Abstract
Sensory information transmitted to the spinal cord dorsal horn is modulated by a complex network of excitatory and inhibitory interneurons. The two main inhibitory transmitters, GABA and glycine, control the flow of sensory information mainly by regulating the excitability of dorsal horn neurons. A presynaptic action of GABA has also been proposed as an important modulatory mechanism of transmitter release from sensory primary afferent terminals. By inhibiting the release of glutamate from primary afferent terminals, activation of presynaptic GABA receptors could play an important role in nociceptive and tactile sensory coding, while changes in their expression or function could be involved in pathological pain conditions, such as allodynia.
Keywords: dorsal horn, pain, GABA, glycine, inhibition
Introduction
The superficial and deep laminae of the spinal cord dorsal horn are under strong inhibitory control, importantly exerted by two neurotransmitters, gamma-aminobutyric acid (GABA) and glycine. These transmitters, released by both local interneurons and inhibitory descending fibers, bind to their cognate anion permeable receptors, GABAA and glycine, respectively. GABA additionally binds to its G protein–coupled receptor, the GABAB receptor. Activation of GABA and glycine receptors depresses neuronal excitation through hyperpolarization of the postsynaptic membrane and/or activation of a shunting conductance. Indeed, application of bicuculline or strychnine (blockers of GABAA and glycine receptors, respectively) to a spinal cord slice preparation shows the effect of ambient GABA and glycine on excitability of dorsal horn neurons (Fig. 1). As we will describe subsequently, GABA can also directly decrease glutamate release from primary afferent fibers (PAFs).1 Behavioral studies in rodents have shown that intrathecal administration of bicuculline or strychnine induces nocifensive responses and lowers nociceptive threshold in rats, while injection of GABA or glycine is antinociceptive under most circumstances.2–4 Furthermore, enhancing GABAA receptor function by spinal application of GABA or a positive allosteric modulator, such as midazolam, depresses noxious stimulus-evoked activity in spinal cord neurons.5,6 Loss of synaptic inhibition is widely accepted as an important factor contributing to the generation and maintenance of chronic pain. Therefore, we will consider recent advances in our understanding of inhibition in the spinal cord dorsal horn.
Figure 1.
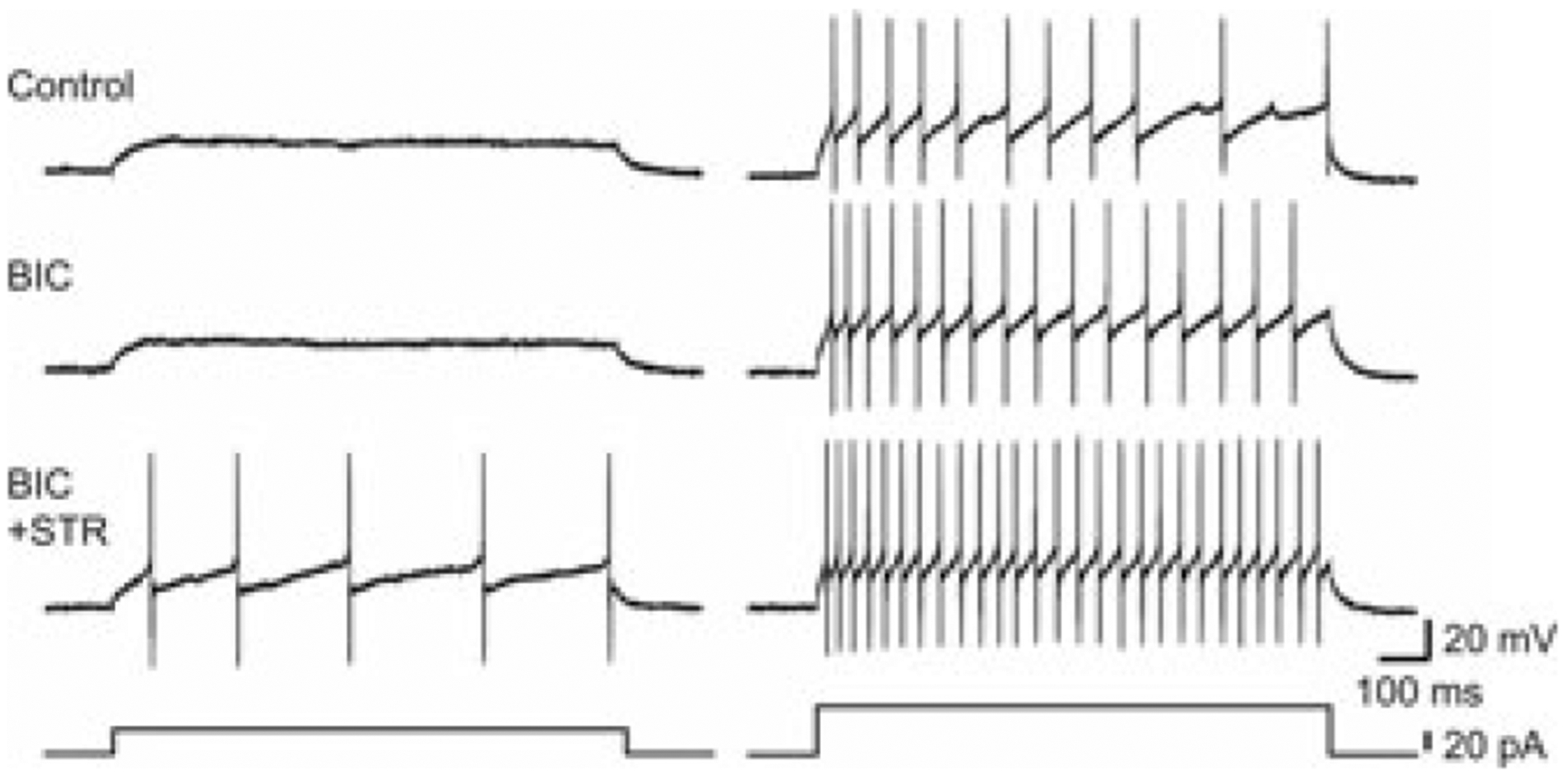
Blockade of GABAA and glycine receptors with bicuculline and strychnine enhances excitability of inhibitory neurons in mouse dorsal horn. Shown are examples of action potentials induced by current injection under three conditions: (1) control; NBQX [10 μM] and AP5 [50 μM]; (2) BIC; NBQX, AP5, and bicuculline [10 μM]; and (3) BIC+STR; NBQX, AP5, bicuculline, and strychnine [1 μM]. Resting membrane potential was kept at −65 mV. Left panel shows responses to a small current injection in the three conditions, and the right panel shows responses to a larger current injection. Bottom traces show injected currents. Taken with permission from Ref. 15.
