Abstract
The immune system protects against viruses and diseases and produces antibodies to kill pathogens. This review presents a brief overview of the immune system regarding its protection of the human body from COVID-19; illustrates the process of the immune system, how it works, and its mechanism to fight virus; and presents information on the most recent COVID-19 treatments and experimental data. Various types of potential challenges for the immunes system are also discussed. At the end of the article, foods to consume and avoid are suggested, and physical exercise is encouraged. This article can be used worldwide as a state of the art in this critical moment for promising alternative solutions related to surviving the coronavirus.
Keywords: Immunity system, COVID-19, Case study, Potential challenges, Data analysis
Introduction
The earth is relaxing but humans are dying. As of April 18, 2020, more than 154,000 have people died, 2.2 million have been affected, and at least 185 countries have been affected by the coronavirus. The world experienced coronavirus for the first time in 2002–2003 through severe acute respiratory syndrome (SARS), and in 2011, Middle East respiratory syndrome (MERS) for the first time. The causative agents for both cases (SARS-CoV and MERS-CoV,) were newly identified coronaviruses of zoonotic origin in the genus Beta coronavirus [1]. The present coronavirus (SARS-CoV-2) COVID-19 appeared for the first time in Wuhan, China, at the end of 2019. People are being affected by human-to-human transmission due to close contact [2,3], and people affected by COVID-19 suffer from severe respiratory illness [4]. People who are elderly and have many comorbidities are the most vulnerable to COVID-19 [5,6].
There is no registered treatment or vaccine for this disease [7]. For the treatment of affected people, limited urgent use of chloroquine and hydroxychloroquine have been approved by the United States Food and Drug Administration. The use of an antiviral drug called Favilavir as a treatment for coronavirus has been approved by the National Medical Products Administration of China. The drug has shown efficacy in treating the disease, with very low side effects in a clinical trial involving 70 patients. The clinical trial has been ongoing in Shenzhen, Guangdong province [8]. This review article reported the recent observations regarding the development of the immunity level in the human body for resisting the coronavirus as an alternative solution before the invention of drugs and vaccinations.
Process of the immune system in the human body
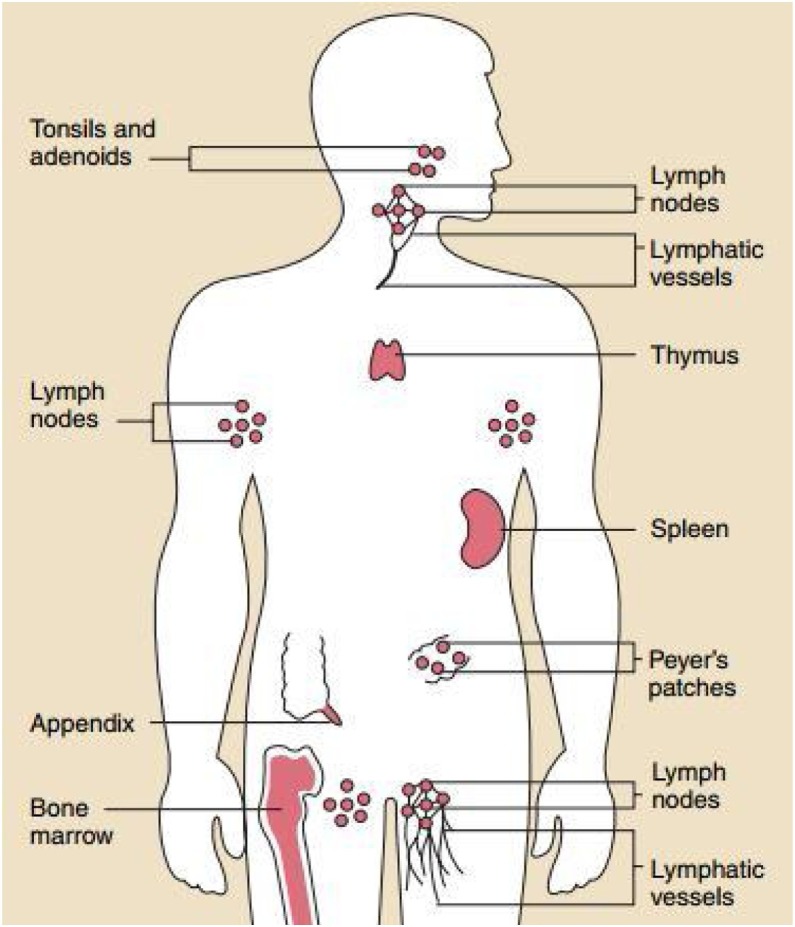
The body contains the organs of the immune system (Fig. 1 ), which protects against diseases [9,10]. It plays a key role to maintain health and pathogenesis. It also protects the body from harmful substances, germs, and cell changes (neoplasm) [11]. The key player in the immune system is the white blood cells, which can travel throughout the body through the blood vessels. To monitor for invading microbes, the body exchanges cells and fluids between blood and lymphatic vessels and enables the lymphatic system. The lymphatic vessels carry lymph. Each lymph node contains specialized compartments where they can encounter antigens. Through the incoming lymphatic vessels, the immune cells and foreign particles enter the lymph nodes. When they are in the bloodstream, they are transported to tissues throughout the body. They continue the cycle all over by patrolling for foreign antigens everywhere and then gradually drift back into the lymphatic system. The immune cells gather, work, and serve to confront antigens in lymph nodes and the spleen’s compartments [12].
Fig. 1.
The organs of the immune system are positioned throughout the body [12].
Impacts of Covid-19 on the human body
COVID-19 is an RNA virus with a crown-like appearance. Its diameter is approximately 60–140 nm. On one side, it has a concave surface with a ridge. It makes a larger binding interface, as well as more contacts with ACE2. It can make better contact with the N-terminal helix of ACE2 and have higher affinity [13]. It is transmitted through respiratory droplets from coughing and sneezing and enters the nasal system by inhaling and starts replicating. ACE2 is the main receptor for the COVID-19 virus [14]. The spike protein (S protein) present on the surface of COVID-19 is pinched inside the host cell binding to the ACE2 receptor. Here, the enzyme furin is present in the host cell and plays a vital role for the virus to enter, which was absent in SARS-CoV [15]. Next, the virus starts to propagate with limited innate immune response and can be detected by nasal swabs. The virus then propagates and reaches the respiratory tract, where it faces a more robust innate immune response. At this stage, the disease is clinically manifest and an innate response cytokine may be predictive of the subsequent clinical course [16]. For beta and lambda infections, viral-infected epithelial cells are a major source [17]. The disease will be mild for 80% of the infected patients and mostly restricted to the upper and conducting airways [18]. With conservative symptomatic therapy, these individuals may be monitored and monitored at home. Approximately 20% of the infected patients develop pulmonary infiltrates and some of these develop very severe disease [19]. The mortality rate of severe patients with COVID-19 can be as high as 49%, based on a recent epidemiological by China CDC [20]. In Wuhan, 292 patients with COVID-19 were studied. Age was the risk factor of patients with a severe condition, as shown by the Lasso algorithm. When the age of patients with a severe condition increased by 5 years, the risk increased by 15.15%. Most of the patients with COVD-19 were elderly patients in the severe group, with basic diseases. Chronic obstructive pulmonary disease, hypertension, malignant tumor, coronary heart disease, and chronic kidney disease were more frequent in the severe group than in the mild group. Of 145 severe cases, 51 patients died (34.69%), and 90.2% of the patients who dies were over 60 years old. Forty patients had basic disease out of 51 deaths (78.43%). Reports have demonstrated that patients aged older than 60 years who have comorbidities, especially hypertension, are at risk for severe disease and death from SARS-CoV-2 infection [[21], [22], [23]].
