Epidemiological investigations have reproducibly found that the ‘Western-style’ dietary habits (typically characterized by low consumption of fruits, vegetables, salads, fish and mono- and poly-unsaturated fatty acids such as fish and olive oil, and high consumption of simple sugars, saturated fat, red meat and processed foods) as one of the main perpetrators for the rising worldwide incidence of chronic diseases 3. From that perspective, a dietary-pattern intervention targeting gut microbiome can provide an effective avenue for the prevention and treatment of such chronic diseases.
Mediterranean diet: the intangible cultural and nutritional dietary pattern
Prompted by this evidence, a move towards a Mediterranean-style diet is exemplified as not only a prudent choice of lifestyle but also as a scientifically accepted mechanism that is able to yield the benefits for management of several human disease pathologies and an overall improvement of health and well-being. In 2013, the Mediterranean diet (hereafter, MD) was also enrolled on the “Representative List of the Intangible Cultural Heritage of Humanity” by the United Nations Educational, Scientific and Cultural Organization (UNESCO) 4. In 406 B.C., Hippocrates, the father of medicine, had already stated: “Let food be your medicine and medicine be your food”. Strikingly, this 20-centuries-old statement has been clearly and consistently validated by the ever-mounting literature indicating that the dietary habits can modulate predisposition to various human gastrointestinal, metabolic, cardiovascular and systemic diseases. A Mediterranean-style diet typifies a nutritionally balanced diet, characterized by intake in high amounts and frequency of important sources of fibers (cereals, vegetables, legumes, fruits and nuts) and chemical ingredients with anti-oxidative properties (vitamins, flavonoids, phytosterols, minerals, terpenes and phenols) ( Table 1) 5. In addition, high proportions of oleic acid, polyphenols and unsaturated fatty acids delivers significant anti-atherogenic and anti-inflammatory properties ( Table 2) 6. Studies have demonstrated that switching to a Mediterranean-style diet demonstrates amelioration in serum inflammation biomarkers as well as their gene expression profile (nutrigenomics), not only in healthy subjects 7. but also in patients with obesity, type-2 diabetes and Crohn's disease ( Table 2) 8– 15. Such dietary habit changes are also accompanied by specific changes in the population level of several gut microbial groups 6, 16– 20. Although the association of diet-microbiome interactions with the host health and disease status remains to be comprehended by means of all-inclusive and mechanistic epidemiological and omics investigations and specific disease related pathologies; however, advancements in novel hypotheses and postulations have already exceeded over and beyond merely a speculation stage. In this context, while concurring that MD represents a promising, efficacious and holistic approach to maintain/restore host heath, it is equitable to believe that (many of) these health benefits of MD are mediated via modulation of host gut microbial clades and their metabolic functions ( Figure 1).
Table 1. Nutritional characteristics of a typical Mediterranean-style dietary pattern.
| Ingredient | Frequency of consumption |
|---|---|
| Variety of unprocessed or minimally processed whole grains,
cereals, and legumes |
Regular staple |
| Variety of fresh fruits, vegetables, and salads | Daily basis (seasonal varieties) |
| Dry fruits, nuts, seeds, honey | Regular basis (generally as snacks) |
| Extra-virgin olive oil; fish oil | Principal source of fat (rich in poly- and mono-saturated fatty acids; low in
saturated fat) |
| Poultry (eggs and chicken) | Low to moderate consumption |
| Sea food (fish, oysters, sea weeds) | Low to moderate consumption |
| Red and processed meats | Very low consumption |
| Unprocessed or minimally processed cheese and yogurt | Low to moderate consumption |
| Wine | Low to moderate (occasionally; particularly with evening meals) |
Figure 1. A diagrammatic overview depicting how the various health beneficial effects of Mediterranean diet might be mediated via positive changes in the gut microbiome composition and the gut microbiota-derived metabolites.
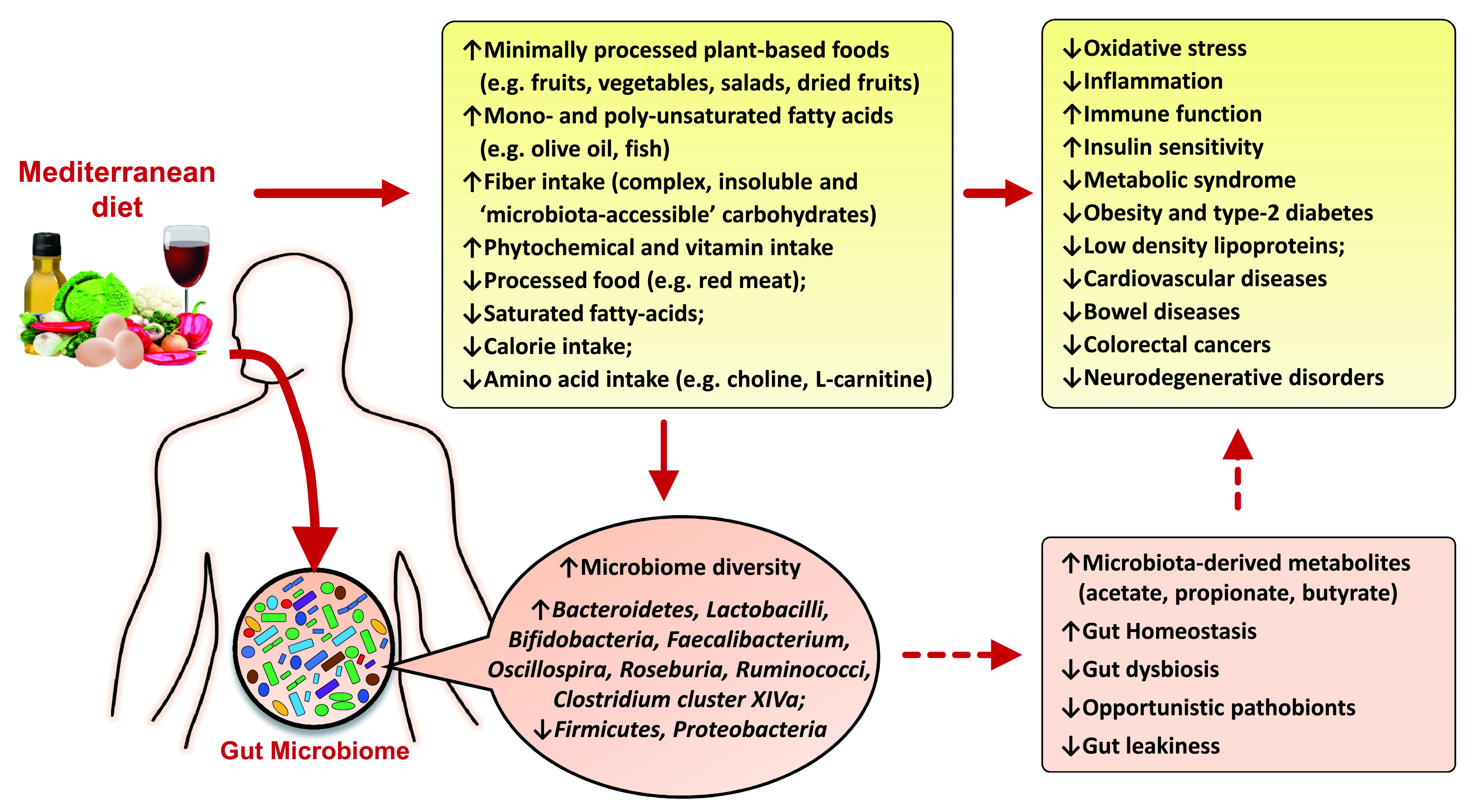
References are shown in square brackets.
