Abstract
Coronavirus Disease 2019 (COVID-19) is a rapidly progressing global pandemic that may present with a variety of cardiac manifestations including, but not limited to, myocardial injury, myocardial infarction, arrhythmias, heart failure, cardiomyopathy, shock, thromboembolism, and cardiac arrest. These cardiovascular effects are worse in patients who have pre-existing cardiac conditions such as coronary artery disease, hypertension, diabetes mellitus, and coagulation abnormalities. Other predisposing risk factors include advanced age, immunocompromised state, and underlying systemic inflammatory conditions. Here we review the cellular pathophysiology, clinical manifestations and treatment modalities of the cardiac manifestations seen in patients with COVID-19.
Keywords: COVID-19; Coronavirus disease 2019; SARS-CoV-2; Cardiovascular; Pathophysiology; Complications, and treatment
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), the virus causing the Coronavirus Disease 2019 (COVID-19) pandemic, has involved more than 7 million cases worldwide. The United States (US) has the highest number of infected patients with more than 2 million cases and 100,000 deaths by the second week of June 2020 [1], [2]. The respiratory symptoms including acute respiratory distress syndrome (ARDS) are well discussed in the literature. However, the extrapulmonary manifestations with likely cellular cytotoxicity is not well studied [3]. The cardiovascular sequela of COVID-19 can cause contractility disorders, arrhythmias, pericardial disease, vascular insufficiency, and sudden cardiac arrest. We sought to review cellular cytotoxicity, clinical symptoms, diagnosis and management of cardiovascular complications in COVID-19.
2. Epidemiology
Shortly after the outbreak of COVID-19 pneumonia in Wuhan, China COVID-19, its causative agent of SARS-CoV-2 was first reported in January 2020 [4]. This outbreak has rapidly spread across China and globally through person to person transmission. The mean incubation period of this virus ranges between 5 and 7 days, therefore the travelers and suspected contacts are advised to quarantine for 14 days. The basic reproduction number ranges from 2.24 to 3.58 and can be as high as 6.47 in intensive social contacts [5]. The most common symptoms at the disease onset include fever, sore throat, cough and myalgia. The infected patients may also present with cardiovascular disease (CVD) like acute coronary syndrome (ACS) and congestive cardiac failure (CHF) [6]. A study of 5700 patients have reported hypertension (56.6%), coronary artery disease (11.1%) and congestive cardiac failure (6.9%) as common underlying co-morbidities in confirmed COVID-19 cases [7]. Another study comprising 44,672 cases reported five-fold increase in case fatality rates in patients with underlying CVD as compared to patients without CVD (10.5% vs 2.3%) [8]. The impact of COVID-19 on the cardiovascular system is evidenced through multiple studies which report myocarditis in 7–17%, heart failure in 24%, arrhythmias in 17% and thrombotic complications in 31% of hospitalised COVID-19 cases [9], [10].
3. Cardiovascular cellular pathogenesis, and complications of COVID-19
The COVID-19 infection is initiated through binding of S-protein of SARS-CoV-2 with the host receptor angiotensin-converting enzyme 2 (ACE2) which mediates its entry into the cells. ACE-2 is highly expressed on the pulmonary epithelial cells, cardiac myocytes and vascular endothelial cells which is responsible for extensive cardiopulmonary symptoms [11]. Upon binding with ACE-2, S-protein cleaves at dibasic arginine site by host protease TMPRSS2 to generate S1 and S2 subunits. The S2 subunit induces membrane fusion and viral endocytosis in the cell. After viral entry into the cell, the viral RNA is released in the cytoplasm where it replicates and processed into virion- containing particles which fuses with the cell membrane to be released for widespread infection. SARS-CoV-2 also internalizes and downregulates the expression of ACE-2 on the cell surface [11]. Since ACE-2 primarily converts angiotensin I and II to cardioprotective peptides, angiotensin 1–9 and angiotensin 1–7; its loss on cell surface may potentiate cardiac damage. Additionally, the loss of ACE-2 on vascular endothelium may exacerbate endothelial dysfunction, inflammation and thrombosis [6], [12]. The ACE-2 expression in vascular endothelial cells is linked to underlying pathological state, age and gender. It’s activity is reduced in vessels with established atherosclerotic plaques and diabetes whereas it is increased in women and young adults due to a potential role of estrogen [13], [14]. Since the ACE-2 levels are downregulated in COVID-19, any underlying factor that diminishes ACE-2 expression compromises the cardioprotective action of Ang 1–7/Ang 1–9, further promoting the vascular damage. The reduced ACE-2 also induces cytokine release through dysregulating renin-angiotensin-aldosterone system, depressing Mas receptor (ACE2/MasR axis) and activating ACE2/bradykinin B1R/DABK axis [15]. These cellular effects are translated into exacerbation of underlying cardiovascular disease or new onset of cardiac symptoms.
The cardiac complications of COVID-19 can be divided into electrical and mechanical dysfunction. The electrical aberrance is seen in arrhythmias whereas pericardial, myocardial, valvular and vascular complications arise due to mechanical dysfunction.
