Abstract
Rationale and Objectives
Chest CT is not suitable for critically ill patients with COVID-19 and lung ultrasound (LUS) may play an important role for these patients. In this study, we summarized the findings of LUS and explore the value of semiquantitative LUS scores in evaluation and follow-up of COVID-19 pneumonia.
Materials and Methods
Retrospectively studied the LUS and chest CT imaging of 128 critically ill patients with COVID-19. The imaging data were reviewed to acquire the LUS and CT scores. The correlation between LUS scores and CT scores were made to evaluate the accuracy of LUS. A cut-off point of LUS score was calculated to distinguish critical-type patients from severe-type patients. LUS follow-up of 72 patients were compared with the gold standard chest CT.
Results
The most common LUS features of COVID-19 pneumonia were crowded or coalescent B-lines with multifocal small consolidations in multi-zone. The mean LUS score was 8.1 points in severe-type patients and 15.7 points in critical-type patients (P<0.05). The correlation between LUS scores and CT scores was high (r=0.891, p<0.01) and it was higher in critical-type patients than that in severe-type patients. The LUS score higher than 10.5 points had a 97.4% sensitivity and 75.0% specificity to distinguish critical-type patients. The consistency of LUS and chest CT in follow-up was 0.596, with higher consistency in diagnosis of lesion progression (Kappa values was 0.774).
Conclusion
Our scoring system provides a more quantitative use of LUS findings and accurate evaluation of lung damage for critically ill patients with COVID-19.
Key words: Lung ultrasound, Evaluation, COVID-19, Pneumonia
INTRODUCTION
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which first appeared in Wuhan in December 2019 (1, 2). As of May 25, 2020, there have been more than 5.0 million confirmed cases in more than 200 countries and territories around the world (3). Given the rapid spread of this virus, the World Health Organization declared that COVID-19 should be characterized as a pandemic on March 11, 2020 (4). Although the incidence rate of severe cases of COVID-19 was lower than that of severe acute respiratory syndrome (SARS) in 2013, COVID-19 is more infectious, and it led to a large number of severe cases in a short amount of time (5). At the peak of the pandemic, there were 9689 patients with severe illness in Wuhan (6). The main clinical manifestations of those critical patients were respiratory distress, fever and other severe systemic symptoms (7). Many critical patients required mechanical ventilation or even extracorporeal membrane oxygenation (ECMO) support (8).
The timely and accurate evaluation of lung lesions is very important in the treatment of patients with COVID-19, and chest computed tomography (CT) is considered the ‘gold-standard’ imaging technique (9). However, CT is not suitable as a follow-up tool for critically ill patients because of the risks of patient transport and infecting others. Bedside chest radiography has many restrictions and may lead to poor-quality X-ray films with low sensitivity, which has been well described (10). Therefore, it is necessary to devise a novel imaging technique to evaluate lung lesions.
Lung ultrasound (LUS) is a highly useful apparatus in the intensive care unit (ICU). It offers a quick, reliable, inexpensive and radiation-free monitoring tool at the patient's bedside (11). LUS is used to evaluate lung disease through the findings obtained when ultrasound waves cross tissues with a varying air/fluid ratio. Many researchers have further highlighted the advantages of LUS in ICUs for the evaluation of patients with respiratory distress (12, 13, 14). However, there have been only a few case reports of bedside LUS application in critical patients with COVID-19 (15, 16, 17). In this study, by analyzing ultrasonic data in 128 cases, we aimed to summarize the findings of LUS and to explore the value of semiquantitative LUS scores in the evaluation and follow-up of critically ill patients with COVID-19.
MATERIALS AND METHODS
This is a subgroup analysis of patients enrolled in a larger ultrasound research project on COVID-19. Our study was approved by the clinical research ethics committee of the hospital (No. WDRY2020-K031) and has been carried out in accordance with The Code of Ethics of the World Medical Association. The data were collected and analyzed to facilitate better clinical decisions and treatment.
Study Population
A total of 128 consecutive critically ill patients who were admitted to the hospital from February 1, 2020 to March 31, 2020 and had a confirmed diagnosis of COVID-19 before their hospitalization were included in the study. The diagnostic criterion was defined as real-time fluorescence polymerase chain reaction revealing the positive detection of COVID-19 in throat swabs or the lower respiratory tract. According to the Diagnosis and Treatment Guidelines for COVID-19 (7th edition) issued by the National Health Commission of China, the severity of the disease was classified into 4 categories. Mild-type patients had mild clinical symptoms and no pulmonary changes on CT imaging. Common-type patients had symptoms of fever and signs of respiratory infection with pneumonia changes on CT imaging. Severe-type patients presented with any one of the following: a. respiratory distress and respiratory rate ≥ 30/min, b. fingertip blood oxygen saturation ≤ 93% in resting conditions, or c. arterial partial pressure of oxygen (PaO2)/oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg= 0.133 kPa). Finally, critical-type patients met any one of the following criteria: a. respiratory failure requiring mechanical ventilation, b. shock, and c. ICU admission requirement due to multiple organ failure. Patients with severe and critical type were enrolled in our study and defined as critically ill patients.
Patients with a history of lung carcinoma, tuberculosis, congenital lung diseases or recent chest surgery were excluded from the study. To rule out the effects of acute heart failure on the lungs, patients with reduced left ventricular ejection fraction (<50%) were also excluded from the study.
