Abstract
To increase the capacity of identifying coronavirus disease 2019 (COVID-19) infection, many Biosafety Level 2 (BSL-2) labs have been established in a short period of time for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid tests all over the world. However, their biosafety has not been evaluated, which could have been the first gateway to SARS-CoV-2 transmission. During 9–11 March 2020, the first comprehensive evaluation of the biosafety in all 89 labs qualified for conducting SARS-CoV-2 tests in Sichuan Province of China was conducted. The degree of compliance with 39 criteria in five categories was evaluated: biosafety requirements for lab activities (14 criteria), sample transfer, acceptance and management (6 criteria), waste management (9 criteria), personnel training and protection (4 criteria), and lab environmental disinfection, emergency plans and accident handling (6 criteria). Our results revealed that, although an overall median compliance rate of 94.6% for 39 criteria, only four of 89 labs met all of them. Criteria in personnel training and protection have been most satisfactorily met, followed by lab environmental disinfection, emergency plans and accident handling. The most severe risk was the lack of automatic doors at the main entrance or in core operation areas, especially among labs in CDC and hospitals. This risk, together with failure for keeping pressure in the core operation areas 25 ± 5 Pa (mainly among labs in the third-party testing agencies), may cause accidental exposure to biological agents from lab activities. Other severe risk included failure for standard labeling of SARS-CoV-2 wastes and lacking regular monitoring of sterilization effects. Our findings would provide experiences and lessons for strengthening lab biosafety in other Chinese provinces, and also serve as an important reference for many other countries where such labs are being or will be quickly built for fighting the COVID-19. The information of lab safety should be considered to be internally linked to the national intelligent syndromic surveillance system (NISSS), for better improving the safety of the labs at the greatest need and facilitating more comprehensive surveillance of risk for disease outbreak.
Keywords: SARS-CoV-2, COVID-19, Biosafety, BSL-2, Lab safety, Nucleic acid test, Environmental risk
1. Introduction
At the end of 2019, a series of pneumonia cases of unknown cause were reported in Wuhan, Hubei Province of China (Coronaviridae Study Group of the International Committee on Taxonomy, 2020). In January 2020, deep sequencing analysis from lower respiratory tract samples identified a new strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as causative agent for that observed pneumonia cluster (Sterpetti, 2020). The viral infection was named as coronavirus disease 2019 (COVID-19) on 11th February 2020, which could develop a severe acute respiratory syndrome with serious clinical symptoms, including fever, dry cough, dyspnea, respiratory disorders, and pneumonia, and may result in progressive respiratory failure and death (Coccia, 2020). The World Health Organization (WHO) declared it as a pandemic on 11th March 2020 when more than 118,000 cases, including over 4000 deaths, were confirmed in 114 countries worldwide (Di Gennaro, 2020). In addition to the human movement resulting in person-to-person contact (Weitz, 2020, Jia and Yang, 2020), the geo-environmental factors have also been identified as determinants of the accelerated diffusion and severity of COVID-19 (Coccia, 2020, Zhou et al., 2020).
To prepare for a long fight against the COVID-19, we need to intensity every link in the chain of COVID-19 control and prevention. Working largely unseen, pathogenic microorganism labs will have been playing a vital role as the first gateway in nucleic acid testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from beginning to the end of this pandemic. To increase the capacity of identifying SARS-CoV-2 infections, many Biosafety Level 2 (BSL-2) labs for testing SARS-CoV-2 have been established or adapted from the existing pathogenic microorganism labs in a short period of time all over the world. However, the potential biological hazards from such rapidly built labs could threaten the safety of lab workers and consequently all citizens, e.g., leakage of infectious agents outside the labs. The biological accidents in pathogenic microorganism labs have occasionally occurred since 1897 (Meyer and Eddie, 1897). According to the US governmental data on biosafety in the labs, during 2008–2012, there had been plenty of accident (e.g., spills, record-keeping errors) occurring between 100 and 275 potential releases of pathogens each year in labs that handle select agents, although few lab workers were reported to be infected (Kaiser, 2014). The largest accident of lab-acquired infections (LAIs) was reported in 1976, with 159 released biological agents causing 4079 infections (Pike, 1976). Recently, the most severe accident was the mishandling of severe acute respiratory syndrome (SARS) virus in March 2004, which has resulted in one death, eight infections, and more than 200 contacts quarantined in a leading SARS research lab in China (Normile, 2004). Although the risk of LAIs is usually lower and the quality and safety measures are higher in diagnostic labs than in research labs, those accidents indicate that biosafety of pathogenic microorganism labs remains a serious concern to the public and community health.