Mechanism of immune systems in the human body against COVID-19
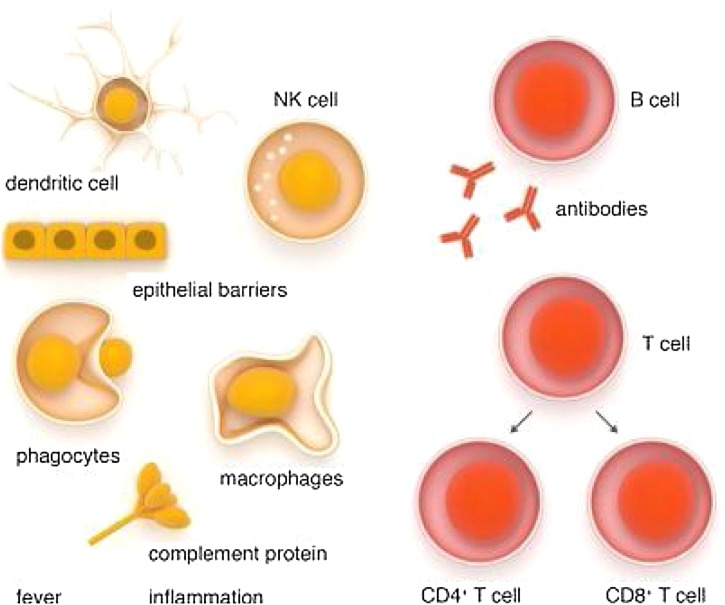
Because there is no registered medicine or vaccine against COVID-19, the immune system is the best defense because it supports the body’s natural ability to defend against pathogens (eg, viruses, bacteria, fungi, protozoan, and worms [24,25]) and resists infections. As long as the immune system is functioning normally, infections such as COVID-19 go unnoticed. The three types of immunity are innate immunity (rapid response), adaptive immunity (slow response), and passive immunity (Fig. 2 ). Passive immunity has two types: natural immunity, received from the maternal side, and artificial immunity, received from medicine. Skin and inflammatory responses begin when the body is affected [26,27]. However, when the body encounters germs or viruses for the first time, the immune system cannot work properly, and illness can occur. This scenario is what has occurred in the case of COVID-19 [28].
Fig. 2.
Innate and adaptive immune system [28].
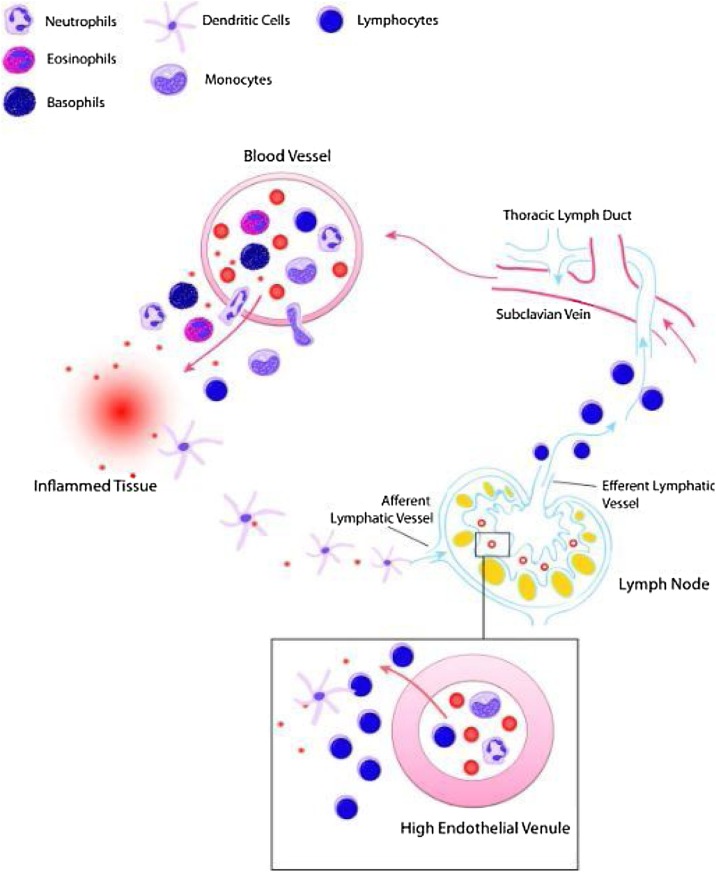
When the cells of the immune system become educated, they complete their jobs by recirculating between central and peripheral lymphoid organs and migrating it and from sites of injury via blood (Fig. 3 ). Blood carries naïve and educated immune cells from one site to another, as it flows throughout the body, and acts as a pipeline for the immune system. The cells again enter into the bloodstream to be transported to tissues throughout the body after exiting these nodes through outgoing lymphatic vessels [29].
Fig. 3.
Blood in the pipeline of the immune system [29].
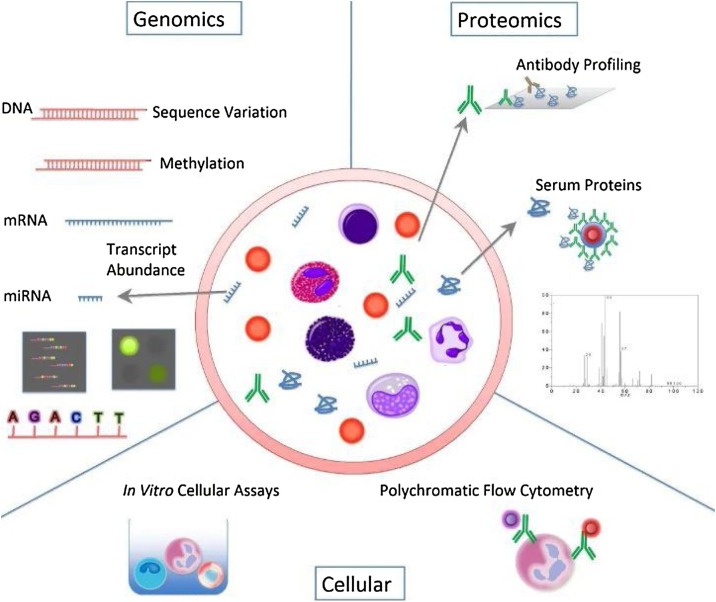
Many molecular and cellular profiling assays are now available for the study of the human immune system (Fig. 4 ). The level of advancement of instruments has increased (e.g., polychromatic flow cytometers have improved). In the fields of genomics and proteomics, major technological breakthroughs have also occurred, creating a unique facility for the study of human beings in health and disease where inherent heterogeneity dictates that large collections of samples be analyzed [29].