Region-specific inhibition by GABA and glycine in the spinal cord dorsal horn
The relative contributions of GABA and glycine to the control of the flow of information in the dorsal horn varies among different laminae and somatosensory modalities. Immunohistochemical studies and, more recently, transgenic mice in which enhanced green fluorescent protein (eGFP) is expressed under the promoter of the gene encoding the enzyme glutamic acid decarboxylase 67 (GAD67), involved in the synthesis of GABA (GAD67-eGFP mice), have shown that GABAergic interneurons are abundant in the spinal cord dorsal horn. This is especially true in superficial laminae,7–10 where nociceptive fibers terminate. A subpopulation of GABAergic interneurons also express glycine (33%, 43%, and 64% of GABAergic neurons in lamina I, II, and III, respectively, express glycine).10,11 In situ hybridization studies and observations from glycine transporter 2 (GlyT2)-eGFP mice have shown that glycinergic neurons are more abundant in the deeper dorsal layers (laminae III–V)12,13 that receive tactile sensory inputs as well as some nociceptive inputs.
Analysis of miniature inhibitory postsynaptic currents (mIPSCs) mediated by GABAA and glycine receptors, reflecting quantal release of these transmitters, has confirmed that the contribution of GABA and glycine to fast synaptic inhibition changes between different laminar regions of the dorsal horn. GABAergic mIPSCs seem to predominate in laminae I and II outer (IIo), while glycine mIPSCs play a dominant role in lamina III.14 Our group has recently characterized two populations of inhibitory interneurons in GAD67-eGFP mice. One group of neurons predominantly receives strongly bicuculline-sensitive mIPSCs with slower decay kinetics (GABA-dominant) while another class of inhibitory neurons predominantly receives fewer bicuculline-sensitive mIPSCs with fast decay kinetics (glycine-dominant).15 Consistent with previous studies,14,16,17 inhibitory interneurons are mainly GABA-dominant in laminae I–IIo, while glycine-dominant neurons are prevalent at the lamina II–III border, as illustrated in Figure 2.
Figure 2.
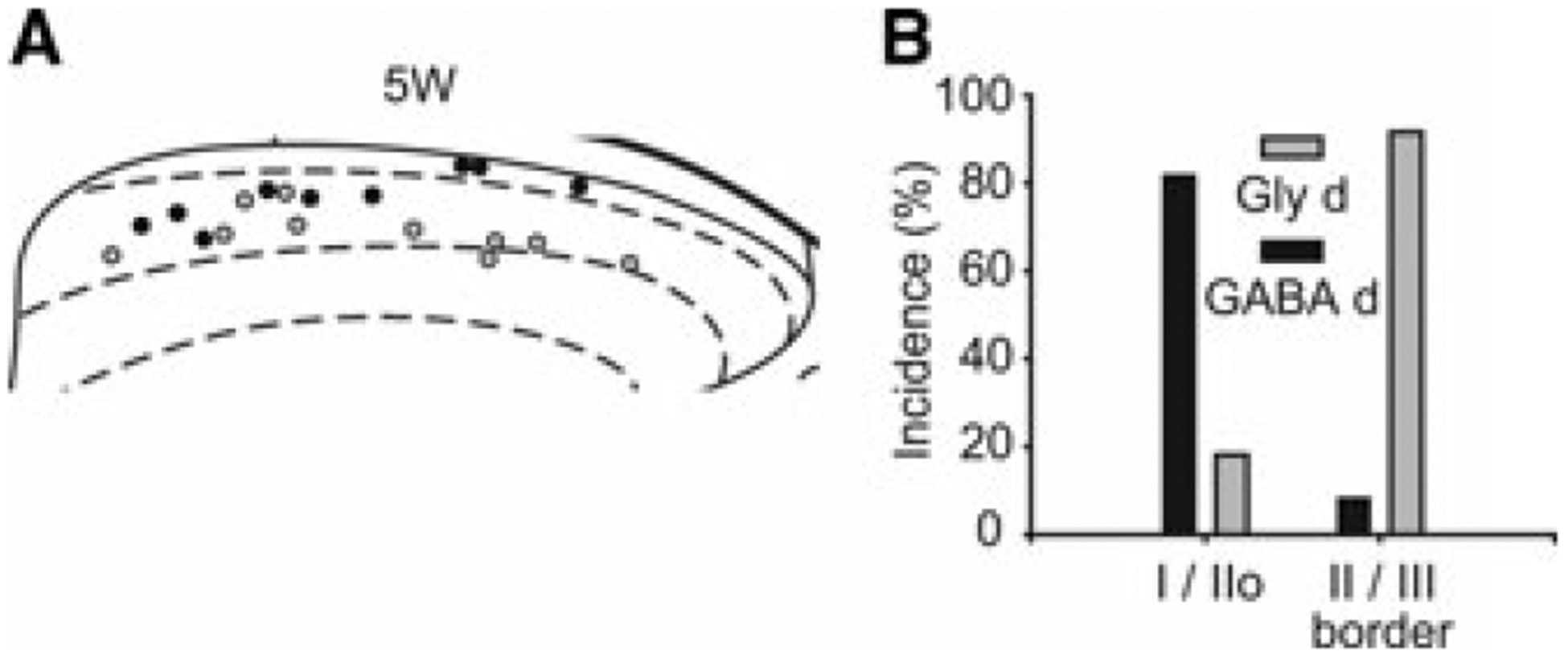
GAD67-eGFP+ neurons have regionally distinct properties of synaptic inhibitory input. Part A shows soma locations of glycine-dominant (Gly-d, gray circles) and GABA-dominant (GABA-d, black circles) neurons recorded from post-natal day 29–32 (5W) mice. Right and upper sides of the schematic diagram show lateral and dorsal edges of the dorsal horn, respectively. Laminae I, II, and III are separated by dotted lines. (B) Gly-d neurons are the major population at the lamina II/III border (n = 12), while GABA-d neurons (black bars) are the major population in lamina I and IIo (n = 11). Bars indicate the incidence of neurons located in laminae I/IIo, and at the laminae II/III border. Taken with permission from Ref. 15.
Extrasynaptic GABAA and glycine receptors mediate tonic currents in dorsal horn neurons,18–20 where they play important roles in regulating neuronal excitability. We have shown in mature mice that tonic GABA currents predominate in GABA-dominant neurons, while tonic glycine currents are critical in regulating the inhibitory tone of glycine-dominant neurons at the lamina II–III border.15 This border area receives inputs from low-threshold mechanosensitive afferents and is critically involved in the generation of dynamic mechanical allodynia.21 Excitatory interneurons expressing the γ isoform of protein