3.1. Electrical dysfunction and arrhythmias
Arrhythmia in COVID-19 can be secondary to electrolyte imbalance, pulmonary disease, medication side effects, activated protein kinase C (PKC), or direct oxidized Ca2+/calmodulin-dependent protein kinase II (CAMKII) [16], [17]. In particular, hypokalemia and hypomagnesemia are commonly seen in COVID-19 due to angiotensin II-mediated urinary excretion and gastrointestinal losses [18]. The hypoxia in ARDS can increase the risk of arrhythmias as evidenced in an experimental study by Clark et al. which reported arrhythmias in 61% of the hypoxic rats [19], [20]. The widely used drugs chloroquine, hydroxychloroquine, azithromycin, and lopinavir/ritonavir can also trigger arrhythmias by prolonging QT [21]. The upregulated angiotensin II and angiotensin II receptor type 1 axis (AngII-AT1R) activates the PKC which interferes with initiation and propagation of action potentials leading to arrhythmias [22], [23]. Another probable mechanism is that Ang II activates NADPH oxidase via phosphorylation and translocation of p47phox [24]. The activated NADPH oxidase increases the reactive oxygen species (ROS) which oxidizes the CaMKII into ox-CaMKII. The ox-CaMKII in turn phosphorylates ryanodine receptor 2 (RyR2) leading to increased diastolic sarcoplasmic Ca2+ leak which triggers delayed after depolarizations and induces atrial and ventricular arrhythmias [17], [24]. The systemic inflammation in COVID-19 may also dysregulate the post-translational modification of cardiac ion channels resulting in arrhythmia [25], [26] It is also noteworthy that viral proteins of SARS-CoV-2, ORF3 and ORF8, activate NLRP3 inflammasomes which inturn promotes atrial fibrillation [27], [28].
3.2. Mechanical dysfunction
3.2.1. Pericardial damage
Elevated angiotensin II levels facilitate an inflammatory cascade via NF-KB, elevated IL-6, TGF-β and vascular endothelial growth factor (VEGF). These cytokines promote serosal inflammation and fibrosis that may present as pericarditis [29], [30], [31], [32]. SARS-CoV-2 can cause pericarditis that may progress to pericardial effusion. Pericarditis associated with pericardial effusion creates physical stress and can further complicate Takotsubo cardiomyopathy [33]. The combined cases of myocarditis and pericarditis cases due to COVID-19 have also been reported [29], [34]. The clinical presentation may overlap with other COVID-19 symptoms like fever, chest pain, and severe hypotension. The diagnosis is made through a combined diagnostic approach including electrocardiography, echocardiography, and chest radiographs.
3.2.2. Myocardial damage
Myocardial dysfunction in the setting of COVID-19 can present as ACS, myocarditis, heart failure, stress cardiomyopathy, and shock. Several mechanisms have been suggested for the myocardial injury associated with COVID-19.
SARS-CoV-2 can induce systemic inflammatory response syndrome (SIRS) through a combination of cytokine release syndrome (CRS) and dysregulated immune activity that leads to myocardial dysfunction [29], [35]. The immune system recognizes SARS-CoV-2 via recognition of intrinsic viral RNA patterns, or the pathogen-associated molecular patterns (PAMPs). This identified pathogen attaches to the pattern recognition receptor (PRRs), which initiate host-immune defense against the virus. The interaction of PAMPs with PRRs in combination with a dysregulated inflammatory response in the setting of sepsis leads to cytokine storm. Cytokines contribute to myocardial damage by facilitating the release of reactive oxygen species (ROS), endogenous nitric oxide, and superoxide anion (Fig. 1). Eventually, damage-associated molecular proteins (DAMPs) like heat-shock proteins, high-mobility group box 1 (HMGB1), histones, and oxidized lipoproteins are released from the damaged myocardium which further activate the inflammation and injure myocytes. These excessive DAMPs create an ongoing vicious inflammatory cycle resulting in septic/COVID-19 cardiomyopathy [36], [37]. The Notch signaling pathway also promotes inflammatory cytokines, facilitates the SARS-CoV-2 infection, and plays a major role in structural cardiac remodeling therefore a therapy directed at modulation of Notch pathway can limit the COVID-19 infection and limit the inadvertent remodeling [38].
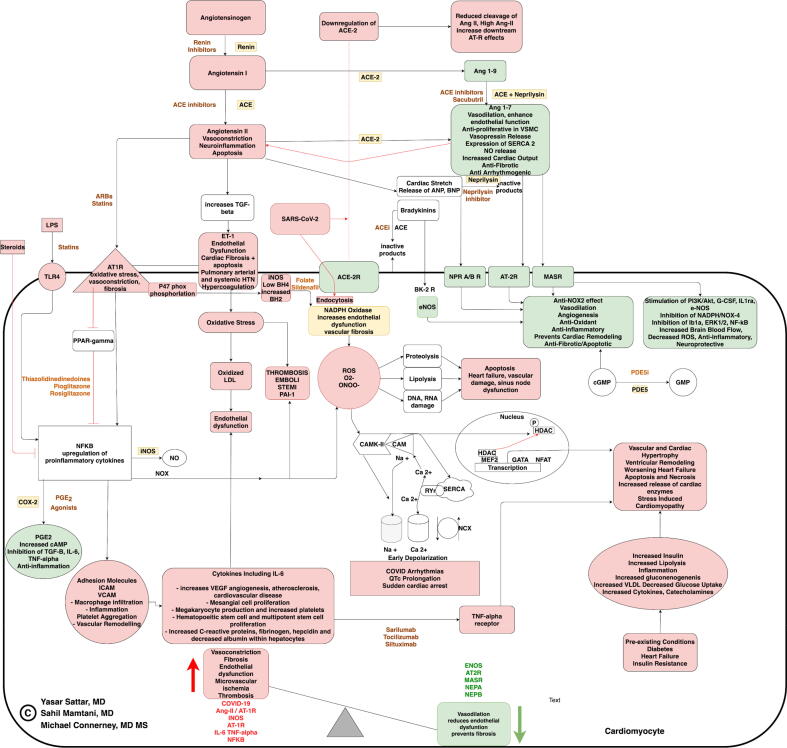
Fig. 1.