Fig. 4.
Immune profiling armamentarium [29].
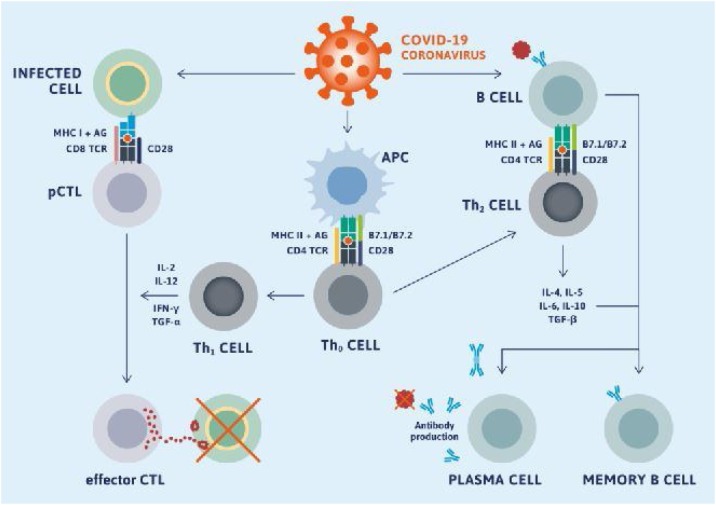
After being affected by virus immune responses to mediate antibody. The B cells are assisted by T cells to differentiate into plasma cells, which then produce antibodies specific to a viral antigen. A neutralizing nature antibody is efficient in fully blocking the virus from entering into host cells to limit the infection and plays a very intense protective role at the later stage of infection and prevents relapse of infection. By contrast, a cellular immunity response can be observed inside the infected cells, which is mediated by T-lymphocytes. The overall adaptive immune response is directed by helper T cells, and cytotoxic T cells play a vital role in the clearance and cleaning of viral-infected cells [30].
Information from SARS-CoV and MERS-CoV may allow the exploration of knowledge to understand how SARS-CoV-2 escapes the host’s immune response, because data on SARS-CoV-2 remain limited. Notably, 80% of the RNA sequence of SARS-CoV and 50% of the RNA sequence of MERS-CoV match the RNA of SARS-CoV-2 [31], and SARS-CoV-2 exhibits additional genomic regions. Compared with SARS-CoV and other closely related coronaviruses, its S protein is 20–30 amino acids longer. Thus, SARS-CoV-2 has similar immune evasion strategies, but an additional mechanism remains undiscovered [32,33].
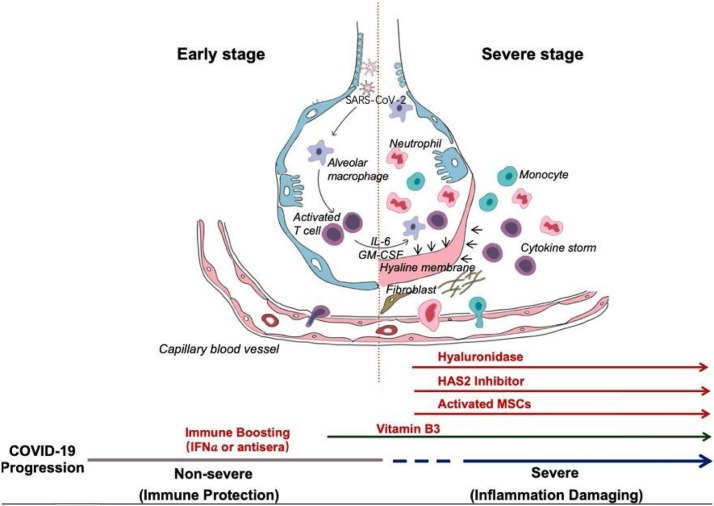
The synopsis of Shi et al. [34] is based on clinical common sense. They proposed some normal approaches for the treatment of patients with COVID-19 (Fig. 5 ). They posited that the two-phase immune defense-based protective phase and inflammation-driven damaging phase division are essential. In the first phase, doctors should attempt to boost immune response, and in the second phase, they should attempt to suppress it. Vitamin B3 should be used immediately after the coughing begins because it is highly lung protective. When breathing difficulty starts, hyaluronidase can be given intratracheally and simultaneously, 4-MU can be used to inhibit HAS2. Clearly, susceptibility information will be provided by HLA typing for strategizing prevention, treatment, vaccination, and clinical approaches.
Fig. 5.
Progression of COVID-19 infection and potential adjuvant interventions [34].
Reasons for failure
The leading cause for mortality of patients with COVID-19 is respiratory failure from acute respiratory distress syndrome [35]. Secondary hemophagocytic lymphohistiocytosis (sHLH) is characterized by fulminant and fatal hypercytokinemia with multiorgan failure, and it is underrecognized. Viral infection triggers sHLH and occurs in 3.7%–4.3% of sepsis cases in adults [36,37]. sHLH, resembled by a cytokine profile, is associated with COVID-19 disease severity, characterized by increased interleukin (IL)-2, IL-7, interferon-inducible protein 10, granulocyte-colony stimulating factor, macrophage inflammatory protein 1-, monocyte chemoattractant protein 1, and tumor necrosis factor- (TNF-) [38]. A recent retrospective fatality predictor’s multicenter study of 150 confirmed COVID-19 cases in Wuhan, China, included elevated ferritin and IL-6, suggesting that mortality might due to virally driven hyperinflammation [39].
Treatment for patients with COVID-19
Research is ongoing worldwide to develop a vaccine against COVID-19. According to a report [40], 115 vaccine candidates are being developed. Among them, 78 are confirmed as active and 37 are unconfirmed; 73 are in exploratory out of 78 confirmed active projects. The most advanced candidates have been moved into clinical development. Table 1 shows the clinical phase vaccine candidates for COVID-19.
Table 1.
Clinical phase vaccine candidates for COVID-19 [40].
| Candidate | Vaccine characteristic | Lead developer | status |
|---|---|---|---|
| mRNA-1273 | LNP-encapsulated mRNA vaccine encoding S protein | Moderna | Phase I (NCT04283461) |
| Ad5-nCoV | Adenovirus type 5 vector that expresses S protein | CanSino Biologicals | Phase I (NCT04313127) |
| INO-4800 | DNA plasmid encoding S protein delivered by electroporation | Inovio Pharmaceuticals | Phase I (NCT04336410) |
| LV-SMENP-DC | DCs modified with the lentiviral vector | Shenzhen Geno-Immune | Phase I (NCT04276896) |
| expressing synthetic minigene based on domains of selected viral proteins; administered with antigen-specific CTLs | Medical Institute | ||
| Pathogen-specific aAPC | aAPCs modified with the lentiviral vector expressing synthetic minigene based on domains of selected viral proteins | Shenzhen Geno-Immune Medical Institute | Phase I (NCT04299724) |
According to another report [41], 108 adults have received a low, middle, or high dose of the vaccine, given as an intramuscular injection. All those adults were not affected by SARS-CoV-2, and their age was in between 18 and 90 years. Their mean age was 36.3, and 51% of them were male and observed for 28 days. Live virus or pseudovirus neutralization assays can detect neutralizing antibodies, in addition to binding antibodies measured by ELISA at approximately 14 days.