Clinical Characteristics
The demographics and baseline characteristics that we collected consisted of gender, age, clinical signs and symptoms (such as fever, cough, shortness of breath, chest pain, fatigue, and loss of appetite), body mass index, blood oxygen saturation and coexisting conditions. After thorough clinical assessment, blood samples were taken to evaluate C-reactive protein (CRP), leucocytes and procalcitonin.
Chest CT Evaluation
All enrolled patients underwent chest CT examination within 48 hours after hospitalization. The extent of lung lesions was evaluated using the CT scoring system adopted by Pan et al (18). Each of the 5 lung lobes was visually scored from 0 to 5: no involvement, scored 0; <5% involvement, scored 1; 5%-25% involvement, scored 2; 26%-49% involvement, scored 3; 50%-75% involvement, scored 4; and >75% involvement, scored 5. The total CT score was the sum of the individual lobar scores (the maximum score=25). All images were interpreted and scored by two senior radiological specialists. These investigators were blinded to the clinical data.
Bedside LUS Examination
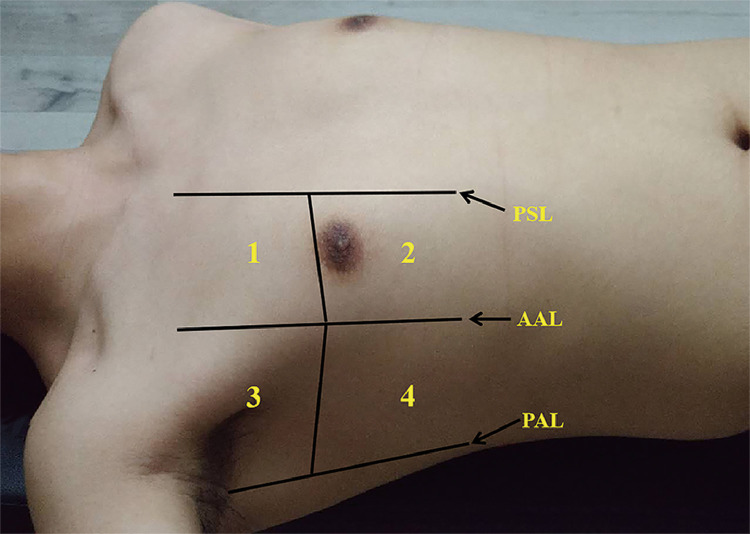
All enrolled patients underwent bedside LUS evaluation within 24 hours after hospitalization. The bedside LUS were performed by 6 sonographers with 2 to 10 years’ experience in ultrasound. The machine used was a GE Vivid™ iq ultrasonography (GE Healthcare, China) equipped with a convex C1-5-RS probe. The frequency was set at 3.5 MHz, the depth was set at 10 cm, and the gain was adjusted to obtain the best possible image so that the reverberation artifact (lung comet) could be clearly detected even if the patient were obese. According to international evidence-based recommendations for point-of-care LUS in the emergency setting (19), the complete eight-zone LUS examination was performed with patients in the supine or near-to-supine position. The chest wall was divided into 8 zones: 2 anterior and 2 lateral zones per side. The anterior chest wall was delineated from the parasternal line to the anterior axillary line, and the 2 lateral chest walls were delineated between the axillary anterior and posterior lines. Superior and inferior zones were divided by the third intercostal space (Figure 1 ). Videoclips were recorded throughout the respiratory cycle for subsequent off-line analysis.
Figure 1.
Schematic diagram of the eight-zone lung ultrasound examination protocol. Each hemithorax is separated into four quadrants: anterior and lateral zones separated by the anterior axillary lines with each zone divided into upper and lower portions by the third intercostal space. PSL: the parasternal line; AAL: the anterior axillary line; PAL: the posterior axillary line.
LUS Scores
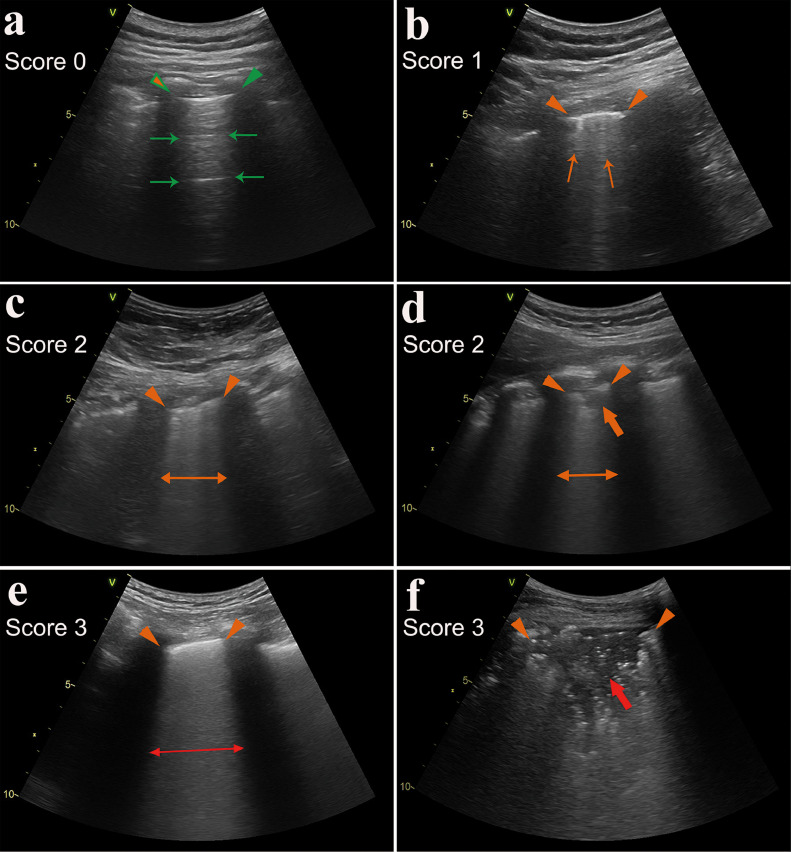
Each zone was scored according to the LUS pattern as follows (20, 21): a normal lung pattern was identified by the presence of normal lung sliding with A-lines or fewer than two isolated B-lines and was scored as 0; the presence of 3 or more well-spaced B-lines presented in a single intercostal space was scored as 1; the presence of crowded B-lines (more than 50% range in a view) with or without consolidation limited to the subpleural space was scored as 2; and the presence of confluent B-lines (approaching 100% range in a view) or a tissue pattern characterized by dynamic air bronchograms that was defined as lung consolidation was scored as 3. The most severe ultrasound finding can be considered representative of the entire zone. The most severe ultrasound finding observed in each zone was recorded and used to calculate the sum of the scores (the maximum score=24). The LUS score characteristics are summarized in Figure 2 . In addition, pneumothorax was defined as the presence of a lung point and absence of lung sliding and B-lines. Pleural effusion was defined as intrapleural anechoic collection.