Flowchart demonstrates the SARS-CoV-2 induced cardiotoxicity. Caption: SARS-CoV-2 binds to the ACE-2 receptor(ACE-2R), leading to downregulation of ACE-2R that leads to pathway shift towards conversion of more angiotensin II that binds to Angiotensin II type 1 receptor (AT1R), Endothelin 1 receptor (ET1) promoting increased reactive oxygen species (ROS) by NADPH oxidase enzyme. NADPH oxidase is also activated by low BH4, High BH2, high iNOS. Increased ROS can cause cell apoptosis causing heart failure worsening, vascular damage, and sinus node dysfunction. Increased ROS can also cause CAMK-II activation causing early depolarization and arrhythmias. Increased ET1 also leads to increased thrombosis by increased endothelial dysfunction by oxidizing low density lipoprotein and PAI-1. Lipopolysaccharide binds to receptor TLR-4 increasing NFKBeta, that can increase IL-6, TNF-alpha, other inflammatory cytokines, and causes diffuse vascular damage and organ dysfunction. Ang 1–7 and Ang 1–9 have a positive role in our body, and can fight against COVID 19 but their levels, and downstream pathways are decreased in COVID-19 patients due to low ACE-2 R levels. eNOS, NPR A/B R, AT 2R, MASR normally counteracts the negative effects of AT1R, ET1 but this balance is disturbed in COVID-19 causing switch of pathway towards AT1R downstream effects. Abbreviations: SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus-2; IL-6: Interleukin 6; Ang II: Angiotensin II; ET1: Endothelin 1; Ang1-9: Angiotensin 1–9; Ang 1–7: Angiotensin 1–7; ACE: Angiotensin Converting Enzyme; AT1R: Angiotensin II receptor type 1; AT2R: Angiotensin II receptor type 2; NPR A/B Natriuretic peptide receptor A/B guanylate cyclase; TNFα: Tumor necrosis factor alpha; INOS: Inducible nitric oxide synthase; ENOS: endothelial nitric oxide synthase; TGF-β: Transforming growth factor beta; ROS: Reactive oxygen species; CAMK-II: calmodulin dependent protein kinase II; CAM: Calmodulin; cGMP: Cyclic guanosine monophosphate; ANP/BNP: Atrial/Brain natriuretic peptide; PDE5/PDE5i: Phosphodiesterase 5 enzyme/inhibitor; BH4: Tetrahydrobiopterin; BH2: Dihydrobiopterin; TLR4: transmembrane lipopolysaccharide receptor 4; ICAM: intercellular adhesion molecule; VCAM: vascular cell adhesion molecule; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; PGE2: Prostaglandin E; MASR: Mitochondrial assembly g protein coupled receptor; NEPA: Neprilysin A receptor; NEPB: Neprilysin B receptor; NPR A/B: Neprilysin receptor A/B; HDAC: Histone Deacetylase; GATA: Globin transcription factor; NFAT: Nuclear factor of activated T-cells; PPAR-γ: Peroxisome proliferator-activated receptor gamma; Ca 2+: Calcium ion; Na +: Sodium ion; NCX: Sodium-Calcium exchanger; MEF2: myocyte-enhancer factor-2; STEMI: ST-segment elevation myocardial infarction. Color coding: Green frames resemble the protective and anti-inflammatory mechanisms, which are imbalanced by the pro-inflammatory COVID-19 state depicted in red causing overt inflammation and cell destruction; Brown color depicts the potential therapeutic targets that can help balance this inflammatory state; Yellow color shows all enzymes involved in RAAS or COVID induced cardiovascular cytotoxicity pathway. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The SARS-CoV-2 may cause direct myocardial cytotoxicity via 3C proteinase-mediated apoptosis, disruption of host protein translational mechanisms, loss of cellular homeostasis, and dysregulated host-immune response [39], [40]. After viral infection of cardiomyocytes, the immune system triggers the infiltration of natural killer cells, macrophages, and lymphocytes. These cells initially confer protective effects, but hyper-activation can worsen the severity of myocarditis and lead to cardiomyopathy [41]. Biopsy specimens in myocarditis have demonstrated infiltration of mononuclear cells in the cardiac myocytes causing focal or diffuse damage [15]. In addition, the hypoxemia in COVID-19 can induce increased intracellular calcium levels which can result in cardiomyocyte damage and eventual cardiomyopathy [42].
COVID-19 can also cause stress cardiomyopathy due to emotional or physical stress on cardiac myocardium in the setting of anxiety and widespread inflammation [43].The myocardial stunning in the setting of catecholamine surge and coronary microvascular dysfunction may be exacerbated by COVID-19 from RAS dysregulation and plaque disruption [44]. Increased catecholamines further worsens the microvascular dysfunction through increasing cytokine production of IL-6 via alpha-1 adrenergic receptor signaling in immune cells [44]. The excessive catecholamines may also worsen the transient hypokinesia through beta2-coupling from Gs to Gi [45]. The combined effects of catecholamine surge and microvascular dysfunction in COVID-19 may result in hypercontraction of the left ventricular apex and outflow tract resulting in stress cardiomyopathy.