At 28 days, dose-dependent antibody responses peaked, with seroconversion documented in 50%–75% of the participants in the middle- and high-dose groups. Moreover, specific T cell responses toward the spike glycoprotein were shown by interferon enzyme-linked immunospot and flow-cytometry. Among 83%–97% of participants, dose-dependent responses were detectable starting from 14 days. The most common adverse effects were fever, fatigue, headache, and muscle pain. One study demonstrated that [42] for the treatment of COVID-19, convalescent plasma therapy is effective. The survival rate of patients with SARS of viral etiology has been improved with this treatment [43]. Pre-donation assessment is performed to ensure compliance with the current regulations for plasma donors [44]. Individuals aged between 18 and 65 years who have recovered and not been infected by COVID-19 for the last 14 days are the convalescent donors. Individuals from the tropical disease areas were also excluded. Plasma, approximately 400–800 mL, was collected from each donor, stored in units of 200 or 250 mL, and frozen within 24 h of collection to be used for further transfusions [45]. The safety of using convalescent plasma is another issue. Any adverse event did not associate during the epidemic of influenza, SARS-CoV, and MARS-CoV but did occur for Ebola. Reports say treatment with convalescent plasma for patients with COVID-19 is safe without any major adverse events [46]. Table 2 shows the associated adverse events to convalescent plasma in different epidemics.
Table 2.
Associated adverse events to convalescent plasma in different epidemics.
| Country | Viral etiology | Adverse events | References |
|---|---|---|---|
| China | COVID-19 | None | [47] |
| China | COVID-19 | None | [48] |
| China | COVID-19 | Self-limited facial erythema in 2/10 patients. No major adverse events. | [49] |
| China | COVID-19 | None | [50] |
| South Korea | COVID-19 | None | [51] |
| China | SARS-CoV | None | [52] |
| China | SARS-CoV | None | [53] |
| China | SARS-CoV | None | [54] |
| Taiwan | SARS-CoV | None | [55] |
| China | SARS-CoV | None | [56] |
| China | SARS-CoV | None | [57] |
| China | SARS-CoV | None | [58] |
| South Korea | MERS-CoV | None | [59] |
| Guinea | Ebola | Nausea, skin erythema, fever. No major adverse events. | [60] |
| China | Influenza A (H1N1) | None | [61] |
| China | Influenza A (H1N1) | None | [62] |
| China | Influenza A (H1N1) | None | [63] |
| China | Influenza A (H1N1) | None | [64] |
Because there is no definite and specific treatment for patients with COVID-19, some antiviral agents are prescribed to the patients, depending on their condition and location. Among the antiviral agents, remdesivir is the most well-known potential drug for the treatment of patients with COVID-19. For the treatment of Ebola virus infection in 2017, Gilead Sciences synthesized and developed remdesivir, and it is a phosphoramidate prodrug of an adenosine C-nucleoside and a broad spectrum antiviral agent [65].
Hydroxychloroquine and chloroquine are other drugs that have a long history of clinical use and similar chemical structures and are often used for the treatment of malaria erythematosus and rheumatoid arthritis [66]. Lopinavir is another drug, which was administered and marketed in combination with ritonavir by Abbott under the brand name Kaletra in 2000. Lopinavir is a protease inhibitor with high specificity with HIV-1 protease [67]. Another drug, umifenovir, was first developed in Russia and used in Russia and China for the treatment of prophylaxis, infections associated with influenza A and B, and other arbovirus [68]. Favipiravir was developed by Fujifilm Toyama Chemical, Japan, in 2014, for treating avian influenza resistant to neuraminidase inhibitors [69]. Oseltamivir is used to treat influenza A and B. This drug targets the neuraminidase distributed on the surface of the influenza virus to inhibit the spread of the influenza virus in the human body [[70], [71], [72]]. Table 3 presents the off-label drugs against SARS-CoV-2 and COVID-19.
Table 3.
Off-label drugs against SARS-CoV-2 and COVID-19 disease [92].
| Drug | Class | Target | Dosage | References |
|---|---|---|---|---|
| Camostat mesilate | Serine protease inhibitor | TMPRSS2 | 200 mg three times daily, for 2 weeks, per oral | [73,74] |
| Nafamostat mesilate | Serine protease inhibitor | TMPRSS2 | 240 mg daily, for 5 days, per oral | [75] |
| Chloroquine phosphate | Antimalarial drug | ACE2 | 250 mg daily until clinical convalescence, per oral | [78,79] |
| Hydroxychloroquine | Antimalarial drug | Endosome, pH elevation | 400 mg loading dose twice daily at day 1, 200 mg twice daily for 4 days, or 600 mg for 6 days, or 400 mg for 5 days, per oral | [[80], [81], [82]] |
| Remdesivir | Antiviral drug | RdRp | 200 mg loading dose at day 1, 100 mg for 9–13 days, per oral or intravenous | [[83], [84], [85]] |
| Lopinavir/ritonavir | Antiviral drug | Viral proteases | 400 mg lopinavir and 100 mg ritonavir twice daily, for 14 days, per oral | [[86], [87], [88]] |
| Umifenovir | Antiviral drug | Membrane fusion, clathrin-mediated endocytosis | 400 mg three times daily, for 9 days, per oral | [89,90] |
| Favipiravir | Antiviral drug | RdRp | 6000 mg loading dose at day 1, 2, 400 mg for days 2–10, per oral | [91] |
Recent observations of COVID-19 treatment improving the immune system: case study
Researchers are attempting to improve the immune system against COVID-19 and here some of the data reviewed. Ten proteins are encoded by the COVID-19 genome; one of them is the S protein, as aforementioned, because a glycoprotein exists in the virus-infected region (Fig. 6 ). The S protein is a significant therapeutic target, ensured its location, and targetable using antibodies [93]. The formation of neutralizing antibodies’ immunization of animals with S protein-oriented vaccines is very effective in preventing infection by homologous coronavirus [94]. If human cells are infected by virus entities, epitopes from any of that viruses’ proteins can theoretically be bound and presented by MHC-1 receptors on host cell surfaces, leading to the stimulation of CD4 and CD8 T cells to provoke antibody-mediated and cell-mediated immune responses.
Fig. 6.
Adaptive immune response against coronavirus requires stimulation of B cell and T cell epitopes [95].
Case study-1
At the University of Copenhagen, researchers net MHC made in-silicon predictions of epitopes presented by 11 MHC-1 alleles that covered approx. 90% of the Asian population. Using that approach, they compiled a list of 100 candidate epitopes for the following MHC alleles—A*0101, A*0201, A*0301, A*11:01, A*2402, B*40:01, C*0401, C*0701, C*0702, and DRB1*0401—resulting in 1100 MHC/peptide binding studies. For the project, they partnered with Intavis and Intavis, who synthesized the COVID-19 epitopes assessed in the study. Using their unique neoscreen technology, they performed in vitro binding studies of the epitopes. The study identified 159 epitopes that stably bind the MHC-1 allele and 22 that bind the tested MHC-II alleles [95]. The relevant data are listed in Table 4, Table 5 .