MD, gut microbiome and host health
The human gut microbiome is comprised of tens of trillions of microbial cells. The association of gut microbiome dysbiosis with various dramatically rising human diseases such as obesity, type-2 diabetes and aging-related diseases like Alzheimer’s disease spurs urgent need for devising effective and safe treatments using gut microbiome modulators. Our dietary practices have an immense effect on many features of gut microbes. One of the primary functions of gut microbiome is to metabolize dietary ingredients in a way that the products of this biotransformation (e.g., SCFAs, vitamins, bioactive derivative compounds and modified plant flavonoids) can benefit various aspects of human health and metabolism 51. Mediterranean-style diets are considered nutritionally balanced and scientifically recommended dietary pattern ( Table 1) 5 that are beneficial for the prevention and/or treatment of obesity, type-2 diabetes, inflammatory disorders and cardiovascular diseases ( Figure 1 and Table 2) 8, 10, 11, 22, 52– 54. On the other hand, consumption of Western-style diets (mainly omnivore-type diets) may lead to a gut microbiome composition that is more associated with several diseases 55. This increasing understanding about the importance of diet-microbiome interactions and their strong influence on human health has led to a novel and timely concept of exploring and developing ‘microbiota-directed’ foods’ that can function by modulating the functional spectrum of the gut microbial community, providing precursor substratum for microbial biotransformation to bioactive molecules beneficial for the host health, or a combination of both of these approaches 56. This could provide novel and potential ways for improving host health by fostering/supporting healthy gut microbial communities, restoring the loss of microbial diversity associated with Western-style diets, and ameliorating the structural and functional dysbiosis associated with various human diseases. One example of this microbiota-directed food is ‘prebiotic’, which is defined as “a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon/gut 57. Indeed, a food can include one or more prebiotics (e.g., plant fibers, inulin, chicory, galacto-oligosaccharides, and others) capable of being processed by selective array of commensal/ beneficial gut microbes, leading to the production of beneficial metabolites like SCFAs. These SCFAs act as key mediators of the beneficial effects of such fibrous diets on host health 51, 58.
Table 2. Different components of a typical Mediterranean-style diet and their association with various health benefits.
| MD ingredient | Positive health
effect |
Possible mechanism(s) | Reference |
|---|---|---|---|
| Fruits, vegetables, fiber, yogurt, probiotics/
prebiotics, vitamins |
Gut homeostasis | ↑Gut homeostasis
↑Beneficial gut bacteria, SCFAs ↑Gut immune system ↓Gut leakiness, opportunistic pathogens ↓Gut and systemic inflammation |
16– 19, 21 |
| PUFA and MUFA, fruits, vegetables, poultry | Cardiovascular health | ↑Gut homeostasis
↑Gut permeability, beneficial gut bacteria, SCFAs ↓Gut dysbiosis ↓Gut leakiness, gut and systemic inflammation -Improved blood lipid and cholesterol profiles, ↑HDL ↓LDL and VLDL ↓Atherosclerosis |
22– 28 |
| Whole grains, fruits, vegetables, fiber, tea,
polyphenols, minerals, vitamins, low- glycemic foods |
Liver diseases | ↑Gut homeostasis
↑Gut permeability, beneficial gut bacteria, SCFAs ↓Gut dysbiosis ↓Gut leakiness, gut and systemic inflammation ↓Liver toxicity and inflammation ↓Oxidative stress |
29, 30 |
| Fruits, vegetables, PUFA and MUFA,
polyphenols, vitamins C and B 12 and folic acid, carotenoids |
Brain diseases | ↑Gut homeostasis
↑Gut permeability, beneficial gut bacteria, SCFAs ↓Gut dysbiosis ↓Gut leakiness, gut and systemic inflammation ↓Gut and brain inflammation ↑Enteric nervous system ↑Memory and cognition ↓Stress, depression and hypertension |
31– 33 |
| PUFA, MUFA, nuts, antioxidants, flavonoids,
yogurt, whole milk |
Asthma and allergy | ↑Immune-potentiation
↓Systemic inflammation ↓Oxidative stress |
34– 37 |
| Fiber, vegetables, MUFA, probiotics/
prebiotics, antioxidants, low-glycemic foods, vitamins |
Diabetes | ↑Gut homeostasis
↑Gut permeability, beneficial gut bacteria, SCFAs ↑Gut immune function ↓Gut leakiness ↑Immune-potentiation ↓Endotoxemia ↓Metabolic syndrome ↓Systemic low-grade inflammation ↓Oxidative stress |
11– 15, 38– 40 |
| Fiber, probiotics, PUFA, MUFA | Kidney diseases | ↑Gut permeability, beneficial gut bacteria, SCFAs
↓Gut leakiness, gut and systemic inflammation ↑Saccharolytic gut bacteria ↓Proteolytic gut bacteria ↓p-cresol indoxyl sulphate |
41– 43 |
| Fresh fruits and vegetables, vitamins C and
E, β-carotene |
Gastric disorders | ↑Gastric homeostasis
↓Opportunistic pathogens ↓Chemical contaminants ↓Oxidative stress ↓Ulcers |
44 |
| Fiber, PUFA/MUFA, probiotics/prebiotics,
antioxidants, vitamins, polyphenols |
Colorectal cancers | ↑Immune-function
↓Low-grade inflammation ↓Oxidative stress |
24, 44– 48 |
| Fiber,
probiotics/prebiotics, omega-3 fatty acids, antioxidants, low-glycemic index, polyphenols |
Obesity/ Weight loss | ↑Gut homeostasis
↑Gut permeability, beneficial gut bacteria, SCFAs ↓Gut leakiness ↑Insulin sensitivity ↑GLP-1, PYY ↑Satiety ↓Colonic transit ↓Fasting glucose, C-peptide ↓IGF-1 ↓Nutrient sensing ↓Oxidative stress |
49, 50 |
GLP-1, glucagon-like peptide 1; HDL, high-density lipoproteins; IGF, insulin-like growth factor; LDL, low-density lipoproteins; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; PYY, peptide YY; SCFAs, short-chain fatty acids; VLDL, very low density lipoproteins.
Higher adherence to MD has been found to be associated with reduced incidences of several chronic diseases, such as obesity, type-2 diabetes, metabolic syndrome, gastrointestinal cancer, cardiovascular diseases, fatty liver diseases, chronic kidney diseases and neurodegenerative diseases like Alzheimer’s disease ( Table 2) 59– 63. Based on different studies, the lowering effects of MD against the incidence of these disorders have been attributed to different MD dietary constituents derived from fruits, vegetables, lean meat, nuts and grains, fibers, mono- and poly-unsaturated fatty acids, vitamins, polyphenols, and antioxidant ( Table 2) 64– 66. Considering that the pathology of the above-mentioned diseases involves a dysbiotic gut microbiome and that the ingredients of the MD closely interact with gut microbial community, it can be suggested that the mechanisms by which MD drives health effects on the host might be mediated by an integrated interplay between the diet and the gut microbiome ( Figure 1). Two main mechanisms underlying these positive effects of MD are (a) beneficial modulation of the gut microbiota (i.e., increased microbial diversity and production of beneficial metabolites like SCFAs), and (b) reduced metabolic endotoxemia by suppressing the growth of gram-negative bacteria and improving the gut barrier integrity by modulating tight junctions and mucus secretion 67. Both of these mechanisms can help in reducing the systemic inflammation, which is the hallmark of many chronic diseases in the human body. Table 2 presents some of the reported beneficial health effects of Mediterranean-style diets and summarizes how different MD components could prevent/ameliorate these disorders by promoting a homeostatic gut microbiome and permeability to maintain the balanced arrays of the bacterial metabolites and the inflammatory molecules across the gut epithelium.
