Abstract
Secondary bacterial infections are commonly associated with prior or concomitant respiratory viral infections. Viral infections damage respiratory airways and simultaneously defects both innate and acquired immune response that provides a favorable environment for bacterial growth, adherence, and facilitates invasion into healthy sites of the respiratory tract. Understanding the molecular mechanism of viral-induced secondary bacterial infections will provide us a chance to develop novel and effective therapeutic approaches for disease prevention. The present study describes details about the secondary bacterial infection during viral infections and their immunological changes.The outcome of discussion avails an opportunity to understand possible secondary bacterial infections associated with novel SARS-CoV-2, presently causing pandemic outbreak COVID-19.
Keywords: Viral infection, Secondary bacterial infection, Immune response, SARS-CoV-2
Introduction
Bacteria and viruses often occupy the same niches, however, interest in their potential collaboration in promoting wellness or disease development has only recently gained attention. The interaction of some bacteria and viruses are well characterized and researchers are typically more interested in the location of the infection than the manner of cooperation [1]. There are two overarching types of bacteria-virus disease causing interactions by direct interactions that in some way aid the viruses, and indirect interactions of aiding bacteria. The virus-promoting direct interactions occur when the virus exploits a bacterial component to facilitate penetration into the host cell [2]. On the other hand, indirect interactions result in increased bacterial pathogenesis as a consequence of viral infection. Enteric viruses mainly utilize the direct pathway, while respiratory viruses indirectly affect bacteria [3]. The causative agent of the current pandemic outbreak, SARS-CoV-2 is also reported to associate with secondary bacterial and fungal coinfectionsas patients showed the symptoms of atypical bacterial pneumonia [[1], [2], [3]]. It has been known that SARS-CoV is the nearest virus to SARS-CoV-2 and their systemic comparison may further help in understanding the disease pathogenesis during COVID-19 and secondary bacterial infections [4]. However, SARS-CoV-2 is reported to evolve in the human body that might affect itsvirulence, infectivity, and transmissibility [5], thus the biology of SARS-CoV-2 further needs to be understood in detail, especially in case of secondary bacterial infections. This review focuses on some key examples of how virus-bacteria interactions impact the infection process across the two organ systems and provide evidence supporting this as an emerging premise in infectious disease pathogenesis.
Human body is inhabited by a diverse microbial community that is collectively coined as commensal microbiota. Recent research has greatly advanced our understanding of how commensal microbiota affects host health. Among the various kinds of pathogenic infections of the host, viral infections constitute one of the most serious public health problems worldwide. During the infection process, viruses may have substantial and intimate interactions with the commensal microbiota [4]. A plethora of evidence suggests that the commensal microbiota regulated by invading viruses through diverse mechanisms, thereby having stimulatory or suppressive roles in viral infections. Furthermore, the integrity of the commensal microbiota can be troubled by invading viruses, causing dysbiosis in the host, and further influencing virus infectivity. In the present article, we discuss current insights into the regulation of viral infection by the commensal microbiota. We also draw attention to the disruption of microbiota homeostasis by several viruses [5].
Viral infections predispose patients to secondary bacterial infections, which often have a more severe clinical course. The mechanisms underlying post-viral bacterial infections are complex and include multifactorial processes mediated by interactions between viruses, bacteria, and the host immune system [6]. Studies over the past 15 years have demonstrated that unique microbial communities reside on the mucosal surfaces of the gastrointestinal tract and the respiratory tract, which has both direct and indirect effects on host defense against viral infections [7]. The antiviral immune responses induced by acute respiratory infections such as influenza are associated with changes in microbial composition and function (“dysbiosis”) in the respiratory and gastrointestinal tract, which in turn may alter subsequent immune function against secondary bacterial infection or alter the dynamics of inter-microbial interactions, thereby enhancing the proliferation of potentially pathogenic bacterial species [4]. Here, we summarize the literature on the interactions between host microbial communities and host defense, including how influenza and other acute respiratory viral infections disrupt these interactions, thereby contributing to the pathogenesis of secondary bacterial infections.
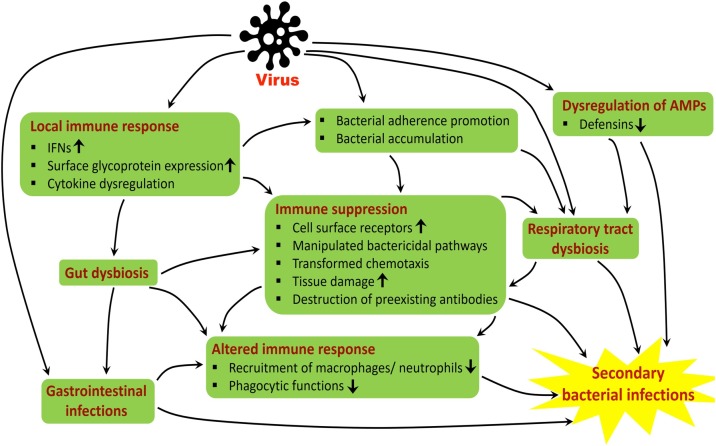
Mechanisms of viral predisposition to secondary bacterial infections
Respiratory viruses are often encouraged, secondary bacterial infections by supplementing the outgrowth opportunistic bacterial pathogens. Viral infections damage the respiratory airway by both histologically and functionally [8]. Cell loss, goblet cell hyperplasia, altered mucus secretion, reduced ciliary beat frequency, dis-coordinated mucociliary clearance function along with reduced oxygen exchange are characteristics of viral infection [9]. These effects are associated with different molecular mechanisms by which the predisposition of the virus occurs in the respiratory tract that facilitates the bacterial infection, simultaneously (Fig. 1 ).
Fig. 1.
Proposed model for viral-induced secondary bacterial infections.