Although it remains unknown how many SARS-CoV-2 LAIs have occurred among the infected health workers (3019 in China as of 11 February 2020) (Wu and McGoogan, 2020), the importance of biosafety in the rapidly built labs can never be overemphasized. In China, before carrying out SARS-CoV-2 tests in a given diagnostic lab, a request accompanied by the required proof documents demonstrating meeting all biosafety regulations must be submitted to the National Health Commission of the People’s Republic of China for approval, in order to have that lab qualified for conducting the corresponding activities (National Health Commission of the People's Republic of China, 2020). However, according to the previous lessons, an array of factors may emerge during the operation of the lab, which could play crucial roles in the exposure to and transmission of LAIs, such as inadequate compliance with existing regulations and procedures, weak personnel protection, and insufficient facilities (e.g., appropriate sterilization or decontamination facilities) (Zhong and Zeng, 2006). With the newly emerging pathogens and an increasing variety of lab materials and facilities, there may be even more potential biosafety risks nowadays. However, biosafety of the newly established pathogenic microorganism labs for SARS-CoV-2 tests has not been evaluated. This study filled this critical gap by investigating the potential biological hazards of those labs in Sichuan Province of China, which could provide experiences and lessons for strengthening biosafety of both diagnostic labs and research labs in other Chinese provinces. Although the regulatory measures are usually different among countries, findings of this study would be an important reference for many countries where similar labs are being or will be quickly built for fighting against the COVID-19, especially the developing countries and regions with incomplete regulations and limited resources.
2. Methods
Sichuan Province is located in the west to Hubei Province and has the fourth largest population in China (around 83 million as of the end of 2019), with about 60,000 traveling back from Wuhan (the early-stage epicenter of the COVID-19 epidemic) during 10–20 January 2020. Jointly by Sichuan Provincial Health Commission, Sichuan Public Health Law Supervision Enforcement, and Sichuan Provincial Center for Disease Control and Prevention (CDC), the first comprehensive evaluation of the biosafety in all 89 BSL-2 labs qualified for conducting nucleic acid testing for SARS-CoV-2 in Sichuan Province was conducted during 9–11 March 2020. Only one qualified BSL-2 lab in Sichuan CDC has existed before January 29, 2020; 88 were later qualified in CDCs at different levels, hospitals, and third-party testing agencies.
Following the Lab Biosafety Guide for COVID-19, issued by the Chinese National Health Commission, the biosafety of 89 BSL-2 labs were evaluated on a basis of 39 criteria in five categories: biosafety requirements for lab activities (14 criteria), sample transfer, acceptance and management (6 criteria), waste management (9 criteria), personnel training and protection (4 criteria), and lab environmental disinfection, emergency plans and accident handling (6 criteria). We examined the completeness of the required facilities and infrastructure and lab records (e.g., for experimental procedures, equipment maintenance, waste disposal), and interviewed lab workers who were engaged in SARS-CoV-2 tests for assessing operational procedures due to inability to access the core operation areas during the SARS-CoV-2 tests. All information was collected via a paper-based questionnaire, entered into computer, and cross-checked by all participating parties. We calculated the degree of compliance to each category (i.e., percentage of meeting different numbers of criteria in each category) among the 89 BSL-2 labs, and the compliance rate to each criterion (i.e., percentage of meeting each criterion) among the 89 BSL-2 labs, as well as compliance rates among the labs established in CDCs, hospitals, and third-party testing agencies, separately.
3. Results
There were two to 20 staffs in each lab, with five working in one lab on average. The daily maximum amount of nucleic acid tests for SARS-CoV-2 in all those labs was 4578. Although an overall median compliance rate of 94.6% for 39 detailed criteria, only four of 89 labs met all of them. In general, criteria in personnel training and protection have been most satisfactorily met (85 out of 89 labs have met all four criteria under that category), followed by lab environmental disinfection, emergency plans and accident handling (Table 1 ). The high compliance rates in those two aspects should have greatly contributed to no reports of LAIs so far during the COVID-19 pandemic, compared to some LAIs happening during the SARS epidemic (Zhong and Zeng, 2006).