Table 4.
Reference peptides.
| Allele | # | Sequence | Reference |
|---|---|---|---|
| A*0101 | 309 | VTEHDTLLY | [96] |
| A*0201 | 42 | VLDFAPPGA | |
| A*0301 | 52 | AVAHKVHLMYK | [97] |
| A*1101 | 315 | AVFDRKSDAK | [98] |
| A*2402 | 288 | AYAQKIFKIL | [99] |
| B*4001 | 417 | REDQWCGSL | [100] |
| C*0102 | 369 | QYDPVAALF | [101] |
| C*0401 | 369 | QYDPVAALF | [102] |
| C*0701 | 211 | YLHARLREL | |
| C*0702 | 70 | NYFNRMFHF | |
| DRB1*0401 | 139 | AKFVAAWTLKAAA | [103] |
Table 5.
Number of epitopes with a minimum 60% stability [95].
| min 60% hits | Enrichment factor | |
|---|---|---|
| A*0101 | 14 | 2.1 |
| A*0201 | 15 | 6.1 |
| A*0301 | 41 | 1.8 |
| A*1101 | 49 | 1.4 |
| A*2402 | 30 | 1.5 |
| B*4001 | 30 | 1.6 |
| C*0102 | 3 | 31 |
| C*0401 | 1 | ND |
| C*0701 | 3 | 5.3 |
| C*0702 | 3 | 16 |
| DRB1*0401 | 22 | 4.2 |
Case study-2 [105]
As of March 13, 2020, outside China, there were 32 countries with more than 100 COVID-19 cases [104]. The highest number of infections was found in seven countries: the United States (n = 2294), France (n = 3671), Germany (n = 3675), Spain (n = 5232), South Korea (n = 8086), Iran (n = 11,364), and Italy (n = 17,660). The number of confirmed cases in the other 25 countries was fewer than 1200 [105]. The related data is presented in Table 3.
The change of R0 and Rt is connected to the proportion of individuals who have immunity in their body to that pathogen in that population. The alternative method of estimating Rt for a pathogen including the population is by multiplying R0 through the proportion of the population of individuals considered non-immune to that pathogen. In this perception, R0 will only have a similar level of Rt if there are no immune persons in the population. It indicates that any partial pre-existing immunity to the infecting elements is able to decrease the number of expected secondary cases emerging.
Whenever this perception is applied in case of herd immunity to control the COVID-19 epidemic, the fatality rate of the coronavirus is between 0.25% and 3.0% of the estimated population (ie, the measured number of people who may die from affecting this virus), but when the population attains the Pcrit herd immunity level, it can be difficult to accept (Table 6 ).
Table 6.
Estimates of SARS-CoV-2 effective reproduction number (Rt) of 32 study countries (as of March 13, 2020), and the minimum proportion (Pcrit, as % of population) necessary to have recovered from COVID-19 with subsequent immunity, to halt the epidemic in that population. [105].
| Study countries | Population infected by COVID-19 | Estimates of effective reproduction number (Rt) (95% CI), (n = 32) | Minimum proportion (%) of total population required to recover from COVID-19 to confer immunity (Pcrit) |
|---|---|---|---|
| Rt >4 | |||
| Bahrain | 210 | 6.64 (5.20, 8.61) | 85.0 |
| Slovenia | 141 | 6.38 (4.91, 8.38) | 84.3 |
| Qatar | 320 | 5.38 (4.59, 6.34) | 81.4 |
| Spain | 5232 | 5.17 (4.98, 5.37) | 80.7 |
| Denmark | 804 | 5.08 (4.60, 5.62) | 80.3 |
| Finland | 155 | 4.52 (3.72, 5.56) | 77.9 |
| Rt (2–4) | |||
| Austria | 504 | 3.97 (3.56, 4.42) | 74.8 |
| Norway | 996 | 3.74 (3.47, 4.04) | 73.3 |
| Portugal | 112 | 3.68 (2.86, 4.75) | 72.8 |
| Czech Republic | 141 | 3.57 (2.88, 4.45) | 72.0 |
| Sweden | 814 | 3.44 (3.20, 3.71) | 70.9 |
| United States | 2294 | 3.29 (3.15, 3.43) | 69.6 |
| Germany | 3675 | 3.29 (3.18, 3.40) | 69.6 |
| Switzerland | 1139 | 3.26 (3.05, 4.78) | 69.3 |
| Brazil | 151 | 3.26 (2.99, 3.55) | 69.3 |
| Netherlands | 804 | 3.25 (3.02, 3.51) | 69.2 |
| Greece | 190 | 3.12 (2.67, 3.67) | 67.9 |
| France | 3661 | 3.09 (2.99, 3.19) | 67.6 |
| Israel | 143 | 3.02 (2.56, 3.59) | 66.9 |
| United Kingdom | 798 | 2.90 (2.72, 3.10) | 65.5 |
| Italy | 17,660 | 2.44 (2.41, 2.47) | 59.0 |
| Canada | 198 | 2.30 (2.07, 2.57) | 56.5 |
| Iceland | 134 | 2.28 (1.90, 2.75) | 56.1 |
| Rt (1–2) | |||
| Iran | 11,364 | 2.00 (1.96, 2.03) | 50.0 |
| Australia | 199 | 1.86 (1.71, 2.03) | 46.2 |
| Belgium | 559 | 1.75 (1.55, 1.97) | 42.9 |
| Malaysia | 197 | 1.74 (1.61, 1.88) | 42.5 |
| Iraq | 101 | 1.67 (1.41,1.97) | 40.1 |
| Japan | 734 | 1.49 (1.44, 1.54) | 32.9 |
| Korea | 8086 | 1.43 (1.42, 1.45) | 30.1 |
| Singapore | 200 | 1.13 (1.06, 1.19) | 11.5 |
| Kuwait | 100 | 1.06 (0.89, 1.26) | 5.66 |
Case study-3
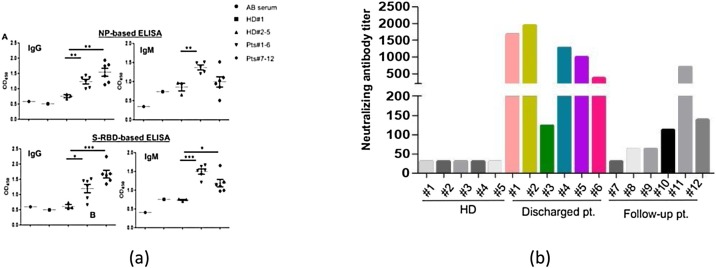
Dong et al. [106] experimented on patients by using various methods. Initially, they used sera, but no significant result was observed. Subsequently, the team focused on NP and S-RBD. To determine optical dilutions, the serum from a patient and human AB serum was titrated. For IgM a dilution of 1:50 and for IgG a dilution of 1:150 were used. Compared with healthy donor groups, NP- and S-RBD-specific IgM and IgG antibodies were both detected in the area of newly discharged patients (Fig. 7 ). Compared with those of healthy donors, Anti-SARS-CoV-2 IgG antibodies were also more clearly observed than IgM in the followed-up patients. These findings indicate that patients with COVID-19 mounted IgG and IgM responses to SARS-CoV-2 proteins, especially NP and S-RBD, and suggest that patients who are infected could maintain their IgG levels, at least for 2 weeks. Because the RBD domain of the S protein has been shown to bind to the human receptor ACE2, the existence of antibodies against it may suggest the neutralization of SARS-CoV-2 infection. To assess that phenomenon, they performed a pseudovirus particle-based neutralization assay. Patients #1, 2, 4, and 5, all within the discharged group, had high levels of neutralizing antibody titers. Those findings demonstrate that most recently discharged patients had protective humoral immunity to SARS-CoV-2. Except for patient #11, the followed-up patients had lower levels of neutralizing antibody titers than the recently discharged other patients, and all were positive except for patient #7, who was negative.