kinase C (PKCγ), located at the ventral border of inner lamina II (lamina IIi), and in lamina III, are essential contributors to mechanical allodynia.22 These PKCγ-expressing interneurons receive projections from low-threshold mechanoreceptors23 and are normally inhibited by glycinergic interneurons.24 Removal of glycine inhibition allows activation of a polysynaptic excitatory pathway triggered by low-threshold mechanical input, leading to the excitation of nociceptive-specific projection neurons in the superficial dorsal horn.21 This is similar to the polysynaptic excitatory pathway between low-threshold Aβ fibers and lamina I projection neurons observed in the presence of bicuculline and strychnine in another study.25 Further experiments will be required to assess the contribution of synaptic and tonic glycine currents in controlling the excitability of inhibitory and excitatory interneurons, both in control animals and in animal models of chronic pain.
Presynaptic modulation of primary afferent terminals
GABAA receptors and primary afferent depolarization
GABA has long been known to be one of the inhibitory transmitters mediating presynaptic inhibition of excitatory transmission in the spinal cord, acting through both ionotropic (GABAA) and G protein–coupled receptors (GABAB). In 1957, Frank and Fuortes26 first proposed the concept of presynaptic inhibition, based on the observation that muscle afferent volleys depressed the size of the monosynaptic excitatory postsynaptic potential of spinal motoneurons produced by other muscle afferents without any changes in the membrane potential or excitability of those motoneurons. This form of presynaptic inhibition is caused by depolarization of the primary afferent terminals (PAD) and is strongly depressed by GABAA receptor antagonists, such as bicuculline, suggesting that GABAergic interneurons can be involved in this mechanism through a polysynaptic circuit.27–29
Histological evidence supporting this hypothesis has been provided by several studies. GABAergic interneurons form axo-axonic synapses in both the ventral and dorsal horns, not only on group Ia, Ib, and II muscle PAFs, but also on cutaneous afferents, particularly those of large diameter.30 As shown in Figure 3, the stimulation of sensory cutaneous afferents could activate a disynaptic circuit in the dorsal horn, producing release of GABA from inhibitory interneurons, causing decrease of glutamate release from the PADs. Electron microscopy studies performed on the superficial dorsal horn from spinal cord preparations of different mammalian species have demonstrated the presence of complex synaptic structures called glomeruli. These are formed by a central terminal of either myelinated or unmyelinated PAFs, and several dendrites and axons.11,31 Axon terminals presynaptic to central terminals of unmyelinated PAFs are predominantly GABAergic, while the majority of those contacting terminals of myelinated PAFs are both GABAergic and glycinergic.32 In lamina III of the rat spinal cord, axons containing GABA, or GABA plus glycine, form a synaptic triadic arrangement, contacting both the terminal of a hair follicle myelinated afferent and a postsynaptic dendrite.33 Parvalbumin-expressing inhibitory interneurons, which predominate in lamina III, have recently been shown to be involved in axo-axonic synapses with nonnociceptive Aδ down hair afferents belonging to synaptic glomeruli, or with larger myelinated fibers such as hair follicle afferents.34
Figure 3.