The increased cardiometabolic demand in systemic infection coupled with hypoxia secondary to pulmonary dysfunction can create myocardial oxygen supply/demand mismatch. These events when accompanied by inflammation induced hypercoagulation, microthrombi and plaque rupture can lead to cardiac ischemia and acute coronary syndrome [46]. It is unclear whether the SARS-CoV-2-mediated myocardial damage will have long-term implications on cardiac function and survival therefore follow up data on survivors is required to better assess the long-term outcomes.
The symptoms of acute coronary syndrome (ACS) include chest pain, dyspnea, and sinus dysfunction which can overlap with COVID-19 symptoms [47]. A case series of 18 COVID-19 patients with STEMI reported that 33% of cases manifested chest pain at the time of ST-elevation. Among these patients, 22% died of myocardial infarction whereas 50% died of non- coronary myocardial injury [48]. The diagnosis of ACS requires elevated troponin along with either echocardiographic or electrocardiographic abnormalities [49]. The ventricular dysfunction in COVID-19 may present with features of heart failure, cardiogenic shock, and myocarditis [50]. Their clinical presentation will overlap with the symptoms of COVID-19 including respiratory distress and hypotension. The diagnosis can only be ascertained by utilizing various diagnostic modalities such as cardiac markers, echocardiography, MRI, and biopsy. A study reports that heart failure is a common manifestation of COVID-19 patients, seen in 23% of affected patients [47]. Cardiogenic shock was reported in a few cases with depressed left ventricular ejection fraction, left ventricular hypokinesis, and elevated cardiac markers [33], [37]. A case of myocarditis showed similar diagnostic features but presented without the typical respiratory features of COVID-19 [51]. Cardiovascular complications may present in isolation without respiratory symptoms in COVID-19.
3.2.3. Valvular damage
The ACE-2 is widely expressed on the stromal fibroblasts of cardiac valves, particularly aortic valves. It is evidenced from the RT-PCR that the ACE-2 expression is downregulated in stenotic valves. The depressed ACE2/Angiotensin-(1–7)/Mas receptor axis may potentiate inflammation, fibrosis and valvular sclerosis [52]. Simultaneously, upregulated AngII-AT1R axis induces inflammatory cytokines like TNF-α and IL-6 amplifier (IL-6 AMP) which further induces pro-inflammatory cytokines and recruits macrophages to create a positive feedback loop of inflammation [53]. These downstream effects of SARS-CoV-2 infection result in valvular damage which may not present acutely due to the slowly progressive nature of cardiac valvular disease. However, periodic follow-up of cardiac function is essential in COVID-19 survivors to timely identify this pathology.
3.2.4. Vascular complications
3.2.4.1. Hypertension
Hypertension is frequently seen in COVID-19 patients however it is unclear whether it is a risk factor for acquiring COVID-19. The unregulated Angiotensin II stimulates vasoconstriction and systemic inflammation which may worsen the underlying hypertension through oxidative stress and endothelial dysfunction. The reactive oxygen species (ROS) like superoxide, interacts with nitric oxide to form peroxynitrite which reduces the availability of NO and inhibits the eNOS activity [54]. The elevated levels of inflammatory markers like CRP and TNF also destabilize eNOS and limit NO production. Since NO has vasodilatory and anti-inflammatory effects, its reduced bioavailability can exacerbate hypertension. The ROS can also impair the vascular structure and function by directly inducing cell damage [54].
3.2.4.2. Thromboembolism
Hypercoagulation is another commonly seen manifestation of the disease [29], [30], [31]. Diffuse cytokine storm, elevated angiotensin II, endothelin-1, plasminogen activator inhibitor 1 (PAI-1), tissue factor, ROS, and extrinsic clotting cascade are all interrelated pathophysiological components of hypercoagulation [55] (Fig. 1). The endothelial cells line the intima of the vasculature and are closely connected by adherents, tight and gap junctions which maintain the endothelial integrity [56]. In SARS-CoV-2, excessive cytokines, ROS and proteases from the activated neutrophils may damage these junctional proteins, resulting in trans-endothelial migration of neutrophils [57]. These neutrophils degranulate and form neutrophil extracellular traps which release DAMPs (DNA and neutrophil elastase) that further damage the junctional proteins and endothelial layer [58]. This leads to greater serum contact with subendothelial tissue, which activates Von-Willebrand Factor, platelet adhesion, and the coagulation cascade. Inflammation leads to increased PAI-1, which increases endothelial fibrin deposition and reduced thrombolysis, ultimately contributing to the hypercoagulable and prothrombotic state of COVID-19 (Fig. 1).
