Summary
Aggregatibacter actinomycetemcomitans is a Gram-negative bacterium associated with localized aggressive periodontitis, as well as other systemic diseases. This organism produces a number of virulence factors, all of which provide some advantage to the bacterium. Several studies have demonstrated that clinical isolates from diseased patients, particularly those of African descent, frequently belong to specific clones of A. actinomycetemcomitans that produce significantly higher amounts of a protein exotoxin belonging to the repeats-in-toxin (RTX) family, leukotoxin (LtxA), while isolates from healthy patients harbor minimally leukotoxic strains. This finding suggests that LtxA might play a key role in A. actinomycetemcomitans pathogenicity. Because of this correlation, much work over the past 30 years has been focused on understanding the mechanisms by which LtxA interacts with and kills host cells. In this article, we review those findings, highlight the remaining open questions, and demonstrate how knowledge of these mechanisms, particularly the toxin’s interactions with lymphocyte function-associated antigen-1 (LFA-1) and cholesterol, enables the design of targeted anti-LtxA strategies to prevent/treat disease.
Keywords: Aggregatibacter actinomycetemcomitans, leukotoxin, repeats-in-toxin, LFA-1
1. Introduction
Periodontitis is a chronic, inflammatory disease characterized by loss of both connective tissue attachment and alveolar bone (Cochran, 2008; Darveau, 2010). Unlike chronic periodontitis, localized aggressive periodontitis (LAP) has been closely linked to specific strains of a single organism, Aggregatibacter actinomycetemcomitans (Zambon, 1985). While A. actinomycetemcomitans was initially considered to be the sole causative agent of LAP (Zambon, 1985; Zambon, Haraszthy, Hariharan, Lally, & Demuth, 1996; Zambon, Slots, & Genco, 1983), some recent work from the Fine lab has hypothesized that A. actinomycetemcomitans may instead promote the development of an alliance of specific microorganisms that cause symptoms of the disease (Fine, Markowitz, Fairlie, Tischio-Bereski, Ferrendiz et al., 2013; Fine, Patil, & Velusamy, 2019). Strains of A. actinomycetemcomitans that have been isolated from patients with periodontal disease tend to produce significant amounts of a protein exotoxin called leukotoxin (LtxA), while strains isolated from healthy patients more often produce much less LtxA (Haraszathy et al., 2000; Haubek et al., 2008; Zambon et al., 1983). In particular, the JP2 clone, which is often found in patients of African descent, differs from other clones, particularly in a 530-bp deletion in the promoter region of the LtxA gene operon, thus resulting in increased LtxA production (Haubek, Poulsen, Westergaard, Gahlen, & Kilian, 1996; Haubek, Poulsen, & Kilian, 2007). This toxin specifically kills human white blood cells (Lally, Golub, & Kieba, 1994; Lally, Hill, Kieba, & Korostoff, 1999), thus limiting the ability of the host to respond to an infection.
LtxA has been described as a “key” virulence factor due to its immunosuppressive activities and the close association with disease (Johansson, 2011), and numerous studies have focused on understanding the mechanisms of LtxA, with the goal of uncovering novel strategies to treat LAP. Traditionally, treatment of LAP has involved scaling and root planing. While this process can be effective in removing A. actinomycetemcomitans from diseased sites initially, the organism is often recovered from treated sites several months after initial treatment (Renvert, Wikström, Dahlén, Slots, & Egelberg, 1990; Sbordone, Ramaglia, Gulletta, & Iacono, 1990). Subsequent treatment with either an additional round of debridement or surgical excision of gingival tissue did not improve outcomes (Renvert, Wikström, Dahlén, Slots, & Egelberg, 1990). The recolonization of A. actinomycetemcomitans has been proposed to be due to the organism’s ability to reside in soft tissues (Blix, Hars, Preus, & Helgeland, 1992; Christersson, Slots, Rosling, & Genco, 1985; Sreenivasan, Meyer, & Fives‐Taylor, 1993). Therefore, systemic antibiotics, particularly macrolides, such as erythromycin, or tetracyclines, are often prescribed in addition to surgical treatment (Feres, Figueiredo, Soares, & Faveri, 2015; Prakasam, Elavarasu, & Natarajan, 2012). However, this treatment is not effective in all patients (Deas & Mealey, 2010; Mombelli, Gmur, Gobbi, & Lang, 1994) for reasons including antibiotic resistance (Saxen & Asikainen, 1993; Walker, 1996). Because of the importance of LtxA in A. actinomycetemcomitans virulence, inhibition of the activity of this toxin could represent a next-generation, anti-virulence approach to the treatment of LAP. While substantial amounts of work over the past forty years have contributed to our strong understanding of this protein toxin, a number of gaps in our understanding remain. Filling these gaps is essential for the development of effective anti-LtxA strategies. In this review, we have highlighted what is known about the mechanisms of LtxA delivery to and entry into host cells, with a focus on those data that will enable discovery of new therapeutic options targeting LtxA.
2. LtxA Expression/Secretion
LtxA is a member of the repeats-in-toxin (RTX) family of proteins (Ludwig & Goebel, 2006). This family shares a number of structural and functional features, including synthesis of four essential proteins from genes organized into a single operon (rtxCABD, in transcriptional order) (Ludwig & Goebel, 2006). These genes encode for the structural protein (rtxA), an acyltransferase (rtxC), and two proteins that are essential components of the secretion machinery (rtxB and rtxD). In A. actinomycetemcomitans, these genes are denoted ltxA, ltxC, ltxB, and ltxD.
The ltxA gene encodes for the LtxA structural protein, consisting of 1,055 amino acids with a molecular weight of 114,000 g/mol. The toxin has been divided into four functional domains, including an N-terminal hydrophobic domain (residues 1–420), a central domain (residues 421–730), a repeat domain (residues 731–900), and a C-terminal domain (residues 901–1055) (Fig 1a). The hydrophobic domain contains most of the hydrophobic amino acids, and has been proposed to play a role in membrane insertion (Lally, Golub, Kieba, Taichman, Rosenbloom et al., 1989), although recent results (Brown, Boesze-Battaglia, Balashova, Mas Gómez, Speicher et al., 2018) call that hypothesis into question, as described in Section 3.6. The repeat domain contains the calcium-binding, glycine-rich amino acid sequence (LXGGXGND) that is characteristic of the RTX toxins. In each of the RTX toxins, this motif is repeated anywhere from less than ten to more than forty times; in LtxA, there are fourteen repeats (Lally et al., 1989). The central and C-terminal domains of LtxA have less sequence similarity to other RTX toxins (Lally et al., 1989), and their specific functions are not entirely clear. However, the C-terminal domain has been proposed to play a role in secretion, as it does for the Escherichia coli α-hemolysin (HlyA) (Koronakis, Koronakis, & Hughes, 1989; Nicaud, Mackman, Gray, & Holland, 1986; Stanley, Koronakis, & Hughes, 1991).
Figure 1. Structure and Secretion of LtxA. (A) Primary structure of LtxA.

LtxA consists of 1,055 amino acids, and has been divided into four functional domains for study. The hydrophobic domain (residues 1–420) consists primarily of hydrophobic residues and contains the reported cholesterol binding site (CRAC336). The central domain (residues 421–730) contains two lysine residues, K562 and K687, that are post-translationally acylated. The repeat domain (residues 731–900) contains fourteen copies of the RTX-specific repeated amino acid motif. The C-terminal domain (residues 901–1055) has been hypothesized to play a role in secretion. Epitopes of the neutralizing anti-LtxA monoclonal antibodies are found in the central, repeat, and C-terminal domains. (B) Type 1 secretion machinery. In A. actinomycetemcomitans, the type 1 secretion machinery consists of the TdeA protein in the outer membrane and the LtxB protein in the inner membrane. The LtxD protein serves as a linker to connect LtxB and TdeA. (C) “Ratcheting” mechanism of secretion. A ratcheting mechanism has been proposed to aid in the secretion of RTX toxins. After translation, the low Ca2+ concentration of the bacterial cytosol permits the toxin to remain in a disordered state, allowing it to pass through the narrow channel of the T1SS. In the extracellular environment, where the Ca2+ concentration is higher, the toxin folds into its active conformation. Continual extracellular folding of the toxin helps to pull the remaining disordered domains through the secretion channel in a ratcheting-like mechanism.
LtxA is expressed as an inactive pro-toxin that requires post-translational acylation by an acyltransferase, encoded by the ltxC gene (Balashova, Shah, Patel, Megalla, & Kachlany, 2009; Lally et al., 1994). This post-translational acylation results in the addition of fatty acids with fourteen to eighteen carbons to two internal lysine residues (K562 and K687). Each of these lysines are preceded by glycine residues (Balashova et al., 2009) and located within the central domain of LtxA (Fong, Tang, Brown, Kieba, Speicher et al., 2011). The fatty acids are attached via amide linkages to the ε-amino groups of the lysines (Lim, Walker, Guo, Pellett, Shabanowitz et al., 2000). Saturated, unsaturated, and hydroxylated fatty acid residues were identified in LtxA produced by two highly leukotoxic strains of A. actinomycetemcomitans, JP2 and HK1651 (Fong et al., 2011). Importantly, a significant amount of heterogeneity was observed in the acylation patterns of LtxA (Fong et al., 2011), but the process of acylation of internal lysine residues by fourteen to eighteen carbon fatty acids is conserved among several RTX toxins, including E. coli HlyA (Lim et al., 2000; Ludwig, Garcia, Bauer, Jarchau, Benz et al., 1996) and Bordetella pertussis adenylate cyclase toxin (CyaA) (Hackett, Guo, Shabanowitz, Hunt, & Hewlett, 1994; Masin, Basler, Knapp, El-Azami-El-Idrissi, Maier et al., 2005). Acylation of the RTX toxins has been demonstrated to be required for host cell toxicity (Basar, Havlicek, Bezouskova, Hackett, & Sebo, 2001; Fong et al., 2011; Masin et al., 2005; Stanley, Packman, Koronakis, & Hughes, 1994), but the exact role of these hydrocarbon chains in host cell toxicity have not been rigorously tested, until recently when O’Brien and colleagues demonstrated, using a comprehensive mass spectrometry-based approach, that the acyl chains of CyaA affect toxin folding and stability (O’Brien, Cannella, Voegele, Raoux-Barbot, Davi et al., 2019). Future work may demonstrate a similar role in other RTX toxins, including LtxA.
An initial study into the function of the ltxB and ltxD genes demonstrated that they share significant sequence similarity with the corresponding genes of the E. coli HylA (hlyB and hlyD) and Pastuerella haemolytica leukotoxin (lktB and lktD) (Lally, Golub, Kieba, Taichman, Decker et al., 1991). Subsequent studies quantified the homology in the protein gene products of the A. actinomycetemcomitans and E. coli B and D genes: LtxB is 83% homologous to E. coli HlyB (Guthmiller, Kolodrubetz, Cagle, & Kraig, 1990) and LtxD is 68% homologous to E. coli HlyD (Guthmiller et al., 1990). HlyB resides in the inner membrane (Hardie, Issartel, Koronakis, Hughes, & Koronakis, 1991) and acts as an ATP binding cassette (ABC) transporter (Young & Holland, 1999). HlyD is a protein that connects the inner and outer membranes (Mackman, Nicaud, Gray, & Holland, 1986). For these reasons, LtxB and LtxD were proposed to form a part of the secretion machinery that enables release of LtxA into the extracellular environment. Mutations in either the ltxB or ltxD genes resulted in a decreased production of LtxA by A. actinomycetemcomitans (Guthmiller, Kolodrubetz, & Kraig, 1995), and recombinant expression of ltxA and ltxC, without ltxB and ltxD, in E. coli resulted in the cytosolic localization of LtxA (Lally et al., 1991), supporting the hypothesized role of these gene products in the secretion of active LtxA.
Additionally, the tdeA gene, located on a separate locus, encodes for a protein that is required for secretion of LtxA (Crosby & Kachlany, 2007). In E. coli, TolC is the outer membrane protein that is involved in HlyA secretion. The HlyD protein acts as an “adaptor” between HlyB and TolC to form a channel through which the toxin can pass (Balakrishnan, Hughes, & Koronakis, 2001). TdeA has been proposed to form the export channel of the secretion machinery in the outer membrane because of the many similarities between TdeA and TolC, including a strong β-sheet propensity and an N-terminal membrane insertion signal (Crosby & Kachlany, 2007). Deletion of tdeA inhibits LtxA secretion, demonstrating its importance in this process (Crosby & Kachlany, 2007). A subsequent genetic screen demonstrated that mutations in either ltxB or ltxD inhibited secretion of LtxA (Isaza, Duncan, Kaplan, & Kachlany, 2008). Together, these results support a model of secretion via the type 1 secretion system, T1SS, in which LtxB in the inner membrane is connected to TdeA in the outer membrane by the LtxD linkage, forming a complete channel across both the inner and outer membranes to enable ATP-dependent secretion of LtxA into the extracellular environment (Fig 1b and c).
Recently, a “ratcheting” mechanism was proposed for the secretion of the RTX toxin B. pertussis CyaA (O’Brien, Perez, Karst, Cannella, Enguéné et al., 2018). In the bacterial cytosol, the calcium concentrations are low and the repeat domain of the toxin exists in a disordered state (Chenal, Guijarro, Raynal, Delepierre, & Ladant, 2009). This unordered conformation allows the toxin to readily pass through the T1SS machinery. Once in the extracellular environment, where the Ca2+ concentration is greater, Ca2+ binds to the repeat domain (Chenal, Karst, Pérez, Wozniak, Baron et al., 2010), enabling this domain of the toxin to adopt a more ordered conformation consisting of parallel β-roll conformation (Chenal et al., 2010). The toxin has biological activity in this folded conformation (Hanski & Farfel, 1985). Similarities between LtxA, CyaA, and many of the RTX toxins suggest that this ratcheting mechanism may be conserved among the family (Fig 1c).
3. Host cell interactions
Upon release, the toxin presumably interacts with specific host cells. Numerous studies over the past several decades have revealed aspects of these mechanisms; however, even with this extensive catalogue of investigations, numerous open questions remain. Model system approaches and gain-of-function mutations have been critical for isolating specific mechanisms and have substantially advanced our understanding of LtxA activity. However, most of these reports focus on a single, specific interaction, making it difficult to place all of the identified interactions into the proper context of cell cytotoxicity. Also complicating the issue is the fact that different cells may be affected by LtxA in different ways. In this section, we compile these findings in an effort to assemble a complete picture of the LtxA-host interaction, and, maybe more importantly, highlight the gaps in the knowledge that remain.
3.1. Host cell types targeted by LtxA
For many years, LtxA has been reported to specifically kill immune cells from humans and Old World primates (Taichman, Simpson, Sakurada, Cranfield, DiRienzo et al., 1987). As a result, the proposed role of LtxA in A. actinomycetemcomitans pathogenesis has been in immunosuppression (Rabie, Lally, & Shenker, 1988). However, recent evidence has been uncovered suggesting that the target of LtxA could be broader than just immune cells. Here, we provide evidence of each of these proposed interactions and a discussion about their putative roles in A. actinomycetemcomitans virulence.