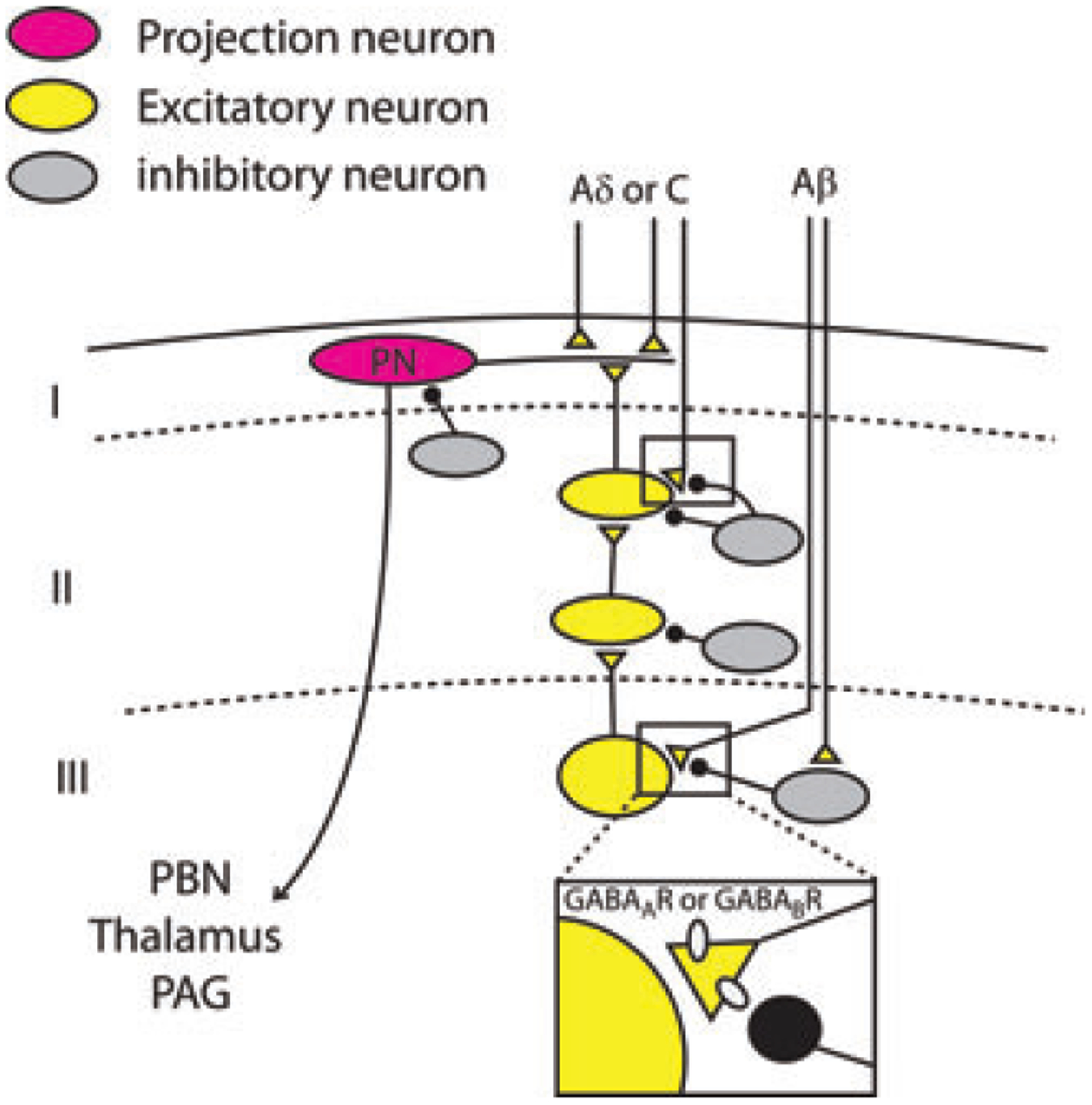
Schematic representation of a proposed polysynaptic excitatory pathway connecting lamina III to lamina I projection neurons. This pathway is normally under powerful inhibitory control mediated by GABA and glycine released from inhibitory neurons. Presynaptic inhibition mediated by GABAA or GABAB receptors in the spinal cord dorsal horn is illustrated in detail for the Aβ fiber synaptic terminal onto a lamina III neuron, but also occurs on nociceptor PAF terminals. Stimulation of cutaneous tactile fibers induces GABA release from inhibitory interneurons, causing the activation of GABAA or GABAB receptors expressed on primary afferent terminals and the inhibition of glutamate release onto lamina III–IV neurons.