Table 1.
Percentage of Biosafety Level 2 (BSL-2) labs for SARS-CoV-2 nucleic acid testing that meet different numbers of criteria under each major category of biosafety regulations.
| Categories | Criteria | Percentage of labs compliance with criteria (n = 89) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 100% |
75–100% |
50–75% |
<50% |
||||||
| N | % | N | % | N | % | N | % | ||
| Biosafety requirements for lab activities | 14 | 28 | 31.5 | 54 | 60.7 | 7 | 7.9 | 0 | 0.0 |
| Sample transfer, acceptance and management | 6 | 56 | 62.9 | 25 | 28.1 | 8 | 9.0 | 0 | 0.0 |
| Waste management | 9 | 40 | 44.9 | 47 | 52.8 | 2 | 2.2 | 0 | 0.0 |
| Personnel training and protection | 4 | 85 | 95.5 | 4 | 4.5 | 0 | 0.0 | 0 | 0.0 |
| Lab environmental disinfection, emergency plans and accident handling | 6 | 73 | 82 | 11 | 12.4 | 5 | 5.6 | 0 | 0.0 |
However, some potential risks still existed. The most severe risk was the lack of automatic doors at the main entrance or in core operation areas (28 out of 89, 31.5%), especially in CDC (17/43, 39.5%) and hospitals (11/36, 30.6%) (Table 2 ). This risk, together with failure for keeping pressure in the core operation areas 25 ± 5 Pa (11/70, 15.7%), especially in labs in the third-party testing agencies (3/8, 37.5%), may cause accidental exposure to biological agents from lab activities. Another severe risk was failure for standard labeling of SARS-CoV-2 wastes (22/89, 24.7%), which often occurred in non-hospital labs, i.e., in the third-party testing agencies (5/10, 50%) and CDCs (12/43, 27.9%); lacking regular monitoring of sterilization effects was a less severe risk (17/89, 19.1%), but still increased potential risk for waste management, especially in CDCs (10/43, 23.3%) (Table 3 ). Two less severe but still serious risks existed in sample transfer, acceptance and management: failure to meet the UN2814 requirements for outer packaging of specimens to be transported (15/89, 16.9%) and lack of double-locker management of specimens by two people (14/89, 15.7%) (Table 3). Some other issues, although generally fine, may still run certain types of agencies into risk. For example, 20.9% (9/43) of CDCs did not classify solid wastes with warning signs; 20% (2/10) of the third-party testing agencies were not equipped with disinfectants for specimen leakage accidents, and did not have certified pressure steam sterilizers and qualified lab workers, and records of storing, lending, and destroying specimens (Table 4 ). A moderate risk that lab workers operated experiments outside the core operation areas of the lab, although observed in only one lab in a third-party testing agency, may cause potential leakage of biological agents and hence should be eradicated.
Table 2.
Percentage of meeting biosafety criteria for lab activities (i.e., compliance rate) among the 89 Biosafety Level 2 (BSL-2) labs for SARS-CoV-2 nucleic acid testing, established in the Centers for Disease Control and Prevention (CDC), hospitals, and third-party testing agencies.
 |
*Not applicable to 19 BSL-2 labs, with 8 in CDCs, 9 in hospitals, and 2 in third-party testing agencies.
Items colored in grey, yellow, orange, and red represent the compliance rates of 90–95%, 85–90%, 80–85%, and <80%, respectively.
Table 3.
Percentage of meeting criteria of sample and waste management (i.e., compliance rate) among the 89 Biosafety Level 2 (BSL-2) labs for SARS-CoV-2 nucleic acid testing, established in the Centers for Disease Control and Prevention (CDC), hospitals, and third-party testing agencies.
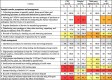 |
Items colored in grey, yellow, orange, and red represent the compliance rates of 90–95%, 85–90%, 80–85%, and <80%, respectively.
Table 4.
Percentage of meeting criteria of personnel training and emergency handling (i.e., compliance rate) among the 89 Biosafety Level 2 (BSL-2) labs for SARS-CoV-2 nucleic acid testing, established in the Centers for Disease Control and Prevention (CDC), hospitals, and third-party testing agencies.
 |
Items colored in grey, yellow, orange, and red represent the compliance rates of 90–95%, 85–90%, 80–85%, and <80%, respectively.