Microbiome-mediated pro-health effects of MD: purported and speculated mechanisms
Extensive literature is available about the health benefits of MD; however, knowledge concerning the impact of MD on gut microbiota-mediated disease outcomes remain limited. The precise mechanism by which MD confers its beneficial effects on host health such as lowering the risk of cardiovascular, metabolic and gastrointestinal diseases and certain cancers remains unclear. However, most of these beneficial effects have long been speculated to be mediated by several interrelated and overlapping factors including cholesterol-lowering effect, protection against oxidative stress and inflammation, modification of hormones/growth factors involved in carcinogenesis, and control/inhibition of nutrient-sensing pathways by restriction of specific amino acids 66. In addition, recent literature suggests that diet has a major impact on gut microbiome and the production of gut microbiota-derived microbial metabolites, which can considerably influence the host metabolic health 68. Ever-mounting metagenomic studies have suggested that specific nutrients, particularly dietary fiber and proteins, exert strong effects on gut microbiota composition as well as on the production of microbial metabolites that further influence multiple features of the host immune and metabolic health 69, 70. For example, trimethylamine N-oxide (TMAO), a gut microbial metabolites from dietary choline and L-carnitine, is known to increase the risk of cardiovascular diseases independently of cardio-metabolic risk factors 71. TMAO, at abnormally high levels, can induce vascular inflammation and prothrombosis by exaggerating platelet hyper-responsiveness to multiple agonists, and could be implicated in the pathogenesis of obesity and type 2 diabetes 72, 73. Given that a typical Mediterranean-style diet contains a proportion of choline and L-carnitine (which are abundant in eggs, red meat, and cheese) that is over 50% lower than a typical Western-style diet, it could be speculated that some of the beneficial effects of MD on host cardio-metabolic health might at least partly be mediated via such microbiome-related mechanisms. On the other hand, studies have shown that a poor adherence to a Mediterranean-style diet is associated with higher urinary TMAO level 16. It is acknowledged that the physiological effects of the gut microbiota on host health are mediated not only by the direct interaction of the microbe with the host but also equivalently by the indirect microbial processes including the production of fermentation metabolites from diet or de novo. Thus, the array of microbial metabolites in the host gut from dietary fermentation is a function of not only the gut microbial ecology but also the substrate such as the fermentation of complex carbohydrates (e.g., fiber) or other plant-based foods, which are abundant in a Mediterranean-style diet, that leads to the production of beneficial SCFAs, in contrast to TMAO, which is frequently observed in people consuming Western-style diets poor in minimally processed plant-based foods and rich in processed red meats.
MD is also rich in complex and insoluble fiber content when compared to a typical Western-style diet. A high intake of dietary fiber is well known to promote the beneficial modulation/maintenance of the gut microbiota with a reduced population of Firmicutes, while increasing that of Bacteroidetes, thereby yielding high levels of SCFAs including butyrate in the gut. These microbiota-derived metabolites including acetate, propionate and butyrate are known to protect against the development of several intestinal, inflammatory and allergic disease, and some of these effects are thought to be mediated via binding of these metabolites to specific G-protein-coupled receptors expressed on enteroendocrine and immune cells 74. Adherence to a Mediterranean-style diet has been shown to reshape the gut microbiota of obese individuals, with increased population of Bacteroides, Prevotella, Roseburia, Ruminococcus, and Faecalibacterium prausnitzii, which are known for their fibrolytic activity, producing SCFAs by metabolizing carbohydrates 18. Notably, Bacteroides fragilis and F. prausnitzii are also known to confer anti-inflammatory effects via inducing CD4+ T cells, the secretors of the anti-inflammatory interleukin-10 75, 76. In addition, the high content of vegetables, legumes, and fruit in a Mediterranean-style diet are also found to be associated with increased intestinal levels of short-chain fatty acids. These reports suggest that the Mediterranean-style diet fosters beneficial bacteria in the gut, which subsequently secretes beneficial metabolites.
Another reason that could underlie the health beneficial effects of Mediterranean-style diet is the lower intake of processed foods, as the Mediterranean dietary pattern is rich in minimally processed food, unlike a Western-style dietary pattern, which contains a much higher proportion of processed plant- and animal-based foods. This minimized intake of processed plant food with restricted calorie intake through MD is also known to confer positive effects on the gut microbiota diversity and composition by protecting/fostering the homeostasis of population levels of several beneficial bacterial groups 77. On the other hand, long-term adherence to a Western-style dietary pattern, which has very low content of complex, insoluble, minimally processed, and microbiota-accessible plant-based fibers and carbohydrates, can lead to a dysbiotic gut microbiota, with various subdominant but important bacterial groups vanishing over generations, and may also negatively influence several important features of the host immune system, thereby increasing the predisposition to various gastrointestinal, metabolic and immune diseases 74, 76, 78. Several studies have shown that the healthier and calorie-restricted dietary patterns with a high proportion of minimally processed plant-based foods can reprogram several important gut microbial functions that are crucial for promoting host health and wellbeing 77, 79.
Emerging evidence shows that the adherence to a MD is associated with higher gut microbial diversity. The CORonary Diet Intervention with Olive Oil and Cardiovascular PREVention (CORDIOPREV) study involving 138 participants with metabolic syndrome and 101 healthy counterparts has reported restoration of the gut microbial dysbiosis in metabolic syndrome patients following long-term adherence to the MD, although the disease persisted 80. Interestingly, the study demonstrated that the MD adherence could restore the patients’ gut microbiota in a similar way to the gut microbiota composition seen in metabolically healthy subjects, by fostering the population of saccharolytic bacterial genera, including Bacteroidetes, Faecalibacterium, Roseburia and Ruminococci, all of which are known to be associated with increased fermentation capacity to produce healthy SCFAs in the gut. A long-term adherence to the MD has also been found to increase the population of Roseburia sp. and Oscillospira sp., in addition to improving insulin sensitivity in obese people 81. Notably, Roseburia is a prominent butyrate-producing genus that has been found to confer anti-inflammatory effects and is generally found to be reduced in type-2 diabetes patients 82, 83. These reports suggest that a MD might be effective in the prevention and management of type-2 diabetes, although more studies are needed to affirm these therapeutic effects in particular reference to their connection with gut microbiota-associated factors.
Altogether, these reports suggest that the MD modulates gut microbiota profiles (such as higher population levels of Bacteroidetes, Clostridium cluster XIVa, Faecalibacterium prausnitzii, Lactobacilli, and Bifidobacteria, or lower Firmicutes) and can influence the diversity, activities and functionalities of various gut bacteria, thereby also fostering healthy metabolites (such as SFCAs) that can confer multiple benefits to the host intestinal, metabolic and immune health. Nevertheless, broader and more inclusive clinical and epidemiological studies assessing the temporal changes in the composition and function of gut microbiota enterotypes are still needed to establish a gut microbiome signature as a marker of MD adherence.