3.1.1. Immune cells
An initial study into the effect of A. actinomycetemcomitans on host cells reported that a cell extract of the Y4 strain of A. actinomycetemcomitans, isolated from a juvenile patient with periodontitis, was toxic to human polymorphonuclear leukocytes (PMNs) and peripheral blood monocytes, but not to human lymphocytes, platelets, or fibroblasts. Additionally, this extract was not toxic to rabbit, mouse, or rat leukocytes or chicken embryo fibroblasts. No hemolytic activity was observed in human erythrocytes. This activity was observed to be due to a protein component, which was then named “leukotoxin” (Taichman, Dean, & Sanderson, 1980). Subsequent work demonstrated that this extract and the toxin component were also active against human monocytes, human leukemic cell lines, and T- and B-cell lines (Simpson, Berthold, & Taichman, 1988; Zambon, DeLuca, Slots, & Genco, 1983). The leukotoxin was not active, at concentrations below 500 ng/mL, against neural cells, human gingival fibroblasts, and murine cells (Simpson et al., 1988). This specific targeting of host cells led to the hypothesis that LtxA acts in an immunosuppressive manner, to promote A. actinomycetemcomitans proliferation (Rabie et al., 1988). While this function has not yet been conclusively implicated in the etiology of LAP, significant additional evidence over the years has accumulated to indicate that LtxA specifically targets human immune cells (Balashova, Dhingra, Boesze-Battaglia, & Lally, 2016; DiFranco, Gupta, Galusha, Perez, Nguyen et al., 2012; Kieba, Fong, Tang, Hoffman, Speicher et al., 2007; Vega, Schober, Kim, Belinka, & Kachlany, 2019).
3.1.2. Erythrocytes
Some early reports suggested the presence of a hemolytic protein produced by certain strains of A. actinomycetemcomitans (Avila-Campos, 1995; Haubek, Dirienzo, Tinoco, Westergaard, López et al., 1997; Kimizuka, Miura, & Okuda, 1996). Balashova et al. reported that beta-hemolysis (complete erythrocyte lysis) by A. actinomycetemcomitans is dependent on the culture composition but independent of blood type. An LtxA mutant strain was not hemolytic, and purified LtxA exhibited dose-dependent lytic behavior against both sheep and human erythrocytes, suggesting that LtxA possesses some hemolytic activity (Balashova, Crosby, Al Ghofaily, & Kachlany, 2006). A subsequent study has also found evidence of hemolytic behavior in LtxA, though at much higher concentrations and longer incubation times than necessary to observe cytotoxic behavior against human immune cells (LFA-1) (Reinholdt, Poulsen, Brinkmann, Hoffmann, Stapulionis et al., 2013).
The ability of LtxA to lyse non-immune cells suggests the toxin possesses multiple mechanisms of cytotoxic activity depending on the target cell. However, there is considerable variation in the relative lysis and incubation time among the studies, which may be a result of differences in erythrocyte concentration or toxin purification protocols. Interestingly, the study by Reinholdt et al. reveals a difference in LtxA sensitivity between sample erythrocytes from different human donors (Reinholdt et al., 2013). Curiously, there is quite a difference between LtxA-induced lysis in human and sheep cells. A cursory examination reveals that humans and sheep erythrocytes contain similar membrane compositions (Virtanen, Cheng, & Somerharju, 1998). This suggests that the mechanism of cell lysis is more complex than a lipid interaction leading to pore formation (Skals, Bjaelde, Reinholdt, Poulsen, Vad et al., 2014), and could involve recognition by LtxA of a glycolipid or other receptor on the erythrocytes. HlyA from E. coli has been shown to target glycophorin, but blocking the carbohydrate rich protein did not inhibit LtxA-induced lysis (Forman, Nishikubo, Han, Le, Balashova et al., 2010). The same study did determine that incubating LtxA with gangliosides could prevent lysis of erythrocytes, but was only partially effective in preventing LtxA-mediated cytotoxicity against THP-1 monocytes at low concentrations and shorter incubation times, suggesting the toxin has a greater activity against immune cells than erythrocytes.
3.1.3. Endothelial cells
One of the key features of LAP is a breakdown of tissue at the site of infection (Shaddox, Gonçalves, Vovk, Allin, Huang et al., 2013). To determine if LtxA is involved in this process, Dietmann and colleagues investigated the effect of LtxA on human microvascular endothelial cells. They found that high doses of LtxA decreased cell proliferation by causing cell cycle arrest in the G2/M phase and induced apoptosis (Dietmann, Millonig, Combes, Couraud, Kachlany et al., 2013). The authors hypothesized that these effects could lead to destruction of the gingival tissue, inhibiting the barrier function of the tissue and therefore promoting invasion of A. actinomycetemcomitans (Dietmann et al., 2013).
3.1.4. Target cell discussion
Although evidence of broad cellular interactions by LtxA exists, it seems most likely that the primary target of LtxA is human immune cells. Reported effects in erythrocytes and fibroblasts require significantly higher toxin concentrations and much longer incubation times than the observed effects in human lymphocytes, thus calling into question the physiological relevance of these interactions. While it can be difficult to compare between different studies, one recent study investigated the effect of LtxA against K562 cells expressing β2 integrins and against human erythrocytes. (K562 cells are erythroleukemic cells that do not endogenously express LFA-1 (Lozzio & Lozzio, 1979).) The authors found the concentration of LtxA (5 μg/mL) that killed approximately 80% of the white blood cells after 1 hr only lysed approximately 10% of the erythrocytes after 2 hrs and 30–50% after 24 hrs (Reinholdt et al., 2013). The observed effects in endothelial cells required 1000 times more LtxA (5 mg/mL) and three times longer incubation times (72 hrs) (Dietmann et al., 2013).
3.2. Membrane interactions of LtxA
A number of bacterial toxins act as “pore-forming” toxins, where the protein toxin disrupts the membrane bilayer in such a way as to connect two volumes that had been previously been separated by the membrane (Gilbert, 2002). The “pore” is a nanometer-sized hole in the membrane structure, which is stabilized by the protein. Several mechanisms of pore formation have been proposed, including the barrel-stave pore, in which the protein oligomerizes to form a protein-lined channel, and the toroidal pore, in which the protein bends the membrane to form a protein- and lipid-lined channel (Serra & Martínez, 2011).
Initially, LtxA was thought to be a pore-forming toxin, killing cells by disrupting the gradients across the membrane (Lear, Furblur, Lally, & Tanaka, 1995; Taichman, Iwase, Lally, Shattil, Cunningham et al., 1991). However, the cell type specificity of the toxin, and the results of traditional pore-formation assays have been inconclusive. For example, a series of osmotic protection experiments demonstrated that large polysaccharides, such as maltose, inhibit LtxA-mediated 51Cr release from HL-60 cells, but this molecule only delayed (not prevented) propidium iodide influx (Iwase, Lally, Berthold, Korchak, & Taichman, 1990). Channel formation by LtxA in planar lipid bilayers was observed, but only when the membrane was broken and reformed in the presence of toxin, a state not likely to occur physiologically. Additionally, this “channel” formation required a thirty times higher concentration of LtxA than that needed to kill host cells (Lear et al., 1995). Similarly, in HL-60 cells, patch clamp experiments revealed evidence of channel formation, but only at very high concentrations (Karakelian, Lear, Lally, & Tanaka, 1998). Thus, little evidence exists to support the hypothesis of the formation of stable pores as the primary means of LtxA-mediated cytotoxicity.
Alternatively, the toxin seems to induce a membrane restructuring, which enables leakage of small molecules, but which does not constitute a permanent, well-defined pore. Brown and colleagues conducted a membrane leakage experiment and determined that LtxA has very little membrane-permeabilization activity. Dye leakage (membrane disruption) was only observed when a significant fraction of the lipids in the membrane was comprised of phosphatidylethanolamine (PE) (Brown, Boesze-Battaglia, Du, Stefano, Kieba et al., 2012). This lipid is unique from phosphatidylcholine (PC), which has a structure in which the polar headgroup and hydrophobic tails have relatively similar cross-sectional areas, giving the individual lipids a “cylindrical” shape, and promoting lamellar phase formation (Jouhet, 2013; van Meer & de Kroon, 2011). The headgroup of PE is smaller, giving the lipid a somewhat “conical” shape, which promotes formation of a curved, inverted hexagonal (HII) phase (van Meer & de Kroon, 2011). These results suggested that LtxA induces a phase change in the lipids, transitioning the membrane from a lamellar (bilayer) phase to a non-lamellar (hexagonal II) phase (Brown et al., 2012). This finding is consistent with observations of membrane restructuring by other RTX toxins, including B. pertussis CyaA, Vibrio cholerae cytolysin, and E. coli α-hemolysin (Bakás, Chanturiya, Herlax, & Zimmerberg, 2006; Martín, Requero, Masin, Konopasek, Goñi et al., 2004; Zitzer, Bittman, Verbicky, Erukulla, Bhakdi et al., 2001).
Although LtxA, and the RTX toxins more generally, are still occasionally referred to as “pore forming toxins,” evidence points instead to a more subtle interaction with the cells, particularly at the low, biologically relevant concentrations likely experienced by host cells. We propose that in its initial interaction with the host cell membrane, LtxA uses this lipid phase change to weaken the membrane and enable partitioning of the toxin into the membrane. Previously observed membrane disruption may be a side effect of LtxA embedding in the membrane, but does not appear to be the mechanism that leads to cell death, at biologically relevant concentrations. Rather, the toxin seems to interact with cells in a specific manner that is mediated by one or several cell surface receptors.
3.3. Receptors of LtxA
Although there are reports of interactions between LtxA and erythrocytes and endothelial cells, the toxin is able to interact with human immune cells at a much lower concentration and with much faster kinetics. This host cell type specificity of LtxA suggests that the toxin recognizes a specific component on human white blood cells that is not present on other cell types. Several studies identified the lymphocyte function-associated antigen-1 (LFA-1) integrin as the functional receptor for LtxA. However, a number of recent studies have identified additional receptors that might be involved in LtxA recognition of other cell types or that might play a supplementary role in the interaction of LtxA with human immune cells. Reported evidence of interactions between LtxA and these receptors is described in detail below.
3.3.1. Lymphocyte function-associated antigen-1 (LFA-1)
The LFA-1 integrin on the surface of leukocytes was the first protein receptor shown to be targeted by LtxA (Lally, Kieba, Sato, Green, Rosenbloom et al., 1997). This interaction is hypothesized to be the basis for a mechanism for A. actinomycetemcomitans to evade an immune response in the host, as LFA-1 expression is specific to leukocytes (Slots & Genco, 1984).
The function of leukocyte integrins, especially LFA-1, and their role in signaling and cell migration have been extensively reviewed elsewhere (Hogg, Patzak, & Willenbrock, 2011; Verma & Kelleher, 2017; Walling & Kim, 2018). Briefly, LFA-1 is a transmembrane glycoprotein that functions as an adhesion and signaling receptor on the surface of leukocytes, which upon activation, binds with the ligand intercellular adhesion molecule 1 (ICAM1) preceding transmigration of the cell into tissue. The integrin is composed of two non-covalently associated subunits: CD11a (αL) and CD18 (β2) (Fig 2a), making it one of the four members of the β2 integrin family. The CD11a subunit is a 129 kDa protein comprised of a 1,063 amino acid extracellular region, a 29 amino acid transmembrane (tm) region, and a 53 amino acid cytoplasmic tail. Within the extracellular region is a domain containing seven four-stranded β-sheet repeats (W1–W7), which folds into the β-propeller domain (Fig 2b). Inserted between β-sheets W2 and W3 is an approximately 200 amino acid domain (αI-domain) tethered to the surface of the β-propeller, which is responsible for the binding of ICAM1 (Hogg et al., 2011; Huang & Springer, 1997; Larson, Corbi, Berman, & Springer, 1989). The smaller CD18 subunit (85 kDa) possesses a βI-like domain in the N-terminal that associates with the αI-domain near the top of the β-propeller, which contains three metal-ion binding sites. The stalk region contains four repeat domains, each containing an eight-cysteine motif, which are exposed during integrin activation (Beglova, Blacklow, Takagi, & Springer, 2002). These domains, the integrin-epidermal growth factor (I-EGF) domains, are thought to regulate the activity of the N-terminal binding sites (Lu, Ferzly, Takagi, & Springer, 2001).
Figure 2. Structure of LFA-1. (A) LFA-1.
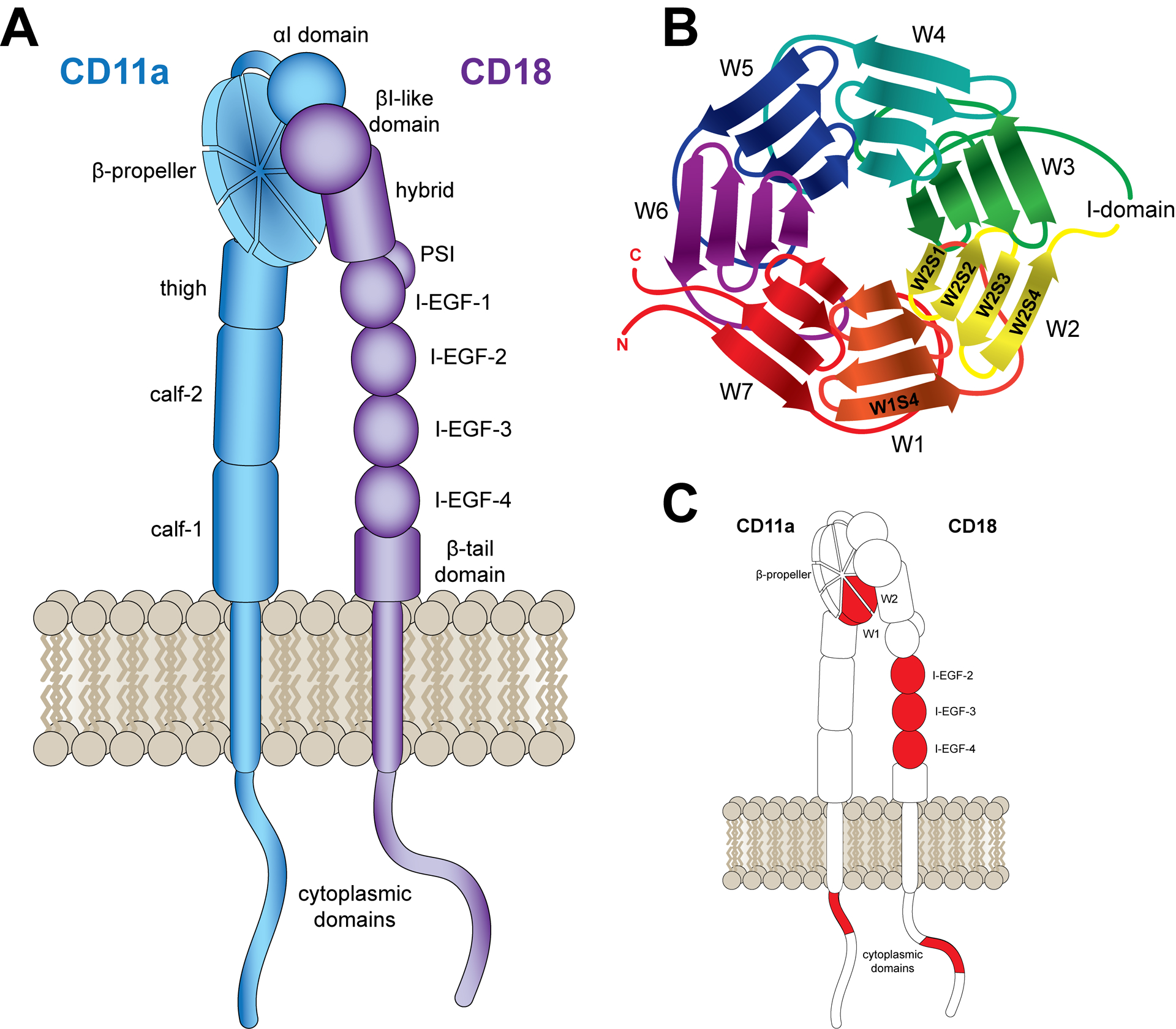
LFA-1 is a heterodimeric integrin composed of CD11a and CD18 subunits. Both subunits contain short cytoplasmic domains, transmembrane domains, and large extracellular domains. (B) CD11a β-propeller. The CD11a β-propeller consists of seven sheets (W1–W7), each comprised of four strands (S1–S4). (C) Reported LtxA Binding Sites. LtxA has been reported to target the CD11a β-propeller, the CD18 I-EGF domains, and the cytoplasmic domains of both CD11a and CD18. Each reported binding site is highlighted in red.