The coagulation profile in COVID-19 patients may show thrombocytopenia, elevated D-dimer, prolonged prothrombin time, international normalized ratio (INR), and short activated partial thromboplastin time (aPTT) [59]. This coagulation disarray predisposes patients to thromboembolic complications. A case study reports an incidence of acute pulmonary embolism with right ventricular failure in a COVID-19 patient which demonstrated elevated D-dimers at 1280 ng/mL [60]. The increased risk of thrombosis further predisposes the risk of disseminated intravascular coagulation (DIC), venous thromboembolism (VTE) and multi-organ failure. There are multiple risk stratification tools to ascertain the risk of VTE in COVID-19 patients including Caprini score, Padua model and IMPROVE [International Medical Prevention Registry on Venous Thromboembolism]. Wang et al., using the Padua model, reported a high risk of VTE in 40% of hospitalized patients with COVID-19 [61]. Another study has shown that 71.4% of the non-survivors of COVID-19 had DIC with elevated D-dimer [47]. Given the high mortality in patients who develop DIC, there is an expert consensus as reviewed by Song et al. regarding the management of coagulopathy in these patients. Current recommendations emphasize the importance of routine monitoring of coagulation parameters and the implementation of scores to diagnose COVID-19 related coagulopathy.
4. Treatment approaches for cardiac complications
A variety of different drug treatments are being investigated in COVID-19 infection despite the lack of definitive evidence about their efficacy. The proposed drug regimens include hydroxychloroquine, azithromycin, lopinavir/ritonavir, tocilizumab, remdesivir and ribavirin [62]. The role of hydroxychloroquine in treating COVID-19 patients with CVD is controversial. It inhibits binding of SARS-CoV-2 with ACE-2 receptor by blocking TMPRSS-2, and inhibits viral entry by altering endosomal pH [63]. This drug has shown positive outcomes in terms of viral clearance however its safety in patients with cardiovascular disease is not clearly established due to potential side effects of polymorphic ventricular tachycardia, long QT-syndrome and increased risk of sudden death [64]. A clinical trial of 81 patients reported high dose of chloroquine is associated with severe arrhythmias and deaths and was therefore halted [65]. Since different drugs used in COVID-19 have a certain risk of pro-arrhythmic side effects, it should be balanced with the anticipated benefit of therapy and administered under close monitoring.
Arrhythmias in COVID-19 are managed based on etiology including repletion of electrolytes, discontinuation of medications causing arrhythmia, managing volume status, or suppressing catecholamine surge in COVID-19 [44], [66], [67], [68]. Brugada syndrome can be treated by alleviating the underlying triggers including fever, or pneumonia, or ICD placement in cases of Brugada-1 [69], [70], [71]. Furthermore, in the setting of shock in COVID-19, the use of vasopressor such as dobutamine, epinephrine or PDE-inhibitors such as milrinone should be avoided due to synergistic proarrhythmic activity of these medications. In hemodynamically stable arrhythmias, beta-blockers should be used and epinephrine should be avoided especially during the catecholaminergic surge in COVID-19 [72].
The pericarditis in COVID-19 is treated with steroids and supportive care however pericardiocentesis may be required in pericardial effusion. The collected fluid should be checked for cytology, autoimmune reactions, cultures and tested for SARS-CoV-2 [51].
The choice of treatment in COVID-19 patients with STEMI depends on the availability of a PCI facility, or fibrinolytic and institutional policy of COVID-19 STEMI cases. Anticoagulant therapies, such as unfractionated heparin or enoxaparin, may also be considered in COVID-19 with STEMI and NSTEMI [73]. In COVID-19 with NSTEMI, the decision to perform PCI depends on the Global registry of acute clinical events (GRACE) score >140, or Thrombolysis in Myocardial infarction (TIMI) >3 or hemodynamic instability [74]. As per the protocol by one Chinese medical center, for stable patients who tested positive for COVID-19 with STEMI, it is reasonable to administer thrombolytics if onset of symptoms is <12 h, pending no contraindications. Elective PCI can be considered once the patients are treated and have recovered from COVID-19. However, in unstable patients and those who do not have severe pneumonia, the recommendation assessed the risks and benefits of early PCI [75]. Since dual antiplatelet therapy (DAPT) after PCI in COVID-19 patients has increased bleeding risk, the new strategy is to reduce the duration of aspirin to 1–3 months and allow prolonged use of P2Y12 inhibitors. The early antiplatelet therapy with P2Y12 antagonists like ticagrelor, is beneficial after PCI in COVID-19 due to the inhibitory effects on platelet activation and intravascular thrombus formation [76]. Furthermore, the management of cardiovascular cardiomyopathy is based on type of manifestation. The treatment of hemodynamically stable heart failure involved optimal medical regimen including aldosterone antagonists, diuretics, and beta-blockers [51], [77]. In patients with cardiogenic shock, iatrogenic agents such as milrinone, a phosphodiesterase 3-inhibitor (PDE-3i) can be used under close observation [51]. There is widespread controversy about the use of ACEi, ARBs, and neprilysin inhibitors (sacubitril) in COVID-19 exacerbated heart failure. ACEi/ARBs can worsen COVID-19 by increasing ACE2 expression which may amplify the chances of viral invasion [78], [79] (Fig. 1). On the contrary, ACE inhibitors can be beneficial by increasing ACE-2 and further opposing the action of angiotensin II receptor type 1 (AT1R) through increasing Ang 1–7 and, Ang 1–9 downstream effects [80] (Fig. 1). A case-control study by Mancia et al. showed no association of risk of COVID-19 with the use of ACEi in overall case patients (odds ratio, 0.96 [95% CI, 0.87 to 1.07]) and those with fatal outcomes (odds ratio, 0.91 [95% CI, 0.69 to 1.21) [81]. However, we still need evidence from different randomized clinical trials to support the use of ACEi/ARBs in these patients. The summary of balance between AT1R and Ang 1–7, Ang 1–9 is shown in Fig. 1.