Figure 2.
Lung ultrasound imaging and the scoring system. (a) A normal lung pattern was identified by the presence of clear pleural line (indicated by green triangular arrowheads) and A-lines (marked with green thin arrows), scored as 0; (b) A small number of B-lines (marked with orange thin arrows), scored as 1; (c-d) The presence of crowded B-lines (indicated by orange thin double arrow) or consolidations limited to the subpleural space (marked with orange thick arrow), scored as 2; (e-f) the presence of confluent B-lines (indicated by red thin double arrow) or mass consolidation with dynamic air bronchograms (marked with red thick arrow), scored as 3. The thickened, irregular and interrupted pleural lines were indicated by orange triangular arrowheads.
The ultrasound images were analyzed and scored by 3 doctors with 3 to 6 years’ experience in LUS. All doctors were blinded to each other and the clinical data. Random ultrasound images of twenty patients were digitally transferred to a computer and analyzed by two independent observers to assess interobserver variability. The two observers were blinded to each other and neither of them were participated in the lung ultrasound examination. Interobserver consistency was defined as the same patient acquired the same lung ultrasound score from two independent observers.
LUS Follow-up
To dynamically assess lung lesions, a follow-up of bedside LUS was performed on all patients every two days after admission or at any time the doctors deemed it necessary. Chest CT was performed again after admission when doctors considered the patient's condition to have deteriorated significantly, when accurate assessment of the lung lesion was needed, or when the patient's condition improved significantly and the patient no longer required care in the ICU. If the CT follow-up was performed more than twice, only the first follow-up was selected for evaluation. Patients with CT scores that had increased ≥ 2 points compared with that at admission were defined as having disease progression. Similarly, a reduction of ≥2 points in the CT score compared with that at admission or a reduction of lung lesion density was defined as disease resolution. Changes in the CT score within 1 point were defined as no change. The bedside LUS scores within 24 hours before the CT examination were compared with those at admission. Similar to the definition of CT, if the LUS score increased or decreased ≥ 2 points compared with that at admission, it was defined as progression or improvement; otherwise, it was defined as no change. The results of LUS in follow-up were evaluated by comparison with the gold standard chest CT to evaluate the accuracy.
Statistical Analysis
Continuous numeric variables are expressed as the mean value ± standard deviation, and dichotomous variables are expressed as the frequency number (%) or median [interquartile range]. Variables were compared between groups using an unpaired Student's t test if the data were normally distributed or the Wilcoxon rank sum test if the data were not normally distributed. The intragroup correlation coefficient (ICC) was used to test the consistency of CT scores and ultrasound scores between two observers, and ICC values > 0.75 represent good repeatability. The distribution of involved lobes and the number of involved lobes in different clinical types were compared by the chi-squared test or Fisher's exact test when sample sizes were small. The relationship between LUS scores and CT scores was studied with Pearson correlations. A receiver operating characteristic curve (ROC) was used to test the ability of CT scores and LUS scores to distinguish critical-type patients from severe-type patients. A kappa consistency check was used to evaluate the consistency of LUS and chest CT in follow-up. Statistical significance was defined at a level of P<0.05. All statistical tests were analyzed with SPSS software (version 20.0).
RESULTS
Demographic and clinical characteristics
The demographic and clinical characteristics are shown in Table 1 . The median age was 65 years (IQR: 55-71; range: 26-94 years), and 75 (58.6%) were male. Patients presented with underlying chronic diseases in 93 cases (72.7%), and the most common underlying chronic diseases were hypertension (44 cases, 34.4%), coronary heart disease (22 cases, 17.2%) and diabetes (19 cases, 14.8%). Regarding the clinical manifestations, fatigue (96.1%), fever (95.3%), and shortness of breath (94.5%) were the most prevalent. A total of 99 (77.3%) patients had decreased oxygen saturation. During hospitalization, 119 (93.0%) and 42 (32.8%) patients demonstrated elevated levels of C-reactive protein and procalcitonin, respectively, while 70 (54.7%) patients had a reduction in leucocytes.