Virus facilitates bacteria adherence and colonization
Epithelial cells of the respiratory tract facilitate bacterial adherence using different mechanisms during viral infection, while the disease severity varies upon virus, bacterial strain, and experimental model used. Respiratory syncytial virus (RSV) reported to bind directly with Haemophilus influenzae and Staphylococcus pneumonia, so enhancing bacterial proximity to the epithelial monolayer and supplementing attachment to the host cell receptors [10]. Interestingly, RSV glycoprotein expressed and localized on the host cell membrane upon infection that is further act as bacterial receptors for pneumococcal binding [11,12]. Respiratory viruses are also able to up-regulate the expression of host cell membrane protein to facilitate their binding [9,10]. Influenza virus, known to induce the production of type I interferons (IFNs) that results in reduced production of CCL2 (chemokine (C-C motif) ligand 2) and so decreased recruitment of macrophages thus results in enhanced colonization of S. pneumonie in mice [11]. Influenza virus also makes mice susceptible to pneumonia caused by Staphylococcus aureus where both virus and bacterial load increased during co-infection [12,13]. RSV, parainfluenzavirus-3, and influenza viruses reported increasing the bacterial adherence upon infection, in both primary and immortalized epithelial cells with different differences [9]. Recently, it has been reported that surface glycoprotein adhesion molecule-1 (ICAM-1) presents up-regulated expression during RSV and adenovirus infection. ICAM-1 presented as a cognate ligand for the Type IV pilus of non-typeable H. influenza induced secondary bacterial infection via adherence promotion [14]. RSV infections also confirmed to increase adherence for S. pneumoniae in human nasopharyngeal cells (HEp-2) and pneumocyte type II cells (A549). The enhanced pneumococcal adherence in epithelial cells results in bacterial accumulation and thus secondary bacterial infection may occur [15,16].
Impaired innate and adaptive immune response
Secondary bacterial infection with viral infections can drastically change the immunity level by the uncontrolled bacterial growth [17,18]. Viral infection impaired the phagocyte functions and induced alveolar macrophages (AM) depletion results in the facilitation of bacterial infection via apoptosis [19]. Infection in AM caused by the influenza virus reported to result in low-level production of cytokines and chemokines which is also required for recruitment and activation of neutrophils [18]. Influenza virus infection can diminish NADPH oxidase-dependent phagocytic bacterial clearance and so increase the chances of secondary bacterial infections [20]. Virus-induced dysregulation of proinflammatory cytokine response played a major role in promoting secondary bacterial infections [21,22]. While type I IFNs are well reported for antiviral and immuno-stimulatory properties, their, inappropriate or excessive production may have harmful effects. Results suggested that IFNs have an important role in the production of specific cytokines like immunosuppressive IL-10 and pro-inflammatory IL-6, suppression of which is key to the linkage between innate and adaptive immune response, involving IL-7 and IL-23, dendritic cells, macrophages, natural killer cells, CD4+ and CD8+, T cells, all these results in the reduced ability to efficiently eliminate bacterial co-infections and thus promote secondary bacterial infections. Dysregulation of antimicrobial peptides is another way used by the upper respiratory tract (URT) viruses to facilitate secondary bacterial infections. Expression of antimicrobial peptides lipocalin2, CAMP, REG3B, S100A8, and S100A9 found down-regulated during URT infections [23]. In another study, RSV infection reported to reduce the gene expression of chinchilla beta-defensin 1(an orthologue of human beta defensin3, hBD-3), and thus RSV infection found to induce 10–100 increase innasopharynx (NP) load of H. influenza [24].
Virus-induced immune suppression
Bacterial superinfections are associated with viral immune suppression condition which is not only limited to the respiratory tract but also related to several organ failures. These immuno-suppressive viruses include especially HIV, cytomegalovirus (CMV), measles virus (MeV), and others. The prime characteristic of HIV infection is the severe reduction of CD4+ T cells that make the patient much more susceptible to develop bacterial superinfections like tuberculosis [25]. It has been reported that HIV infection ease the Mycobacterium infection along with various characteristics to macrophages that constrains bacterial clearance which promotes bacterial colonization with disease development. These HIV associated features to macrophages comprise the up-regulation of cell surface receptors for Mycobacterium entry, manipulation of bactericidal pathways used by macrophages, transformed chemotaxis, induction of immune response causing Th1/Th2 imbalance and apoptotic response that mediated by diminished tumor necrosis factor [26]. CMV infection is also connected with immune system paralysis that is characterized by enhanced IL-10 and NFkβ production, lymphopenia, reduction of IFN-γ producing T cells, and increase tissue damage by dysregulated cytokine production. Immune response during CMV infection is generated a favorable environment for secondary bacterial infections [[27], [28], [29]]. MeV infection is another example of immunosuppressive characteristics that are associated with a secondary bacterial infection. MeV reported to have associated with several weeks of immune suppression, loss of delayed-type hypersensitivity responses, and thus increased susceptibility to secondary bacterial infections [30]. It has been reported that MeV infection destroys pre-existing antibodies that increase the vulnerability to pathogens that result in secondary bacterial infections [31].
Exploitation of bacteria through viral infection
The majority of reported direct bacteria-virus interactions are associated with infection in the gastrointestinal tract caused by viruses. In body system, commensal bacteria are considered the first line of defense against invading pathogens by out-competing their disease-promoting counterparts and limiting tissue accessibility. Undoubtedly, enteric viruses encounter these large numbers of diverse commensal bacteria, but rather than always preventing infection, some viruses evolved to exploit this contactfacilitating the disease developmental process [32].