Fig. 7.
Detection of antibody responses to recombinant SARS-CoV-2 proteins in patients with COVID-19. (A) Serological responses of 12 patients with COVID-19 to recombinant NP (top) and S-RBD (bottom). The experiment was performed in duplicate. (B) Measurement of neutralizing antibody titers by pseudovirus-based assay. The experiment was performed in triplicate. NP, nucleocapsid protein. S-RBD receptor binding domain of spike protein. HD, healthy donor. Pt, patient. HD#1, serum was collected in 2018. HD#2–4, the sera were from close contact. *P < **P [106].
Potential challenges in immune system development
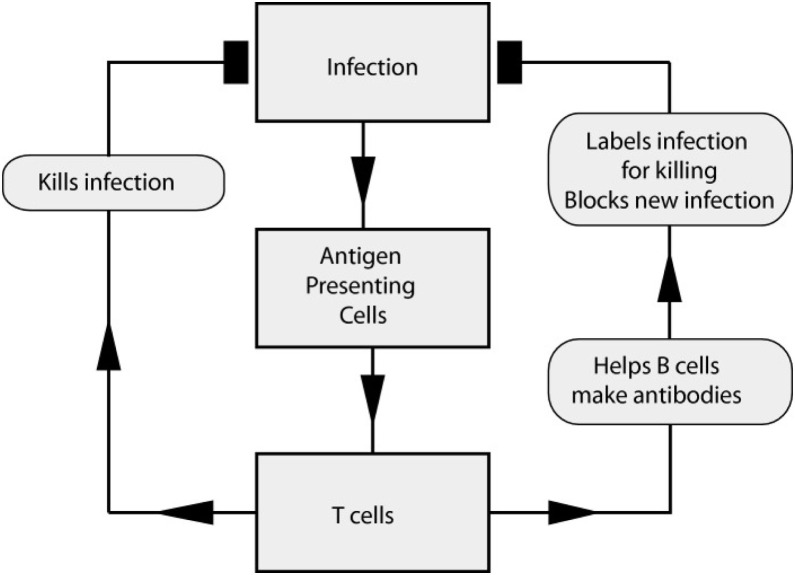
An effective immune system must have the ability to interpret changes in the world around it and respond properly; however, it must overcome challenges to work in different environments with different pathogens (Fig. 8 ). Most of the time, the immune system encounters something new and considers it harmless, but in some cases, that response can be dangerous. An efficient immune system must have the ability to distinguish this. It should have the ability to adapt to strange environmental changes to fight against infections. A healthy immune system has symbiotic microbial farms and reacts to a harmful infection. When pathogens enter the body, they attempt to use it as a host, and the immune system poses many threats. A different door is used for every infection to enter a cell, and blocking these routes of entry can stop an infection before it begins. The immune system neutralizes an infection by producing antibodies; however, this must be conducted at the proper time. An immune system—ideally—must stop an infection before it has established a foothold in the body [[107], [108], [109], [110], [111]].
Fig. 8.
The adaptive immune response to infection [111].
Suggested food, vaccination, drugs, and supplementary for the immune system for COVID-19
According to the World Health Organization, healthy foods and hydration are vital. Individuals consuming a well-balanced diet are healthier with a strong immune system and have a reduced risk of chronic illness, infectious diseases. Vitamins and minerals are vital. Vitamin B, insoluble in water, protects from infection. Vitamin C protects from flu-like symptoms [112]. Insufficient vitamin D and vitamin E can lead to coronavirus infection [113]. Vitamin D can be found in sunlight, and vitamin E can be found in, for example, oil, seeds, and fruits. Insufficient iron and excess iron can lead to risk [114,115]. Zinc is necessary for maintaining the immune system [116]. Food rich in protein should be the top priority because it has immune properties (immunoglobulin production) and potential antiviral activity [[117], [118], [119]]. Therefore, in a regular meal, individuals should eat fruit, vegetables, legumes, nuts, whole grains, and foods from animal sources (Fig. 9 ). Food from plants containing vitamin A should be consumed, and 8–10 cups of water should be drunk daily. Malnutrition is dangerous for patients with COVID-19 and thus proper nutrition should be provided [120,121]. Fruit juice, tea, and coffee can also be consumed. Too much caffeine, sweetened fruit juices, fruit juice concentrates, syrups, fizzy drinks, and still drinks must be avoided. Unsaturated fats, white meats, and fish should be consumed. Saturated fat, red meat, more than 5 g salt per day, and industry processed food should be avoided [122]. Along with diet, physical activity is another factor. Individuals should be active and perform physical exercise regularly to boost the immune system and should have proper sleep [123]. Although there is no registered medicine for COVID-19 treatment, hydroxychloroquine and remdesivir are prescribed and are partially effective [124].<-- -->
Fig. 9.
Nutrition advice for adults during the COVID-19 outbreak [122].
Conclusions
This review on boosting the immune system is a potential resource for the treatment of patients with COVID-19. The process and mechanism of the immune system can be a good source of knowledge for immune system development. Further research could focus on the most recent observations regarding COVID-19 treatment. If the potential challenges can be overcome, this would be a substantial achievement. Finally, nutrition (eg, dietary recommendations) to boost the immune system should be explored and recommended because no registered medicine is available for COVID-19 treatment.
Funding
No funding sources.
Competing interests
The authors declared that there is no conflict of interest.
Ethical approval
Not required.
References
- 1.Eakachai Prompetchara, Chutitorn Ketloy, Tanapat Palaga. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020 doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 2.Yan-Rong Guo, Qing-Dong Cao, Zhong-Si Hong, Yuan-Yang Tan, Shou-Deng Chen, Hong-Jun Jin, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak — an update on the status. Mil Med Res. 2020 doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus infected pneumonia. N Engl J Med. 2020;19 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok K.O., Lai F., Wei W.I., Wong S.Y.S., Tang J.W.T. Herd immunity — estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.bbc.com/news/science-environment-51892402.
- 7.Lei Zhang, Yunhui Liu. Potential interventions for novel coronavirus in China: a systematic review. J Med Virol. 2020 doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/.
- 9.https://en.wikipedia.org/wiki/Immunesystem.
- 10.https://kidshealth.org/en/parents/immune.html.