Non-human primates (NHPs): an ideal model to elucidate diet-microbiome interactions in human health and disease
Given the profound impact of diet on the diversity and composition of host gastrointestinal microbiome 68, 84– 86, the gut microbiome can be illustrated as a valuable biomarker of long-term intake of healthy or unhealthy diets. Therefore, it is imperative to elucidate whether, how and to what extent these long-term dietary patterns can influence the composition of the gut microbiota and how this could affect the production of beneficial microbial metabolites. As mentioned above, diet shapes the gut microbiome by supplying specific substrates that differentially foster the growth of specific gut microbial communities 87– 89; this diet-microbiome network is universally consistent in human and animal studies 85, 87, 90, 91. Specifically, the majority of diet-microbiome-targeted studies focus on high-fat, high-sugar, low-fiber vs. low-fat, low-sugar, high-fiber diets, with particular reference to nutrition- and gut-related maladies, including obesity, endotoxemia, insulin resistance, type-2 diabetes, and other metabolic syndromes 92, 93. Several animal models, including those in mice, hamsters, rats, guinea pigs and zebrafish are used for investigating diet-microbiome interactions, but, given the specific and prominent impact of dietary patterns on the gut microbiome, these models might not invariably be truly translatable to human milieus owing to far-reaching dissimilarities in their dietary regimen, age, body size and environmental elements. Accordingly, novel models such as NHPs are being sought for investigations of human diets and their interactions with gut microbiome, to decipher better understanding in human inference 92, 94– 100. In addition, while it is challenging to precisely control and monitor dietary patterns through questionnaires in human interventions, studies performed on small animals are disadvantaged by short-term diet intervention periods. To overcome these limitations, NHPs represent an excellent model for investigating the diet-microbiome interactions and their impact on host health. Their physiological and phylogenetic closeness to humans makes them a perfect and biologically relevant animal model for human context to examine diet-microbiome connections as well as for studying the relation of this network with different nutrition- and gut-related diseases 95, 96, 99– 102. In one such endeavor, we recently performed a study wherein we demonstrated the effect of a long-term Western- vs. Mediterranean-style diet intake on the gut microbiome composition in NHPs (Cynomolgus monkeys; Macaca fascicularis) 21. Previous reports have demonstrated that the gut microbiome of NHPs resembles more closely to those of primates than other animals 103. The human gut largely harbors microbes belonging to nine different bacterial divisions, viz. Firmicutes, Bacteroides, Actinobacteria, Proteobacteria, Verrucomicrobia, Fusobacteria, Spirochaetes, Cyanobacteria, and VadinBE97 104, 105. Interestingly, our NHP data also demonstrated Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Verrucomicrobia, Fibrobacteres, Spirochaetes, Cyanobacteria, and Tenericutes as most abundant bacterial phyla 21. This again validates that the NHP microbiome studies can yield important indications about specific features of these bacterial clades in the human gut and provide unique opportunities to investigate diet-microbiome interactions.
Effect of MD on NHP microbiome: similar findings as seen in human and rodent studies
Our data obtained feeding a MD to NHPs also demonstrated that MD boosted the gut microbiome diversity and promoted the carriage of several important bacterial groups, including Bacteriodes, Prevotella, Lactobacillus, Faecalibacterium, Clostridium and Oscillospira 21, again corroborating that the host diet can influence gut microbiome diversity and composition. Notably, similar effects as seen in the NHP cohort have also previously been seen in human studies ( Figure 2). For example, diets rich in fiber and unsaturated fatty acids are known to help maintaining a healthy and diverse gut microbiome. Our NHP data also demonstrated higher alpha-diversity in NHPs consuming the MD, an effect that has also been observed in several other studies on humans or animal animals 68, 78, 99, 106 and can be attributed to a higher proportion of fiber in the MD. In addition, our NHP study also demonstrated higher Bacteroidetes-Firmicutes ratio and higher abundance of Clostridium and Prevotella in NHPs on MD. Similar results have been reported by previous studies performed in humans and other mammals reporting that MD (and specifically the higher fiber intake and less intake of high-glycemic index sugars) positively alters the gut microbiota, with an increased Bacteroides, Clostridium and Prevotella population 8, 88, 99, 107– 109 whereas a low-fiber, high-fat and high-sugar diet is linked with increased Firmicutes and reduced Bacteroides population 88. Another interesting observation was the increased abundance of genus Oscillospira in MD-fed NHPs. Oscillospira, a genus from the Ruminococcaceae family, is usually found prevalent in the gastrointestinal tract of ruminants consuming diets rich in complex plant fibers and hence is regarded as genus adapted to vegetable-rich diets, such as Mediterranean-style diets 110, 111.
Figure 2. Effects of Mediterranean diet on the population of major gut bacterial groups that have been reported consistently in different studies involving non-human primates and human subjects.
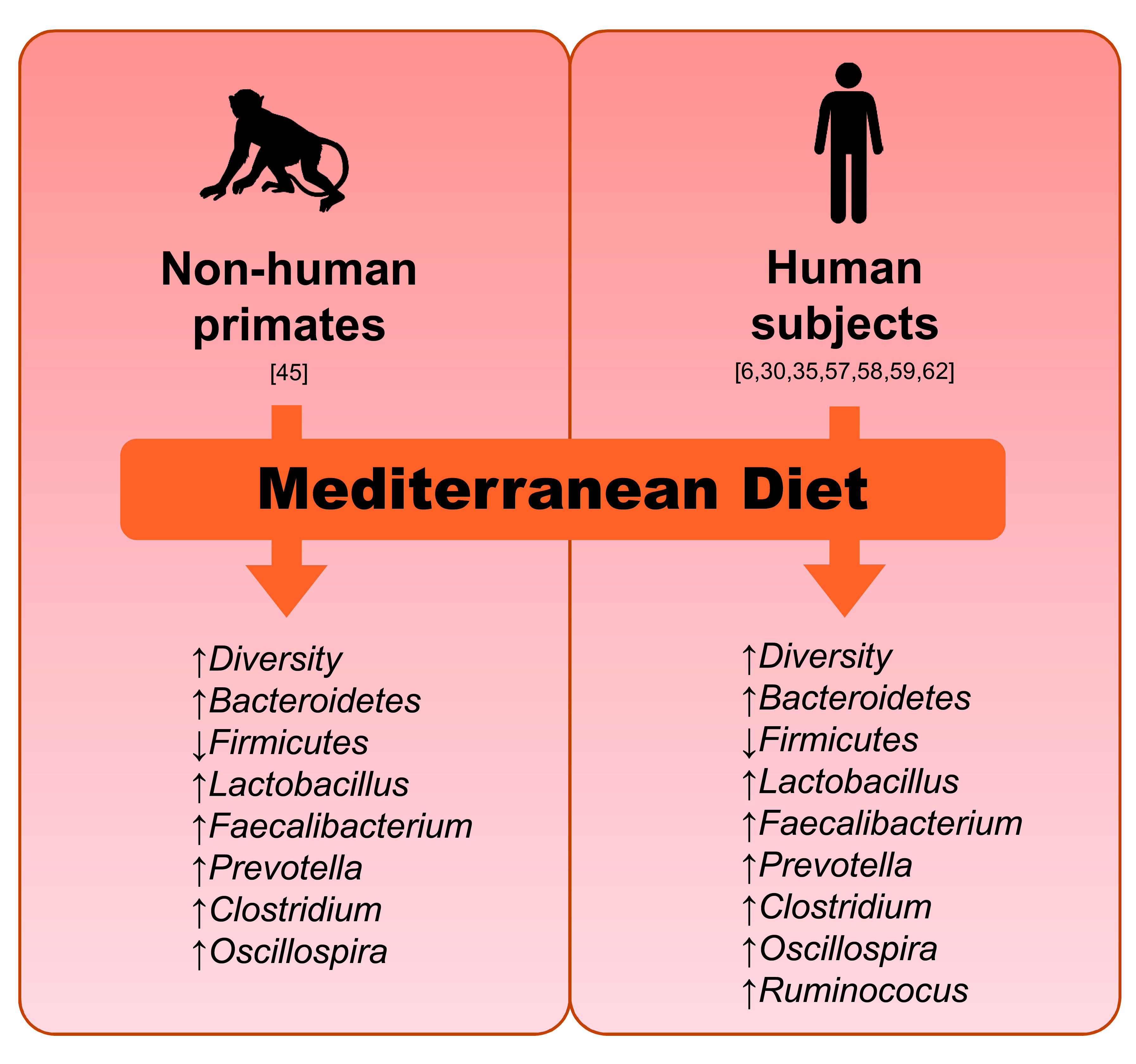
Another interesting observation from this NHP study was the increased abundance of Faecalibacterium prausnitzii, one of the beneficial butyrate-producer previously reported to be found in higher numbers in humans consuming a Mediterranean-style diet 111– 113. Various human and small animal studies have reproducibly reported fostering of a population of beneficial bacterial groups, including Lactobacillus sp 114– 116. and Faecalibacterium sp 117. following consumption of diets that are rich in complex carbohydrates, such as prebiotics. In addition, omega-3 fatty acids, which are present in higher ratio in Mediterranean-style diet, can also stimulate the intestinal population of several beneficial bacteria including lactobacilli that typically inhabit the distal gut, a primary site for the metabolism of mono- and poly-unsaturated fatty acids 9, 118, 119. Interestingly, in a recent study on these NHPs, we demonstrated that the consumption of MD also leads to an increased Lactobacillus abundance and increased bacterially processed bioactive compounds in the mammary glands compared with Western diet-fed monkeys, clearly indicating that the influence of MD on the microbiome reaches far outside the gut in distal sites such as the mammary glands and could also establish an alternative pathway for breast cancer prevention 120. Conversely, high-fat diets are also known to reduce the intestinal carriage of lactobacilli 93, 121, 122. In addition, we have recently reported that MD also protects these NHPs against hepatosteatosis while reducing BMI and body fat 123. Hence, these common findings between NHP and human studies clearly demonstrate and advocate that NHPs can prove to be an excellent experimental animal model to examine the diet-microbiome interaction in context to human health and disease.