Under in vitro conditions, viruses may able to directly bind to their target cells and undergo replication. However, this strategy may prove problematic in the gastrointestinal tract whereasa large number of bacteria occupy tissue surfaces, directly competing for receptor binding sites, and reducing the likelihood of pathogenic bacterial proliferation or virus attachment. Other components, like mucus or enzymatic secretions, may also interfere or assist the infection process. To circumvent this, rather than compete for host cell-binding sites, some viruses can utilize bacterial ligands to enhance their association with more accessible host cells, initiating infection. This same strategy may be employed by some viruses that may not exclusively target the host’s epithelial cells rather uses bacteria to assist infection of other cell types in addition to or exclusive of epithelial cells [33,34].
Although the nature of the interaction remains the same, there may be additional benefits to viruses with bacteria interactions other than direct disease progression. Studies have also shown that association with fecalmicrobiota increased poliovirus environmental fitness and stability, as exposure to bacteria or their polysaccharides decreased the efficacy of virus inactivation by heat and bleach, potentially aiding viral survival in the environment [35]. This observation was further supported by the higher susceptibility to inactivation with heat observed in a poliovirus mutant that did not bind LPS as efficiently. Furthermore, the introduction of these bacterial polysaccharide components has been found to enhance wild type poliovirus binding to its host cells expressing its receptor [35]. In short, gastrointestinal microbiota not only increase poliovirus infectivity but may also promote virus transfer to the next host. Thus, there are numerous ways that direct viral interaction with bacteria aid viral pathogenesis, and this topic is an emerging area of study in microbiology.
Bacterial species often benefit from viral infections, the virus-induced disease state can allow normally harmless bacteria to become opportunistically pathogenic. Under normal, healthy circumstances, direct competition between microbes limits pathogen invasion by saturating colonization sites, priming barrier immunity to produce antimicrobials, and increasing the immune response to invading microorganisms [36]. When microbial populations are disrupted, niches previously inaccessible to invading pathogens become available, and the microenvironment where native microbiota previously was outcompeted their disease-causing counterparts are compromised. The overall ways viruses aid bacteria pathogenesis include a complex combination of cellular receptor up-regulation, disruption of the epithelial layers, displacement of commensal bacteria, and immune system suppression [37]. Arguably, the interactions between influenza viruses and pathogenic bacteria (i.e., S. pneumoniae, S. aureus, and H. influenzae) remain the best-studied within the human body, and they exemplify some of these mechanisms with both viruses and bacteria benefitting from the relationship. Influenza viruses not only damage the host epithelium (e.g., apoptosis), they also provide potential binding sites for bacteria through three mechanisms such as neuraminidase cleavage of sialic acid from host cells, bacterial host receptor up-regulation, and host regeneration of the common bacterial receptors fibrin and fibrinogen [38,39]. This pattern of host damage is common amongst upper respiratory tract viruses and bacteria [40,41], and these interactions are summarized in Table 1 . Thus, these numerous dynamics that exist between the influenza virus and bacteria exemplify the multiple complex ways of viral infection that can indirectly assist bacterial infection [42,43].
Table 1.
List of secondary bacterial infections and their immune responses during viral infection.
| Viral infection | Secondary bacterial disease | Bacterial name | Immune response |
|---|---|---|---|
| Influenza | Pneumonia, Otitis media, Sinusitis, meningitis | S. pneumonia, S. aureus, S. pyrogenes, M.catarrhalis, N.meningitids | loss of ciliary function, produce INF, Mucus response.Destroy phagocytic cell, produce cytokines including TNF-α, IL-6, and pro-IL-1β, |
| Coronavirus | Pneumonia | H. influenzae | activate the innate immune response or induce costimulatory signals for adaptive immunity |
| Adenovirus | Pneumonia | S. pneumonia, H. influenza,S. aureus, M.catarrhalis | Produce cytokines, loss of ciliary function, produce INF, Mucus response. |
| Measles virus | Otitis media, Pneumonia, Tracheobronchitis. | S. pneumonia, H. influenzae,S. aureus, | activate the innate immune response, produce cytokines including TNF-α, IL-6, and pro-IL-1β, Mucus response. |
| Human rhinovirus | Pneumonia, Otitis media, Sinusitis, | S. pneumonia, H. influenzae,S. aureus | Alters in pulmonary immune response, produce cytokines, inflammatory cell recruitment. |
| Parainfluenza virus | Pneumonia | S. pneumoniae | Destroy phagocytic cell, produce cytokines including TNF-α, IL-6, and pro-IL-1β. |
| Respiratory tract viral infection | Pneumonia, lungs problem | S. pneumonia,S. aureus,H. influenzae, pseudomonas. | Mucus response, IFN secretion, epithelial cell death, cytokine release, loss of ciliary function. |
| UTI viral infection BK Virus, Herpes virus | UTI infection is occur | E. Coli, K. pneumoniae, P. aeruginosa | chemokines and cytokines such as CXCL8, CCL2, interleukins (IL-6, IL-8, IL-10, IL-17A), and granulocyte colony stimulating factor (G-CSF) are release. |
Secondary infection of bacteria for viral infection
Epidemiological and clinical research clearly says that bacterial secondary infection can significantly increase after viral infections [44]. As an example, Up to 75% of that influenza-infected patient that goes on to acquire pneumonia, and they are establishedthat have bacterial co-infection [45]. Bacterial secondary infection after influenza infectionlooks to occur normally. Data revealed that upto 65% of the laboratory establishedthat cases of influenza infection showed bacterial secondary infection. However, Klein et al. [46] established through a meta-analysis that this figure ranged between 11 and 35%. In the site of influenza epidemic or pandemic bacterial secondary infection can have an alarming situation that is predominantly risk to immune-compromised or immune-suppressed patients. Immunosuppression is related to more severe illness and much of a higher risk of mortality from a secondary bacterial infection [47]. During pandemic Swine influenza in 2009, there was an arising patient in hospital pneumonia cases as a consequence of secondary bacterial pneumonia, which was recognized in 29–55% of mortalities [[48], [49], [50]].