4. Implications and limitations
According to these results from our comprehensive evaluation, there were various biosafety risks in many aspects of those newly established labs for SARS-CoV-2 tests, and any problem could lead to unpredictable biosafety accidents. Early detection of these problems can effectively reduce or even avoid these potential risks. In addition to overcoming those problems, it is more important to have deeper reflection on how to strengthen the current biosafety system, in order to avoid such biological hazards fundamentally and cost-effectively. We suggest to strengthen the biosafety system in the following aspects:
-
(1)
Tightening procedures for qualifying labs on the basis of all biosafety requirements before approval. Any inadequate protection in microbiology activities, waste disposal, and storage and transportation of biological agents would result in potential consequences of LAIs. For example, failure to keep lower pressure in the core operation areas and lack of automatic doors at the main entrance and core operation areas in some labs were revealed from this study. Therefore, even during the emergency period, the rapid establishment or qualification of pathogenic microorganism labs needs to check all basic requirements of BSL-2 labs before carrying out any microbiology activity.
-
(2)
Strengthening enforcement of the existing regulations and procedures and continuous training for personnel protection. The existing regulations on general biosafety of pathogenic microorganism labs of China (e.g., a technical guidance for lab testing of 2019-nCoV infection (National Health Commission of the People's Republic of China, 2020), although not legally mandatory, were formulated based on the previous lab accidents, LAIs, and opinions of peers. Therefore, strict compliance with these regulations and procedures can effectively avoid many known infection threats. For example, failure to conduct standard labeling of SARS-CoV-2 wastes in some labs was found in this study. Mistakes made by lab workers have accounted for about 78% of the underlying causes of LAIs (Wurtz, 2016), and most risks of biological hazards for humans could be significantly reduced through proper training on the use of appropriate techniques, containment devices, and facilities in correct procedures. Therefore, routine training and strict supervision for personnel protection is an important guarantee for biosafety.
-
(3)
Speeding up the progress of the institutionalized biosafety. Biotechnology is a “double-edged sword”, benefiting and also bringing potential risk to humans. Thus, it is necessary to prioritize biosafety by law. “Regulations on the Biosafety Management of Pathogenic Microbe Labs” were amended in 2018 in China, which, however, has not been effective as the law. When conflicts occur between the regulations and the law, the law still holds more power than do regulations. The COVID-19 pandemic should urge all countries without complete institutionalization of biosafety to step up the efforts for systematically establishing or improving the nationwide control and prevention capacity of biosafety risk, especially low- and middle-income countries.
-
(4)
Establishing the real-time reporting systems of LAIs and the network surveillance systems of BSL-2 labs. The actual risk of LAIs is usually difficult to quantify due to lack of information, which has disabled evidence-based control measures and regulations. The network surveillance systems for COVID-19 nucleic acid tests can monitor and collect information from many aspects of lab activities (microbiology activities in the lab) and environments (e.g., quality of lab facilities). Such public health infrastructures will permanently be important reserves of emergency handling capacity in one country, for both COVID-19 re-emergence and other emerging infectious diseases in the future. For example, 21 of 89 BSL-2 labs in Sichuan Province have been adapted from the labs that are part of the Chinese National Influenza Surveillance Network, which have unprecedentedly speeded up the construction during the emergency.
-
(5)
The course “biosafety in the lab” should be a compulsory course of all relevant disciplines in the university (e.g., life science, biomedicine, clinical medicine, and preventive medicine), in order to improve safety awareness and capacity of relevant technicians who are or will be engaged in lab work, and to reduce potential biosafety accidents in the future, such as safe operation, disposal of solid wastes, and lab emergency handling.
There are some limitations of this survey, which should be improved in future for a better evaluation of the biosafety of labs established or qualified in response to emerging infectious diseases. First, lab directors were interviewed for answering all operation-related questions, which is more subjective than by observation and might overlook some incompliance with safety regulations during practical operations. In future, an alternative way of evaluating those aspects could be observing mock operations, which can be set up ahead of time in mock core operation areas. Second, due to the urgency of this survey, we could not conduct an in-depth interview for more open questions, which might help to identify potential reasons underlying the incompliance. Third, for the same reason above, we could not systematically examine all historical records of all labs since the COVID-19 outbreak. Therefore, we could not have the total number of SARS-CoV-2 tests in each lab, which might have related to the degree of compliance with safety regulations, and also have enabled us to quantify the potential risk of incompliance for better the current emergency plans.