The mechanisms of presynaptic inhibition mediated by PAD have largely been elucidated.30 Primary sensory neurons exhibit a higher intracellular concentration of chloride than central neurons. This is due to the high expression of the transporter NKCC1, which transports Cl−, Na+, and K+ into the cell, and low expression of KCC2, which transports Cl− and K+ out of the cell.35–37 For this reason, the chloride equilibrium potential in dorsal root ganglion neurons (DRGs) is about −30 mV. Thus, the opening of GABAA receptors causes the efflux of Cl− and depolarization of the terminals. Inactivation of voltage-dependent sodium and calcium channels, caused by the terminal depolarization38 and the shunting effect due to opening of GABAA receptors, impairs the propagation of action potentials along PAFs into the terminals, and decreases the release of glutamate. Suprathreshold depolarizations can potentially produce the opposite effect by eliciting action potentials in spinal PAF terminals, possibly triggering dorsal root reflexes that may contribute to neurogenic inflammation.39 The upregulation of the GABA system on DRG neurons40 and/or the increase of NKCC1 transporter activity41 could contribute to the shift from presynaptic inhibition to dorsal reflexes and pain sensitization.
Studies performed during the last decade have suggested the need for additional mechanisms for PAD. PAF terminals express other synaptic receptors able to depolarize the terminals. Glutamatergic ionotropic AMPA, NMDA, and kainate receptors have been detected at the central terminals of PAFs. Activation of these receptors causes depolarization of PAFs in the rat spinal cord.42–44 Furthermore, the observation that PAD is not completely blocked by inhibition of synaptic transmission suggests that it could be partly mediated by either spillover of transmitters released from PAFs or a dendroaxonic reciprocal synaptic microcircuit.45,46 At the glomerular level, several dendrites contacting PAF central terminals contain clear vesicles, so these could be involved in the generation of PAD.10,47
The subunit composition of presynaptic GABAA receptors expressed on PAF terminals has recently been investigated. Central terminals of primary afferents in mouse dorsal horn express four α subunits (α1–3, α5), with a prevalence of α2 or α3 on C-fibers, while myelinated Aδ and Aβ fibers are co-labeled in roughly equal proportion with each subunit.48 Mice with nociceptors (DRG neurons expressing SNS [or Nav1.8] channels) selectively lacking the GABAAα2 subunits exhibit reduced potentiation of dorsal root potentials and impaired thermal and mechanical antihyperalgesia by diazepam in a model of inflammatory pain,49 confirming a role of the α2 subunit in regulating sensitization in models of inflammatory and neuropathic pain.50,51
Although glycine is present in several axon terminals of GABAergic neurons presynaptic to PAFs (see previous discussion), strychnine does not block PAD or presynaptic inhibition,27,52,53 and glycine does not directly depolarize PAFs.54 Furthermore, glycine receptors have not been detected on PAF terminals,55 so the putative presynaptic role of glycine on PAF central terminals is still controversial. It is possible that the major site of action of glycine, released by inhibitory interneurons, is on postsynaptic dendrites, belonging to glomeruli or triadic arrangements.
GABAB receptors
Metabotropic GABAB receptors are expressed both in DRGs and spinal cord dorsal horn, particularly in the superficial laminae.56,57 Their activation produces antinociceptive effects: treatment with the GABAB agonist baclofen induces dose-dependent inhibition of C-fiber and pinch-evoked activity of rat wide dynamic range neurons in vivo,58 and reverses hypersensitivity of these neurons to mechanical stimuli after spinal cord ischemia.59
Exogenous activation of GABAB receptors by baclofen inhibits glutamate and substance P release from PAFs in spinal cord dorsal horn60 by acting on presynaptic voltage-dependent calcium channels. A postsynaptic effect of baclofen has also been observed in rat superficial and deep dorsal horn, consisting of the generation of an outward current mediated by potassium channels.57,61,62 GABAB receptors are also involved in the depression of GABA release from dorsal horn neurons: in lamina I, paired pulse depression of evoked IPSCs is decreased by an antagonist of GABAB receptors, while it is not affected by GABAA antagonists.63 Because GABAB receptors have a higher sensitivity for GABA than GABAA receptors do, they could be activated even under conditions of low extracellular concentrations of GABA. The endogenous effect of GABAB receptors in modulating glutamate release from PAFs in lamina II has recently been investigated:64 blockade of GABAB receptors facilitates the evoked action potential–dependent synaptic responses and increases neuronal excitability after dorsal root stimulation.
Function of GABA-mediated presynaptic inhibition in the dorsal horn, and future perspectives
The first synapse in the somatosensory pathway, that is, the synapse between nociceptive or tactile PAFs and dorsal horn neurons, is modulated by several mechanisms of presynaptic inhibition. Here we have illustrated some aspects of the inhibition mediated by GABA receptors. Both ionotropic GABAA and metabotropic GABAB receptors exert an inhibitory action on glutamate release from PAFs, involving different cellular mechanisms. Despite the large number of studies regarding GABA-mediated presynaptic inhibition in dorsal horn, several aspects remain to be elucidated.