Relationship between pathological changes and host responses
Viral infections are different with specific characteristics those are developed in the infected area and for that reason, bacterial colonization occurs. Bacteria can easily adhere and penetrated the infected region of the host cell [51]. Influenza virus produces neuraminidase, which also helps to increase the adhesion of some bacterial species by eliminating sialic acid which helps to depict the host cell receptors [39,52]. Some other bacteria such as Streptococci have sialic acid which allows for direct attachment with the viral hemagglutinin (HA) and this is expressed by influenza-infected host cells [52,53]. When the viral infection occurs they damaged the host cell by directly or inflammation of immune cell responses. The wound or damaged tissue welcomes the bacterial cells which can easily adhere and this viral infection increased by bacterial adhesion. For example, the contacts of apical receptors like integrins help the adhesion of bacteria such as S. aureus and Pseudomonas aeruginosa [[54], [55], [56], [57]]. At the onset of viral infection, the host inflammatory responses are the reason for the up-regulation in the expression of host receptor molecules and other molecules that bacteria can use as a receptor [52,58]. As an example, Cundell et al. [59] reported that increased performance of the G-protein-coupled platelet-activating factor (PAF) is utilized by some bacteria such as S. pneumoniae, for their attachment and colonization in the endothelial cells [59,60]. In difference, it has been recommended that the platelet-activating factor (PAF) receptor does not disturb the preliminary bacterial adherence and colonization but is more involved with secondary bacterial transition or spread into the blood and facilitates the growth of invasive disease [61].
The respiratory viruses enter through the upper respiratory tract and these virus-infected in the acute infection of the respiratory tract. When the influenza virus or other viral infections are beginning in the respiratory tract than they might influence the secondary bacterial infection such as pneumonia by changing the microbial composition in the upper respiratory tract. For that secondary infection, there is developed the growth of pathogens and large bacterial loads has occurred in the lower respiratory tract [61]. Acute viral infection and secondary bacterial colonization have a bidirectional relationship.
Viral factors implicated in severity of infection
Humans are constantly exposed to viral agents with endogenous or exogenous in origin, but few of them are the cause of significant pathogenecity or severety of the diseases. Viruses are require host cells to replicate and assemble infectious offspring. The character of the viral factors are more important which imparted in the disease progression and tissue damage [62]. Recently, Miller et al. [63], demonstrated the role of viral factors showed their severity in infant respiratory tract illness. Both, host and viral risk factors are necessary to understand which may help to develop the disease prevention strategies. When a viral strain move between hosts is accountable to new symptoms appearance or stably adapted to new hosts. Viruses are able to initiate infection, and strat their replication by the help of specific virulence factors [64]. It is well documented that different viral strains posses variable viral factors and therefore different degree of patheogenecity observed with different virulence [65]. One of the most important viral factor is located in their genome to defeat the host barriers, such as a point mutation in 5′ untranslated region (5′ UTR) of polio vaccine strain but the strain without mutation shows the greater pathogenecity in hosts [64]. Thus, viral genome is important factor to control the tropism, virus entry to host, shedding and transmission.
Viruses are able to developed immunomodulation pathway to undermine the host immune response. The virus-encoded decoy receptors are targeted to cytokines and chemokines of host cells [66]. Usually, primary bacterial clearance needs innate immunity with bacterial phagocytosis by local AM. The phagocytic activity of CD11c + alveolar macrophages in broncho alveolar lavage fluid was lower. To define the effect of interferon c (IFNc) were immunized with exogenous IFNc which inhibited alveolar macrophage-mediated phagocytosis of pneumococci both in vitro and in vivo. There was also repressed the external expression of the scavenger receptor which is responsible for phagocytosis of pneumococci by alveolar macrophages. Thirdly, interferon improved the surface expression of MHC class II antigen for that region interferon c inhibits bacterial phagocytosis and destroys innate defense against pneumococcal infection. in vivo treatment with the interferon c specific antibody had little consequence on the progress of viral infection but prohibited increased susceptibility to pneumococcal infection by developed macrophage expression of inflammatory cytokines and inhibiting pre-regulation of MHC class II expression [67]. The post-viral bacterial infection mechanism is very complex because different multifunctional interaction occurs between the virus, bacteria, and also host immune system. The pathogenesis of the respiratory super-infection has been endorsed by the direct mucosal or epithelial damage by the influenza virus. This viral infection increased the bacterial colonization of the upper and lower respiratory tracts. The host immune responses are the main cause of increased susceptibility to secondary bacterial infections. Another way, modern research suggests that our microbial populations be present in on our mucosal surfaces and probably affect our immune responses which create the characters of ecological relations among hosts and pathogens. The past 10 years result revealed the complex relationship of secondary microbial infections in the host body during viral infection [68]. In the time of secondary infection, different bacteria affect the physical substance in the host body, number of genes, and metabolic activities in different organ systems. Actually, respiratory viral infections can decrease the host immune responses, for that reason microbial growth conditions in the URT, LRT is high. When the influenza virus attacks host, body induced antiviral interferon pathways but this immune system is inadequate during host defense against secondary bacterial infections, therefore the pathogenic bacteria can easily proliferate and infected the host cell. Thus, molecular basis of virulence is polygenic in nature by consideration of viral factors. Therefore, viruses are efficiently altering the host cell response and exhibit greater pathogenicity [69].