To identify LFA-1 as the receptor for LtxA, Lally and colleagues used an immunoaffinity purification procedure, employing a monoclonal antibody that inhibits LtxA-mediated cytotoxicity (Lally et al., 1997). The proteins from detergent-solubilized host cell membranes that bound to the immunoaffinity column were identified to be the CD11a and CD18 subunits of LFA-1. Antibodies against these two subunits were found to inhibit LtxA-mediated cytotoxicity, and cloning of the two subunits into a cell line that does not express LFA-1 and is not susceptible to LtxA resulted in toxin-susceptible cells (Lally et al., 1997). Kachlany and colleagues later correlated the sensitivity of many immune cell lines to LtxA with their expression levels of LFA-1 and found that these two factors are related. Additionally, they observed that cells expressing a constitutively active form of LFA-1 were ten times more sensitive to LtxA than those expressing wildtype LFA-1, indicating that LtxA binds to the active form of LFA-1 (Kachlany, Schwartz, Balashova, Hioe, Tuen et al., 2010). Subsequent work validated this finding by demonstrating that chemical activation of LFA-1 enhances susceptibility of THP-1 cells to LtxA (DiFranco et al., 2012).
3.3.1.1. Binding site of LtxA on LFA-1
Investigations into the potential sites on LFA-1 recognized by LtxA have identified several domains on both subunits. Each of the studies have provided essential information into the LtxA binding, but none has definitively demonstrated where LtxA binds. Taken together, they reveal a complex relationship that we hypothesize requires both subunits of LFA-1.
CD11a subdomain.
Investigations into the domains of CD11a required for LtxA cytotoxic activity point to a region of the β-propeller. A series of mutations on Jβ2.7 cells revealed that LtxA cytotoxic activity is dependent on β-sheets 1 and 2 in the β-propeller (Kieba et al., 2007). The Jβ2.7 cell line is a mutant of the Jn.9 Jurkat T cell clone (Cherry, Weber, & Klickstein, 2001) that does not translate CD11a (Weber, York, Springer, & Klickstein, 1997). Although CD18 mRNA is present in the cell, evidence suggests that both subunits are required for cell surface expression of CD18 (Weber et al., 1997), a finding that was recently verified using knockout Jurkat E6.1 mutants (Vega et al., 2019). Thus, the Jβ2.7 cells express neither CD11a nor CD18; as a result, the cells are insensitive to LtxA (Kieba et al., 2007). Transfection of Jβ2.7 cells with human CD11a cDNA (Jβ2.7/hCD11a) restored LFA-1 expression and LtxA vulnerability (Kieba et al., 2007). When hCD11a was replaced by bovine CD11a (Jβ2.7/bCD11a), the cells were susceptible to LtxA, but when the cells were transfected with murine CD11a (Jβ2.7/mCD11a) they were not susceptible to LtxA. These results suggest that a critical LtxA binding domain is located on the hCD11a subunit. To pinpoint the location of this subdomain, the researchers produced additional Jβ2.7 mutants, making murine substitutions in the human sequence. Cells expressing a CD11a mutant consisting of the murine β-sheets 1 and 2 (Fig 2c), were not susceptible to LtxA, demonstrating the presence of a binding site for LtxA in β-sheets 1 and 2 of hCD11a.
CD18 subdomain.
While the β-propeller of the CD11a subunit was shown to be required for LtxA activity, other results suggest that additional binding sites might exist. Bovine lymphocyte BL3 cells, which express bovine CD11a and CD18 (bCD11a and bCD18 respectively) are not susceptible to LtxA, but replacement of the bCD18 subunit with the human equivalent (hCD18), rendered the cells susceptible to LtxA, indicating that the CD18 subunit plays an important role in LtxA-mediated cytotoxicity as well as the CD11a subunit (Kieba et al., 2007).
Dileepan and colleagues made a series of mutant K562 cells to identify the specific domains in CD18 responsible for LtxA activity. K562 cells do not natively express LFA-1 and are therefore not susceptible to LtxA activity (Taichman et al., 1991). The researchers found that LtxA was able to kill K562 mutants expressing bCD11a with hCD18 but not those expressing hCD11a with bCD18 (Dileepan, Kachlany, Balashova, Patel, & Maheswaran, 2007), suggesting the presence of an LtxA binding site in the CD18 subunit of LFA-1 (Fig 2c). To pinpoint the specific regions of CD18 involved in the LtxA interaction, the authors made a series of mutations to the CD18 subunit, substituting bovine sequences in human CD18 and vice versa. Interestingly, they reported that three of the cysteine-rich integrin epidermal growth factor-like (I-EGF) domains (I-EGF-2–I-EGF-4) together play a critical role in the cell’s vulnerability to LtxA. This finding may explain why LtxA only targets LFA-1 in the active configuration (DiFranco et al., 2012), as the I-EGF domains would be inaccessible when LFA-1 is in the inactive (low affinity) state (Beglova et al., 2002).
Several additional investigations also point to the β2 subdomain as being the target for LtxA. LtxA showed significant toxicity toward P/5 cells (Dileepan et al., 2007), which is a K562 cell mutant that expresses the integrin p150/95, consisting of CD11c (αx) and CD18 subunits (Ortlepp, Stephens, Hogg, Figdor, & Robinson, 1995). Similarly, Reinholdt and colleagues used K562 mutants expressing LFA-1 and two other β2 integrins, CD11b/CD18 (Mac-1) and CD11c/CD18 (p150,95) to investigate the role of CD18 in the susceptibility of cells to LtxA. They report that the expression of any of the three integrins transferred remarkably comparable LtxA susceptibility to K562 cells (Reinholdt et al., 2013). Along the same line, Ristow and colleagues performed a series of mutations to investigate integrin targets of LtxA (Ristow, Tran, Schwartz, Pankratz, Mehle et al., 2019). In this study, the researchers made knockouts of U-937 cells, a human monocyte cell line that natively expresses β2 integrins. They found that knockout of the α-integrins CD11b, CD11c, or CD11d in these cells did not significantly alter LtxA-mediated cytotoxicity, relative to the wildtype cells. Interestingly, the CD11a knockout cells were marginally, but significantly, more resistant to LtxA, suggesting that LtxA has a slight preference for LFA-1 over the other β2 integrins.
Knockouts in lymphocytes without CD18 were found to not be susceptible to LtxA, as were knockouts without CD11a (Vega et al., 2019). The authors noted that deletion of each individual subunit prevented cell surface expression of the other. A similar requirement for both subunits in LFA-1 function was reported previously (Weber et al., 1997). Because of this, Vega et al. concluded that “[in] lymphocytes, both CD11a and CD18 are critical for LtxA to induce its toxic effects (Vega et al., 2019).”
Like LtxA, a number of other RTX toxins have been reported to bind to CD18, including E. coli α-HlyA, B. pertussis CyaA, Pasteurella haemolytica leukotoxin (Lkt), and Actinobacillus pleuropneumoniae ApxIII (Bergh, Zecchinon, Fett, & Desmecht, 2009; Guermonprez, Khelef, Blouin, Rieu, Ricciardi-Castagnoli et al., 2001; Li, J., Clinkenbeard, & Ritchey, 1999; Morova, Osicka, Masin, & Sebo, 2008; Ristow et al., 2019).
Cytoplasmic domains.
Nygren and colleagues hypothesized that LtxA might interact with the cytosolic domains of LFA-1 because of evidence that immediately after LtxA internalization, the toxin is located near the plasma membrane. The authors determined that LtxA has a strong binding affinity for regions of the cytosolic domains on both CD11a and CD18 (Nygren, Balashova, Brown, Kieba, Dhingra et al., 2018) (Fig 2c). Specifically, LtxA binds to peptides representative of an N-terminal region of the CD11a cytosolic domains and to a peptide representing an intermediate region of CD18 with affinity similar to that of LtxA–β-propeller peptide binding. However, additional evidence suggests that although LtxA seems to have a strong affinity for the cytoplasmic domains of LFA-1, these interactions may not play a significant role in LtxA-mediated cytotoxicity. Dileepan et al. demonstrated that K562 cells expressing bCD11a along with CD18 containing a bovine extracellular domain and human transmembrane and cytosolic domains were not susceptible to LtxA (Dileepan et al., 2007). Additionally, Jβ2.7 hybrid mutants expressing human β-propeller substitution on murine CD11a were found to be vulnerable to LtxA, while a murine β-propeller substitution on hCD11a was not (Kieba et al., 2007). Most recently, deletions in the cytoplasmic tail of CD18 did not affect LtxA activity (Ristow et al., 2019). Thus, despite the strong affinity of LtxA for the cytosolic domains of LFA-1, this mutation data suggests that these regions are not as important as the extracellular domains in conveying susceptibility to LtxA activity.
3.3.1.2. LFA-1 binding site discussion
The results discussed here have revealed the importance of both LFA-1 subunits for LtxA activity, suggesting that there may be critical binding sites on both. Efforts to elucidate the location of the binding sites have been hampered by the observation that both subunits be present for either to be expressed on the surface of the cell (Vega et al., 2019). Consequently, any further study may require an extensive catalog of point mutations while being mindful of the impact the substitutions may have on the structural conformation of the proteins. Further complicating the investigations is that the specific domain of LtxA that interacts with LFA-1 is not known. Earlier work identified three epitopes that were critical to LtxA activity: one in the central region, one in the repeat region and one in the C-terminal region (Lally et al., 1994). It is likely that one, some, or all of these epitopes are involved in the recognition and binding of LtxA to LFA-1, but this has not yet been demonstrated experimentally.
3.3.2. P2 receptors
Reports of LtxA-mediated hemolysis prompted studies to identify a receptor for LtxA on erythrocytes. Munkgaard and colleagues hypothesized that like the E. coli α-HlyA, LtxA interacts with P2X receptors in an ATP-dependent manner to mediate lysis. The authors observed that certain pharmacological P2X antagonists, including pyridoxalphosphate‐6‐azolphenyl‐2′,4′‐disulfonic acid (PPADS), inhibited LtxA-mediated hemolysis (Munksgaard, Vorup‐Jensen, Reinholdt, Söderström, Poulsen et al., 2012). A subsequent study identified a similar effect in human monocytes (THP-1 cells) (Fagerberg, Jakobsen, Skals, & Praetorius, 2016). While these reports suggest a role for the P2X receptors in LtxA-mediated lysis of erythrocytes and monocytes, it is important to note that P2X inhibitors have been demonstrated to interfere directly with oligomerization of the Staphylococcus aureus α-toxin (Schwiering, Husmann, & Hellmann, 2017) another toxin that had been reported to require P2X receptor activation (Skals, Leipziger, & Praetorius, 2011). Preliminary evidence from our lab has likewise indicated that PPADS interacts directly with LtxA, significantly altering the toxin’s secondary structure and affinity for P2X-free membranes. We propose that additional experimental controls must be conducted before definitive connections can be made between P2X receptors and LtxA.
3.3.3. Fas receptor
DiFranco and colleagues recently demonstrated that LtxA kills lymphoma cells in a caspase-8-dependent manner. The authors then screened a number of receptors and proteins that can activate caspase-8, and identified the death receptor, Fas, as an essential component in the LtxA-mediated killing of these cells (DiFranco, Johnson-Farley, Bertino, Elson, Vega et al., 2015). The researchers also observed decreased antibody binding when cells were pretreated with the toxin, suggesting that LtxA binds directly with the Fas receptor. In a recent investigation by Vega et al., the researchers further identified the importance of the Fas receptor for LtxA cytotoxicity in lymphocytes (Vega et al., 2019). They found that Fas−/− mutant Jurkat E6.1 cells were not significantly susceptible to low concentrations of LtxA, even over durations up to 72 hrs. In particular, deletion of Fas appeared to inhibit LtxA-mediated caspase activation and disruption of the mitochondrial membrane. The authors verified that although Fas and LFA-1 colocalize on the membrane, deletion of Fas did not affect expression or cell surface localization of LFA-1 (Vega et al., 2019). This report demonstrates that both Fas and LFA-1 are involved in LtxA-mediated cytotoxicity in lymphocytes; while not yet studied, Fas may play an important role in LtxA-mediated cytotoxicity against other immune cell types as well.
3.3.4. Receptor discussion
Although several functional receptors for LtxA have been identified, the strongest and most complete evidence indicates that LFA-1 is essential to the interaction of LtxA with host immune cells. The Fas receptor appears to play a supporting role in this interaction, although the exact details of this interaction remain to be determined. We expect that future work will further elucidate these interactions, leading to a clearer picture of this initial interaction of LtxA with host cells.
3.4. Interaction of LtxA with cholesterol
One of the critical interactions between LtxA and host cells is the binding to cholesterol, a common characteristic of toxins targeting animal cells (Palmer, 2004), and specifically, the RTX toxins (Bumba, Masin, Fiser, & Sebo, 2010; Osickova, Balashova, Masin, Sulc, Roderova et al., 2018; Vazquez, Maté, Bakás, Fernández, Malchiodi et al., 2014). Removal of cholesterol from the host cell membrane with methyl-β-cyclodextrin significantly inhibited the ability of LtxA to kill the cell (Brown, Koufos, Balashova, Boesze-Battaglia, & Lally, 2016; Fong, Pacheco, Otis, Baranwal, Kieba et al., 2006).
Using SPR, Brown and colleagues determined that LtxA’s affinity for cholesterol-containing membranes was approximately four orders of magnitude greater than for cholesterol-free membranes. The binding of LtxA to cholesterol was then determined to occur through a cholesterol recognition/amino acid consensus (CRAC) site in the N-terminal domain of LtxA (Brown, Balashova, Epand, Epand, Bragin et al., 2013). Conserved among several RTX proteins (Brown et al., 2013), the CRAC binding motif, in general, is a consensus pattern consisting of: -(L/V)-(X)1–5-Y-(X)1–5-(R/K)-, where X1–5 is a sequence containing up to five amino acids (Li, H. & Papadopoulos, 1998). Although this sequence motif is found in a wide variety of proteins, the presence of the sequence does not automatically imply that the protein binds cholesterol (Li, H. & Papadopoulos, 1998). Analysis of the sequence of LtxA reveals that it actually contains two CRAC motifs: CRAC336 and CRAC503 (Brown et al., 2013). To determine which of the two putative binding sites is/are responsible for cholesterol recognition by the toxin, the authors produced two full LtxA mutants where the central tyrosine (Y) of each CRAC domain was mutated to a proline (P). Of the two mutations, only the protein resulting from the Y336P substitution failed to kill Jn.9 cells, indicating that the CRAC336 domain mediates LtxA’s interaction with cholesterol.