Synaptic responses generated by glutamate release from PAFs onto dorsal horn neurons are known to undergo a strong short-term depression (see Ref. 65). We have observed that, in rat lamina III, the level of depression is variable from one postsynaptic cell to another (unpublished observation). This likely reflects (1) different PAF properties and/or (2) different synaptic circuits recruited by dorsal root stimulation. The roles of GABAA and GABAB receptors in short-term depression have not been established. We hypothesize that during PAF repetitive stimulation, the activation of presynaptic GABAA and GABAB receptors could contribute to shaping the pattern of postsynaptic response amplitudes, both in superficial and deep dorsal horn. Application of bicuculline attenuates paired pulse depression of postsynaptic responses evoked on dorsal horn neurons by low-threshold afferent stimulation in vivo.66 Preliminary results obtained in our laboratories indicate that the GABAA agonist muscimol is able to modulate glutamate release from Aβ fibers onto rat spinal lamina III neurons and increase the paired pulse ratio of evoked EPSCs. Thus, synaptic depression at central terminals of PAFs is modified by receptor-mediated presynaptic inhibition. This could, in turn, affect the firing pattern of dorsal horn neurons and the sensory coding process. A characterization of the postsynaptic neurons involved in this mechanism (i.e., inhibitory versus excitatory interneurons) would also be critical in understanding the organization and function of presynaptic inhibition in the dorsal horn.
Another important consideration is how presynaptic modulation is affected by the plastic changes that occur during chronic pain. Some studies suggest that peripheral inflammation induces an increase of PAD on PAFs (both nociceptive and nonnociceptive). Application of inflammatory agents on DRGs induces a rapid increase of intracellular Cl− concentration through the upregulation of the NKCC1 transporter.67 After persistent peripheral inflammation, GABA-induced depolarization on DRGs increases, partially due to the inhibition of voltage-dependent potassium currents40 and to enhanced GABAA receptor function67 in addition to elevated intracellular chloride. The potentiation of PAD during inflammatory pain could contribute to hyperalgesia by enhancing dorsal root reflexes in nociceptors.39,68,69
Modulation of PAD on low-threshold afferents could be involved in the generation of allodynia. If glutamate release from low-threshold mechanoreceptors increased due to augmented excitability of presynaptic terminals, this could help drive the excitatory polysynaptic pathway revealed with use of GABAA and glycine receptor antagonists, as proposed by Torsney and MacDermott (Fig. 3). However, enhanced PAD could also mediate enhanced presynaptic inhibition. Correspondingly, a recent study has proposed an opposite role for PAD in inflammatory pain.49 Mice lacking benzodiazepine-sensitive α2-GABAA receptors in primary nociceptors showed reduced antihyperalgesia in response to intrathecally injected diazepam in an inflammatory pain model. These results suggest that facilitation of GABAA receptor activation on spinal nociceptor terminals could exert an analgesic action. A more extensive characterization of the dorsal horn synaptic circuits and neuronal types involved in PAD and presynaptic inhibition will be important to clarify these discrepancies and understand the role of presynaptic GABAA receptors in chronic pain.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.MacDermott AB, Role LW & Siegelbaum SA. 1999. Presynaptic ionotropic receptors and the control of transmitter release. Annu. Rev. Neurosci 22: 443–485. [DOI] [PubMed] [Google Scholar]
- 2.Beyer C, Roberts LA & Komisaruk BR. 1985. Hyperalgesia induced by altered glycinergic activity at the spinal cord. Life Sci. 37: 875–882. [DOI] [PubMed] [Google Scholar]
- 3.Roberts LA, Beyer C & Komisaruk BR. 1986. Nociceptive responses to altered GABAergic activity at the spinal cord. Life Sci. 39: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 4.Yaksh TL 1989. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain 37: 111–123. [DOI] [PubMed] [Google Scholar]
- 5.Clavier N, Lombard MC & Besson JM. 1992. Benzodiazepines and pain: effects of midazolam on the activities of nociceptive non-specific dorsal horn neurons in the rat spinal cord. Pain 48: 61–71. [DOI] [PubMed] [Google Scholar]
- 6.Sumida T et al. 1995. Intravenous midazolam suppresses noxiously evoked activity of spinal wide dynamic range neurons in cats. Anesth. Analg 80: 58–63. [DOI] [PubMed] [Google Scholar]
- 7.Daniele CA & MacDermott AB. 2009. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J. Neurosci 29: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin BJ et al. 1975. Immunocytochemical localization of glutamate decarboxylase in rat spinal cord. J. Comp. Neurol 164: 305–321. [DOI] [PubMed] [Google Scholar]
- 9.Oliva AA Jr. et al. 2000. Novel hippocampal interneuronal subtypes identified using transgenic mice that express green fluorescent protein in GABAergic interneurons. J. Neurosci 20: 3354–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todd AJ & Sullivan AC. 1990. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J. Comp. Neurol 296: 496–505. [DOI] [PubMed] [Google Scholar]
- 11.Todd AJ 1990. An electron microscope study of glycine-like immunoreactivity in laminae I-III of the spinal dorsal horn of the rat. Neuroscience 39: 387–394. [DOI] [PubMed] [Google Scholar]
- 12.Hossaini M, French PJ & Holstege JC. 2007. Distribution of glycinergic neuronal somata in the rat spinal cord. Brain Res. 1142: 61–69. [DOI] [PubMed] [Google Scholar]