Altogether, these findings from our cohort of the MD-fed NHPs demonstrate that the positive modulation of the gut microbiome is concomitant with the beneficial effects of MD on host health and hint that this microbiome modulation might even at least in part also mediate/underlie the health effects of MD, particularly given that the majority of the MD-induced changes in the gut microbiome including enhanced microbial diversity and fostering of several beneficial bacterial groups are known to be beneficial for host intestinal, metabolic and overall health 21. However, although the health benefits of MD have been studied extensively, research on MD’s impact on microbiome-mediated disease outcomes still remains undetermined. For instance, it is known that MD consumption leads to gut microbiome alterations; however, the magnitude to which these alterations occur may be potentially impacted by multiple factors such as the study duration, host age and lifestyle habits, specific disease predisposition or severity, level of dietary adherence, etc. which otherwise remain to be explicated and hence would be prerequisite to pinpoint the factors contributing to the outcome of MD on gut microbiome and deliver conclusive data on the role of gut microbiome in MD’s beneficial health outcomes 124, 125. As a result, according to the existing literature, the examination of gut microbial composition could not be endorsed as a stand-alone tool for the health effects of MD. To this end, systemic approaches that incorporate gut metagenomics, transcriptomics and metabolomics could help elucidate the complex association of MD-microbiome interrelationship with host health.
Perspectives on the future
Recent evidences from animal and human studies are beginning to elucidate the mechanisms underlying the pro-health effects of the traditional MD. The metabolism and fermentation of plant-based foods packed with complex fibers, a wide variety of vitamins and phytochemicals, could also play an important role in promoting host metabolic health. Although more research is needed, specific dietary patterns (particularly, the Mediterranean-style diet) have been found to have a strong influence on the gut microbiome composition and metabolic function, suggesting that there is an opportunity to prevent and treat various lifestyle-related disorders based on gut microbiota outcomes. Given the plasticity of the gut microbiota, it can be speculated that such Mediterranean-style nutritional intervention can positively affect host health, either by maintaining a physiologically homeostatic gut microbiota configuration or by reversing the gut dysbiosis state; this positive modulation of the gut microbiome could be considered as a potential and holistic target for non-pharmacological interventions in a variety of disease states. The potentiality of the gut microbiome is tremendous. It can be easily and rapidly modulated in a natural, non-invasive, non-pharmacologic way, i.e., via diets and other dietary supplements like probiotics and prebiotics. The ever-mounting knowledge of the gut microbiome as acquired by the use of remarkable high-throughput sequencing and omics tools in combination with the data from various in vitro and in vivo experimental models is shedding a new light on the key mechanisms through which diet-microbiome crosstalk in the gut can modulate potential risk factors of several human diseases. This wealth of knowledge is certainly going to offer potential avenues for decoding the complexities and functionalities of the diet-microbiome interface and for developing personalized dietary strategies to determine the healthiest dietary pattern for the personalized host.
In conjunction with gut microbial composition in response to diets like MD and the Western diet, it is becoming evident that the microbial metabolites produced by gut microbiome and dietary interactions plays an important role to regulate host metabolism and physiology 126. MD feeding enhances abundance of probiotics or beneficial bacteria like lactobacilli 21, however, future studies focusing on determining how the increased abundance of lactobacilli benefit host health will be important to conclude the mechanistic views. It is difficult to pinpoint exact molecular mechanism(s) by which diets and ingredients can impact human health; however, several layers of mechanistic studies like systemic, organ- and cell-based mechanism should be the focus of future research. Such studies can provide important information to include the vital ingredients that not only enhance the impact of diets like MD, but can easily be tailored for personalized nutritional approaches. Combining gut microbiome, and metabolic response (like the glycemic index) of diets on individual basis are attractive area of current research, that can extend valuable outcome in the field of diet-microbiome interactions to benefit human health.
Data availability
No data are associated with this article.
Funding Statement
The authors gratefully acknowledge funding support for this work from the Center for Diabetes, Obesity and Metabolism, the National Institutes of Health funded the Wake Forest Alzheimer's Disease Research Center (funded by P30AG049638); OAIC Pepper Center (P30AG021332); R01AG058829; R01DK099164; R01AG018915; R01HL122393; R01HL087103 and the Clinical and Translational Science Center (Clinical Research Unit, funded by UL1TR001420), all at Wake Forest School of Medicine. We also acknowledge support from Department of Defense funding (Grant numbers: GRANT12726940 and W81XWH-18-1-0118).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Brown K, DeCoffe D, Molcan E, et al. : Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4(8):1095–1119. 10.3390/nu4081095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maslowski KM, Mackay CR: Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. 10.1038/ni0111-5 [DOI] [PubMed] [Google Scholar]
- 3. Devereux G: The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6(11):869–74. 10.1038/nri1958 [DOI] [PubMed] [Google Scholar]
- 4. UNESCO: Mediterranean diet.2013. Reference Source [Google Scholar]
- 5. Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, et al. : Diet and overall survival in elderly people. BMJ. 1995;311(7018):1457–1460. 10.1136/bmj.311.7018.1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Del Chierico F, Vernocchi P, Dallapiccola B, et al. : Mediterranean diet and health: food effects on gut microbiota and disease control. Int J Mol Sci. 2014;15(7):11678–11699. 10.3390/ijms150711678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellett S, et al. : in Nutrigenomics and nutrigenetics in functional foods and personalized nutrition. (CRC Press).2016;286–297. [Google Scholar]
- 8. Marlow G, Ellett S, Ferguson IR, et al. : Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn's disease patients. Hum Genomics. 2013;7:24. 10.1186/1479-7364-7-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaliannan K, Wang B, Li XY, et al. : Omega-3 fatty acids prevent early-life antibiotic exposure-induced gut microbiota dysbiosis and later-life obesity. Int J Obes (Lond). 2016;40(6):1039–42. 10.1038/ijo.2016.27 [DOI] [PubMed] [Google Scholar]
- 10. Lopez-Legarrea P, Fuller NR, Zulet MA, et al. : The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac J Clin Nutr. 2014;23(3):360–368. 10.6133/apjcn.2014.23.3.16 [DOI] [PubMed] [Google Scholar]
- 11. Salas-Salvadó J, Bulló M, Babio N, et al. : Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34(1):14–19. 10.2337/dc10-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salas-Salvadó J, Bulló M, Estruch R, et al. : Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. 2014;160(1):1–10. 10.7326/M13-1725 [DOI] [PubMed] [Google Scholar]