Our lab has recently used a peptide based on the CRAC336 sequence to characterize the interaction of these amino acids with cholesterol. The affinity of this peptide for a series of model membranes containing cholesterol, desmosterol, dihydrocholesterol, or cholesteryl chloride was measured using SPR (Koufos, Chang, Rasti, Krueger, & Brown, 2016). Each of these sterols differs from cholesterol in the structure of their acyl chain, ring structure, or headgroup, respectively. Substitution of the hydroxyl group on cholesterol significantly decreased the affinity of the CRAC336WT peptide, suggesting that the CRAC motif of LtxA may recognize the hydroxyl headgroup of cholesterol. Additionally, the peptide did not significantly disrupt membrane packing, indicating that the peptide does not penetrate into the hydrophobic region of the bilayer. Together, these investigations support a model in which the interaction of LtxA with the lipids of a cell membrane occurs through binding of the CRAC336 motif and the hydroxyl headgroup of cholesterol.
In addition to acting as a secondary receptor for the toxin, cholesterol appears to promote LFA-1 clustering on the host cell membrane after LtxA binding (Fong et al., 2006). We have hypothesized that LtxA binds to both cholesterol and LFA-1 simultaneously, using distinct binding sites, where the cholesterol binding site (CRAC336) is located in the N-terminal, hydrophobic domain (as described above), and the LFA-1 binding site is located in the central/C-terminal part of the toxin, consistent with the epitopes of the neutralizing antibodies (Lally et al., 1994). It is the combined physical interactions of the toxin, cholesterol, and LFA-1 that then drive the reported clustering of the toxin on the host cell membrane into cholesterol- and LFA-1-rich lipid rafts.
However, the relationship between each of these interactions and their place in the broader LtxA cytotoxic pathway remains largely unknown. A model proposed for the CyaA toxin from B. pertussis theorizes that the toxin binds to the CD11b/CD18 integrin receptor, then interacts with the membrane initially forming a conducting pathway for the influx of Ca2+ ions into the cell (Bumba et al., 2010). A similar interaction could feasibly occur with LtxA, initially binding to LFA-1, which then guides it to the membrane. The β-propeller region of CD11a is proximal to the I-domain, where the binding of ICAM1 takes place. This could conceivably be where the initial LtxA interaction occurs, then transitioning to the I-EGF domains on CD18 to position the toxin near the membrane. Local regions of lipids immediately surrounding membrane proteins are enriched in non-bilayer lipids, especially phosphatidylethanolamine (PE), acting as intermediates between the protein and bilayer (de Kruijff, 1997). The LtxA-lipid interaction may then transition the membrane to the HII phase leading to leakage allowing Ca2+ into the cell, which is consistent with the finding of an increased cytosolic Ca2+ concentration after LtxA addition to K562 mutants expressing LFA-1, but not K562 wildtype (Dileepan et al., 2007). The binding to cholesterol may then facilitate the movement of LtxA into lipid rafts where it is endocytosed.
3.5. Structural changes in LtxA
LtxA, like other secreted protein toxins, has an interesting property of being both water soluble and membrane active (Lally et al., 1999), which implies that the protein undergoes a conformational change upon membrane interaction to expose previously sequestered hydrophobic domains. Circular dichroism (CD) spectral analysis revealed only subtle changes in the secondary structure of LtxA after interactions with LFA-1-free membranes (liposomes). In solution, the toxin’s secondary structure consists of approximately 12% α-helix and 31% unordered structures while the remaining 57% signify β-sheet and turn structures (Walters, Brown, Edrington, Baranwal, Du et al., 2013). A small but significant increase in the presence of α-helices coupled with a decrease in the fraction of unordered structures was observed after LtxA was incubated with n-dodecylphosphocholine (DPC) membranes (Walters et al., 2013). However, a significant decrease in the α-helix conformations were observed when LtxA was incubated with Jn.9 cell lipid extracts, which the authors attributed to the mixed lipid composition of the natural membrane. While these reported changes are statistically significant, they are quite small, suggesting that the secondary structure of LtxA does not experience large changes upon membrane interaction, in the absence of LFA-1.
Rather, large-scale changes in the tertiary structure of the protein in a model membrane environment appear to regulate the dual solubility of the toxin. To investigate more global conformational changes, Brown and colleagues employed tryptophan quenching, trypsin digest and mass spectrometry, and immunoblotting to identify the domains of the toxin exposed to solution when the toxin is in an aqueous environment, compared to when it is embedded in a membrane (Brown et al., 2018). Tryptophan quenching is a commonly used biophysical technique to identify regions of a protein that are accessible to an aqueous quencher, often potassium iodide, thus indicating which regions of the protein are exposed to solution. LtxA contains five tryptophan residues, one in the hydrophobic domain, three in the central domain and one in the C-terminal domain (Fig 1a). In buffer, the authors observed an extent of quenching equivalent to three of the five tryptophan residues being exposed to solution. Upon interaction with model membranes, regardless of lipid composition, only two of the toxin’s tryptophan residues were exposed to the aqueous environment. To more specifically identify which domains were exposed, the researchers incubated LtxA with model membranes, and used trypsin to digest the solution-exposed domains of LtxA; mass spectrometry was then used to identify the peptides derived from the digested domains of the toxin. They concluded that the toxin forms a U-shaped conformation where the N-terminal hydrophobic region and the C-terminal region reside on the outside of the membrane, and the central and repeat regions are inside. An immunoblot, using a panel of monoclonal antibodies with epitopes located in varying domains of the toxin, confirmed this result (Brown et al., 2018). Interestingly, the N-terminal hydrophobic domain, so named because it contains a majority of the hydrophobic residues of LtxA, was not located within the membrane. This domain has been hypothesized to play a role in membrane insertion due to the hydrophobicity (Lally et al., 1999), however, these results indicate that this region of the protein actually resides outside of the membrane, likely adopting a conformation in which the hydrophobic residues are sequestered.
It is important to note that, for technical reasons, these studies were conducted in model membrane (liposomal) systems, which lack LFA-1. It is quite possible that the interaction of LtxA with LFA-1 alters these membrane-mediated changes.
3.6. Internalization of LtxA
The interaction of LtxA with LFA-1 and cholesterol appears to initiate an intracellular cascade of events that ultimately leads to apoptosis (Fig 3). While the entire mechanism is not yet known, it is important to note that evidence points to differing pathways in monocytes versus lymphocytes; however, the requirement of LFA-1 is consistent between both cell types (DiFranco et al., 2012; DiFranco et al., 2015).
Figure 3. Proposed Mechanisms of LtxA Secretion and Internalization by Host Cells.

LtxA is released from the A. actinomycetemcomitans cell via the T1SS machinery. This “free” toxin associates with LFA-1 and cholesterol on human immune cell membranes. The LtxA-mediated increase in intracellular calcium concentration activates calpain, which cleaves LFA-1 from talin, enabling clustering of LtxA, cholesterol, and LFA-1 into lipid rafts. Subsequently, the toxin complex is endocytosed, and LtxA is released into the cytosol by disrupting the lysosomal membrane. Alternatively, some of the secreted LtxA re-associates with the bacterial cell membrane, and as a result is released in association with the surface of OMVs. These OMVs are recognized and internalized by host cells in a currently undefined pathway.
3.6.1. Intracellular interactions
Upon interaction with host cells, LtxA initiates an increase in the cytosolic Ca2+ concentration. Fong and colleagues identified an elevated cytosolic concentration of Ca2+ in Jn.9 cells within 60 sec of LtxA addition, and a similar increase was observed in Jβ2.7/hCD11a cells. Remarkably, Jβ2.7 cells, which are not killed by LtxA, also exhibited an increase in Ca2+ concentration, suggesting that this signaling is not initiated by an interaction with LFA-1 (Fong et al., 2006). Additional studies have demonstrated the increase of cytosolic Ca2+ in K562 mutants expressing integrins containing human CD18, but increases were not detected in control K562 cells (Dileepan et al., 2007). In the same study, cells that did not exhibit an increase in Ca2+ concentration were also not susceptible to LtxA activity. The seemingly contradictory results with Fong and colleagues correlating a rise in cytosolic Ca2+ with LtxA susceptibility may be simply an artifact of the cell lines used in the studies. However, the data suggests a complex interaction leading to increases in intracellular Ca2+ concentration that currently remains unknown.
The increase in cytosolic Ca2+ concentration sets up downstream consequences, leading to the cleavage of talin, releasing LFA-1 from the cytoskeleton anchor. Talin is a protein that connects the cytoplasmic domain of β-subunits of integrins to actin. The linking of the integrin to F-actin through talin-binding triggers a conformational change in the integrin into a high-affinity state as part of the inside-out signaling pathway (Campbell & Ginsberg, 2004) which was initiated by an upstream event such as chemokine receptor or T cell receptor (TCR) stimulation (Hogg et al., 2011). The LtxA-mediated increase in cytosolic Ca2+ concentration pathway activates calpain, an enzyme that cleaves talin. Thus, calpain-mediated cleavage of talin releases LFA-1 from the cytoskeleton, enabling it to move laterally in the membrane and cluster in cholesterol-rich lipid raft-like regions (Fig 3). The importance of this interaction in LtxA-mediated cytotoxicity was demonstrated using calpeptin, a calpain inhibitor; calpeptin inhibited LtxA activity in a dose-dependent manner (Fong et al., 2006). This suggests that the clustering of LFA-1 plays an important role in LtxA activity. Since lipid rafts are rich in cholesterol (Simons & Ehehalt, 2002) these results demonstrate that the inhibition of LtxA-mediated cytotoxicity due to depletion of cholesterol in the cell membrane (Brown et al., 2016; Fong et al., 2006) may be result in two consequences: a reduction in the possible binding sites of the toxin and an inhibition in the clustering of LFA-1.
3.6.2. Mechanism of internalization
While the details of LtxA internalization are unknown, there is evidence that the toxin localizes to lysosomes in monocytes, suggesting that, in these cells, the toxin is internalized by endocytosis (Fig 3). However, the transport pathway trafficking LtxA to the lysosome and its binding status with LFA-1 within the lysosome remains unknown. There is no evidence that LFA-1 is sorted to the lysosome, nor is this integrin known to mediate the transport of ligands to the lysosome. This suggests that, in the lysosome, either LtxA is no longer bound to LFA-1 or the toxin-integrin complex is transported together by a previously uncharacterized pathway. Two investigations both report a decrease in the cytosolic pH in toxin-treated cells, suggesting that LtxA disrupts the lysosomal membrane (Balashova et al., 2016; DiFranco et al., 2012). As previously discussed, LtxA is known to disrupt lipid membranes, so if LtxA were to dissociate from LFA-1 after being internalized, it is feasible that it could then mediate damage to the lysosomal membrane, leading to release of lysosomal components into the cytosol. As a result, cell death is caused by either the release of toxic components into the cytosol or the activation of caspases by the cathepsin-mediated pathway (Balashova et al., 2016). These intracellular interactions remain an important area of future study.
4. Association and trafficking of LtxA via outer membrane vesicles
When LtxA was first discovered, there was some controversy over whether the protein was secreted into the supernatant or associated with the bacterial cell membrane. Initial reports suggested that the toxin was cell surface associated (Berthold, Forti, Kieba, Rosenbloom, Taichman et al., 1992; Lally et al., 1991; Ohta, Kato, Kokeguchi, Hara, Fukui et al., 1991). For example, an initial LtxA purification protocol used the outer membrane-disrupting antibiotic, polymyxin B (Tsai, Shenker, DiRienzo, Malamud, & Taichman, 1984). However, subsequent discovery of the ltxB and ltxD genes, which encoded for proteins that appeared to be quite similar to HlyB and HlyD, known to play a role in the secretion of the E. coli HlyA (Guthmiller et al., 1990; Guthmiller et al., 1995), suggested that LtxA was indeed a secreted protein rather than a cell surface-associated protein. Later work found that LtxA can associate with the bacterial cell surface in a manner that is mediated by nucleic acids (Ohta et al., 1991) and is dependent on culture time, pH and composition, as well as strain (Johansson, Claesson, Hänström, & Kalfas, 2003; Kachlany, Fine, & Figurski, 2000). Treatment with DNase and RNase effectively removed most of the LtxA from the cell surface, indicating that the toxin is located on the surface of the cell, as neither enzyme is able to penetrate the bacterial membrane (Ohta, Hara, Fukui, Kurihara, Murayama et al., 1993; Ohta et al., 1991). This finding resulted in a new model in which LtxA is secreted via the T1SS across the inner and outer membranes; after secretion, some of the toxin re-associates with the outer surface of the outer membrane through interactions with cell surface components, including DNA (Fig 3).
This localization on the bacterial cell surface allows LtxA to be secreted via outer membrane vesicles (OMVs), spherical blebs derived from the Gram-negative bacterial outer membrane (OM), with diameters ranging from less than 100 nm to more than 400 nm (Bonnington & Kuehn, 2014; Kuehn & Kesty, 2005; Mayrand & Grenier, 1989). These vesicles have been implicated in the delivery to host cells of bacterial toxins including those produced by enterotoxigenic Escherichia coli (ETEC) (Horstman & Kuehn, 2000; Kesty & Kuehn, 2004; Wai, Takade, & Amako, 1995), Helicobacter pylori (Fiocca, Necchi, Sommi, Ricci, Telford et al., 1999; Parker, Chitcholtan, Hampton, & Keenan, 2010), and Vibrio cholerae (Chatterjee & Chaudhuri, 2013; Rasti & Brown, 2019; Rasti, Schappert, & Brown, 2018; Zavan, Bitto, Johnston, Greening, & Kaparakis‐Liaskos, 2019). A number of RTX toxins, including the B. pertussis CyaA (Donato, Goldsmith, Paddock, Eby, Gray et al., 2012; Hozbor, Rodriguez, Fernandez, Lagares, Guiso et al., 1999), E. coli HlyA (Aldick, Bielaszewska, Uhlin, Humpf, Wai et al., 2009; Balsalobre, Silvan, Berglund, Mizunoe, Uhlin et al., 2006), and Kingella kingae toxin (RtxA) (Maldonado, Wei, Kachlany, Kazi, & Balashova, 2011) have been shown to be biologically active after release in an OMV-associated form.
Toxin-containing OMVs produced by A. actinomycetemcomitans were first identified in the 1980’s. Both strains Y4 and N27 were observed to form vesicles that were more toxic to host cells than purified lipopolysaccharide (LPS) (Holt, Tanner, & Socransky, 1980; Nowotny, Behling, Hammond, Lai, Listgarten et al., 1982). While the specific presence of LtxA was not observed, the authors reported that protease treatment inhibited the toxicity of the OMVs, demonstrating that the OMVs contained some type of toxic protein (Nowotny et al., 1982). Another report demonstrated that the most leukotoxic strains of A. actinomycetemcomitans produced more vesicles than those strains that express little LtxA (Lai, Listgarten, & Hammond, 1981). A later immunocytochemical analysis demonstrated that LtxA was located on the OMV surface (Berthold et al., 1992). This surface localization of LtxA on the OMV was validated by subsequent reports that trypsin, which is unable to access the vesicle lumen, completely digests OMV-associated LtxA (Nice, Balashova, Kachlany, Koufos, Krueger et al., 2018), and the demonstration that treatment of the OMVs with DNase, which likewise can only access surface-associated LtxA, is able to release LtxA from the OMV surface (Ohta et al., 1991). Release of the RTX toxins via association with OMVs seems to be highly conserved among this family of toxins, as this mechanism of toxin release has been reported for B. pertussis CyaA, K. kingae RtxA, V. cholerae cytolysin, and E. coli α-hemolysin (Balsalobre et al., 2006; Donato et al., 2012; Elluri, Enow, Vdovikova, Rompikuntal, Dongre et al., 2014; Maldonado et al., 2011).