Table 1.
Clinical characteristics of patients with COVID-19
| Clinical characteristics | All Patients (n=128) | Disease type |
||
|---|---|---|---|---|
| Severe (n=52) | Critical (n=76) | P value | ||
| Age, years | 65.0 (55.0-71.0) | 60.0 (49.0-67.0) | 68.0 (58.0-77.0) | <0.01 |
| Male sex, n (%) | 75 (58.6%) | 29 (55.8%) | 46 (60.5%) | 0.59 |
| Time from illness onset to hospital admission, days | 12.0 (8.0–14.0) | 11.0 (8.0–13.0) | 12 (8.0–15.0) | 0.41 |
| Signs and symptoms | ||||
| Fever, n (%) | 122 (95.3%) | 46 (88.5%) | 76 (100.0%) | <0.01 |
| Cough, n (%) | 92 (71.9%) | 39 (75.0%) | 53 (69.7%) | 0.52 |
| Shortness of breath, n (%) | 121 (94.5%) | 45 (86.5%) | 76 (100.0%) | <0.01 |
| Chest pain/tightness, n (%) | 117 (91.4%) | 44 (84.6%) | 73 (96.0%) | 0.05 |
| Fatigue, n (%) | 123 (96.1%) | 47 (90.4%) | 76 (100.0%) | 0.01 |
| Loss of appetite, n (%) | 119 (93.0%) | 43 (82.7%) | 76 (100.0%) | <0.01 |
| Body mass index>28 kg/m2, n (%) | 44 (34.4%) | 19 (36.5%) | 25 (32.9%) | 0.71 |
| Blood saturation of oxygen, % | 82.2±16.6 | 89.4±4.7 | 75.7±17.2 | <0.01 |
| Coexisting conditions | ||||
| Hypertension, n (%) | 44 (34.4%) | 15 (28.8%) | 29 (38.2%) | 0.28 |
| Coronary heart disease, n (%) | 22 (17.2%) | 7 (13.5%) | 15 (19.7%) | 0.36 |
| Diabetes, n (%) | 19 (14.8%) | 5 (9.6%) | 14 (18.4%) | 0.17 |
| Chronic obstructive pulmonary disease, n (%) | 8 (6.3%) | 3 (5.8%) | 5 (6.6%) | 0.85 |
| Laboratory tests | ||||
| C-reactive protein increased | 119 (93.0%) | 43 (82.7%) | 76 (100.0%) | <0.01 |
| Leucocytes decreased | 70 (54.7%) | 20 (38.5%) | 50 (65.8%) | <0.01 |
| Procalcitonin level increased | 42 (32.8%) | 8 (15.4%) | 34 (44.7%) | <0.01 |
Each value represents the medians (interquartile range), means ± SD or the numbers (%).
Clinical treatment
All patients were given oxygen therapy. Thirty-eight patients used noninvasive mechanical ventilation, 31 patients used invasive mechanical ventilators, 4 patients used ECMO, and 42 patients were sent to the ICU. As of April 25th, 2020, 7 patients remained in the hospital, 84 had been discharged, and 37 patients had died.
CT characters and scores
The CT characteristics and lesion distribution on admission are summarized in Table 2 . The median interval between symptom onset and CT examination was 12 (8-15) days. The most frequent CT features were ground-glass opacity (96.1%), followed by consolidation (75.8%) and crazy-paving pattern (ground-glass opacity with superimposed inter- and intralobular septal thickening, 60.9%). Most patients had bilateral and multifocal involvement. The involvement of the peripheral lung was observed in all patients. In severe-type patients, an average of 3.0 lobes were involved, while in critical-type patients, an average of 4.2 lobes were involved. The mean CT score was 9.2 points in severe-type patients and 17.5 points in critical-type patients, and there was a significant difference between patients of different types (P<0.01). The consistency of CT quantitative scores between two observers showed good repeatability, with an ICC of 0.963 (95% confidence interval 0.958–0.975).
Table 2.