- 11.https://www.ncbi.nlm.nih.gov/books/NBK279364/.
- 12.http://www.imgt.org/IMGTeducation/Tutorials/ImmuneSystem/UK/theimmunesystem.pdf.
- 13.Shang J., Ye G., Shi K., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wan Y., Shang J., Graham R., et al. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. 2020. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N.L., Chan P.K., Wong C.K., et al. Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem. 2005;51:2333–2340. doi: 10.1373/clinchem.2005.054460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hancock A.S., Stairiker C.J., Boesteanu A.C., et al. Transcriptome analysis of infected and bystander type 2 alveolar epithelial cells during influenza A virus infection reveals in vivo Wnt pathway downregulation. J Virol. 2018;92 doi: 10.1128/JVI.01325-18. e01325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, McGoogan JM. Characteristics of and important lessons from the coro-navirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020 [in press]. [DOI] [PubMed]
- 19.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D., Hu B., Hu C., Chang Hu, Zhu F., Liu X., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [Published online 7 February 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 doi: 10.1016/S0140-6736(20)30211-7. (January) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):49. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin Yin, Zhen Fu, Jiao Xie, Jixing Zhang, et al. 2020. April Analysis of risk factors of severe COVID-19 patients. [Google Scholar]
- 24.https://www.britannica.com/science/immune-system.
- 25.https://www.ncbi.nlm.nih.gov/books/NBK279397/.
- 26.https://www.khanacademy.org/science/high-school-biology/hs-human-body-systems/hs-the-immune-system/a/hs-the-immune-system-review.
- 27.https://en.wikibooks.org/wiki/HumanPhysiology/TheImmuneSystem.
- 28.Chaussabel D., Pascual V., Banchereau J. Assessing the human immune system through blood transcriptomics. BMC Biol. 2010;8:84. doi: 10.1186/1741-7007-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.https://gulfnews.com/world/3-types-of-immunity-your-best-defence-vs-coronavirus-1.1583917783603?slide=1.
- 30.Swatantra Kumar, Rajni Nyodu, Maurya Vimal K., Saxena Shailendra K. Coronavirus disease 2019 (COVID-19) 2020. Host immune response and immunobiology of human SARS-CoV-2 infection; pp. 43–53. [Google Scholar]
- 31.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 33.Susanna Felsenstein, Herbert Jenny A., McNamara Paul S., Hedrich Chris-tian M. COVID-19: immunology and treatment options. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y., Wang Y., Shao C., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020 doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan Qm, Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos-Casals M., Brito-Zeron P., Lopez-Guillermo A., Khamashta M.A., Bosch X. Adult haemophagocytic syndrome. Lancet. 2014;383:1503–1516. doi: 10.1016/S0140-6736(13)61048-X. [DOI] [PubMed] [Google Scholar]
- 37.Karakike E., Giamarellos-Bourboulis E.J. Macrophage activation-like syn-drome: a distinct entity leading to early death in sepsis. Front Immunol. 2019;10:55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puja Mehta, McAuley Daniel F., Michael Brown, Emilie Sanchez, Tat-tersall Rachel S., Manson Jessica J., et al. COVID-16: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)30628-0. (March (10229)):P1033–034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Tung Thanh, Zacharias Andreadakis, Arun Kumar, Gómez Román Raúl, Stig Tollefsen, Melanie Saville, et al. 2020. The COVID-19 vaccine development land-scape. [Google Scholar]
- 41.Nelson Lee, Allison McGeer. The starting line for the COVID-19 vaccine devel-opment. Lancet. 2020 [Google Scholar]
- 42.Mingxiang Ye, Dian Fu, Yi Ren, Faxiang Wang, Dong Wang, Fang Zhang, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mair-Jenkins J., Saavedra -Campos M., Baillie J.K., et al. The effectiveness of con-valescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan Y., Shang J., Graham R., et al. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hancock A.S., Stairiker C.J., Boesteanu A.C., et al. Transcriptome analysis of infected and bystander type 2 alveolar epithelial cells during influenza A virus infection reveals in vivo Wnt pathway downregulation. J Virol. 2018;92 doi: 10.1128/JVI.01325-18. e01325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manuel Rojas, Yhojan Rodríguezab, Monsalvea Diana M., Acosta Ampudia Yeny, Bernardo Camachoc, Esteban Gallod Juan, et al. Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun Rev. 2020;19(July (7)) doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bin Zhang, Shuyi Liu, Tan Tan., Wenhui Huang, Yuhao Dong, Luyan Chen, et al. 2020. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection chest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with Covid-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F., et al. Treatment with convalescent plasma for patients with COVID-19 in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., et al. Use of convalescent plasma therapy in two patients with COVID-19 with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35:e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soo Y.O.Y., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K.S., et al. Retrospective comparison of convalescent plasma with continuing high-dose methyl-prednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng Y., Wong R., Soo Y.O.Y., Wong W.S., Lee C.K., Ng M.H.L., et al. Use of conva-lescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nie Q.-H.-H., Luo X.-D.-D., Hui W.-L.-L. Advances in clinical diagnosis and treatment of severe acute respiratory syndrome world. J Gastroenterol. 2003;9:1139–1143. doi: 10.3748/wjg.v9.i6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yeh K.-M.-M., Chiueh T.-S.-S., Siu L.K., Lin J.-C.-C., Chan P.K.S., Peng M.-Y.-Y., et al. Experi-ence of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou X., Zhao M., Wang F., Jiang T., Li Y., Nie W., et al. Epidemiologic features, clinical diagnosis and therapy of first cluster of patients with severe acute res-piratory syndrome in Beijing area. Zhonghua Yi Xue Za Zhi. 2003;83:1018–1022. [PubMed] [Google Scholar]
- 57.Kong L. Letter to editor. Transfus Apher Sci. 2003;29:101. doi: 10.1016/S1473-0502(03)00109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong V.W.S., Dai D., Wu A.K.L., Sung J.J.Y. Treatment of severe acute respiratory syndrome with convalescent plasma Hong Kong. Med J. 2003;9:199–201. [PubMed] [Google Scholar]
- 59.Ko J.-H.-H., Seok H., Cho S.Y., Ha Y.E., Baek J.Y., Kim S.H., et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 60.van Griensven J., Edwards T., de Lamballerie X., Semple M.G., Gallian P., Baize S., et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hung I.F., To K.K., Lee C.-K.-K., Lee C.-K.-L., Chan K., Yan W.-W.-W., et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;(52):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan K.K.C., Lee K.L., Lam P.K.N., Law K.I., Joynt G.M., Yan W.W. Hong Kong’s experi-ence on the use of extracorporeal membrane oxygenation for the treatment of influenza A (H1N1) Hong Kong Med J. 2010;16:447–454. [PubMed] [Google Scholar]
- 63.Yu H., Gao Z., Feng Z., Shu Y., Xiang N., Zhou L., et al. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS One. 2008;3:e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kong L.K., Zhou B.P. Successful treatment of avian influenza with convalescent plasma. Hong Kong Med J. 2006;12:489. [PubMed] [Google Scholar]
- 65.Siegel D., Hui H.C., Doerffler E., Clarke M.O., Chun K., Zhang L., et al. Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J Med Chem. 2017;60(5):1648–1661. doi: 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- 66.Rynes R. Antimalarial drugs in the treatment of rheumatological diseases. Rheumatology. 1997;36(7):799–805. doi: 10.1093/rheumatology/36.7.799. [DOI] [PubMed] [Google Scholar]
- 67.https://www.accessdata.fda.gov/drugsatfdadocs/label/2019/021251s058slp. KALETRA (lopinavir and ritonavir) tablet.12/2019.