Kato et al. demonstrated that A. actinomycetemcomitans OMVs released by strain JP2, a highly leukotoxic strain, are enriched in LtxA relative to the outer membrane. While the lipid and protein compositions of the OMVs were similar to those of the outer membrane, the OMVs contained some proteins and lipids that were not detected in the OM (Kato, Kowashi, & Demuth, 2002). The OMVs were much more toxic to host cells than OMVs produced by strain 652, a minimally leukotoxic strain; additionally, JP2 OMVs were more toxic than a corresponding mass of purified OM (Brogan, Lally, Poulsen, Kilian, & Demuth, 1994), indicating that JP2 OMVs contain more LtxA than does the OM (Kato et al., 2002). This paper importantly demonstrates that not only is LtxA released in a biologically active form in association with OMVs, but that it is actively sorted to the vesicles from the OM. While the exact process enabling this sorting has not been identified, it is likely due to variations in the affinity of LtxA for certain membrane components. Both Porphyromonas gingivalis and Pseudomonas aeruginosa OMVs have been reported to contain more negatively charged LPS than the bacterial cell, as well as differential protein compositions (Haurat, Aduse-Opoku, Rangarajan, Dorobantu, Gray et al., 2011; Kadurugamuwa & Beveridge, 1995). It is quite possible that at least one of the lipids detected in JP2 OMVs but not OM (Kato et al., 2002) is an LPS variant that is found only in the OMVs, to which LtxA has a preferential affinity.
Although A. actinomycetemcomitans OMVs are enriched in LtxA, a subsequent report indicated that LtxA is not required for OMV association with host cells (Demuth, James, Kowashi, & Kato, 2003). JP2 and 652 OMVs, as well as OMVs produced by an isogenic, leukotoxin-deficient strain of JP2, associated with the cytoplasmic membrane of HL-60 cells to a similar extent within two min (Demuth et al., 2003). We have recently reported that JP2 OMVs are delivered to host (THP-1) cells in a cholesterol- and LFA-1-independent manner (Nice et al., 2018), unlike free LtxA, which requires both cholesterol (Brown et al., 2013; Fong et al., 2006) and LFA-1 (DiFranco et al., 2012; Dileepan et al., 2007; Kieba et al., 2007). This finding is important as it suggests that OMV packaging may permit or even promote delivery of LtxA to cells that do not express LFA-1, i.e., non-immune cells. In fact, OMVs produced by strain D7SS (serotype a), have been observed in perinuclear locations within both HeLa and human gingival fibroblast (HGF) cells (Rompikuntal, Thay, Khan, Alanko, Penttinen et al., 2012; Thay, Damm, Kufer, Wai, & Oscarsson, 2014). Although the authors did not specifically investigate LtxA localization, these reports suggest that the toxin’s association with OMVs, which are internalized in an LFA-1-independent manner, could enable delivery to cells lacking this receptor. However, this hypothesis remains currently untested.
The mechanism by which A. actinomycetemcomitans OMVs are internalized has not been fully elucidated; however, inhibition of endosome acidification by monensin prevented OMV internalization, suggesting that the vesicles are endocytosed (Thay et al., 2014). In much of the OMV literature, consensus is often lacking regarding mechanisms of internalization. Pharmacological inhibitors, which work well to inhibit free toxin internalization often have only small effects on OMV internalization. In some cases, multiple inhibitors have been shown to each slightly inhibit OMV internalization. This phenomenon has recently been suggested to be due to OMV heterogeneity. Specifically, the mechanism of H. pylori OMV uptake was found to depend on both OMV size and composition, both of which varied throughout growth of the bacteria (Turner, Bitto, Steer, Lo, D’Costa et al., 2018; Zavan et al., 2019). Thus, consensus within the OMV field is building toward a model in which bacteria secrete different types of OMVs, all of which are internalized by different mechanisms.
OMV purification is generally accomplished by collecting the bacterial supernatant at the late exponential phase. The OMVs are then purified from the supernatant using ultracentrifugation. The resulting OMV sample contains OMVs produced throughout growth of the bacteria, which likely differ both physically (size, charge, etc.) and chemically (protein, lipid, nucleic acid composition). Often, the OMVs are further separated using density gradient ultracentrifugation or size exclusion chromatography (SEC). These techniques are useful for separating OMVs based on size; still, compositional and functional differences in the OMVs likely exist that will require development of new methods to collect, separate, and analyze OMVs, to enable a more complete understanding of the role of these vesicles in A. actinomycetemcomitans pathogenesis.
Both compositional and size differences in A. actinomycetemcomitans OMVs have been reported. Rompikuntal and colleagues collected OMVs produced by A. actinomycetemcomitans strain D7SS, separated them by density gradient ultracentrifugation, and probed for the presence of LtxA and cytolethal distending toxin (Cdt) in each vesicle population. LtxA and outer membrane protein A (OmpA) were found in OMVs collected in lighter layers (larger OMVs), while Cdt was concentrated in OMVs found in heavier layers (smaller OMVs) (Rompikuntal et al., 2012). We have similarly found that A. actinomycetemcomitans strain JP2 produces at least two populations of OMVs, one highly abundant population of small OMVs (less than 100 nm in diameter), and a less abundant population of large OMVs (greater than 300 nm in diameter) (Nice et al., 2018). Our preliminary evidence indicates that LtxA is concentrated in the “large” OMVs and excluded from the “small” OMVs, leading us to hypothesize that each population of OMV likely serves entirely different purposes. Investigations of the mechanism by which LtxA-containing OMVs are internalized and the fate of the internalized OMV-LtxA are ongoing projects in the field and will have important implications in the development of anti-LtxA strategies.
5. Anti-LtxA strategies
Antibiotic resistance is increasingly becoming more prevalent among pathogenic bacteria, and A. actinomycetemcomitans has already shown resistance to common antibiotic treatments (Al-Zubaydi, Dabija-Wolter, Mohammed, & Bolstad, 2017; Oettinger-Barak, Dashper, Catmull, Adams, Sela et al., 2013). This necessitates the exploration of other approaches to combat bacterial infection. A number of researchers have focused on inhibiting the function of virulence factors that otherwise give the bacteria an advantage over the host or competing bacteria. LtxA represents an ideal anti-virulence target, as it is more prevalent in disease-associated strains of A. actinomycetemcomitans (Haraszathy et al., 2000; Haubek et al., 2008; Zambon et al., 1983), and inhibition of its immunosuppressive activity would eliminate the colonization advantage provided to the bacteria by the toxin. Our lab, as well as some others, have focused on designing strategies to inhibit LtxA delivery to host cells, by building on the current understanding of the targets of LtxA on host cells. The detailed knowledge of the mechanisms used by LtxA to kill host cells that has been gathered over the last few decades, as described above, has enabled the design of these targeted anti-virulence strategies.
5.1. Receptor blocking
As discussed throughout this work, LtxA has a variety of receptors used to target host cells. Since both cholesterol and LFA-1 have been established to be required for cytotoxicity, preventing the toxin from interacting with either may provide a way to inhibit the toxin’s activity. Receptor blocking is a relatively straightforward method to compete with the toxin for binding sites on the host, or bind to the toxin itself, rendering it inert.
5.1.1. Cholesterol inhibition
LtxA has been shown to interact with the hydroxyl group on cholesterol through a CRAC domain located in the toxin’s N-terminal region (Brown et al., 2016). The initial studies into this interaction revealed that a peptide based on the CRAC336 motif could inhibit LtxA cytotoxicity in Jn.9 cells (Brown et al., 2013). Subsequent investigations have built upon this finding to design and synthesize a peptide that inhibits LtxA activity by blocking the binding of the toxin with cholesterol, internalization, and cytotoxicity (Brown et al., 2016; Koufos et al., 2016). We demonstrated that the peptide competes with the toxin for potential binding sites, interrupting the interaction of LtxA with cholesterol. It is well established that many toxins and pathogens recognize and bind cholesterol on the host cell; however, development of strategies to inhibit this interaction are often not viable, as cholesterol is an important component of many cellular functions. The advantage of the CRAC336 peptide is that it seems to interact with cholesterol on the cell surface, where it is less likely to impact cellular signaling. In fact, treatment of THP-1 cells with therapeutically relevant concentrations of the CRAC336 peptide for over 65 days resulted in no difference in viability compared to an untreated control (Koufos et al., 2016).
5.1.2. LFA-1 inhibition
Making use of the previous work that demonstrated the domains of CD11a targeted by LtxA (Kieba et al., 2007), we synthesized a series of peptides based on the last β-strand in the first β-sheet (W1S4) and all four β-strands in the second β-sheet (W2S1–W2S4). We found that four of the five peptides inhibited LtxA cytotoxicity in THP-1 cells (Krueger, Hayes, Chang, Yutuc, & Brown, 2018). This suggests that the peptides bind to LtxA, and inhibit the toxin’s interaction with CD11a on the host cells by occupying the binding site on the toxin. LtxA has been shown to not react with cells expressing murine CD11a (Kieba et al., 2007), so as a control, we synthesized peptides corresponding to the analogous regions of the murine integrin. We found that mW1S4 did not inhibit LtxA activity, but surprisingly, the mW2S1 and mW2S2 peptides did, suggesting the W1S4 region of the β-propeller may control the species specificity of the toxin. Comparing the binding affinities of LtxA to the both species of the W1S4 peptides, we found that LtxA has only negligible binding to the mW1S4 peptide while binding to the hW1S4 peptide with an equilibrium dissociation constant (KD) of approximately 10−8 M. These results were consistent with fluorescent imaging of the interaction of labeled LtxA and labeled CD11a on THP-1 cells. We found that toxin pretreated with human LFA-1-based peptides colocalized with CD11a to a significantly less extent than did toxin alone, suggesting that the peptides act by specifically inhibiting LtxA binding to LFA-1. Although conflicting results about whether the binding site for LtxA on LFA-1 resides on the CD11a or CD18 subunit, our results indicate that CD11a-based peptides are sufficient to block LtxA activity. Importantly, these peptides bind to the receptor binding site on the toxin and thus would not interfere with normal immune function of the target cells.
5.2. Catechins and small molecules
During a routine confocal microscopy experiment, we discovered that pre-treatment of Jn.9 cells with DRAQ5, a commonly used nuclear stain, prevented LtxA internalization into, but not association with, the host cells. This anthracycline was originally developed as a cancer therapeutic because of its ability to cause cell cycle arrest (Chen, Hsieh, Chang, & Chung, 2004; Hellin, Bentires-Alj, Verlaet, Benoit, Gielen et al., 2000; Kim, Lee, & Kim, 2009). Cardiotoxicity has limited the use of this class of drugs for this purpose, but DRAQ5 has been repurposed as a nuclear stain due to its targeting of the cell nucleus through binding to DNA, as well as its ability to cross both the plasma and nuclear membranes. Several anthracyclines were shown to have strong effects on plasma membrane phase behavior (Jedrzejczak, Koceva-Chyla, Gwozdzinski, & Jozwiak, 1999; Kaye & Merry, 1985; Pacilio, Florio, Pagnini, Crispino, Claudio et al., 1998), and we likewise found that DRAQ5 alters the host cell membrane property in a manner that might inhibit LtxA from embedding in the bilayer (Webb, Koufos, & Brown, 2016). Of course, toxicity of this compound limits its use as an anti-LtxA drug, but the work demonstrated that small molecules have potential as anti-toxin agents.
Catechins are polyphenols commonly found in foods like green tea, cocoa, red wine, along with a variety of fruits and vegetables, and are attributed to numerous health benefits due to their antioxidant and antimicrobial properties (Taylor, Hamilton-Miller, & Stapleton, 2005; Yamagata, Tagami, & Yamori, 2015). Inspired by a report of inhibition of LtxA activity by catechins (Kawashima, 2011), as well as inhibition of A. actinomycetemcomitans OMVs (Saito, Tsuzukibashi, & Takada, 2012), we conducted a study to understand the mechanism of this inhibition (Chang, Huang, Lin, & Brown, 2019). This study investigated the inhibitory effects of six catechins. Of the six, epigallocatechin gallate (EGCg) was found to have the strongest effect, but remarkably, five of the catechins were shown to significantly inhibit cytotoxicity in THP-1 cells when the toxin was pretreated with the catechins. We attributed the anti-toxin effects to catechin-induced changes to the LtxA structure. After treatment with EGCg, the toxin’s conformation was found to have decreased in α-helix structure by approximately 70% compared to untreated toxin. Further investigation revealed that several of the catechins, notably the galloylated catechins, significantly reduced colocalization of LtxA with model lipid membranes containing cholesterol. This suggests that the structural conformation changes caused by the catechins disrupt the binding of the toxin to cholesterol, and as a result, inhibit LtxA-mediated cytotoxicity. Similarly, extracts of the leaves and twigs of Psidium guajava (guava) were observed to inhibit LtxA activity through direct interactions with the toxin rather than the host cell (Kwamin, Gref, Haubek, & Johansson, 2012). These extracts are rich in catechins and other polyphenolic molecules (Barbalho, Farinazzi-Machado, Goulart, Brunnati, Machado et al., 2012).
5.3. Future Potential of Anti-LtxA Strategies
The results described above demonstrate that strategies can be designed, using knowledge of the processes by which LtxA recognizes host cells, to inhibit these essential steps and prevent LtxA function. We, and others, have hypothesized that by inhibiting the activity of this particular toxin, the virulence of the bacteria would be reduced resulting in decreased severity of infection. Advantages of such a strategy include slower development of resistance due to decreased selective pressure (compared to antibiotics) and a targeted treatment that would not impact healthy microbiota. Additionally, like other Gram negative bacteria, the number of effective antibiotics is limited due to the dual membrane of the bacterium as well as the numerous efflux and neutralization strategies; by targeting an exoprotein, these defensive strategies of the bacteria would be rendered ineffective. However, it is important to note that in vivo support of these hypotheses has not yet been obtained.
While the potential for anti-virulence strategies in the treatment of LAP is strong, a number of limitations has stymied the translation of these therapies from the bench to the clinic. First, like all virulence factors, LtxA expression is not constant; rather it is a function of environmental cues. Thus, a drug aimed at inhibiting LtxA could not be administered in a similar manner as an antibiotic. Targeted and timed release strategies would be essential to ensure that the drug is delivered only when necessary. Fortunately, this is an active field of research, and numerous potentially useful drug delivery designs have been developed. Additionally, these molecules would need to be administered within the gingival pocket, where A. actinomycetemcomitans resides, and would need to resist elimination through the numerous clearance forces within the oral cavity, such as swallowing and mastication. Again, developments in drug delivery strategies will prove essential for this purpose. Finally, one of the primary roadblocks in the translation of anti-virulence strategies, in general, has been the lack of a reliable assay to measure in vivo functionality. Unlike traditional antibiotics, where the effectiveness of treatment can be easily assessed by bacterial number, the determination of the effectiveness of any anti-virulence strategy will likely need to be targeted to the specific application. Bacterial clearance could still be a relevant parameter, but would likely occur on a much slower timescale, as it relies on host immunity. Other factors, such as disease severity and inflammatory response might be more relevant, but quantifiable metrics would need to be tailored to each specific application (Maura, Ballok, & Rahme, 2016). We anticipate that these limitations will be overcome in the near future, enabling the development of clinically useful anti-virulence strategies for the treatment of LAP.