Lung ultrasound and chest CT findings of patients on admission
| Total (128) | Severe type (52) | Critical type (76) | P value | |
|---|---|---|---|---|
| Lung ultrasound findings | ||||
| Thickening and irregularity of pleural lines | 122 (95.3%) | 46 (88.5%) | 76 (100.0%) | <0.01 |
| B-lines in a variety of patterns | 128 (100.0%) | 52 (100.0%) | 76 (100.0%) | >0.05 |
| Confluent B-lines | 85 (66.4%) | 23 (44.2%) | 62 (81.6%) | <0.01 |
| Small consolidations limited to the subpleural space | 64 (50.0%) | 19 (36.5%) | 45 (59.2%) | 0.01 |
| Mass consolidations characterized by dynamic air bronchograms | 6 (4.7%) | 1 (1.9%) | 5 (6.6%) | 0.40 |
| Pleural effusions | 12 (9.4%) | 2 (3.8%) | 10 (13.2%) | 0.12 |
| Pneumothorax | 2 (1.6%) | 0 (0.0%) | 2 (2.6%) | 0.51 |
| Involved zones detected by ultrasound | ||||
| 1-2 zones | 7 (5.5%) | 6 (11.5%) | 1 (1.3%) | 0.02 |
| 3-4 zones | 27 (21.1%) | 20 (38.5%) | 9 (11.8%) | <0.01 |
| 5-6 zones | 46 (35.9%) | 19 (36.5%) | 27 (35.5%) | 0.91 |
| 7-8 zones | 39 (30.5%) | 7 (13.5%) | 39 (51.3%) | <0.01 |
| Lung ultrasound score | 12.6±4.8 | 8.1±3.4 | 15.7±2.6 | <0.01 |
| Chest CT findings | ||||
| Ground glass opacification | 123 (96.1%) | 47 (90.4%) | 76 (100.0%) | 0.01 |
| Consolidation | 97 (75.8%) | 30 (57.7%) | 67 (88.2%) | <0.01 |
| Crazy-paving pattern | 78 (60.9%) | 24 (46.2%) | 54 (71.0%) | <0.01 |
| Pleural effusions | 12 (9.4%) | 2 (3.8%) | 10 (13.2%) | 0.12 |
| Pneumothorax | 3 (2.3%) | 0 (0.0%) | 3 (3.9%) | 0.27 |
| Lesion distribution found by CT | ||||
| 1 lobe involved | 4 (3.1%) | 4 (7.7%) | 0 (0.0%) | 0.02 |
| 2 lobes involved | 20 (15.6%) | 13 (25.0%) | 7 (9.2%) | 0.02 |
| 3 lobes involved | 33 (25.8%) | 21 (40.4%) | 12 (15.8%) | <0.01 |
| 4 lobes involved | 26 (20.3%) | 9 (17.3%) | 17 (22.4%) | 0.49 |
| 5 lobes involved | 45 (35.2%) | 5 (9.6%) | 40 (52.6%) | <0.01 |
| Periphery involvement | 128 (100.0%) | 52 (100%) | 76 (100.0%) | >0.05 |
| Multifocal involvement | 125 (97.7%) | 49 (94.2%) | 76 (100.0%) | 0.07 |
| Bilateral involvement | 119 (93.0%) | 43 (82.7%) | 76 (100.0%) | <0.01 |
| CT score | 14.1±5.0 | 9.2±2.5 | 17.5±3.2 | <0.01 |
Bedside LUS findings and scores
The LUS findings were positive in all patients, but the degree of severity varied among patients. Typical LUS findings included the following: thickening and irregularity of the pleural line, an increase in B lines to different degrees and different extents, small multifocal consolidation limited to the subpleural space, and mass consolidations with dynamic air bronchograms. Pleural effusions were uncommon, and pneumothoraxes were rare in this study. The findings and scores of bedside LUS are summarized in Table 2. The most common LUS features of COVID-19 pneumonia were crowded or coalescent B-lines with small multifocal consolidation in multiple zones.
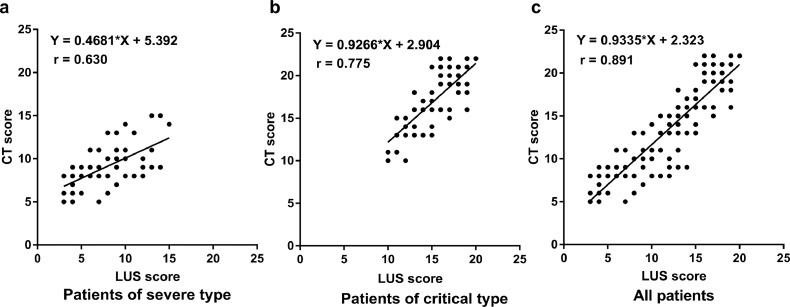
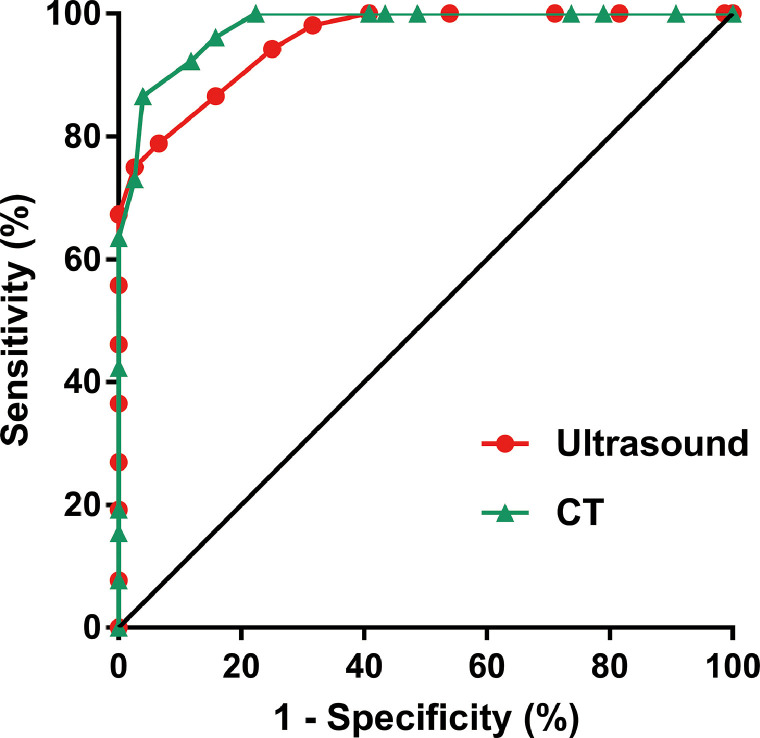
The LUS scores between the two observers showed good consistency, with an ICC of 0.928 (95% confidence interval 0.916–0.942). The mean LUS score was 8.1 points in severe-type patients and 15.7 points in critical-type patients, which were significantly different (P<0.05). Pearson correlation analysis revealed a high correlation between LUS scores and CT scores (r=0.891, p<0.01). The correlation of LUS scores and CT scores in critical-type patients was much higher than that in severe-type patients, as shown in Figure 3, Figure 4 . ROC analysis showed that LUS scores higher than 10.5 points and CT scores higher than 12.0 points could effectively distinguish critical-type patients from severe-type patients, with sensitivities of 97.4% (95% CI: 90.8% to 99.7%) and 96.1% (95% CI: 88.9% to 99.2%) and specificities of 75.0% (95% CI: 61.1% to 86.0%) and 86.5% (95% CI: 74.2% to 94.4%), respectively. The areas under the curve (AUC) of LUS scores and CT scores were 0.950 and 0.974, respectively, as shown in Figure 5 .