In patients with refractory cardiogenic shock in the setting of COVID-19, Veno-Arterial (VAV) extracorporeal membrane oxygenation (ECMO) can be helpful [82], [83]. Many COVID-19 patients present with acute respiratory distress syndrome and pulmonary embolism which can increase the right ventricular afterload resulting in impaired right ventricular function. Echocardiogram and other advanced imaging modalities can play a significant role in determining the right ventricular dysfunction and ascertain the prognosis [84].
COVID myocarditis can be treated with immunosuppressants including steroids, cyclosporine, azathioprine, and immunoglobulins [85], [86]. IL-6 antagonists can be efficacious as they inhibit cytokine storms in COVID-19 [87]. The steroid use is controversial as Chen et al. suggested that steroids might not be helpful in viral myocarditis however few reports have shown favorable outcomes in fulminant COVID-19 cases [88], [89]. In addition, a placebo-controlled trial has shown safety of intravenous immunoglobulin (IVIG) in idiopathic dilated cardiomyopathy or biopsy-proven viral myocarditis with no added benefit in improvement of LV function [90]. IVIG can be considered a salvage therapy in fulminant myocarditis with hemodynamic instability [83]. Preliminary studies about the use of convalescent plasma have also demonstrated efficacy in critical COVID-19 infection [91]. Statins also have a potential benefit in limiting cardiovascular complications in COVID-19 through inhibiting Toll-like receptors (TLR)-NF-κB pathway. It’s use may also counter the hyperlipidemic side-effects of other antiviral drugs used in COVID-19 [92].
5. Treatment approaches for vascular complications
Multiple studies have supported the use of ACEi and ARBs in hypertension due to their potential to improve the clinical symptoms and reduce mortality. A study reported a lower rate of severe disease and IL-6 levels in COVID-19 patients with hypertension who were being treated with ACEi /ARBs. Hence, these drugs may prevent the worsening of blood pressure due to inhibition of inflammatory response [93]. The International Society on Thrombosis and Haemostasis (ISTH) recommends VTE prophylaxis in hospitalised COVID-19 patients. In non-ICU hospitalised cases, standard or intermediate dose of low molecular weight heparin (LMWH) is preferred for thromboprophylaxis however in ICU patients, a combined approach of prophylactic or intermediate-dose LMWH with mechanical compression device is advised after assessing the bleeding risk. The duration of post-discharge thromboprophylaxis in COVID-19 patients ranges between 14 and 30 days [94]. A retrospective study has shown reduced 28-day mortality in COVID-19 patients with elevated D-dimers who used heparin as compared to non-heparin users. (p = 0.017). Hence, prophylaxis with low molecular weight heparin may have a role in preventing COVID-19 associated thrombotic complications and mortality [95]. In cases of diagnosed VTE, parenteral anticoagulation with LMWH is preferred in an in-patient setting whereas direct oral anticoagulants (DOAC) are given after hospital discharge. The total duration of treatment in diagnosed VTE is at least 3 months [94]. There is no evidence that antiplatelets increase the risk of severe COVID-19 hence it should be continued for conditions like recent MI or PCI within the last 3 months. The decision for antiplatelet therapy is individualised on a case to case basis depending on the platelet count, bleeding risk and drug-drug interaction [96].
6. Conclusion
COVID-19 infection has been linked to myocardial injury, leading to severe disease progression. Although incredible efforts are being done to accurately comprehend the mechanism of myocardial injury, it is prudent to expand the randomized controlled data. Cardiologists should have a high suspicion of myocardial injury secondary to COVID-19 infection so that rigorous primary and secondary prevention can be ensured to limit morbidity and mortality.
Disclosure
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
None.
References
- 1.CDC. Cases of Coronavirus Disease (COVID-19) in the U.S., 2020.
- 2.JHU, COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU), 2020.