Role of inflammatory responses in disease severity
The epithelial cells and mucosal immune cells in lung airway are the hotspot of respiratory viruses. These primary cells are getting first infected with viral agents and immediately strat to produce a range of mediators such as type I interferon (IFN), proinflammatory cytokines, and chemokines, which are the major molecules for the development of inflammation process in infected area. These are key factors in virus control molecules until vaccines are not available. It is well documented that infections induce the excessive production of reactive oxidative species (ROS), which are one of the importantat inflammatory mediators [70]. and consequences of several adverse physiological responses [71]. The expression of genes involved in respoiratory process in host, their biogenesis signalling and antioxidant enzymes are regulated at the onset of viral infection. Overproduction of ROS along with mitochondrial damage increase the imflammation process in infected zone [72]. The entry of viral agents into lung and their colonization trigger for acute exacerbations of chronic obstructive pulmonary disease (AECOPD). A details study on patients with AECOPD revealed the elevation level of IL-6, TNF-α, and MCP-1 which are also increased several folds during coinfection [73].
Co-infections of viral and bacterial pathogens increase the disease susceptibility and outcome is more severe with higher mortality. In healthy lung tissue, neutrophils, monocytes, macrophages, dendritic cells, natural killer (NK), and other innate lymphocyte (ILC), B and T cells are found in abundance where AM are mostly abundant. After establishment of infection both pathogen-associated molecular pattern (PAMPs) and damage-associated molecular patterns (DAMPs) can engage in pattern recognition receptors (PRRs) to active the cellular signalling process for the production of soluble interferons (IFNs) to limit the replication process of invading pathogens. Interestingly, this PRR ligation [e.g., toll-like receptors (TLRs) 2 and 4] start to induce the overproduction of cytokines and chemokines which regulate the activation and recruitment of inflammatory molecules in the lung [74]. Recognition of PAMPs by specific PRRs have substantial impact on disease susceptibility and increased pathogen transmission. Therefore, it is important to accurately tune the immune and inflammatory mechanisms to restore the lung tissues from maximum damage [75].
SARS-CoV-2 and secondary bacterial infection
Like other well studied respiratory viral infections including the 1918 influenza outbreak [76] and 2009 H1N1 pandemic [77], the current pandemic outbreak of SARS-CoV-2 is also reported to associate with secondary bacterial infections, thus poor outcomes and fatalities [2,78]. Recent studies reported a low rate of associated secondary bacterial and fungal infections with COVID-19 [3], however, SARS-CoV-2 is a novel coronavirus that suggested as proximally originated [79] and thus we can’t ignore the fact that our current knowledge SARS-CoV-2 is very limited. Very recently, cohort studies of tuberculosis, sequelae, and COVID-19 co-infection revealed SARS-CoV-2 associated infection in patients with TB, however, the number of patients were less and no associated disease pathogenesis need to be determined [80,81]. Further investigation and detailed studies are needed to explore the role of latent or active TB associated with COVID-19 disease severity and progression [82]. Another cohort study of 11 patients suggested that the cases with pre-existing chronic obstructive pulmonarydisease or complicated with secondary bacterial pneumonia as the most severe outcome cases of COVID-19 [83].
SARS-CoV-2 is very much similar to the SARS-CoV, though it caused ongoing pandemic outbreak COVID-2. SARS-CoV has been already reported to regulate immune function-related gene expression in human monocytes. Immune-related gene expression suggested that SARS-CoV infection downregulatesIFN-α/β-inducible and cathepsin/proteasome geneswhile differential regulated genes include, TLR/TLR-signaling, cytokine/cytokine receptor-related, chemokine/chemokine receptor-related, lysosome-related, MHC/chaperon-related, and fibrosis-related genes [84]. In a different study, SARS-CoV also reported suppressing type 1 IFN production [85]. Downregulation and differential regulation of immune genes suggested a possible encouraging environment for secondary bacterial infections. Additionally, SARS patients has been reported to have secondary bacterial infections and confirmed the presence of different bacteria in various human samples, including Escherichia coli, K. pneumoniae, P. aeruginosa, Methicillin-Resistant S. aureus (MRSA), etc. [86]. During the SARS outbreak, increased rates of MRSA infections have been reported in SARS patients that further suggested the role of secondary bacterial infections in viral disease pathogenesis [87].
A recent study, confirmed SARS-CoV-2 infection in pregnant women and studied the clinical features and neonatal outcomes. The study suggested timely use of antibiotics might prevent COVID-19 associated secondary bacterial infections and can reduce mortality and complications during pregnancy [88].
SARS-CoV-2 RNA has also been found in stool samples ofCOVID-19 patients [89]. This raises the question of gastrointestinal infection of SARS-CoV-2 and also suggested a possible fecal-oral route of disease transmission. Further, high expression levels of ACE2 mRNA in the gastrointestinal system, avail indirect evidence of SARS-CoV-2 infection [90,91]. Preliminary studies suggested gastrointestinal infection [92,93] and strong interaction of SARS-CoV-2 with the gastrointestinal system that has high microbiome diversity and possible chances of immune suppression and secondary bacterial infections. Additionally, a recent study reviewed the secondary bacterial and fungal infection in coronavirus patients and suggested guidelines for the use and prescription of antimicrobial in SARS-CoV, SARS-CoV-2, and related viral infections [3]. However, further, clinical studies may provide future directions for SARS-CoV-2 and related secondary bacterial infections.
Conclusion
Viral infections avail bacterial infections by using multiple strategies, including providing a more susceptible site for adhesion, altering immune response, and invasive infection by cell and tissue damage. Secondary bacterial infections then make the clinical outcomes more severe that is an alarming situation in concern of public health. However, antibiotics can diminish the effect of secondary bacterial infections, in-depth understanding of the viral-host, bacterial-host, and viral-bacterial interaction is important to fight against these infections. Especially in the focus of rapid increasing antibiotic resistance and adaptation of microbes may lead to escape from vaccine-induced immunity. The eventual goal of understanding the molecular pathogenesis of viral infection associated secondary bacterial infections is to development of more efficient diagnostics and therapeutic strategies. Further, it becomes an important fight against the current or future potential of pandemics and the emergence of novel viruses like SARS-CoV-2.
Funding
No funding sources.