- 13. Martínez-González MA, de la Fuente-Arrillaga C, Nunez-Cordoba JM, et al. : Adherence to Mediterranean diet and risk of developing diabetes: prospective cohort study. BMJ. 2008;336(7657):1348–1351. 10.1136/bmj.39561.501007.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Esposito K, Maiorino MI, Di Palo C, et al. : Adherence to a Mediterranean diet and glycaemic control in Type 2 diabetes mellitus. Diabet Med. 2009;26(9):900–907. 10.1111/j.1464-5491.2009.02798.x [DOI] [PubMed] [Google Scholar]
- 15. Tortosa A, Bes-Rastrollo M, Sanchez-Villegas A, et al. : Mediterranean diet inversely associated with the incidence of metabolic syndrome: the SUN prospective cohort. Diabetes Care. 2007;30(11):2957–2959. 10.2337/dc07-1231 [DOI] [PubMed] [Google Scholar]
- 16. De Filippis F, Pellegrini N, Vannini L, et al. : High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–1821. 10.1136/gutjnl-2015-309957 [DOI] [PubMed] [Google Scholar]
- 17. Mitsou EK, Kakali A, Antonopoulou S, et al. : Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr. 2017;117(12):1645–1655. 10.1017/S0007114517001593 [DOI] [PubMed] [Google Scholar]
- 18. Haro C, García-Carpintero S, Rangel-Zúñiga OA, et al. : Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol Nutr Food Res. 2017;61(12):1700300. 10.1002/mnfr.201700300 [DOI] [PubMed] [Google Scholar]
- 19. Garcia-Mantrana I, Selma-Royo M, Alcantara C, et al. : Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front Microbiol. 2018;9:890. 10.3389/fmicb.2018.00890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pignanelli M, Just C, Bogiatzi C, et al. : Mediterranean Diet Score: Associations with Metabolic Products of the Intestinal Microbiome, Carotid Plaque Burden, and Renal Function. Nutrients. 2018;10(6): pii: E779. 10.3390/nu10060779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagpal R, Shively CA, Appt SA, et al. : Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Front Nutr. 2018;5:28. 10.3389/fnut.2018.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Estruch R, Ros E, Salas-Salvadó J, et al. : Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–1290. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 23. Georgousopoulou EN, Kastorini CM, Milionis HJ, et al. : Association between Mediterranean diet and non-fatal cardiovascular events, in the context of anxiety and depression disorders: a case/case-control study. Hellenic J Cardiol. 2014;55(1):24–31. [PubMed] [Google Scholar]
- 24. Pauwels EK: The protective effect of the Mediterranean diet: focus on cancer and cardiovascular risk. Med Princ Pract. 2011;20(2):103–111. 10.1159/000321197 [DOI] [PubMed] [Google Scholar]
- 25. Kaliora AC, Dedoussis GV, Schmidt H: Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187(1):1–17. 10.1016/j.atherosclerosis.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 26. Dontas AS, Zerefos NS, Panagiotakos DB, et al. : Mediterranean diet and prevention of coronary heart disease in the elderly. Clin Interv Aging. 2007;2(1):109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panagiotakos DB, Dimakopoulou K, Katsouyanni K, et al. : Mediterranean diet and inflammatory response in myocardial infarction survivors. Int J Epidemiol. 2009;38(3):856–866. 10.1093/ije/dyp142 [DOI] [PubMed] [Google Scholar]
- 28. Pérez-Jiménez F, López-Miranda J, Mata P: Protective effect of dietary monounsaturated fat on arteriosclerosis: beyond cholesterol. Atherosclerosis. 2002;163(2):385–398. 10.1016/S0021-9150(02)00033-3 [DOI] [PubMed] [Google Scholar]
- 29. Abenavoli L, Di Renzo L, Boccuto L, et al. : Health benefits of Mediterranean diet in nonalcoholic fatty liver disease. Expert Rev Gastroenterol Hepatol. 2018;12(9):873–881. 10.1080/17474124.2018.1503947 [DOI] [PubMed] [Google Scholar]
- 30. Salamone F, Li Volti G, Titta L, et al. : Moro orange juice prevents fatty liver in mice. World J Gastroenterol. 2012;18(29):3862–8. 10.3748/wjg.v18.i29.3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Féart C, Samieri C, Allès B, et al. : Potential benefits of adherence to the Mediterranean diet on cognitive health. Proc Nutr Soc. 2013;72(1):140–152. 10.1017/S0029665112002959 [DOI] [PubMed] [Google Scholar]
- 32. Féart C, Samieri C, Rondeau V, et al. : Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. JAMA. 2009;302(6):638–648. 10.1001/jama.2009.1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valls-Pedret C, Lamuela-Raventós RM, Medina-Remón A, et al. : Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29(4):773–782. 10.3233/JAD-2012-111799 [DOI] [PubMed] [Google Scholar]
- 34. Garcia-Marcos L, Castro-Rodriguez JA, Weinmayr G, et al. : Influence of Mediterranean diet on asthma in children: a systematic review and meta-analysis. Pediatr Allergy Immunol. 2013;24(4):330–338. 10.1111/pai.12071 [DOI] [PubMed] [Google Scholar]
- 35. Arvaniti F, Priftis KN, Papadimitriou A, et al. : Adherence to the Mediterranean type of diet is associated with lower prevalence of asthma symptoms, among 10-12 years old children: the PANACEA study. Pediatr Allergy Immunol. 2011;22(3):283–289. 10.1111/j.1399-3038.2010.01113.x [DOI] [PubMed] [Google Scholar]
- 36. Castro-Rodriguez JA, Garcia-Marcos L, Sanchez-Solis M, et al. : Olive oil during pregnancy is associated with reduced wheezing during the first year of life of the offspring. Pediatr Pulmonol. 2010;45(4):395–402. 10.1002/ppul.21205 [DOI] [PubMed] [Google Scholar]
- 37. Chatzi L, Apostolaki G, Bibakis I, et al. : Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax. 2007;62(8):677–83. 10.1136/thx.2006.069419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marrazzo G, Barbagallo I, Galvano F, et al. : Role of dietary and endogenous antioxidants in diabetes. Crit Rev Food Sci Nutr. 2014;54(12):1599–1616. 10.1080/10408398.2011.644874 [DOI] [PubMed] [Google Scholar]
- 39. Paniagua JA, de la Sacristana AG, Sánchez E, et al. : A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J Am Coll Nutr. 2007;26(5):434–444. 10.1080/07315724.2007.10719633 [DOI] [PubMed] [Google Scholar]
- 40. Rocca AS, LaGreca J, Kalitsky J, et al. : Monounsaturated fatty acid diets improve glycemic tolerance through increased secretion of glucagon-like peptide-1. Endocrinology. 2001;142(3):1148–1155. 10.1210/endo.142.3.8034 [DOI] [PubMed] [Google Scholar]
- 41. Montemurno E, Cosola C, Dalfino G, et al. : What would you like to eat, Mr CKD Microbiota? A Mediterranean Diet, please! Kidney Blood Press Res. 2014;39(2–3):114–123. 10.1159/000355785 [DOI] [PubMed] [Google Scholar]
- 42. Mekki K, Bouzidi-bekada N, Kaddous A, et al. : Mediterranean diet improves dyslipidemia and biomarkers in chronic renal failure patients. Food Funct. 2010;1(1):110–115. 10.1039/c0fo00032a [DOI] [PubMed] [Google Scholar]
- 43. Ranganathan N, Ranganathan P, Friedman EA, et al. : Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther. 2010;27(9):634–647. 10.1007/s12325-010-0059-9 [DOI] [PubMed] [Google Scholar]
- 44. Praud D, Bertuccio P, Bosetti C, et al. : Adherence to the Mediterranean diet and gastric cancer risk in Italy. Int J Cancer. 2014;134(12):2935–2941. 10.1002/ijc.28620 [DOI] [PubMed] [Google Scholar]
- 45. Donovan MG, Selmin OI, Doetschman TC, et al. : Mediterranean Diet: Prevention of Colorectal Cancer. Front Nutr. 2017;4:59. 10.3389/fnut.2017.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Couto E, Boffetta P, Lagiou P, et al. : Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br J Cancer. 2011;104(9):1493–9. 10.1038/bjc.2011.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cappellani A, Zanghì A, Di Vita M, et al. : Strong correlation between diet and development of colorectal cancer. Front Biosci (Landmark Ed). 2013;18:190–198. [DOI] [PubMed] [Google Scholar]
- 48. Biondi A, Fisichella R, Fiorica F, et al. : Food mutagen and gastrointestinal cancer. Eur Rev Med Pharmacol Sci. 2012;16(9):1280–1282. [PubMed] [Google Scholar]
- 49. Shai I, Schwarzfuchs D, Henkin Y, et al. : Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–241. 10.1056/NEJMoa0708681 [DOI] [PubMed] [Google Scholar]