Figure 3.
The correlation of lung ultrasound scores and CT scores in patients of different types. High correlation was observed between lung ultrasound scores and CT scores in all patients (c). The correlation was higher in critical-type patients (b) than in severe-type patients (a).
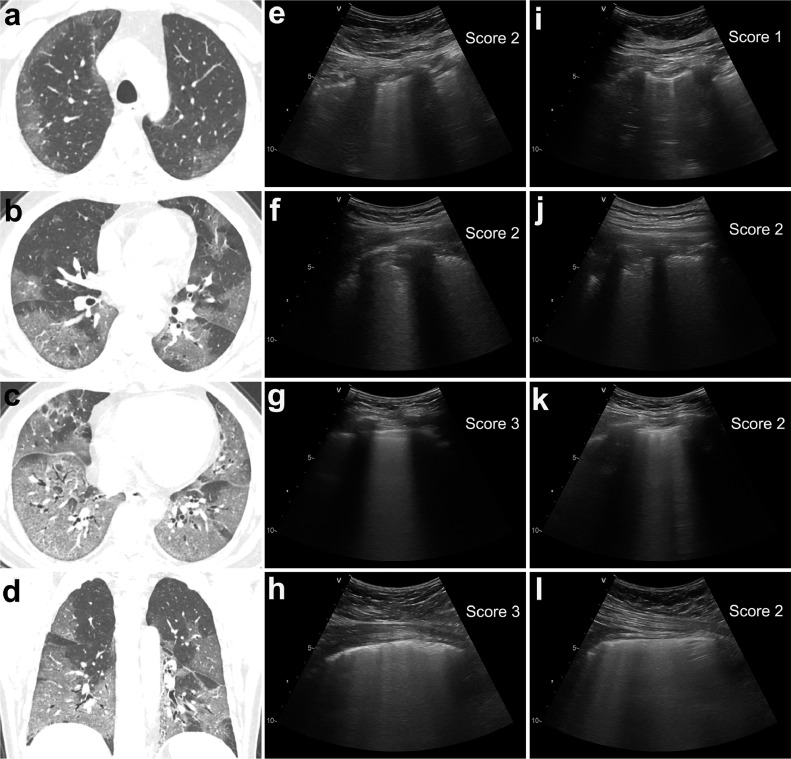
Figure 4.
Chest CT and lung ultrasound imaging of a critical-type patient. (a-d) Chest CT showed that all five lobes of the bilateral lungs were involved in this patient, and the total CT score was 18 points. (e-l) The lung ultrasound findings in eight zones. e: right anterior upper zone, f: right anterior lower zone, g: right posterior upper zone, h: right posterior lower zone, i: left anterior upper zone, j: left anterior lower zone, k: left posterior upper zone, l: left posterior lower zone. The total LUS score was 17 points, which was very approximate to the CT score.
Figure 5.
Lung ultrasound and chest CT scores for the diagnosis of critical-type patients. ROC analysis showed that the cut-off point of 10.5 in LUS score had a sensitivity of 97.4% and a specificity of 75.0% to distinguish critical-type patients from severe-type patients. The area under the curve of LUS scores and CT scores were 0.950 and 0.974, respectively.
LUS in follow-up
Repeat chest CT was performed in 72 patients during their hospitalization based on clinical needs. Chest CT confirmed that there were 51 patients with lesion progression, 5 patients without change and 16 patients with improvement. The follow-up of LUS showed that there were 48 patients with lesion progression, 13 patients without change and 11 patients with improvement. The follow-up results of chest CT and LUS are summarized in Table 3 . The consistency of LUS and chest CT in follow-up was 0.596. The consistency of LUS and chest CT in the diagnosis of lesion progression was much higher than that in the diagnosis of lesion improvement and no change (Kappa values were 0.774, 0.593 and 0.259, respectively). An increase of 2 points in the LUS scores had a sensitivity of 95.8% and specificity of 79.1% to predict the progression of lung lesions.
Table 3.