6. Conclusions and Perspectives
The important role of LtxA in A. actinomycetemcomitans pathogenicity has made the toxin an attractive target for scientific study. The information arising from these studies has enabled the design of anti-virulence strategies, which have potential use as antibiotic-free therapeutics. However, while many advances in our understanding of the mechanisms of this toxin have been achieved, significant gaps remain. As our understanding of the basic mechanisms of this toxin advance, we anticipate that even more therapeutic targets will be identified to promote the development of newer, more effective treatment strategies for LAP.
Acknowledgements
This work was supported by the National Institutes of Health (DE027769 (ACB), DE025275 (ACB), and DE026962 (EK)) and the National Science Foundation (1554417 (ACB)). This work is dedicated to Dr. Edward T. Lally for his significant impact on the field, both scientifically and through his dedicated guidance and mentorship of us and so many others working in oral microbiology and immunology.
References
- Al-Zubaydi SS, Dabija-Wolter G, Mohammed MMA, & Bolstad AI (2017). The effect of metronidazole plus amoxicillin or metronidazole plus penicillin V on a periodontal biofilm model. An in vitro study. Journal of Oral Microbiology, 9, 1325270. doi: 10.1080/20002297.2017.1325270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldick T, Bielaszewska M, Uhlin BE, Humpf H-U, Wai SN, & Karch H (2009). Vesicular stabilization and activity augmentation of enterohaemorrhagic Escherichia coli haemolysin. Molecular Microbiology, 71(6), 1496–1508. doi: 10.1111/j.1365-2958.2009.06618.x [DOI] [PubMed] [Google Scholar]
- Avila-Campos MJ (1995). Haemolytic activity of Actinobacillus actinomycetemcomitans strains on different blood types. Journal of the São Paulo Institute of Tropical Medicine, 37(3), 215–217. doi: 10.1590/S0036-46651995000300006 [DOI] [PubMed] [Google Scholar]
- Bakás L, Chanturiya A, Herlax V, & Zimmerberg J (2006). Paradoxical lipid dependence of pores formed by the Escherichia coli α-hemolysin in planar phospholipid bilayer membranes. Biophysical Journal, 91(10), 3748–3755. doi: 10.1529/biophysj.106.090019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan L, Hughes C, & Koronakis V (2001). Substrate-triggered recruitment of the TolC channel-tunnel during type I export of hemolysin by Escherichia coli. Journal of Molecular Biology, 313(3), 501–510. doi: 10.1006/jmbi.2001.5038 [DOI] [PubMed] [Google Scholar]
- Balashova NV, Crosby JA, Al Ghofaily L, & Kachlany SC (2006). Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infection and Immunity, 74(4), 2015–2021. doi: 10.1128/iai.74.4.2015-2021.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashova NV, Dhingra A, Boesze-Battaglia K, & Lally ET (2016). Aggregatibacter actinomycetemcomitans leukotoxin induces cytosol acidification in LFA-1 expressing immune cells. Molecular Oral Microbiology, 31, 106–114. doi: 10.1111/omi.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balashova NV, Shah C, Patel JK, Megalla S, & Kachlany SC (2009). Aggregatibacter actinomycetemcomitans LtxC is required for leukotoxin activity and initial interaction between toxin and host cells. Gene, 443(1–2), 42–47. doi: 10.1016/j.gene.2009.05.002 [DOI] [PubMed] [Google Scholar]
- Balsalobre C, Silvan JM, Berglund S, Mizunoe Y, Uhlin BE, & Wai SN (2006). Release of the type I secreted α-haemolysin via outer membrane vesicles from Escherichia coli. Molecular Microbiology, 59(1), 99–112. doi: 10.1111/j.1365-2958.2005.04938.x [DOI] [PubMed] [Google Scholar]
- Barbalho SM, Farinazzi-Machado FMV, Goulart R. d. A., Brunnati ACS, Machado AM, Ottoboni B, & Nicolau CCT (2012). Psidium guajava (guava): A plant of multipurpose medicinal applications. Medicinal & Aromatic Plants, 1(4), 1000104. doi: 10.4172/2167-0412.1000104 [DOI] [Google Scholar]
- Basar T, Havlicek V, Bezouskova S, Hackett M, & Sebo P (2001). Acylation of lysine 983 is sufficient for toxin activity of Bordetella pertussis adenylate cyclase. Journal of Biological Chemistry, 276, 348–354. doi: 10.1074/jbc.M006463200 [DOI] [PubMed] [Google Scholar]
- Beglova N, Blacklow SC, Takagi J, & Springer TA (2002). Cysteine-rich module structure reveals a fulcrum for integrin rearrangement upon activation. Nature Structural Biology, 9(4), 282–287. doi: 10.1038/nsb779 [DOI] [PubMed] [Google Scholar]
- Bergh PGACV, Zecchinon LLM, Fett T, & Desmecht D (2009). Porcine CD18 mediates Actinobacillus pleuropneumoniae ApxIII species-specific toxicity. Veterinary Research, 40(4), 33. doi: 10.1051/vetres/2009016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthold P, Forti D, Kieba IR, Rosenbloom J, Taichman NS, & Lally ET (1992). Electron immunocytochemical localization of Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiology and Immunology, 7(1), 24–27. doi: 10.1111/j.1399-302X.1992.tb00015.x [DOI] [PubMed] [Google Scholar]
- Blix IJS, Hars R, Preus HR, & Helgeland K (1992). Entrance of Actinobacillus actinomycetemcomitans into HEp‐2 cells in vitro. Journal of Periodontology, 63(9), 723–728. doi: 10.1902/jop.1992.63.9.723 [DOI] [PubMed] [Google Scholar]
- Bonnington KE, & Kuehn MJ (2014). Protein selection and export via outer membrane vesicles. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1843(8), 1612–1619. doi: 10.1016/j.bbamcr.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogan JM, Lally ET, Poulsen K, Kilian M, & Demuth DR (1994). Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: Analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infection and Immunity, 62(2), 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Balashova NV, Epand RM, Epand RF, Bragin A, Kachlany SC, … Lally ET (2013). Aggregatibacter actinomycetemcomitans leukotoxin utilizes a cholesterol recognition/amino acid consensus site for membrane association. Journal of Biological Chemistry, 288(32), 23607–23621. doi: 10.1074/jbc.M113.486654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Boesze-Battaglia K, Balashova NV, Mas Gómez N, Speicher K, Tang H-Y, … Lally ET (2018). Membrane localization of the repeats-in-toxin (RTX) leukotoxin (LtxA) produced by Aggregatibacter actinomycetemcomitans. PLOS ONE, 13(10), e0205871. doi: 10.1371/journal.pone.0205871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Boesze-Battaglia K, Du Y, Stefano FP, Kieba IR, Epand RF, … Lally ET (2012). Aggregatibacter actinomycetemcomitans leukotoxin cytotoxicity occurs through bilayer destabilization. Cellular Microbiology, 14(6), 869–881. doi: 10.1111/j.1462-5822.2012.01762.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AC, Koufos E, Balashova NV, Boesze-Battaglia K, & Lally ET (2016). Inhibition of LtxA toxicity by blocking cholesterol binding with peptides. Molecular Oral Microbiology, 31(1), 94–105. doi: 10.1111/omi.12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumba L, Masin J, Fiser R, & Sebo P (2010). Bordetella adenylate cyclase toxin mobilizes its β2 integrin receptor into lipid rafts to accomplish translocation across target cell membrane in two steps. PLOS Pathogens, 6(5), e1000901. doi: 10.1371/journal.ppat.1000901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell ID, & Ginsberg MH (2004). The talin–tail interaction places integrin activation on FERM ground. Trends in Biochemical Sciences, 29(8), 429–435. doi: 10.1016/j.tibs.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Chang EH, Huang J, Lin Z, & Brown AC (2019). Catechin-mediated restructuring of a bacterial toxin inhibits activity. Biochimica et Biophysica Acta (BBA) - General Subjects, 1863(1), 191–198. doi: 10.1016/j.bbagen.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, & Chaudhuri K (2013). Vibrio cholerae O395 outer membrane vesicles modulate intestinal epithelial cells in a NOD1 protein-dependent manner and induce dendritic cell-mediated Th2/Th17 cell responses. Journal of Biological Chemistry, 288(6), 4299–4309. doi: 10.1074/jbc.M112.408302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Hsieh WT, Chang WC, & Chung JG (2004). Aloe-emodin induced in vitro G2/M arrest of cell cycle in human promyelocytic leukemia HL-60 cells. Food and Chemical Toxicology, 42(8), 1251–1257. doi: 10.1016/j.fct.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Chenal A, Guijarro JI, Raynal B, Delepierre M, & Ladant D (2009). RTX calcium binding motifs are intrinsically disordered in the absence of calcium: Implication for protein secretion. Journal of Biological Chemistry, 284(3), 1781–1789. doi: 10.1074/jbc.M807312200 [DOI] [PubMed] [Google Scholar]
- Chenal A, Karst JC, Pérez ACS, Wozniak AK, Baron B, England P, & Ladant D (2010). Calcium-induced folding and stabilization of the intrinsically disordered RTX domain of the CyaA toxin. Biophysical Journal, 99(11), 3744–3753. doi: 10.1016/j.bpj.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry LK, Weber KS, & Klickstein LB (2001). A dominant Jurkat T cell mutation that inhibits LFA-1-mediated cell adhesion is associated with increased cell growth. Journal of Immunology, 167(11), 6171–6179. doi: 10.4049/jimmunol.167.11.6171 [DOI] [PubMed] [Google Scholar]
- Christersson LA, Slots J, Rosling BG, & Genco RJ (1985). Microbiological and clinical effects of surgical treatment of localized juvenile periodontitis. Journal of Clinical Periodontology, 12(6), 465–476. doi: 10.1111/j.1600-051X.1985.tb01382.x [DOI] [PubMed] [Google Scholar]
- Cochran DL (2008). Inflammation and bone loss in periodontal disease. Journal of Periodontology, 79(8), 1569–1576. doi: 10.1902/jop.2008.080233 [DOI] [PubMed] [Google Scholar]
- Crosby JA, & Kachlany SC (2007). TdeA, a TolC-like protein required for toxin and drug export in Aggregatibacter (Actinobacillus) actinomycetemcomitans. Gene, 388(1–2), 83–92. doi: 10.1016/j.gene.2006.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP (2010). Periodontitis: A polymicrobial disruption of host homeostasis. Nature Reviews Microbiology, 8, 481–490. doi: 10.1038/nrmicro2337 [DOI] [PubMed] [Google Scholar]
- de Kruijff B (1997). Lipids beyond the bilayer. Nature, 386(6621), 129–130. doi: 10.1038/386129a0 [DOI] [PubMed] [Google Scholar]
- Deas DE, & Mealey BL (2010). Response of chronic and aggressive periodontitis to treatment. Periodontology 2000, 53(1), 154–166. doi: 10.1111/j.1600-0757.2009.00334.x [DOI] [PubMed] [Google Scholar]
- Demuth D, James D, Kowashi Y, & Kato S (2003). Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cellular Microbiology, 5(2), 111–121. doi: 10.1046/j.1462-5822.2003.00259.x [DOI] [PubMed] [Google Scholar]
- Dietmann A, Millonig A, Combes V, Couraud P-O, Kachlany SC, & Grau GE (2013). Effects of Aggregatibacter actinomycetemcomitans leukotoxin on endothelial cells. Microbial Pathogenesis, 61–62, 43–50. doi: 10.1016/j.micpath.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco KM, Gupta A, Galusha LE, Perez J, Nguyen T, Fineza CD, & Kachlany SC (2012). Leukotoxin (leukothera®) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. Journal of Biological Chemistry, 287(21), 17618–17627. doi: 10.1074/jbc.M111.314674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco KM, Johnson-Farley N, Bertino JR, Elson D, Vega BA, Belinka BA, & Kachlany SC (2015). LFA-1-targeting leukotoxin (LtxA; leukothera®) causes lymphoma tumor regression in a humanized mouse model and requires caspase-8 and Fas to kill malignant lymphocytes. Leukemia Research, 39(6), 649–656. doi: 10.1016/j.leukres.2015.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileepan T, Kachlany SC, Balashova NV, Patel J, & Maheswaran SK (2007). Human CD18 is the functional receptor for Aggregatibacter actinomycetemcomitans leukotoxin. Infection and Immunity, 75(10), 4851–4856. doi: 10.1128/iai.00314-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato GM, Goldsmith CS, Paddock CD, Eby JC, Gray MC, & Hewlett EL (2012). Delivery of Bordetella pertussis adenylate cyclase toxin to target cells via outer membrane vesicles. FEBS Letters, 586, 459–465. doi: 10.1016/j.febslet.2012.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elluri S, Enow C, Vdovikova S, Rompikuntal PK, Dongre M, Carlsson S, … Wai SN (2014). Outer membrane vesicles mediate transport of biologically active Vibrio cholerae cytolysin (vcc) from V. Cholerae strains. PLOS ONE, 9(9), e106731. doi: 10.1371/journal.pone.0106731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg SK, Jakobsen MR, Skals M, & Praetorius HA (2016). Inhibition of P2X receptors protects human monocytes against damage by leukotoxin from Aggregatibacter actinomycetemcomitans and α-hemolysin from Escherichia coli. Infection and Immunity, 84(11), 3114–3130. doi: 10.1128/iai.00674-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feres M, Figueiredo LC, Soares GMS, & Faveri M (2015). Systemic antibiotics in the treatment of periodontitis. Periodontology 2000, 67(1), 131–186. doi: 10.1111/prd.12075 [DOI] [PubMed] [Google Scholar]
- Fine DH, Markowitz K, Fairlie K, Tischio-Bereski D, Ferrendiz J, Furgang D, … Dewhirst FE (2013). A consortium of Aggregatibacter actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis is present in sites prior to bone loss in a longitudinal study of localized aggressive periodontitis. Journal of Clinical Microbiology, 51(9), 2850–2861. doi: 10.1128/jcm.00729-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine DH, Patil AG, & Velusamy SK (2019). Aggregatibacter actinomycetemcomitans (Aa) under the radar: Myths and misunderstandings of Aa and its role in aggressive periodontitis. Frontiers in Immunology, 10(728), 728. doi: 10.3389/fimmu.2019.00728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, Cover TL, & Solcia E (1999). Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. Journal of Pathology, 188(2), 220–226. doi: [DOI] [PubMed] [Google Scholar]