- 50. Kaaks R, Bellati C, Venturelli E, et al. : Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) Randomised Trial. Eur J Clin Nutr. 2003;57(9):1079–88. 10.1038/sj.ejcn.1601647 [DOI] [PubMed] [Google Scholar]
- 51. Koh A, De Vadder F, Kovatcheva-Datchary P, et al. : From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165(6):1332–1345. 10.1016/j.cell.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 52. Santoro A, Pini E, Scurti M, et al. : Combating inflammaging through a Mediterranean whole diet approach: the NU-AGE project's conceptual framework and design. Mech Ageing Dev. 2014;136–137:3–13. 10.1016/j.mad.2013.12.001 [DOI] [PubMed] [Google Scholar]
- 53. Craig WJ: Health effects of vegan diets. Am J Clin Nutr. 2009;89(5):1627S–1633S. 10.3945/ajcn.2009.26736N [DOI] [PubMed] [Google Scholar]
- 54. Craig WJ: Nutrition concerns and health effects of vegetarian diets. Nutr Clin Pract. 2010;25(6):613–620. 10.1177/0884533610385707 [DOI] [PubMed] [Google Scholar]
- 55. Albenberg LG, Wu GD: Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146(6):1564–1572. 10.1053/j.gastro.2014.01.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barratt MJ, Lebrilla C, Shapiro HY, et al. : The Gut Microbiota, Food Science, and Human Nutrition: A Timely Marriage. Cell Host Microbe. 2017;22(2):134–141. 10.1016/j.chom.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gibson GR, Roberfroid MB: Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. 10.1093/jn/125.6.1401 [DOI] [PubMed] [Google Scholar]
- 58. Joseph J, Depp C, Shih PB, et al. : Modified Mediterranean Diet for Enrichment of Short Chain Fatty Acids: Potential Adjunctive Therapeutic to Target Immune and Metabolic Dysfunction in Schizophrenia? Front Neurosci. 2017;11:155. 10.3389/fnins.2017.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schwingshackl L, Schwedhelm C, Galbete C, et al. : Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients. 2017;9(10): pii: E1063. 10.3390/nu9101063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grosso G, Marventano S, Yang J, et al. : A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit Rev Food Sci Nutr. 2017;57(15):3218–3232. 10.1080/10408398.2015.1107021 [DOI] [PubMed] [Google Scholar]
- 61. Schwingshackl L, Missbach B, König J, et al. : Adherence to a Mediterranean diet and risk of diabetes: a systematic review and meta-analysis. Public Health Nutr. 2015;18(7):1292–1299. 10.1017/S1368980014001542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sofi F, Abbate R, Gensini GF, et al. : Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92(5):1189–1196. 10.3945/ajcn.2010.29673 [DOI] [PubMed] [Google Scholar]
- 63. Mitrou PN, Kipnis V, Thiébaut AC, et al. : Mediterranean dietary pattern and prediction of all-cause mortality in a US population: results from the NIH-AARP Diet and Health Study. Arch Intern Med. 2007;167(22):2461–2468. 10.1001/archinte.167.22.2461 [DOI] [PubMed] [Google Scholar]
- 64. Jin Q, Black A, Kales SN, et al. : Metabolomics and Microbiomes as Potential Tools to Evaluate the Effects of the Mediterranean Diet. Nutrients. 2019;11(1): pii: E207. 10.3390/nu11010207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhao L, Zhang F, Ding X, et al. : Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science. 2018;359(6380):1151–1156. 10.1126/science.aao5774 [DOI] [PubMed] [Google Scholar]
- 66. Tosti V, Bertozzi B, Fontana L: Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J Gerontol A Biol Sci Med Sci. 2018;73(3):318–326. 10.1093/gerona/glx227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bailey MA, Holscher HD: Microbiome-Mediated Effects of the Mediterranean Diet on Inflammation. Adv Nutr. 2018;9(3):193–206. 10.1093/advances/nmy013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. David LA, Maurice CF, Carmody RN, et al. : Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Richards JL, Yap YA, McLeod KH, et al. : Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clin Transl Immunology. 2016;5(5):e82. 10.1038/cti.2016.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clemente JC, Ursell LK, Parfrey LW, et al. : The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148(6):1258–1270. 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tang WH, Wang Z, Levison BS, et al. : Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schugar RC, Shih DM, Warrier M, et al. : The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017;19(12):2451–2461. 10.1016/j.celrep.2017.05.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhu W, Gregory JC, Org E, et al. : Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165(1):111–124. 10.1016/j.cell.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thorburn AN, Macia L, Mackay CR: Diet, metabolites, and "western-lifestyle" inflammatory diseases. Immunity. 2014;40(6):833–842. 10.1016/j.immuni.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 75. Round JL, Mazmanian SK: Inducible Foxp 3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(27):12204–12209. 10.1073/pnas.0909122107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mazmanian SK, Liu CH, Tzianabos AO, et al. : An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. 10.1016/j.cell.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 77. Griffin NW, Ahern PP, Cheng J, et al. : Prior Dietary Practices and Connections to a Human Gut Microbial Metacommunity Alter Responses to Diet Interventions. Cell Host Microbe. 2017;21(1):84–96. 10.1016/j.chom.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sonnenburg ED, Smits SA, Tikhonov M, et al. : Diet-induced extinctions in the gut microbiota compound over generations. Nature. 2016;529(7585):212–215. 10.1038/nature16504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dey N, Wagner VE, Blanton LV, et al. : Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell. 2015;163(1):95–107. 10.1016/j.cell.2015.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, et al. : CORonary Diet Intervention with Olive oil and cardiovascular PREVention study (the CORDIOPREV study): Rationale, methods, and baseline characteristics: A clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low-fat diet on cardiovascular disease in coronary patients. Am Heart J. 2016;177:42–50. 10.1016/j.ahj.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haro C, Montes-Borrego M, Rangel-Zúñiga OA, et al. : Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J Clin Endocrinol Metab. 2016;101(1):233–242. 10.1210/jc.2015-3351 [DOI] [PubMed] [Google Scholar]
- 82. Karlsson FH, Tremaroli V, Nookaew I, et al. : Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. 10.1038/nature12198 [DOI] [PubMed] [Google Scholar]
- 83. Qin J, Li Y, Cai Z, et al. : A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- 84. Carmody RN, Gerber GK, Luevano JM, Jr, et al. : Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. 10.1016/j.chom.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yatsunenko T, Rey FE, Manary MJ, et al. : Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. 10.1038/nature11053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Flint HJ: The impact of nutrition on the human microbiome. Nutr Rev. 2012;70 Suppl 1:S10–S13. 10.1111/j.1753-4887.2012.00499.x [DOI] [PubMed] [Google Scholar]