Follow-up of LUS and chest CT in 72 patients with COVID-19
| Chest CT |
||||
|---|---|---|---|---|
| Lung ultrasound | Lesion progression | No change | Lesion resolution | Total |
| Lesion progression | 46 (63.9%) | 1 (1.4%) | 1 (1.4%) | 48 (66.7%) |
| No change | 4 (5.6%) | 3 (4.2%) | 6 (4.3%) | 13 (18.0%) |
| Lesion improvement | 1 (1.4%) | 1 (1.4%) | 9 (12.5%) | 11 (15.3%) |
| Total | 51 (70.8%) | 5 (7.0%) | 16 (22.2%) | 72 (100.0%) |
DISCUSSION
We used an LUS score for the first time, to our knowledge, to evaluate and follow-up pneumonia in critically ill patients with COVID-19. We found that the LUS of these patients showed certain characteristics, including the thickening and irregularity of the pleural line, increase in B lines, small multifocal consolidation limited to the subpleural space and mass consolidations with dynamic air bronchograms. These characteristics can be clearly seen on ultrasound and can be easily calculated to form semiquantitative scores. Our study showed that semiquantitative LUS scores were highly correlated with chest CT scores and could be effectively used to evaluate the lung lesions of patients with severe pneumonia. An LUS score higher than 10.5 points highly suggested that the patient was in critical condition. There was a high consistency between LUS scores and chest CT scores in the follow-up of critically ill patients, especially for patients with lesion progression.
The discovery that SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) binds to angiotensin converting enzyme (ACE)-2, which is highly expressed in the lower airways, explained why SARS-CoV-2 causes acute respiratory distress syndrome (ARDS) and respiratory failure (22). Severe respiratory distress caused by lung damage is the leading cause of death in patients with COVID-19. The timely and accurate evaluation of lung lesions is very important for the clinical management of these patients. For critically ill patients, available modalities today mainly include chest radiographs and the gold-standard chest CT. The choice of modality is based not only on the clinic need but also on local resources and patient condition. Some realities that have to be considered, such as the high contagiousness of SARS-CoV-2 and the risk of transporting unstable patients with hypoxemia and hemodynamic failure, may greatly affect the feasibility of lung disease investigation (23).
LUS is a convenient imaging modality that is simple, noninvasive, repeatable, cost-effective and independent of the operator's experience. Its increasing popularity and supporting research data substantiate its role as an emerging technique for bedside chest imaging in critical care (23). Many studies have confirmed the important role of LUS in the ICU (11,12,14). Our study showed that LUS is an excellent monitoring tool for patients with COVID-19, especially critically ill patients. First, all patients had confirmed SARS-CoV-2 infection before admission, and the role of bedside LUS for these patients was in evaluating the severity of lung lesions rather than forming a diagnosis. Second, most COVID-19 pneumonia cases present with peripulmonary and subpleural involvement in the early stage (24). This pathological feature makes it easy to detect by ultrasound. These characteristics of COVID-19 pneumonia provide an ideal application condition for LUS. Our study confirmed that the positive rate of bedside LUS in critically ill patients with COVID-19 was 100%. The crowding of B lines was observed in all patients, and consolidation in a variety of patterns were observed in most patients.
In this study, we used semiquantitative ultrasound scores to represent the involvement of both lungs. At present, there is no uniform standard of LUS scores for adult applications. The chest wall has been divided into 6 points (BLUE protocol), 6 zones, 8 zones, 12 zones, or 28 zones in different studies on the basis of the specific circumstances (19, 25, 26). The BLUE protocol only selects six points to represent the condition of both lungs, which will miss many lesions. The 28-zone protocol is too complicated for bedside application. In our study, all patients were critically ill, and it was difficult to change their position for LUS examination of the back. Therefore, we chose the 8-zone protocol recommended for critical patients by the World Interactive Network Focused on Critical UltraSound (WINFOCUS). It was interesting that there was a good linear correlation between the LUS scores based on the 8-zone protocol and the CT scores based on the 5-lung-lobe protocol. Although the LUS results are related to the degree of aeration of the lung's outer and subpleural layers and are completely different from the chest CT results, they can also effectively reflect the condition of lung involvement. Our study indicated that the more severe the lung damage was, the more accurate the LUS scores. This result was mainly related to the pathological characteristics of severe COVID-19 pneumonia—diffuse distribution of lesions and extensive peripheral involvement. The more severe the lung lesion, the more likely it is to involve the surrounding areas, and the less likely it is to be missed by ultrasound evaluation. Further ROC analysis showed that LUS scores higher than 10.5 points could effectively distinguish critical-type patients from severe-type patients. In addition, we found the clinical diagnosis of critical-type usually lagged behind LUS findings. In 18 cases of severe-type patients, they did not have critical clinical symptoms while their LUS scores were higher than 10.5 points. There were 14 cases of them suddenly deteriorated into critical-type within 4 days. The high LUS score was valuable for early warning of critical-type patients.
Bedside LUS has a significant advantage over gold-standard chest CT for convenient patient follow-up. Ultrasound has no radiation, and the examination can be performed anytime and anywhere. More importantly, LUS follow-up does not necessitate the transport of the patient or a change in the patient's body position, which can ensure the safety of critically ill patients. However, the accuracy of ultrasonic follow-up in COVID-19 pneumonia has not been reported. Our results showed that the follow-up of LUS and chest CT had different reliability in patients with different clinical outcomes. There was a high consistency between LUS and CT in the diagnosis of lesion progression (kappa values 0.774), though there was moderate and poor consistency in the diagnoses of lesion improvement and no change (kappa values were 0.593 and 0.259, respectively). In some patients whose clinical symptoms were significantly improved after active treatment, although the density of the lesion was reduced on CT, the scope of the lesion was not significantly changed in the short time period. Therefore, LUS scores may not be sensitive enough to detect these subtle improvements. This study indicated that LUS scores were more accurate in suggesting disease progression than in measuring improvement and stabilization. An increase of 2 points in the LUS scores had a high sensitivity and specificity to predict the progression of lung lesions.