- Fong KP, Pacheco CMF, Otis LL, Baranwal S, Kieba IR, Harrison G, … Lally ET (2006). Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cellular Microbiology, 8(11), 1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong KP, Tang HY, Brown AC, Kieba IR, Speicher DW, Boesze-Battaglia K, & Lally ET (2011). Aggregatibacter actinomycetemcomitans leukotoxin is post-translationally modified by addition of either saturated or hydroxylated fatty acyl chains. Molecular Oral Microbiology, 26(4), 262–276. doi: 10.1111/j.2041-1014.2011.00617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Nishikubo JB, Han RK, Le A, Balashova N, & Kachlany SC (2010). Gangliosides block Aggregatibacter actinomycetemcomitans leukotoxin (LtxA)-mediated hemolysis. Toxins, 2(12), 2824–2836. doi: 10.3390/toxins2122824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert RJC (2002). Pore-forming toxins. CMLS Cellular and Molecular Life Sciences, 59(5), 832–844. doi: 10.1007/s00018-002-8471-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guermonprez P, Khelef N, Blouin E, Rieu P, Ricciardi-Castagnoli P, Guiso N, … Leclerc C (2001). The adenylate cyclase toxin of Bordetella pertussis binds to target cells via the αmβ2 integrin (Cd11b/Cd18). Journal of Experimental Medicine, 193(9), 1035–1044. doi: 10.1084/jem.193.9.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller JM, Kolodrubetz D, Cagle MP, & Kraig E (1990). Sequence of the lktB gene from Actinobacillus actinomycetemcomitans. Nucleic Acids Research, 18(17), 5291–5291. doi: 10.1093/nar/18.17.5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthmiller JM, Kolodrubetz D, & Kraig E (1995). Mutational analysis of the putative leukotoxin transport genes in Actinobacillus actinomycetemcomitans. Microbial Pathogenesis, 18(5), 307–321. doi: 10.1006/mpat.1995.0028 [DOI] [PubMed] [Google Scholar]
- Hackett M, Guo L, Shabanowitz J, Hunt DF, & Hewlett EL (1994). Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science, 266, 433–435. doi: 10.1126/science.7939682 [DOI] [PubMed] [Google Scholar]
- Hanski E, & Farfel Z (1985). Bordetella pertussis invasive adenylate cyclase. Partial resolution and properties of its cellular penetration. Journal of Biological Chemistry, 260(9), 5526–5532. [PubMed] [Google Scholar]
- Haraszathy VI, Hariharan G, Tinoco EMB, Cortelli JR, Lally ET, Davis E, & Zambon JJ (2000). Evidence for the role of highly leukotoxic Actinobacillus actinomycetemcomitans in the pathogenesis of localized juvenile and other forms of early-onset periodontitis. Journal of Periodontology, 71, 912–922. doi: 10.1902/jop.2000.71.6.912 [DOI] [PubMed] [Google Scholar]
- Hardie KR, Issartel JP, Koronakis E, Hughes C, & Koronakis V (1991). In vitro activation of Escherichia coli prohaemolysin to the mature membrane-targeted toxin requires HlyC and a low molecular-weight cytosolic peptide. Molecular Microbiology, 5, 1669–1679. doi: 10.1111/j.1365-2958.1991.tb01914.x [DOI] [PubMed] [Google Scholar]
- Haubek D, Poulsen K, Westergaard J, Dahlen G, & Kilian M (1996). Highly Toxic Clone of Actinobacillus actinomycetemcomitans in Geographically Widespread Cases of Juvenile Periodontitis in Adolescents of African Origin. Journal of Clinical Microbiology, 34(6), 1576–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubek D, Dirienzo JM, Tinoco EM, Westergaard J, López NJ, Chung CP, … Kilian M (1997). Racial tropism of a highly toxic clone of Actinobacillus actinomycetemcomitans associated with juvenile periodontitis. Journal of Clinical Microbiology, 35(12), 3037–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubek D, Poulsen K, & Kilian M (2007). Microevolution and Patterns of Dissemination of the JP2 Clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Infection and Immunity, 75(6), 3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubek D, Ennibi O-K, Poulsen K, Væth M, Poulsen S, & Kilian M (2008). Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet, 371(9608), 237–242. doi: 10.1016/S0140-6736(08)60135-X [DOI] [PubMed] [Google Scholar]
- Haurat MF, Aduse-Opoku J, Rangarajan M, Dorobantu L, Gray MR, Curtis MA, & Feldman MF (2011). Selective sorting of cargo proteins into bacterial membrane vesicles. Journal of Biological Chemistry, 286, 1269–1271. doi: 10.1074/jbc.M110.185744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellin AC, Bentires-Alj M, Verlaet M, Benoit V, Gielen J, Bours V, & Merville MP (2000). Roles of nuclear factor-κB, p53, and p21/WAF1 in daunomycin-induced cell cycle arrest and apoptosis. Journal of Pharmacology and Experimental Therapeutics, 295(3), 870–878. [PubMed] [Google Scholar]
- Hogg N, Patzak I, & Willenbrock F (2011). The insider’s guide to leukocyte integrin signalling and function. Nature Reviews Immunology, 11(6), 416–426. doi: 10.1038/nri2986 [DOI] [PubMed] [Google Scholar]
- Holt SC, Tanner AC, & Socransky SS (1980). Morphology and ultrastructure of oral strains of Actinobacillus actinomycetemcomitans and Haemophilus aphrophilus. Infection and Immunity, 30(2), 588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstman AL, & Kuehn MJ (2000). Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. Journal of Biological Chemistry, 275, 12489–12496. doi: 10.1074/jbc.275.17.12489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozbor D, Rodriguez ME, Fernandez J, Lagares A, Guiso N, & Yantorno O (1999). Release of outer membrane vesicles from Bordetella pertussis. Current Microbiology, 38(5), 273–278. doi: 10.1007/PL00006801 [DOI] [PubMed] [Google Scholar]
- Huang C, & Springer TA (1997). Folding of the β-propeller domain of the integrin αL subunit is independent of the I domain and dependent on the β2 subunit. Proceedings of the National Academy of Sciences of the United States of America, 94(7), 3162–3167. doi: 10.1073/pnas.94.7.3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaza MP, Duncan MS, Kaplan JB, & Kachlany SC (2008). Screen for leukotoxin mutants in Aggregatibacter actinomycetemcomitans: Genes of the phosphotransferase system are required for leukotoxin biosynthesis. Infection and Immunity, 76(8), 3561–3568. doi: 10.1128/IAI.01687-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M, Lally ET, Berthold P, Korchak HM, & Taichman NS (1990). Effects of cations and osmotic protectants on cytolytic activity of Actinobacillus actinomycetemcomitans leukotoxin. Infection and Immunity, 58(6), 1782–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrzejczak M, Koceva-Chyla A, Gwozdzinski K, & Jozwiak Z (1999). Changes in plasma membrane fluidity of immortal rodent cells induced by anticancer drugs doxorubicin, aclarubicin and mitoxantrone. Cell Biology International, 23(7), 497–506. doi: 10.1006/cbir.1999.0399 [DOI] [PubMed] [Google Scholar]
- Johansson A (2011). Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins, 3(12), 242–259. doi: 10.3390/toxins3030242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Claesson R, Hänström L, & Kalfas S (2003). Serum-mediated release of leukotoxin from the cell surface of the periodontal pathogen Actinobacillus actinomycetemcomitans. European Journal of Oral Sciences, 111, 209–215. doi: 10.1034/j.1600-0722.2003.00030.x [DOI] [PubMed] [Google Scholar]
- Jouhet J (2013). Importance of the hexagonal lipid phase in biological membrane organization. Frontiers in Plant Science, 4, 494. doi: 10.3389/fpls.2013.00494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachlany SC, Fine DH, & Figurski DH (2000). Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infection and Immunity, 68(11), 6094–6100. doi: 10.1128/iai.68.11.6094-6100.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachlany SC, Schwartz AB, Balashova NV, Hioe CE, Tuen M, Le A, … Rao J (2010). Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leukemia Research, 34(6), 777–785. doi: 10.1016/j.leukres.2009.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa JL, & Beveridge TJ (1995). Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: A novel mechanism of enzyme secretion. Journal of Bacteriology, 177(14), 3998–4008. doi: 10.1128/jb.177.14.3998-4008.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakelian D, Lear JD, Lally ET, & Tanaka JC (1998). Characterization of Actinobacillus actinomycetemcomitans leukotoxin pore formation in HL60 cells. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1406(2), 175–187. doi: 10.1016/S0925-4439(98)00002-7 [DOI] [PubMed] [Google Scholar]
- Kato S, Kowashi Y, & Demuth DR (2002). Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microbial Pathogenesis, 32(1), 1. doi: 10.1006/mpat.2001.0474 [DOI] [PubMed] [Google Scholar]
- Kawashima Y (2011). Effects of catechin gallate on bactericidal action and leukotoxic activity of Aggregatibacter actinomycetemcomitans. International Journal of Oral-Medical Sciences, 10(1), 20–24. doi: 10.5466/ijoms.10.20 [DOI] [Google Scholar]
- Kaye S, & Merry S (1985). Tumour cell resistance to anthracyclines - A review. Cancer Chemotherapy and Pharmacology, 14, 96–103. doi: 10.1007/BF00434344 [DOI] [PubMed] [Google Scholar]
- Kesty NC, & Kuehn MJ (2004). Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. Journal of Biological Chemistry, 279(3), 2069–2076. doi: 10.1074/jbc.M307628200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieba IR, Fong KP, Tang HY, Hoffman KE, Speicher DW, Klickstein LB, & Lally ET (2007). Aggregatibacter actinomycetemcomitans leukotoxin requires β-sheets 1 and 2 of the human CD11a β-propeller for cytotoxicity. Cellular Microbiology, 9(11), 2689–2699. doi: 10.1111/j.1462-5822.2007.00989.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Lee YS, & Kim DK (2009). Doxorubicin exerts cytotoxic effects through cell cycle arrest and Fas-mediated cell death. Pharmacology, 84(5), 300–309. doi: 10.1159/000245937 [DOI] [PubMed] [Google Scholar]
- Kimizuka R, Miura T, & Okuda K (1996). Characterization of Actinobacillus actinomycetemcomitans hemolysin. Microbiology and Immunology, 40, 717–723. doi: 10.1111/j.1348-0421.1996.tb01132.x [DOI] [PubMed] [Google Scholar]
- Koronakis V, Koronakis E, & Hughes C (1989). Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO Journal, 8(2), 595–605. doi: 10.1002/j.1460-2075.1989.tb03414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos E, Chang EH, Rasti ES, Krueger E, & Brown AC (2016). Use of a cholesterol recognition amino acid consensus peptide to inhibit binding of a bacterial toxin to cholesterol. Biochemistry, 55(34), 4787–4797. doi: 10.1021/acs.biochem.6b00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger E, Hayes S, Chang EH, Yutuc S, & Brown AC (2018). Receptor-based peptides for inhibition of leukotoxin activity. ACS Infectious Diseases, 4(7), 1073–1081. doi: 10.1021/acsinfecdis.7b00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, & Kesty NC (2005). Bacterial outer membrane vesicles and the host-pathogen interaction. Genes and Development, 19, 2645–2655. doi: 10.1101/gad.1299905 [DOI] [PubMed] [Google Scholar]
- Kwamin F, Gref R, Haubek D, & Johansson A (2012). Interactions of extracts from selected chewing stick sources with Aggregatibacter actinomycetemcomitans. BMC Research Notes, 5, 203. doi: 10.1186/1756-0500-5-203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Listgarten MA, & Hammond BF (1981). Comparative ultrastructure of leukotoxic and non-leukotoxic strains of Actinobacillus actinomycetemcomitans. Journal of Periodontal Research, 16(4), 379–389. doi: 10.1111/j.1600-0765.1981.tb00989.x [DOI] [PubMed] [Google Scholar]
- Lally ET, Golub EE, & Kieba IR (1994). Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. Journal of Biological Chemistry, 269(49), 31289–31295. [PubMed] [Google Scholar]
- Lally ET, Golub EE, Kieba IR, Taichman NS, Decker S, Berthold P, … Rosenbloom J (1991). Structure and function of the B and D genes of the Actinobacillus actinomycetemcomitans leukotoxin complex. Microbial Pathogenesis, 11(2), 111–121. doi: 10.1016/0882-4010(91)90004-T [DOI] [PubMed] [Google Scholar]
- Lally ET, Golub EE, Kieba IR, Taichman NS, Rosenbloom J, Rosenbloom JC, … Demuth DR (1989). Analysis of the Actinobacillus actinomycetemcomitans leukotoxin gene. Delineation of unique features and comparison to homologous toxins. Journal of Biological Chemistry, 264(26), 15451–15456. [PubMed] [Google Scholar]
- Lally ET, Hill RB, Kieba IR, & Korostoff J (1999). The interaction between RTX toxins and target cells. Trends in Microbiology, 7(9), 356–361. doi: 10.1016/S0966-842X(99)01530-9 [DOI] [PubMed] [Google Scholar]
- Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, … Billings PC (1997). RTX toxins recognize a β2 integrin on the surface of human target cells. Journal of Biological Chemistry, 272(48), 30463–30469. doi: 10.1074/jbc.272.48.30463 [DOI] [PubMed] [Google Scholar]
- Larson RS, Corbi AL, Berman L, & Springer T (1989). Primary structure of the leukocyte function-associated molecule-1 αsubunit: An integrin with an embedded domain defining a protein superfamily. Journal of Cell Biology, 108, 703–712. doi: 10.1083/jcb.108.2.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear JD, Furblur UG, Lally ET, & Tanaka JC (1995). Actinobacillus actinomycetemcomitans leukotoxin forms large conductance, voltage-gate ion channels when incorporated into planar lipid bilayers. Biochimica et Biophysica Acta (BBA) - Biomembranes, 1238(1), 34–41. doi: 10.1016/0005-2736(95)00086-I [DOI] [PubMed] [Google Scholar]
- Li H, & Papadopoulos V (1998). Peripheral-type benzodiazepine receptor function in cholesterol transport. Identification of a putative cholesterol recognition/interaction amino acid sequence and consensus pattern. Endocrinology, 139(12), 4991–4997. doi: 10.1210/endo.139.12.6390 [DOI] [PubMed] [Google Scholar]
- Li J, Clinkenbeard KD, & Ritchey JW (1999). Bovine CD18 identified as a species specific receptor for Pasteurella haemolytica leukotoxin. Veterinary Microbiology, 67(2), 91–97. doi: 10.1016/S0378-1135(99)00040-1 [DOI] [PubMed] [Google Scholar]
- Lim KB, Walker CRB, Guo L, Pellett S, Shabanowitz J, Hunt DF, … Hackett M (2000). Escherichia coli α-hemolysin (HlyA) is heterogeneously acylated in vivo with 14-, 15-, and 17-carbon fatty acids. Journal of Biological Chemistry, 275(47), 36698–36702. doi: 10.1074/jbc.C000544200 [DOI] [PubMed] [Google Scholar]
- Lozzio BB, & Lozzio CB (1979). Properties and usefulness of the original K-562 human myelogenous leukemia cell line. Leukemia Research, 3(6), 363–370. doi: 10.1016/0145-2126(79)90033-X [DOI] [PubMed] [Google Scholar]
- Lu C, Ferzly M, Takagi J, & Springer TA (2001). Epitope mapping of antibodies to the C-terminal region of the integrin β2 subunit reveals regions that become exposed upon receptor activation. Journal of Immunology, 166(9), 5629–5637. doi: 10.4049/jimmunol.166.9.5629 [DOI] [PubMed] [Google Scholar]