- 13.Zeilhofer HU et al. 2005. Glycinergic neurons expressing enhanced green fluorescent protein in bacterial artificial chromosome transgenic mice. J. Comp. Neurol 482: 123–141. [DOI] [PubMed] [Google Scholar]
- 14.Inquimbert P, Rodeau JL & Schlichter R. 2007. Differential contribution of GABAergic and glycinergic components to inhibitory synaptic transmission in lamina II and laminae III-IV of the young rat spinal cord. Eur. J. Neurosci 26: 2940–2949. [DOI] [PubMed] [Google Scholar]
- 15.Takazawa T & MacDermott AB. 2010. Glycinergic and GABAergic tonic inhibition fine tune inhibitory control in regionally distinct subpopulations of dorsal horn neurons. J. Physiol 588: 2571–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allain AE et al. 2006. Expression of the glycinergic system during the course of embryonic development in the mouse spinal cord and its co-localization with GABA immunoreactivity. J. Comp. Neurol 496: 832–846. [DOI] [PubMed] [Google Scholar]
- 17.Todd AJ et al. 1996. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J. Neurosci 16: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ataka T & Gu JG. 2006. Relationship between tonic inhibitory currents and phasic inhibitory activity in the spinal cord lamina II region of adult mice. Mol. Pain 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell EA et al. 2007. GABAA and glycine receptor-mediated transmission in rat lamina II neurones: relevance to the analgesic actions of neuroactive steroids. J. Physiol 583: 1021–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi A, Mashimo T & Uchida I. 2006. GABAergic tonic inhibition of substantia gelatinosa neurons in mouse spinal cord. Neuroreport 17: 1331–1335. [DOI] [PubMed] [Google Scholar]
- 21.Miraucourt LS et al. 2009. Glycine inhibitory dysfunction induces a selectively dynamic, morphine-resistant, and neurokinin 1 receptor-independent mechanical allodynia. J. Neurosci 29: 2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malmberg AB et al. 1997. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science 278: 279–283. [DOI] [PubMed] [Google Scholar]
- 23.Neumann S et al. 2008. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J. Neurosci 28: 7936–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miraucourt LS, Dallel R & Voisin DL. 2007. Glycine inhibitory dysfunction turns touch into pain through PKCgamma interneurons. PLoS ONE 2: e1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torsney C & MacDermott AB. 2006. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J. Neurosci 26: 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank K & Fuortes MGF. 1957. Presynaptic and Postsynaptic Inhibition of Monosynaptic Reflexes. Fed. Proc 16: 39–40. [Google Scholar]
- 27.Eccles JC,Schmidt R&Willis WD. 1963. Pharmacological studies on presynaptic inhibition. J. Physiol 168: 500–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feltz P & Rasminsky M. 1974. A model for the mode of action of GABA on primary afferent terminals: depolarizing effects of GABA applied iontophoretically to neurones of mammalian dorsal root ganglia. Neuropharmacology 13: 553–563. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher JP, Higashi H & Nishi S. 1978. Characterization and ionic basis of GABA-induced depolarizations recorded in vitro from cat primary afferent neurones. J. Physiol 275: 263–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudomin P & Schmidt RF. 1999. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res 129: 1–37. [DOI] [PubMed] [Google Scholar]
- 31.Bernardi PS et al. 1995. Synaptic interactions between primary afferent terminals and GABA and nitric oxide-synthesizing neurons in superficial laminae of the rat spinal cord. J. Neurosci 15: 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todd AJ 1996. GABA and glycine in synaptic glomeruli of the rat spinal dorsal horn. Eur. J. Neurosci 8: 2492–2498. [DOI] [PubMed] [Google Scholar]
- 33.Watson AH, Hughes DI & Bazzaz AA. 2002. Synaptic relationships between hair follicle afferents and neurones expressing GABA and glycine-like immunoreactivity in the spinal cord of the rat. J. Comp. Neurol 452: 367–380. [DOI] [PubMed] [Google Scholar]
- 34.Hughes DI et al. 2012. Morphological, neurochemical and electrophysiological features of parvalbumin-expressing cells: a likely source of axo-axonic inputs in the mouse spinal dorsal horn. J. Physiol 590: 3927–3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Leefmans FJ et al. 1988. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J. Physiol 406: 225–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price TJ, Hargreaves KM & Cervero F. 2006. Protein expression and mRNA cellular distribution of the NKCC1 cotransporter in the dorsal root and trigeminal ganglia of the rat. Brain Res. 1112: 146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung KW et al. 2000. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J. Neurosci 20: 7531–7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham B & Redman S. 1994. A simulation of action potentials in synaptic boutons during presynaptic inhibition. J. Neurophysiol 71: 538–549. [DOI] [PubMed] [Google Scholar]
- 39.Willis WD Jr. 1999. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp. Brain Res 124: 395–421. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Lu SG & Gold MS. 2012. Persistent inflammation increases GABA-induced depolarization of rat cutaneous dorsal root ganglion neurons in vitro. Neuroscience 220: 330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price TJ, Cervero F & de Koninck Y. 2005. Role of cationchloride-cotransporters (CCC) in pain and hyperalgesia. Curr. Top Med. Chem 5: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardoni R et al. 2004. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J. Neurosci 24: 2774–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikeda H, Kiritoshi T & Murase K. 2008. Effect of excitatory and inhibitory agents and a glial inhibitor on optically-recorded primary-afferent excitation. Mol. Pain 4: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CJ et al. 2002. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron 35: 135–146. [DOI] [PubMed] [Google Scholar]