Conflict of interests
None declared.
Ethical approval
Not required.
Acknowledgement
SMM is grateful to IIT-Kharagpur for providing necessary facilities and support.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., et al. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Z., Xiao Y., Kang L., Ma W., Shi L., Zhang L., et al. Genomic diversity of severe acute respiratory syndrome–coronavirus 2 in patients with coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCullers J.A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. 2014;12(4):252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 7.Hament J.M., Aerts P.C., Fleer A., Van Dijk H., Harmsen T., Kimpen J.L.L., et al. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr Res. 2005;58(6):1198–1203. doi: 10.1203/01.pdr.0000188699.55279.1b. [DOI] [PubMed] [Google Scholar]
- 8.Avadhanula V., Wang Y., Portner A., Adderson E. Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein. J Med Microbiol. 2007;56(Pt. 9):1133–1137. doi: 10.1099/jmm.0.47086-0. [DOI] [PubMed] [Google Scholar]
- 9.Avadhanula V., Rodriguez C.A., DeVincenzo J.P., Wang Y., Webby R.J., Ulett G.C., et al. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J Virol. 2006;80(4):1629–1636. doi: 10.1128/JVI.80.4.1629-1636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith C.M., Sandrini S., Datta S., Freestone P., Shafeeq S., Radhakrishnan P., et al. Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a a new paradigm in respiratory infection. Am J Respir Crit Care Med. 2014;190(2):196–207. doi: 10.1164/rccm.201311-2110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura S., Davis K.M., Weiser J.N. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest. 2011;121(9):3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iverson A.R., Boyd K.L., McAuley J.L., Plano L.R., Hart M.E., McCullers J.A. Influenza virus primes mice for pneumonia from Staphylococcus aureus. J Infect Dis. 2011;203(6):880–888. doi: 10.1093/infdis/jiq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith A.M., Adler F.R., Ribeiro R.M., Gutenkunst R.N., McAuley J.L., McCullers J.A., et al. Kinetics of coinfection with influenza a virus and Streptococcus pneumoniae. PLoS Pathog. 2013;9(3):e1003238. doi: 10.1371/journal.ppat.1003238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novotny L.A., Bakaletz L.O. Intercellular adhesion molecule 1 serves as a primary cognate receptor for the type IV pilus of nontypeable Haemophilus influenzae. Cell Microbiol. 2016;18(8):1043–1055. doi: 10.1111/cmi.12575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen D.T., Louwen R., Elberse K., Van Amerongen G., Yüksel S., Luijendijk A., et al. Streptococcus pneumoniae enhances human respiratory syncytial virus infection in vitro and in vivo. PLoS One. 2015;10(5):e0127098. doi: 10.1371/journal.pone.0127098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hament J.M., Aerts P.C., Fleer A., Van Dijk H., Harmsen T., Kimpen J.L.L., et al. Enhanced adherence of Streptococcus pneumoniae to human epithelial cells infected with respiratory synctial virus. Pediatr Res. 2004;55(6):972–978. doi: 10.1203/01.PDR.0000127431.11750.D9. [DOI] [PubMed] [Google Scholar]
- 17.Lijek R.S., Weiser J.N. Co-infection subverts mucosal immunity in the upper respiratory tract. Curr Opin Immunol. 2012;24(4):417–423. doi: 10.1016/j.coi.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braciale T.J., Sun J., Kim T.S. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12(4):295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghoneim H.E., Thomas P.G., McCullers J.A. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol. 2013;191(3):1250–1259. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun K., Metzger D.W. Influenza infection suppresses NADPH oxidase–dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J Immunol. 2014;192(7):3301–3307. doi: 10.4049/jimmunol.1303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta D., Petes C., Gee K., Basta S. The role of virus infection in deregulating the cytokine response to secondary bacterial infection. J Interferon Cytokine Res. 2015;35(12):925–934. doi: 10.1089/jir.2015.0072. [DOI] [PubMed] [Google Scholar]
- 22.Davidson S., Maini M.K., Wack A. Disease-promoting effects of type i interferons in viral, bacterial, and coinfections. J Interferon Cytokine Res. 2015;35(4):252–264. doi: 10.1089/jir.2014.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson K.M., Kolls J.K., Alcorn J.F. The immunology of influenza virus-associated bacterial pneumonia. Curr Opin Immunol. 2015;34:59–67. doi: 10.1016/j.coi.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGillivary G., Mason K.M., Jurcisek J.A., Peeples M.E., Bakaletz L.O. Respiratory syncytial virus-induced dysregulation of expression of a mucosal Β-defensin augments colonization of the upper airway by non-typeable Haemophilus influenzaec. Cell Microbiol. 2009;11(9):1399–1408. doi: 10.1111/j.1462-5822.2009.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vittor A.Y., Garland J.M., Gilman R.H. Molecular diagnosis of TB in the HIV positive population. Ann Glob Health. 2014;80(6):476–485. doi: 10.1016/j.aogh.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Bell L.C.K., Noursadeghi M. Pathogenesis of HIV-1 and mycobacterium tuberculosis co-infection. Nat Rev Microbiol. 2018;16(2):80–90. doi: 10.1038/nrmicro.2017.128. [DOI] [PubMed] [Google Scholar]