- 68.Boriskin Y.S., Leneva I.A., Pecheur E.I., Polyak S.J. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15(10):997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 69.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad, Ser B, Phys Biol Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McClellan K., Perry C.M. Oseltamivir Drugs. 2001;61(2):263–283. doi: 10.2165/00003495-200161020-00011. [DOI] [PubMed] [Google Scholar]
- 71.Whitley R.J., Hayden F.G., Reisinger K.S., Young N., Dutkowski R., Ipe D., et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20(2):127–133. doi: 10.1097/00006454-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 72.Wu R., Wang L., Kuo H.D., et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020 doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease receptor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sai J.K., Suyama M., Kubokawa Y., Matsumura Y., Inami K., Watanabe S. Efficacy of camostat mesilate against dyspepsia associated with non-alcoholic mild pancreatic disease. J Gastroenterol. 2010;45(3):335–341. doi: 10.1007/s00535-009-0148-1. 1988 98–104. [DOI] [PubMed] [Google Scholar]
- 75.Hirota M., Shimosegawa T., Kitamura K., Takeda K., Takeyama Y., Mayumi T. Continuous regional arterial infusion versus intravenous administration of the protease inhibitor nafamostat mesilate for predicted severe acute pan-creatitis: a multicenter, randomized, open-label, phase 2 trial. J Gastroenterol. 2020;55(3):342–352. doi: 10.1007/s00535-019-01644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao X., Ye F., Zhang M., Cui C., Huang B., Nui P. In vitro antiviral activity and pro-jection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloro-quine and azithromycin as a tratment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. (March) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRXiv. 2020 (March) [Google Scholar]
- 81.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coro-naviruses. Sci Transl Med. 2017;9(396) doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulangu S., Dodd L.E., Davey R.T., Thsihani Mbaya O., Proschan M., Mukadi D.A. Randomized controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A. Compassion-ate use of remdesivir for patients with severe COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007016. (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.H.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chan J.F., Yao Y., Yeung M.L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon- 1b improves outcome of MERS-CoV infec-tion in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sissoko D., Laouenan C., Folkesson E., MLebing´ A.B., Beavogui A.H., Baize S. Exper-imental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016:13. doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McKee Dwight L., Ariane Sternberg, Ulrike Stange, Stefan Laufer, Cord Naujokat. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. 2020;157(July) doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Traggiai E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., et al. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Z.-Y.-Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K., et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.https://www.immunitrack.com/free-coronavirus-report-for-download/?fbclid=IwAR0mcaK8nHCDsBJjQ4ea-d50j9X95ykDCd0OYPbs28QtzrY21kcR-Y9Cnek.
- 94.http://www.iedb.org/epitope/71290.
- 95.https://www.iedb.org/epitope/419554.
- 96.https://www.iedb.org/epitope/5316.
- 97.https://www.iedb.org/epitope/5731.
- 98.https://www.iedb.org/epitope/53476.
- 99.http://www.iedb.org/epitope/52886.
- 100.http://www.iedb.org/epitope/52886.
- 101.https://www.iedb.org/epitope/2192.
- 102.ProMED-mailCOVID-19 update (39): global, more countries, stability, mitiga-tion impact WHO; 2020. Archive No. 20200314.7088746.
- 103.On Kwok Kin, Florence Lai, In Wei Wan, Shan Wong Samuel Yeung, Tang Julian W.T. Herd immunity – estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.027. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen Dong, Ling Ni, Fang Ye, Meng-Li Chen, Yu Feng, Qiang Deng Yong, et al. Characterization of anti-viral immunity in recovered individuals infected by SARS-CoV-2. medRxiv. 2020 [Google Scholar]
- 105.Abbas A.K., Lichtman A.H., Pillai S. Elsevier; Amsterdam: 2016. Basic immunology: functions and disorders of the immune system. [Google Scholar]
- 106.Davis DM . Penguin; London: 2013. The compatibility gene. [Google Scholar]
- 107.Paul W.E. Johns Hopkins University Press; Baltimore: 2015. Immunity. [Google Scholar]
- 108.Mukherjee S. Scribner; New York: 2010. The emperor of all maladies: a biography of Cancer. [Google Scholar]
- 109.Nicholson Lindsay B. Essays in Biochemistry. 2016. The immune system. [Google Scholar]
- 110.Field C.J., Johnson I.R., Schley P.D. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002;71:16–32. [PubMed] [Google Scholar]
- 111.Nonnecke B.J., McGill J.L., Ridpath J.F., Sacco R.E., Lippolis J.D., Reinhardt T.A. Acute phase response elicited by experimental bovine diarrhea virus (BVDV) infec-tion is associated with decreased vitamin D and E status of vitamin-replete preruminant calves. J Dairy Sci. 2014;97:5566–5579. doi: 10.3168/jds.2014-8293. [DOI] [PubMed] [Google Scholar]
- 112.Wessling-Resnick M. Crossing the Iron Gate: why and how transferrin recep-tors mediate viral entry. Annu Rev Nutr. 2018;38:431–458. doi: 10.1146/annurev-nutr-082117-051749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jayaweera J., Reyes M., Joseph A. Childhood iron deficiency anemia leads to recurrent respiratory tract infections and gastroenteritis. Sci Rep. 2019;9:12637. doi: 10.1038/s41598-019-49122-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Maares M., Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 115.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., et al. A rapid advice guide-line for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;(7):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cross M.L., Gill H.S. Immunomodulatory properties of milk. Br J Nutr. 2000;84:S81–S89. doi: 10.1017/s0007114500002294. [DOI] [PubMed] [Google Scholar]
- 117.Ng T.B., Cheung R.C., Wong J.H., Wang Y., Ip D.T., Wan D.C., et al. Antiviral activities of whey proteins. Appl Microbiol Biotechnol. 2015;99:6997–7008. doi: 10.1007/s00253-015-6818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Norman K., Pichard C., Lochs H., Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5–15. doi: 10.1016/j.clnu.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 119.Schuetz P., Fehr R., Baechli V., Geiser M., Deiss M., Gomes F., et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393:2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 120.http://www.emro.who.int/nutrition/nutrition-infocus/nutrition-advice-for-adults-during-the-covid-19outbreak.html?fbclid=IwAR0mcaK8nHCDsBJjQ4ead50j9X95ykDCd0OYPbs28QtzrY21kcR-Y9Cnek.
- 121.Liz Meszaros. How to boost your immune system during the COVID-19 pan-demic. Intern Med. 2020 [Google Scholar]
- 122.https://time.com/5819965/coronavirus-treatments-research/.