- 87. Turnbaugh PJ, Ridaura VK, Faith JJ, et al. : The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14–16ra14. 10.1126/scitranslmed.3000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. De Filippo C, Cavalieri D, Di Paola M, et al. : Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–14696. 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moschen AR, Wieser V, Tilg H: Dietary Factors: Major Regulators of the Gut's Microbiota. Gut Liver. 2012;6(4):411–6. 10.5009/gnl.2012.6.4.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ou J, Carbonero F, Zoetendal EG, et al. : Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98(1):111–120. 10.3945/ajcn.112.056689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Suzuki Y, Ikeda K, Sakuma K, et al. : Association between Yogurt Consumption and Intestinal Microbiota in Healthy Young Adults Differs by Host Gender. Front Microbiol. 2017;8:847. 10.3389/fmicb.2017.00847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kišidayová S, Váradyová Z, Pristas P, et al. : Effects of high- and low-fiber diets on fecal fermentation and fecal microbial populations of captive chimpanzees. Am J Primatol. 2009;71(7):548–557. 10.1002/ajp.20687 [DOI] [PubMed] [Google Scholar]
- 93. Cani PD, Bibiloni R, Knauf C, et al. : Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- 94. McCord AI, Chapman CA, Weny G, et al. : Fecal microbiomes of non-human primates in Western Uganda reveal species-specific communities largely resistant to habitat perturbation. Am J Primatol. 2014;76(4):347–354. 10.1002/ajp.22238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Amato KR, Martinez-Mota R, Righini N, et al. : Phylogenetic and ecological factors impact the gut microbiota of two Neotropical primate species. Oecologia. 2016;180(3):717–733. 10.1007/s00442-015-3507-z [DOI] [PubMed] [Google Scholar]
- 96. Amato KR, Yeoman CJ, Cerda G, et al. : Variable responses of human and non-human primate gut microbiomes to a Western diet. Microbiome. 2015;3:53. 10.1186/s40168-015-0120-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gomez A, Rothman JM, Petrzelkova K, et al. : Temporal variation selects for diet-microbe co-metabolic traits in the gut of Gorilla spp. ISME J. 2016;10(2):514–26. 10.1038/ismej.2015.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gomez A, Petrzelkova K, Yeoman CJ, et al. : Gut microbiome composition and metabolomic profiles of wild western lowland gorillas ( Gorilla gorilla gorilla) reflect host ecology. Mol Ecol. 2015;24(10):2551–2565. 10.1111/mec.13181 [DOI] [PubMed] [Google Scholar]
- 99. Clayton JB, Vangay P, Huang H, et al. : Captivity humanizes the primate microbiome. Proc Natl Acad Sci U S A. 2016;113(37):10376–10381. 10.1073/pnas.1521835113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hale VL, Tan CL, Niu K, et al. : Diet Versus Phylogeny: a Comparison of Gut Microbiota in Captive Colobine Monkey Species. Microb Ecol. 2018;75(2):515–527. 10.1007/s00248-017-1041-8 [DOI] [PubMed] [Google Scholar]
- 101. Jasinska AJ, Schmitt CA, Service SK, et al. : Systems biology of the vervet monkey. ILAR J. 2013;54(2):122–143. 10.1093/ilar/ilt049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Phillips KA, Bales KL, Capitanio JP, et al. : Why primate models matter. Am J Primatol. 2014;76(9):801–827. 10.1002/ajp.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ley RE, Hamady M, Lozupone C, et al. : Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–1651. 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Bäckhed F, Ley RE, Sonnenburg JL, et al. : Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. 10.1126/science.1104816 [DOI] [PubMed] [Google Scholar]
- 105. Ley RE, Peterson DA, Gordon JI: Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. 10.1016/j.cell.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 106. Amato KR, Yeoman CJ, Kent A, et al. : Habitat degradation impacts black howler monkey ( Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013;7(7):1344–53. 10.1038/ismej.2013.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sánchez-Villegas A, Delgado-Rodríguez M, Alonso A, et al. : Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66(10):1090–1098. 10.1001/archgenpsychiatry.2009.129 [DOI] [PubMed] [Google Scholar]
- 108. Ley RE: Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26(1):5–11. 10.1097/MOG.0b013e328333d751 [DOI] [PubMed] [Google Scholar]
- 109. Wu GD, Chen J, Hoffmann C, et al. : Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mackie RI, Aminov RI, Hu W, et al. : Ecology of uncultivated Oscillospira species in the rumen of cattle, sheep, and reindeer as assessed by microscopy and molecular approaches. Appl Environ Microbiol. 2003;69(11):6808–6815. 10.1128/aem.69.11.6808-6815.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Haro C, Montes-Borrego M, Rangel-Zúñiga OA, et al. : Two Healthy Diets Modulate Gut Microbial Community Improving Insulin Sensitivity in a Human Obese Population. J Clin Endocrinol Metab. 2016;101(1):233–242. 10.1210/jc.2015-3351 [DOI] [PubMed] [Google Scholar]
- 112. Haro C, Garcia-Carpintero S, Alcala-Diaz JF, et al. : The gut microbial community in metabolic syndrome patients is modified by diet. J Nutr Biochem. 2016;27:27–31. 10.1016/j.jnutbio.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 113. Miquel S, Martín R, Rossi O, et al. : Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16(3):255–261. 10.1016/j.mib.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 114. Videla S, Vilaseca J, Antolín M, et al. : Dietary inulin improves distal colitis induced by dextran sodium sulfate in the rat. Am J Gastroenterol. 2001;96(5):1486–1493. [DOI] [PubMed] [Google Scholar]
- 115. Costabile A, Klinder A, Fava F, et al. : Whole-grain wheat breakfast cereal has a prebiotic effect on the human gut microbiota: a double-blind, placebo-controlled, crossover study. Br J Nutr. 2008;99(1):110–120. 10.1017/S0007114507793923 [DOI] [PubMed] [Google Scholar]
- 116. Ben XM, Zhou XY, Zhao WH, et al. : Supplementation of milk formula with galacto-oligosaccharides improves intestinal micro-flora and fermentation in term infants. Chin Med J (Engl). 2004;117(6):927–931. [PubMed] [Google Scholar]
- 117. Ramirez-Farias C, Slezak K, Fuller Z, et al. : Effect of inulin on the human gut microbiota: stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br J Nutr. 2009;101(4):541–550. 10.1017/S0007114508019880 [DOI] [PubMed] [Google Scholar]
- 118. Pusceddu MM, El Aidy S, Crispie F, et al. : N-3 Polyunsaturated Fatty Acids (PUFAs) Reverse the Impact of Early-Life Stress on the Gut Microbiota. PLoS One. 2015;10(10):e0139721. 10.1371/journal.pone.0139721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Robertson RC, Seira Oriach C, Murphy K, et al. : Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun. 2017;59:21–37. 10.1016/j.bbi.2016.07.145 [DOI] [PubMed] [Google Scholar]
- 120. Shively CA, Register TC, Appt SE, et al. : Consumption of Mediterranean versus Western Diet Leads to Distinct Mammary Gland Microbiome Populations. Cell Rep. 2018;25(1):47–56.e43. 10.1016/j.celrep.2018.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Caesar R, Tremaroli V, Kovatcheva-Datchary P, et al. : Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015;22(4):658–668. 10.1016/j.cmet.2015.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lecomte V, Kaakoush NO, Maloney CA, et al. : Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015;10(5):e0126931. 10.1371/journal.pone.0126931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Shively CA, Appt SE, Vitolins MZ, et al. : Mediterranean versus Western Diet Effects on Caloric Intake, Obesity, Metabolism, and Hepatosteatosis in Nonhuman Primates. Obesity (Silver Spring). 2019;27(5):777–784. 10.1002/oby.22436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cani PD: Human gut microbiome: hopes, threats and promises. Gut. 2018;67(9):1716–1725. 10.1136/gutjnl-2018-316723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gutiérrez-Díaz I, Fernández-Navarro T, Sánchez B, et al. : Mediterranean diet and faecal microbiota: a transversal study. Food Funct. 2016;7(5):2347–2356. 10.1039/c6fo00105j [DOI] [PubMed] [Google Scholar]
- 126. Yadav H, Jain S, Bissi L, et al. : Gut microbiome derived metabolites to regulate energy homeostasis: how microbiome talks to host. Metabolomics. 2016;6:e150 10.4172/2153-0769.1000e150 [DOI] [Google Scholar]