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhai P., Ding Y., Wu X., Long J., Zhong Y., Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents. 2020;55:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020;318:H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the Chinese Center for disease control and prevention. JAMA. 2020;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 9.Long B., Brady W.J., Koyfman A., Gottlieb M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–80):e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 pandemic. J. Med. Virol. 2020;92(7):770–775. doi: 10.1002/jmv.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tikellis C., Thomas M.C. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012;2012:256294. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalra S.P., Chen C.L., Kumar M.S. Differential response to methionine-enkephalin in basal hypothalamus and preoptic area following hypothalamic deafferentation. Brain Res. 1981;215:410–413. doi: 10.1016/0006-8993(81)90526-6. [DOI] [PubMed] [Google Scholar]
- 16.Lakkireddy D.R., Chung M.K., Gopinathannair R., Patton K.K., Gluckman T.J., Turagam M. Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the heart rhythm society COVID-19 task force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Circulation. 2020 doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purohit A., Rokita A.G., Guan X., Chen B., Koval O.M., Voigt N. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19) Ann. Clin. Biochem. 2020;57:262–265. doi: 10.1177/0004563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.JAMA. 1963;184:167-. [Google Scholar]
- 20.Sharma S. Acute respiratory distress syndrome. BMJ Clin. Evid. 2010;2010:1511. [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira J.C., Mochly-Rosen D., Boutjdir M. Regulation of cardiac excitability by protein kinase C isozymes. Front. Biosci. (Schol. Ed.) 2012;4:532–546. doi: 10.2741/283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007;292(1):C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 24.Dusi S., Della Bianca V., Grzeskowiak M., Rossi F. Relationship between phosphorylation and translocation to the plasma membrane of p47phox and p67phox and activation of the NADPH oxidase in normal and Ca(2+)-depleted human neutrophils. Biochem. J. 1993;290(Pt 1):173–178. doi: 10.1042/bj2900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagu B., Charpentier F., Toumaniantz G. Identifying potential functional impact of mutations and polymorphisms: linking heart failure, increased risk of arrhythmias and sudden cardiac death. Front. Physiol. 2013;4:254. doi: 10.3389/fphys.2013.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinelli F.R., Pecani A., Conti F., Mancini R., Alessandri C., Valesini G. Post-translational modifications in rheumatoid arthritis and atherosclerosis: Focus on citrullination and carbamylation. J. Int. Med. Res. 2016;44:81–84. doi: 10.1177/0300060515593258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuen K.S., Ye Z.W., Fung S.Y., Chan C.P., Jin D.Y. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020;10:40. doi: 10.1186/s13578-020-00404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao C., Veleva T., Scott L., Jr., Cao S., Li L., Chen G. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cizgici A.Y., Zencirkiran Agus H., Yildiz M. COVID-19 myopericarditis: it should be kept in mind in today's conditions. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020 doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramasamy V., Mayosi B.M., Sturrock E.D., Ntsekhe M. Established and novel pathophysiological mechanisms of pericardial injury and constrictive pericarditis. World J. Cardiol. 2018;10:87–96. doi: 10.4330/wjc.v10.i9.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montecucco F., Mach F., Pende A., Schindler T.H., da Silva R.F., Vuilleumier N. Inflammatory cardiovascular risk biomarkers: update on novelties and limitations. Mediators Inflamm. 2012;2012:515692. doi: 10.1155/2012/515692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabbagh M.F., Aurora L., D'Souza P., Weinmann A.J., Bhargava P. Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua A., O'Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakihana Y., Ito T., Nakahara M., Yamaguchi K., Yasuda T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J. Intensive Care. 2016;4:22. doi: 10.1186/s40560-016-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.S. Hendren Nicholas, H. Drazner Mark, B. Bozkurt, J.L.T. Cooper, Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation.0. [DOI] [PMC free article] [PubMed]
- 38.Rizzo P., Vieceli Dalla Sega F., Fortini F., Marracino L., Rapezzi C., Ferrari R. COVID-19 in the heart and the lungs: could we “Notch” the inflammatory storm? Basic Res. Cardiol. 2020;115:31. doi: 10.1007/s00395-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung G., Luo H., Qiu Y., Yang D., McManus B. Myocarditis. Circ. Res. 2016;118:496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 40.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 41.Bracamonte-Baran W., Cihakova D. Cardiac autoimmunity: myocarditis. Adv. Exp. Med. Biol. 2017;1003:187–221. doi: 10.1007/978-3-319-57613-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 43.Minhas A.S., Scheel P., Garibaldi B., Liu G., Horton M., Jennings M. Takotsubo syndrome in the setting of COVID-19 infection. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.M.F. Konig, M. Powell, V. Staedtke, R.-Y. Bai, D.L. Thomas, N. Fischer, et al., Targeting the catecholamine-cytokine axis to prevent SARS-CoV-2 cytokine storm syndrome. medRxiv. 2020:2020.04.02.20051565.
- 45.Komamura K., Fukui M., Iwasaku T., Hirotani S., Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J. Cardiol. 2014;6:602–609. doi: 10.4330/wjc.v6.i7.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.I. Basu-Ray, N.K. Almaddah, A. Adeboye, M.P. Soos, Cardiac Manifestations Of Coronavirus (COVID-19). StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC., 2020. [PubMed]