5. Conclusions
It has been warned in the spring of 2020 that the COVID-19 pandemic outside China was still escalating with the unclear moment of reaching the peak. In particular, the current and future waves of infections have and will have been inevitably hitting the most vulnerable regions in the world (e.g., Africa and Latin America) (Jia and Yang, 2020), where many countries had weaker capacity of nucleic acid testing for SARS-CoV-2 and biosafety prevention. In the face of rapid growth of COVID-19 patients, most, if not all, countries have established or are establishing nucleic acid testing labs for SARS-CoV-2 in a short period of time, so they may have encountered or will encounter many similar or even more biosafety problems. Experiences from China will be crucial to continuing strengths and overcoming those potential risks during the procedures for ensuring the lab biosafety, especially in middle and low-income countries that cannot afford consequences resulting from avoidable risks as do high-income countries. Therefore, there is an urgent need of involvement of multiple disciplines and international collaboration to build more robust biosafety systems for the improved biosafety. More prospectively, the information of lab safety (e.g., raw data, evaluation results) should be considered to be internally linked to the national intelligent syndromic surveillance system (NISSS), which could help different levels of CDCs better coordinate and allocate limited resources for targeted investigations and interventions to improve the safety of the labs at the greatest need, as well as facilitate more comprehensive surveillance of risk for disease outbreak (Jia and Yang, 2020).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank Sichuan Provincial Health Commission, Sichuan Public Health Law Supervision Enforcement, and Sichuan Center for Disease Control and Prevention for their organization of this timely evaluation across the province. We also thank the International Institute of Spatial Lifecourse Epidemiology (ISLE) for the research support.
Handling Editor: Frederic Coulon
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2020.105964.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Coccia M. Factors determining the diffusion of COVID-19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci. Total Environ. 2020;729:138474. doi: 10.1016/j.scitotenv.2020.138474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gennaro F. Coronavirus Diseases (COVID-19) current status and future perspectives: a narrative review. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P., Yang S. Time to spatialize epidemiology in China. Lancet Global Health. 2020;8 doi: 10.1016/S2214-109X(20)30120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P., Yang S. Are we ready for a new era of high-impact and high-frequency epidemics? Nature. 2020;580:321. doi: 10.1038/d41586-020-01079-0. [DOI] [PubMed] [Google Scholar]
- Jia Peng, Yang Shujuan. China needs a national intelligent syndromic surveillance system. Nature Med. 2020;26:990. doi: 10.1038/s41591-020-0921-5. [DOI] [PubMed] [Google Scholar]
- Kaiser J. The catalyst. Science. 2014;345:1112–1115. doi: 10.1126/science.345.6201.1112. [DOI] [PubMed] [Google Scholar]
- Meyer K.F., Eddie B. Laboratory infections due to Brucella. J. Infect. Dis. 1897;68:24–32. [Google Scholar]
- National Health Commission of the People's Republic of China Technical guidance for laboratory testing of 2019-nCoV infection (Third Edition) Biosaf. Health. 2020;2(1) doi: 10.1016/j.bsheal.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normile D. Infectious diseases. Mounting lab accidents rainse SARS fears. Science. 2004;304:659–661. doi: 10.1126/science.304.5671.659. [DOI] [PubMed] [Google Scholar]
- Pike R.M. Laboratory-associated infections: summary and analysis of 3921 cases. Health Lab Sci. 1976;13:105–114. [PubMed] [Google Scholar]
- Sterpetti A.V. Lessons learned during the COVID-19 virus pandemic. J. Am. Coll. Surg. 2020;230:1092–1093. doi: 10.1016/j.jamcollsurg.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz J.S. Modeling shield immunity to reduce COVID-19 epidemic spread. Nat. Med. 2020;26:849–854. doi: 10.1038/s41591-020-0895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and preventionexternal icon. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Wurtz N. Survey of laboratory-acquired infections around the world in biosafety level 3 and 4 laboratories. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1247–1258. doi: 10.1007/s10096-016-2657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N., Zeng G. What we have learnt from SARS epidemics in China. Bmj. 2006;333:389–391. doi: 10.1136/bmj.333.7564.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Yang S., Jia P. Towards precision management of cardiovascular patients with COVID-19 to reduce mortality. Prog. Cardiovasc. Dis. 2020 doi: 10.1016/j.pcad.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