- 27.Limaye A.P., Kirby K.A., Rubenfeld G.D., Leisenring W.M., Bulger E.M., Neff M.J., et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Omari A., Aljamaan F., Alhazzani W., Salih S., Arabi Y. Cytomegalovirus infection in immunocompetent critically ill adults: literature review. Ann Intensive Care. 2016;6(1):110. doi: 10.1186/s13613-016-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alyazidi R., Murthy S., Slyker J.A., Gantt S. The potential harm of cytomegalovirus infection in immunocompetent critically ill children. Front Pediatr. 2018;6:96. doi: 10.3389/fped.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin D.E. Measles virus-induced suppression of immune responses. Immunol Rev. 2010;236:176–189. doi: 10.1111/j.1600-065X.2010.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mina M.J., Kula T., Leng Y., Li M., De Vries R.D., Knip M., et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science. 2019;366(6465):599–606. doi: 10.1126/science.aay6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berkhout B. With a little help from my enteric microbial friends. Front Med. 2015;2:30. doi: 10.3389/fmed.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karst S.M., Wobus C.E. A working model of how noroviruses infect the intestine. PLoS Pathog. 2015;11(2):e1004626. doi: 10.1371/journal.ppat.1004626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Racaniello V.R. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Robinson C.M., Jesudhasan P.R., Pfeiffer J.K. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe. 2014;15(1):36–46. doi: 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khosravi A., Mazmanian S.K. Disruption of the gut microbiome as a risk factor for microbial infections. Curr Opin Microbiol. 2013;16(2):221–227. doi: 10.1016/j.mib.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullers J.A. Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev. 2006;19(3):571–582. doi: 10.1128/CMR.00058-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lillebaek T., Dirksen A., Vynnycky E., Baess I., Thomsen V.Ø, Andersen ÅB. Stability of DNA patterns and evidence of Mycobacterium tuberculosis reactivation occurring decades after the initial infection. J Infect Dis. 2003;188(7):1032–1039. doi: 10.1086/378240. [DOI] [PubMed] [Google Scholar]
- 39.McCullers J.A., Bartmess K.C. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187(6):1000–1009. doi: 10.1086/368163. [DOI] [PubMed] [Google Scholar]
- 40.Murphy T.F., Bakaletz L.O., Smeesters P.R. Microbial interactions in the respiratory tract. Pediatr Infect Dis J. 2009;28(10 Suppl):S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- 41.Bosch A.A.T.M., Biesbroek G., Trzcinski K., Sanders E.A.M., Bogaert D. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013;9(1):e1003057. doi: 10.1371/journal.ppat.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendaus M.A., Jomha F.A., Alhammadi A.H. Virus-induced secondary bacterial infection: a concise review. Ther Clin Risk Manag. 2015;11:1265–1271. doi: 10.2147/TCRM.S87789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hament J.-M., Kimpen J.L., Fleer A., Wolfs T.F. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol. 1999;26(3-4):189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 44.Gupta R.K., George R., Nguyen-Van-Tam J.S. Bacterial pneumonia and pandemic influenza planning. Emerg Infect Dis. 2008;14(8):1187–1192. doi: 10.3201/eid1408.070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zambon M.C. The pathogenesis of influenza in humans. Rev Med Virol. 2001;11(4):227–241. doi: 10.1002/rmv.319. [DOI] [PubMed] [Google Scholar]
- 46.Klein E.Y., Monteforte B., Gupta A., Jiang W., May L., Hsieh Y.H., et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respi Viruses. 2016;10(5):394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rice T.W., Rubinson L., Uyeki T.M., Vaughn F.L., John B.B., Miller R.R., et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40(5):1487–1498. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza a (H1N1)—United States, May–August 2009. Morb Mortal Wkly Rep. 2009;58(38):1071–1074. [PubMed] [Google Scholar]
- 49.Gill J.R., Sheng Z.M., Ely S.F., Guinee D.G., Beasley M.B., Suh J., et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134(2):235–243. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinberger D.M., Simonsen L., Jordan R., Steiner C., Miller M., Viboud C. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. J Infect Dis. 2012;205(3):458–465. doi: 10.1093/infdis/jir749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selinger D.S., Reed W.P., McLaren L.C. Model for studying bacterial adherence to epithelial cells infected with viruses. Infect Immun. 1981;32(2):941–944. doi: 10.1128/iai.32.2.941-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peltola V.T., McCullers J.A., Fink R.J., Fedson D.S. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. 2004;23(1 suppl):s87–s97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 53.Okamoto S., Kawabata S., Nakagawa I., Okuno Y., Goto T., Sano K., et al. Influenza a virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J Virol. 2003;77(7):4104–4112. doi: 10.1128/JVI.77.7.4104-4112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanford B.A., Shelokov A., Ramsay M.A. Bacterial adherence to virus-infected cells: a cell culture model of bacterial superinfection. J Infect Dis. 1978;137(2):176–181. doi: 10.1093/infdis/137.2.176. [DOI] [PubMed] [Google Scholar]
- 55.Davison V.E., Sanford B.A. Staphylococcus aureusAdherence of to influenza A virus-infected Madin–Darby canine kidney cell cultures. Infect Immun. 1981;32(1):118–126. doi: 10.1128/iai.32.1.118-126.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bucior I., Pielage J.F., Engel J.N. Pseudomonas aeruginosa Pili and Flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012;8(4):e1002616. doi: 10.1371/journal.ppat.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith A.M., McCullers J.A. Secondary bacterial infections in influenza virus infection pathogenesis. Curr Top Microbiol Immunol. 2014;385:327–356. doi: 10.1007/82_2014_394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hakansson A., Kidd A., Wadell G., Sabharwal H., Svanborg C. Adenovirus infection enhances in vitro adherence of Streptococcus pneumoniae. Infect Immun. 1994;62(7):2707–2714. doi: 10.1128/iai.62.7.2707-2714.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cundell D.R., Gerard N.P., Gerard C., Idanpaan-Heikkila I., Tuomanen E.I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377(6548):435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 60.van der Sluijs K.F., van der Poll T., Lutter R., Juffermans N.P., Schultz M.J. Bench-to-bedside review: bacterial pneumonia with influenza – pathogenesis and clinical implications. Crit Care. 2010;14(2):219. doi: 10.1186/cc8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mccullers J.A., Iverson A.R., Murray P.J. The platelet activating factor receptor is not required for exacerbation of bacterial pneumonia following influenza. Scand J Infect Dis. 2008;40(1):11–17. doi: 10.1080/00365540701477568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.MacLachlan N.J., Dubovi E.J. Pathogenesis of viral infections and diseases. Fenner’s Vet Virol. 2011:43–74. [Google Scholar]