- 47.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B. ST-segment elevation in patients with Covid-19 — a case series. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atri D., Siddiqi H.K., Lang J., Nauffal V., Morrow D.A., Bohula E.A. COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl. Sci. 2020 doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang Y., Chen T., Mui D., Ferrari V., Jagasia D., Scherrer-Crosbie M. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020 doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X., Yu S. Cardiac valves: another “Disaster-Hit Area” of COVID-19 patients? Heart Lung. 2020 doi: 10.1016/j.hrtlng.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dinh Q.N., Drummond G.R., Sobey C.G., Chrissobolis S. Roles of inflammation, oxidative stress, and vascular dysfunction in hypertension. Biomed. Res. Int. 2014;2014:406960. doi: 10.1155/2014/406960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wallez Y., Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 57.Bermejo-Martin J.F., Martin-Fernandez M., Lopez-Mestanza C., Duque P., Almansa R. Shared features of endothelial dysfunction between sepsis and its preceding risk factors (aging and chronic disease) J. Clin. Med. 2018;7 doi: 10.3390/jcm7110400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomar B., Anders H.J., Desai J., Mulay S.R. Neutrophils and neutrophil extracellular traps drive necroinflammation in COVID-19. Cells. 2020;9 doi: 10.3390/cells9061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ullah W., Saeed R., Sarwar U., Patel R., Fischman D.L. COVID-19 complicated by acute pulmonary embolism and right-sided heart failure. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7:e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCreary E.K., Pogue J.M. Coronavirus disease 2019 treatment: a review of early and emerging options. Open Forum Infect. Dis. 2020;7:ofaa105. doi: 10.1093/ofid/ofaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quiros Roldan E., Biasiotto G., Magro P., Zanella I. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol. Res. 2020;158:104904. doi: 10.1016/j.phrs.2020.104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roden D.M., Harrington R.A., Poppas A., Russo A.M. Considerations for drug interactions on QTc in exploratory COVID-19 (coronavirus disease 2019) treatment. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 65.Borba M.G.S., Val F.F.A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a Randomized Clin. Trial. JAMA Netw. Open. 2020;3:e208857-e. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PubMed] [Google Scholar]
- 66.Lippi G., South A.M., Henry B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 COVID-19. Ann. Clin. Biochem. 2020 doi: 10.1177/0004563220922255. 4563220922255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kazory A., Ronco C., McCullough P.A. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proc. (Bayl. Univ. Med. Cent.) 2020:1–6. doi: 10.1080/08998280.2020.1754700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang T., Roden D.M. Extracellular potassium modulation of drug block of IKr. Implications for torsade de pointes and reverse use-dependence. Circulation. 1996;93:407–411. doi: 10.1161/01.cir.93.3.407. [DOI] [PubMed] [Google Scholar]
- 69.Chang David, Saleh Moussa, Garcia-Bengo Youssef, Choi Evan, Epstein Laurence, Willner Jonathan. COVID-19 infection unmasking brugada syndrome. HeartRhythm Case Rep. 2020;6(5):237–240. doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sorgente A., Capulzini L., Brugada P. The known into the unknown: Brugada syndrome and COVID-19. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu C.I., Postema P.G., Arbelo E., Behr E.R., Bezzina C.R., Napolitano C. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bellamy D., Nuthall G., Dalziel S., Skinner J.R. Catecholaminergic polymorphic ventricular tachycardia: the cardiac arrest where epinephrine is contraindicated. Pediatr. Crit. Care Med. 2019;20:262–268. doi: 10.1097/PCC.0000000000001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.G. Stefanini Giulio, M. Montorfano, D. Trabattoni, D. Andreini, G. Ferrante, M. Ancona, et al. ST-Elevation Myocardial Infarction in Patients with COVID-19: Clinical and Angiographic Outcomes. Circulation. [DOI] [PMC free article] [PubMed]
- 74.Mahmud E., Dauerman H.L., Welt F.G., Messenger J.C., Rao S.V., Grines C. Management of acute myocardial infarction during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020 doi: 10.1016/j.jacc.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng J., Huang J., Pan L. How to balance acute myocardial infarction and COVID-19: the protocols from Sichuan Provincial People's Hospital. Intensive Care Med. 2020;1–3 doi: 10.1007/s00134-020-05993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou X., Li Y., Yang Q. Antiplatelet therapy after percutaneous coronary intervention in patients with COVID-19: implications from clinical features to pathologic findings. Circulation. 2020;141:1736–1738. doi: 10.1161/CIRCULATIONAHA.120.046988. [DOI] [PubMed] [Google Scholar]
- 77.ACC. Management of the Hospitalized COVID-19 Patient With Acute Cardiomyopathy or Heart Failure. Cardiology Magazine. ACC: ACC, 2020.
- 78.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 80.HFSA/ACC/AHA. Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. ACC News Story2020. [DOI] [PMC free article] [PubMed]
- 81.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020 doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zeng J.H., Liu Y.X., Yuan J., Wang F.X., Wu W.B., Li J.X. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020 doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Y., Li H., Zhu S., Xie Y., Wang B., He L. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC: Cardiovascular Imag. 2020:3423. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mason J.W., O'Connell J.B., Herskowitz A., Rose N.R., McManus B.M., Billingham M.E. A clinical trial of immunosuppressive therapy for myocarditis. The myocarditis treatment trial investigators. N. Engl. J. Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 87.Chau V.Q., Oliveros E., Mahmood K., Singhvi A., Lala A., Moss N. The imperfect cytokine storm: severe COVID-19 with ARDS in patient on durable LVAD support. JACC: Case Rep. 2020::424. doi: 10.1016/j.jaccas.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen H.S., Wang W., Wu S.N., Liu J.P. Corticosteroids for viral myocarditis. Cochrane Database Syst. Rev. 2013;Cd004471 doi: 10.1002/14651858.CD004471.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Belhamissi H., Ferro M., Clement C., Dupont P., Roux L. Pulmonary embolism with massive hepatic cytolysis syndrome. Apropos of a case. Ann. Fr. Anesth. Reanim. 1982;1:521–523. doi: 10.1016/s0750-7658(82)80095-6. [DOI] [PubMed] [Google Scholar]
- 90.McNamara Dennis M., Holubkov R., Starling Randall C., Dec G.W., Loh E., Torre-Amione G. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 91.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castiglione V., Chiriacò M., Emdin M., Taddei S., Vergaro G. Statin therapy in COVID-19 infection. Eur. Heart J. Cardiovasc. Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tadic M., Cuspidi C., Mancia G., Dell'Oro R., Grassi G. COVID-19, hypertension and cardiovascular diseases: should we change the therapy? Pharmacol. Res. 2020;158:104906. doi: 10.1016/j.phrs.2020.104906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Spyropoulos A.C., Levy J.H., Ageno W. Scientific and standardization committee communication: clinical guidance on the diagnosis, prevention and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Atallah B., Mallah S.I., AlMahmeed W. Anticoagulation in COVID-19. Eur. Heart J. Cardiovasc. Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]