- 45.Russo RE, Delgado-Lezama R & Hounsgaard J. 2000. Dorsal root potential produced by a TTX-insensitive micro-circuitry in the turtle spinal cord. J. Physiol 528(Pt 1): 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shreckengost J et al. 2010. Bicuculline-sensitive primary afferent depolarization remains after greatly restricting synaptic transmission in the mammalian spinal cord. J. Neurosci 30: 5283–5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hiura A, Ishizuka H & Villalobos EL. 1991. Gabaergic neurons in the mouse superficial dorsal horn with special emphasis on their relation to primary afferent central terminals. Arch. Histol. Cytol 54: 195–206. [DOI] [PubMed] [Google Scholar]
- 48.Paul J, Zeilhofer HU & Fritschy JM. 2012. Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J. Comp. Neurol 520: 3895–3911. [DOI] [PubMed] [Google Scholar]
- 49.Witschi R et al. 2011. Presynaptic alpha2-GABAA receptors in primary afferent depolarization and spinal pain control. J. Neurosci 31: 8134–8142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knabl J et al. 2008. Reversal of pathological pain through specific spinal GABA(A) receptor subtypes. Nature 451: 330–336. [DOI] [PubMed] [Google Scholar]
- 51.Munro G et al. 2011. A question of balance—positive versus negative allosteric modulation of GABA(A) receptor subtypes as a driver of analgesic efficacy in rat models of inflammatory and neuropathic pain. Neuropharmacology 61: 121–132. [DOI] [PubMed] [Google Scholar]
- 52.De Groat WC, Lalley PM & Saum WR. 1972. Depolarization of dorsal root ganglia in the cat by GABA and related amino acids: antagonism by picrotoxin and bicuculline. Brain Res. 44: 273–277. [DOI] [PubMed] [Google Scholar]
- 53.Levy RA & Anderson EG. 1972. The effect of the GABA antagonists bicuculline and picrotoxin on primary afferent terminal excitability. Brain Res. 43: 171–180. [DOI] [PubMed] [Google Scholar]
- 54.Barker JL & Nicoll RA. 1973. The pharmacology and ionic dependency of amino acid responses in the frog spinal cord. J. Physiol 228: 259–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mitchell K, Spike RC & Todd AJ. 1993. An immunocytochemical study of glycine receptor and GABA in laminae I-III of rat spinal dorsal horn. J. Neurosci 13: 2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Towers S et al. 2000. GABAB receptor protein and mRNA distribution in rat spinal cord and dorsal root ganglia. Eur. J. Neurosci 12: 3201–3210. [DOI] [PubMed] [Google Scholar]
- 57.Yang K, Wang D & Li YQ. 2001. Distribution and depression of the GABA(B) receptor in the spinal dorsal horn of adult rat. Brain Res. Bull 55: 479–485. [DOI] [PubMed] [Google Scholar]
- 58.Dickenson AH, Brewer CM & Hayes NA. 1985. Effects of topical baclofen on C fibre-evoked neuronal activity in the rat dorsal horn. Neuroscience 14: 557–562. [DOI] [PubMed] [Google Scholar]
- 59.Hao JX et al. 1992. Baclofen reverses the hypersensitivity of dorsal horn wide dynamic range neurons to mechanical stimulation after transient spinal cord ischemia; implications for a tonic GABAergic inhibitory control of myelinated fiber input. J. Neurophysiol 68: 392–396. [DOI] [PubMed] [Google Scholar]
- 60.Malcangio M & Bowery NG. 1993. Gamma-aminobutyric acidB, but not gamma-aminobutyric acidA receptor activation, inhibits electrically evoked substance P-like immunoreactivity release from the rat spinal cord in vitro. J. Pharmacol. Exp. Ther 266: 1490–1496. [PubMed] [Google Scholar]
- 61.Allerton CA, Boden PR & Hill RG. 1989. Actions of the GABAB agonist, (−)-baclofen, on neurones in deep dorsal horn of the rat spinal cord in vitro. Br. J. Pharmacol 96: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kangrga I, Jiang MC & Randic M. 1991. Actions of (−)-baclofen on rat dorsal horn neurons. Brain Res. 562: 265–275. [DOI] [PubMed] [Google Scholar]
- 63.Chery N & De Koninck Y. 2000. GABA(B) receptors are the first target of released GABA at lamina I inhibitory synapses in the adult rat spinal cord. J. Neurophysiol 84: 1006–1011. [DOI] [PubMed] [Google Scholar]
- 64.Yang K & Ma H. 2011. Blockade of GABA(B) receptors facilitates evoked neurotransmitter release at spinal dorsal horn synapse. Neuroscience 193: 411–420. [DOI] [PubMed] [Google Scholar]
- 65.Wan YH & Hu SJ. 2003. Short-term depression at primary afferent synapses in rat substantia gelatinosa region. Neuroreport 14: 197–200. [DOI] [PubMed] [Google Scholar]
- 66.De Koninck Y & Henry JL. 1994. Prolonged GABAA-mediated inhibition following single hair afferent input to single spinal dorsal horn neurones in cats. J. Physiol 476: 89–100. [PMC free article] [PubMed] [Google Scholar]
- 67.Funk K et al. 2008. Modulation of chloride homeostasis by inflammatory mediators in dorsal root ganglion neurons. Mol. Pain 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin Q, Wu J & Willis WD. 1999. Dorsal root reflexes and cutaneous neurogenic inflammation after intradermal injection of capsaicin in rats. J. Neurophysiol 82: 2602–2611. [DOI] [PubMed] [Google Scholar]
- 69.Weng HR & Dougherty PM. 2005. Response properties of dorsal root reflexes in cutaneous C fibers before and after intradermal capsaicin injection in rats. Neuroscience 132: 823–831. [DOI] [PubMed] [Google Scholar]