- 63.Miller A.L., Gerard C., Schaller M., Gruber A.D., Humbles A.A., Lukacs M.W. Deletion of CCR1 attenuates pathophysiologic responses during respiratory syncytial virus infection. J Immunol. 2006;176(4):2562–2567. doi: 10.4049/jimmunol.176.4.2562. [DOI] [PubMed] [Google Scholar]
- 64.Albrecht T., Fons M., Boldogh I.R., Alan S. In: Medical Microbiology. 4th ed.) Samuel Baron., editor. University of Texas Medical Branch at Galveston; Galveston (TX): 1996. ISBN 0963117211. PMID 21413282. [Google Scholar]
- 65.Fuentes-González A.M., Contreras-Paredes A., Manzo-Merino J., Lizano M. The modulation of apoptosis by oncogenic viruses. Virol J. 2013;10:182. doi: 10.1186/1743-422X-10-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felix J., Savvides S. Mechanisms of immunomodulation by mammalian and viral decoy receptors: insights from structures. Nat Rev Immunol. 2017;17:112–129. doi: 10.1038/nri.2016.134. [DOI] [PubMed] [Google Scholar]
- 67.Akiko I., Ellen F.F., Ryan D.M. Early local immune defenses in the respiratory tract. Nat Rev Immunol. 2017;17(1):7–20. doi: 10.1038/nri.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denise E.M., David W.C., Stuart C. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041. doi: 10.3389/fmicb.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.John C.K., Jeffery K.T. The role of viral, host, and secondary bacterial factors in influenza pathogenesis. Am J Pathol. 2015;185(6):1528–1536. doi: 10.1016/j.ajpath.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yilong F., Andy Y., Peck G.S., Francesca B., Pei-yong S., Maxime H. Modulation of inflammation and pathology during dengue virus infection by p38 MAPK inhibitor SB203580. Antiviral Res. 2014;110:151–157. doi: 10.1016/j.antiviral.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Banerjee D., Mandal S.M., Das A., Hegde M.L., Das S., Bhakat K.K., et al. Preferential repair of oxidized base damage in the transcribed genes of mammalian cells. J Biol Chem. 2011;286(8):6006–6016. doi: 10.1074/jbc.M110.198796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shitao L., Bishi F., Meshram Chetan D. Innate immune and inflammatory responses to respiratory viruses. Mediators Inflamm. 2019 doi: 10.1155/2019/3146065. Article ID 3146065, 2 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jingtong Z., Yue S., Lingxin X., Weijie Z., Ying L., Peter G.G., et al. The expression of IL-6, TNF-α, and MCP-1 in respiratory viral infection in acute exacerbations of chronic obstructive pulmonary disease. J Immunol Res. 2017 doi: 10.1155/2017/8539294. Article ID 8539294, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iwasaki A., Pillai P.S. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14:315–328. doi: 10.1038/nri3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wenting Z., Qinghua H., Weiwei X., Yue Z., Jiang P., Huan X., et al. Advances in anti-tumor treatments targeting the CD47/SIRPα Axis. Front Immunol. 2020;11:18. doi: 10.3389/fimmu.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.MacIntyre C.R., Chughtai A.A., Barnes M., Ridda I., Seale H., Toms R., et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis. 2018;18(1):637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Virological. 2020;26(4):450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Motta I., Centis R., D’Ambrosio L., García-García J.-M., Goletti D., Gualano G., et al. Tuberculosis, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26(4):233–240. doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tadolini M., Codecasa L.R., Jé-Mía García-García, Blanc F.-X., Borisov S., Alffenaar J.-W., et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56(1):2001398. doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu Y., Bi L., Chen Y., Wang Y., Fleming J., Yu Y., et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. MedRxiv. 2020 doi: 10.1101/2020.03.10.20033795. [DOI] [Google Scholar]
- 83.Dong X., Cao Y. yuan, Lu X. xia, Zhang J. jin, Du H., Yan Y. qin, et al. Eleven faces of coronavirus disease 2019. Allergy Eur J Allergy Clin Immunol. 2020;75(7):1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hu W., Yen Y.T., Singh S., Kao C.L., Wu-Hsieh B.A. SARS-CoV regulates immune function-related gene expression in human monocytic cells. Viral Immunol. 2012;25(4):277–888. doi: 10.1089/vim.2011.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Siu K.L., Chan C.P., Kok K.H., Chiu-Yat Woo P., Jin D.Y. Suppression of innate antiviral response by severe acute respiratory syndrome coronavirus M protein is mediated through the first transmembrane domain. Cell Mol Immunol. 2014;11(2):141–149. doi: 10.1038/cmi.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tan F.L.S., Loo W.L., Tan S.G., Wong C.Y., Tan Y.-M. Severe acute respiratory syndrome in surgical patients: a diagnostic dilemma. ANZ J Surg. 2005;75:21–26. doi: 10.1111/j.1445-2197.2005.03285.x. [DOI] [PubMed] [Google Scholar]
- 87.Yap F.H.Y., Gomersall C.D., Fung K.S.C., Ho P.-L., Ho O.-M., Lam P.K.N., et al. Increase in methicillin-resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis. 2004;39(4):511–516. doi: 10.1086/422641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1-2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 91.Yan R., Zhang Y., Guo Y., Xia L., Zhou Q. Structural basis for the recognition of the 2019-nCoV by human ACE2. BioRxiv. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao Q.Y., Chen Y.X., Fang J.Y. Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2019;21(3):125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]