Whereas our study shows the advantages of bedside LUS, it must be acknowledged that our research may have some limitations. First, it should be recognized that LUS is a surface imaging technique, it owes its accuracy to the fact that nearly all lung pathologies relevant to the critically ill have a peripheral manifestation [24]. COVID-19 is a highly contagious disease, and its treatment has been coordinated by the government. The patients transferred to our hospital usually were in a more serious condition than other patients with COVID-19. The more severe the pneumonia was, the more accurate the LUS evaluation. The inclusion of a larger number of critically ill patients might affect the representativeness of a study with a less seriously ill patient population. Second, the LUS examinations were all performed in the supine position, and it was difficult to change the position of critical patients, which would certainly lead to the lesions located in the posterior lung being missed. Third, to verify the consistency of LUS and CT in follow-up, we only analyzed the patients who underwent CT follow-up, which could not reflect the follow-up effect of LUS in the whole population over the whole course of the disease.
CONCLUSIONS
In conclusion, our scoring system allows the more quantitative use of LUS findings and provides promising applications in critically ill patients with COVID-19. The LUS scores were well correlated with the CT findings and could effectively distinguish critical-type patients from severe-type patients. The follow-up of LUS and chest CT had high consistency in patients with progressive disease. Bedside LUS has the potential to become a reliable tool for dynamic lung monitoring in intensive care and to play an important role in the absence of CT scans.
Declaration of Competing Interest
None
Funding
This work was supported by National Natural Science Foundation of China (Grant Numbers: 81971624 and 81901759) and Natural Science Foundation of Hubei Province (Grant Numbers: 2019CFB461).
REFERENCES
- 1.Zhu N, Zhang D, Wang W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Guan X, Wu P, Wang X. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://systems.jhu.edu
- 4.WHO main website. https://www.who.int
- 5.Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020;20:e102–e107. doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Update on New Coronavirus Pneumonia as at 24:00 Chinese Standard Time on February 19, 2020. National Health Commission of the People's Republic of China. http://www.nhc.gov.cn/xcs/yqtb/202002/4dcfcb9b74ea4a408fc1d56d4db61f93.shtml
- 7.Wang D, Hu B, Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung M, Bernheim A, Mei X. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xirouchaki N, Magkanas E, Vaporidi K. Lung Ultrasound in Critically Ill Patients: Comparison With Bedside Chest Radiography. Intensive Care Med. 2011;37:1488–1493. doi: 10.1007/s00134-011-2317-y. [DOI] [PubMed] [Google Scholar]
- 11.Brogi E, Bignami E, Sidoti A. Could the use of bedside lung ultrasound reduce the number of chest x-rays in the intensive care unit? Cardiovasc Ultrasound. 2017;15:23. doi: 10.1186/s12947-017-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashton-Cleary DT. Is thoracic ultrasound a viable alternative to conventional imaging in the critical care setting? Br J Anaesth. 2013;111:152–160. doi: 10.1093/bja/aet076. [DOI] [PubMed] [Google Scholar]
- 13.See KC, Ong V, Tan YL. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22:203. doi: 10.1186/s13054-018-2105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mojoli F, Bouhemad B, Mongodi S. Lung Ultrasound for Critically Ill Patients. Am J Respir Crit Care Med. 2019;199:701–714. doi: 10.1164/rccm.201802-0236CI. [DOI] [PubMed] [Google Scholar]
- 15.Soldati G, Smargiassi A, Inchingolo R. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J Ultrasound Med. 2020 doi: 10.1002/jum.15284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buonsenso D, Piano A, Raffaelli F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 17.Peng QY, Wang XT, Zhang LN, Chinese Critical Care Ultrasound Study Group (CCUSG) Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;12:1–2. doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan F, Ye T, Sun P. Time Course of Lung Changes On Chest CT During Recovery From 2019 Novel Coronavirus (COVID-19) Pneumonia. Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volpicelli G, Elbarbary M, Blaivas M. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS) International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 20.Soummer A, Perbet S, Brisson H. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40:2064–2072. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- 21.Soldati G, Smargiassi A, Inchingolo R. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19: A Simple, Quantitative, Reproducible Method. J Ultrasound Med. 2020 doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi GP, Sanga V, Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9:e57278. doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Via G, Storti E, Gulati G. Lung ultrasound in the ICU: from diagnostic instrument to respiratory monitoring tool. Minerva Anestesiol. 2012;78:1282–1296. [PubMed] [Google Scholar]
- 24.Xu X, Yu C, Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest. 2015;147:1659–1670. doi: 10.1378/chest.14-1313. [DOI] [PubMed] [Google Scholar]
- 26.Buessler A, Chouihed T, Duarte K. Accuracy of Several Lung Ultrasound Methods for the Diagnosis of Acute Heart Failure in the ED: A Multicenter Prospective Study. Chest. 2020;157:99–110. doi: 10.1016/j.chest.2019.07.017. [DOI] [PubMed] [Google Scholar]