- Ludwig A, Garcia F, Bauer S, Jarchau T, Benz R, Hoppe J, & Goebel W (1996). Analysis of the in vivo activation of hemolysin (HlyA) from Escherichia coli. Journal of Bacteriology, 178(18), 5422–5430. doi: 10.1128/jb.178.18.5422-5430.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, & Goebel W (2006). Structure and mode of action of RTX toxins In Alouf JE& Popoff MR(Eds.), The comprehensive sourcebook of bacterial protein toxins (3rd ed, pp. 547–569). New York, NY: Elsevier. [Google Scholar]
- Mackman N, Nicaud J-M, Gray L, & Holland IB (1986). Secretion of haemolysin by Escherichia coli In Wu HC& Tai PC(Eds.), Protein secretion and export in bacteria. Current topics in microbiology and immunology (Vol. 125, pp. 159–181). Berlin, Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Wei R, Kachlany SC, Kazi M, & Balashova NV (2011). Cytotoxic effects of Kingella kingae outer membrane vesicles on human cells. Microbial Pathogenesis, 51(1–2), 22–30. doi: 10.1016/j.micpath.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín C, Requero M-A, Masin J, Konopasek I, Goñi FM, Sebo P, & Ostolaza H (2004). Membrane restructuring by Bordetella pertussis adenylate cyclase toxin, a member of the RTX toxin family. Journal of Bacteriology, 186(12), 3760–3765. doi: 10.1128/JB.186.12.3760-3765.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masin J, Basler M, Knapp O, El-Azami-El-Idrissi M, Maier E, Konopasek I, … Sebo P (2005). Acylation of lysine 860 allows tight binding and cytotoxicity of Bordetella adenylate cyclase on CD11b-expressing cells. Biochemistry, 44(38), 12759–12766. doi: 10.1021/bi050459b [DOI] [PubMed] [Google Scholar]
- Maura D, Ballok AE, & Rahme LG (2016). Considerations and caveats in anti-virulence drug development. Current Opinion in Microbiology, 33, 41–46. doi: 10.1016/j.mib.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D, & Grenier D (1989). Biological activities of outer membrane vesicles. Canadian Journal of Microbiology, 35(6), 607–613. doi: 10.1139/m89-097 [DOI] [PubMed] [Google Scholar]
- Mombelli A, Gmur R, Gobbi C, & Lang NP (1994). Actinobacillus actinomycetemcomitans in adult periodontitis. II. Characterization of isolated strains and effect of mechanical periodontal treatment. Journal of Periodontology, 65(9), 827–834. doi: 10.1902/jop.1994.65.9.827 [DOI] [PubMed] [Google Scholar]
- Morova J, Osicka R, Masin J, & Sebo P (2008). RTX cytotoxins recognize β2 integrin receptors through N-linked oligosaccharides. Proceedings of the National Academy of Sciences of the United States of America, 105(14), 5355–5360. doi: 10.1073/pnas.0711400105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munksgaard PS, Vorup‐Jensen T, Reinholdt J, Söderström CM, Poulsen K, Leipziger J, … Skals M (2012). Leukotoxin from Aggregatibacter actinomycetemcomitans causes shrinkage and P2X receptor‐dependent lysis of human erythrocytes. Cellular Microbiology, 14(12), 1904–1920. doi: 10.1111/cmi.12021 [DOI] [PubMed] [Google Scholar]
- Nicaud JM, Mackman N, Gray L, & Holland IB (1986). The C-terminal, 23 kDa peptide of E. coli haemolysin 2001 contains all the information necessary for its secretion by the haemolysin (Hly) export machinery. FEBS Letters, 204(2), 331–335. doi: 10.1016/0014-5793(86)80838-9 [DOI] [PubMed] [Google Scholar]
- Nice JB, Balashova NV, Kachlany SC, Koufos E, Krueger E, Lally ET, & Brown AC (2018). Aggregatibacter actinomycetemcomitans leukotoxin is delivered to host cells in an LFA-1-independent manner when associated with outer membrane vesicles. Toxins, 10(10), 414. doi: 10.3390/toxins10100414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny A, Behling UH, Hammond B, Lai CH, Listgarten M, Pham PH, & Sanavi F (1982). Release of toxic microvesicles by Actinobacillus actinomycetemcomitans. Infection and Immunity, 37(1), 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren P, Balashova NV, Brown A, Kieba I, Dhingra A, Boesze-Battaglia K, & Lally ET (2018). Aggregatibacter actinomycetemcomitans leukotoxin causes activation of LFA-1. Cellular Microbiology, e12967. doi: 10.1111/cmi.12967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien DP, Perez ACS, Karst J, Cannella SE, Enguéné VYN, Hessel A, … Chenal A (2018). Calcium-dependent disorder-to-order transitions are central to the secretion and folding of the CyaA toxin of Bordetella pertussis, the causative agent of whooping cough. Toxicon, 149, 37–44. doi: 10.1016/j.toxicon.2018.01.007 [DOI] [PubMed] [Google Scholar]
- O’Brien DP, Cannella SE, Voegele A, Raoux-Barbot D, Davi M, Douche T, … Chenal A (2019). Post-translational acylation controls the folding and functions of the CyaA RTX toxin. FASEB Journal, 33(9), 10065–10076. doi: 10.1096/fj.201802442RR [DOI] [PubMed] [Google Scholar]
- Oettinger-Barak O, Dashper SG, Catmull DV, Adams GG, Sela MN, Machtei EE, & Reynolds EC (2013). Antibiotic susceptibility of Aggregatibacter actinomycetemcomitans JP2 in a biofilm. Journal of Oral Microbiology, 5, 20320. doi: 10.3402/jom.v5i0.20320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Hara H, Fukui K, Kurihara H, Murayama Y, & Kato K (1993). Association of Actinobacillus actinomycetemcomitans leukotoxin with nucleic acids on the bacterial cell surface. Infection and Immunity, 61(11), 4878–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Kato K, Kokeguchi S, Hara H, Fukui K, & Murayama Y (1991). Nuclease-sensitive binding of an Actinobacillus actinomycetemcomitans leukotoxin to the bacterial cell surface. Infection and Immunity, 59(12), 4599–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortlepp S, Stephens PE, Hogg N, Figdor CG, & Robinson MK (1995). Antibodies that activate β2 integrins can generate different ligand binding states. European Journal of Immunology, 25(3), 637–643. doi: 10.1002/eji.1830250302 [DOI] [PubMed] [Google Scholar]
- Osickova A, Balashova N, Masin J, Sulc M, Roderova J, Wald T, … Osicka R. (2018). Cytotoxic activity of Kingella kingae RtxA toxin depends on post-translational acylation of lysine residues and cholesterol binding. Emerging Microbes & Infections, 7(1), 1–15. doi: 10.1038/s41426-018-0179-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacilio C, Florio S, Pagnini U, Crispino A, Claudio PP, Pacilio G, & Pagnini G (1998). Modification of membrane fluidity and depolarization by some anthracyclines in different cell lines. Anticancer Research, 18(6A), 4027–4034. [PubMed] [Google Scholar]
- Palmer M (2004). Cholesterol and the activity of bacterial toxins. FEMS Microbiology Letters, 238, 281–289. doi: 10.1016/j.femsle.2004.07.059 [DOI] [PubMed] [Google Scholar]
- Parker H, Chitcholtan K, Hampton MB, & Keenan JI (2010). Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infection and Immunity, 78(12), 5054–5061. doi: 10.1128/iai.00299-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakasam A, Elavarasu SS, & Natarajan RK (2012). Antibiotics in the management of aggressive periodontitis. Journal of Pharmacy & Bioallied Sciences, 4(Suppl 2), S252–S255. doi: 10.4103/0975-7406.100226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabie G, Lally ET, & Shenker BJ (1988). Immunosuppressive properties of Actinobacillus actinomycetemcomitans leukotoxin. Infection and Immunity, 56(1), 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasti ES, & Brown AC (2019). Cholera toxin encapsulated within several Vibrio cholerae O1 serotype Inaba outer membrane vesicles lacks a functional B-subunit. Toxins, 11(4). doi: 10.3390/toxins11040207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasti ES, Schappert ML, & Brown AC (2018). Association of Vibrio cholerae 569B outer membrane vesicles with host cells occurs in a GM1-independent manner. Cellular Microbiology, 20, e12828. doi: 10.1111/cmi.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinholdt J, Poulsen K, Brinkmann CR, Hoffmann SV, Stapulionis R, Enghild JJ, … Vorup-Jensen T (2013). Monodisperse and LPS-free Aggregatibacter actinomycetemcomitans leukotoxin: Interactions with human β2 integrins and erythrocytes. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1834(2), 546–558. doi: 10.1016/j.bbapap.2012.12.004 [DOI] [PubMed] [Google Scholar]
- Renvert S, Wikström M, Dahlén G, Slots J, & Egelberg J (1990). Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. Journal of Clinical Periodontology, 17(6), 345–350. doi: 10.1111/j.1600-051X.1990.tb00029.x [DOI] [PubMed] [Google Scholar]
- Renvert S, Wikström M, Dahlén G, Slots J, & Egelberg J (1990). On the inability of root debridement and periodontal surgery to eliminate Actinobacillus actinomycetemcomitans from periodontal pockets. Journal of Clinical Periodontology, 17(6), 351–355. doi: 10.1111/j.1600-051X.1990.tb00030.x [DOI] [PubMed] [Google Scholar]
- Ristow LC, Tran V, Schwartz KJ, Pankratz L, Mehle A, Sauer J-D, & Welch RA (2019). The extracellular domain of the β2 integrin β subunit (CD18) is sufficient for Escherichia coli hemolysin and Aggregatibacter actinomycetemcomitans leukotoxin cytotoxic activity. mBio, 10(4), e01459–01419. doi: 10.1128/mBio.01459-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen A-M, Asikainen S, … Oscarsson J (2012). Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infection and Immunity, 80(1), 31–42. doi: 10.1128/iai.06069-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Tsuzukibashi O, & Takada K (2012). Anticytotoxic effect of green tea catechin on Aggregatibacter actinomycetemcomitans vesicles. International Journal of Oral-Medical Sciences, 11(2), 101–105. doi: 10.5466/ijoms.11.101 [DOI] [Google Scholar]
- Saxen L, & Asikainen S (1993). Metronidazole in the treatment of localized juvenile periodontitis. Journal of Clinical Periodontology, 20(3), 166–171. doi: 10.1111/j.1600-051X.1993.tb00339.x [DOI] [PubMed] [Google Scholar]
- Sbordone L, Ramaglia L, Gulletta E, & Iacono V (1990). Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. Journal of Periodontology, 61(9), 579–584. doi: 10.1902/jop.1990.61.9.579 [DOI] [PubMed] [Google Scholar]
- Schwiering M, Husmann M, & Hellmann N (2017). P2X-receptor antagonists inhibit the interaction of S. aureus hemolysin A with membranes. Toxins, 9(10), 332. doi: 10.3390/toxins9100332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra MD, & Martínez MT (2011). Pore‐forming toxins. In eLS (Ed.). [Google Scholar]
- Shaddox LM, Gonçalves PF, Vovk A, Allin N, Huang H, Hou W, … Wallet SM (2013). LPS-induced inflammatory response after therapy of aggressive periodontitis. Journal of Dental Research, 92(8), 702–708. doi: 10.1177/0022034513495242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, & Ehehalt R (2002). Cholesterol, lipid rafts, and disease. Journal of Clinical Investigation, 110(5), 597–603. doi: 10.1172/JCI16390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson DL, Berthold P, & Taichman NS (1988). Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infection and Immunity, 56(5), 1162–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skals M, Bjaelde RG, Reinholdt J, Poulsen K, Vad BS, Otzen DE, … Praetorius HA (2014). Bacterial RTX toxins allow acute ATP release from human erythrocytes directly through the toxin pore. Journal of Biological Chemistry, 289(27), 19098–19109. doi: 10.1074/jbc.M114.571414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skals M, Leipziger J, & Praetorius HA (2011). Haemolysis induced by α-toxin from Staphylococcus aureus requires P2X receptor activation. Pflügers Archiv: European Journal of Physiology, 462(5), 669. doi: 10.1007/s00424-011-1010-x [DOI] [PubMed] [Google Scholar]
- Slots J, & Genco RJ (1984). Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: Virulence factors in colonization, survival, and tissue destruction. Journal of Dental Research, 63(3), 412–421. doi: 10.1177/00220345840630031101 [DOI] [PubMed] [Google Scholar]
- Sreenivasan PK, Meyer DH, & Fives‐Taylor PM (1993). Factors influencing the growth and viability of Actinobacillus actinomycetemcomitans. Oral Microbiology and Immunology, 8(6), 361–369. doi: 10.1111/j.1399-302X.1993.tb00612.x [DOI] [PubMed] [Google Scholar]
- Stanley P, Koronakis V, & Hughes C (1991). Mutational analysis supports a role for multiple structural features in the C-terminal secretion signal of Escherichia coli haemolysin. Molecular Microbiology, 5(10), 2391–2403. doi: 10.1111/j.1365-2958.1991.tb02085.x [DOI] [PubMed] [Google Scholar]
- Stanley P, Packman LC, Koronakis V, & Hughes C (1994). Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science, 266(5193), 1992–1996. doi: 10.1126/science.7801126 [DOI] [PubMed] [Google Scholar]
- Taichman NS, Dean RT, & Sanderson CJ (1980). Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infection and Immunity, 28(1), 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taichman NS, Iwase M, Lally ET, Shattil SJ, Cunningham ME, & Korchak HM (1991). Early changes in cytosolic calcium and membrane potential induced by Actinobacillus actinomycetemcomitans leukotoxin in susceptible and resistant target cells. Journal of Immunology, 147(10), 3587. [PubMed] [Google Scholar]
- Taichman NS, Simpson DL, Sakurada S, Cranfield M, DiRienzo J, & Slots J (1987). Comparative studies on the biology of Actinobacillus actinomycetemcomitans leukotoxin in primates. Oral Microbiology and Immunology, 2, 97–104. doi: 10.1111/j.1399-302X.1987.tb00270.x [DOI] [PubMed] [Google Scholar]
- Taylor PW, Hamilton-Miller JMT, & Stapleton PD (2005). Antimicrobial properties of green tea catechins. Food Science and Technology Bulletin, 2, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thay B, Damm A, Kufer TA, Wai SN, & Oscarsson J (2014). Aggregatibacter actinomycetemcomitans outer membrane vesicles are internalized in human host cells and trigger NOD1- and NOD2-dependent NF-κB activation. Infection and Immunity, 82(10), 4034–4046. doi: 10.1128/iai.01980-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CC, Shenker BJ, DiRienzo JM, Malamud D, & Taichman NS (1984). Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infection and Immunity, 43(2), 700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L, Bitto NJ, Steer DL, Lo C, D’Costa K, Ramm G, … Kaparakis-Liaskos M (2018). Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Frontiers in Immunology, 9, 1466. doi: 10.3389/fimmu.2018.01466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, & de Kroon AIPM (2011). Lipid map of the mammalian cell. Journal of Cell Science, 124, 5–8. doi: 10.1242/jcs071233 [DOI] [PubMed] [Google Scholar]
- Vazquez RF, Maté SM, Bakás LS, Fernández MM, Malchiodi EL, & Herlax VS (2014). Novel evidence for the specific interaction between cholesterol and α-haemolysin of Escherichia coli. Biochemical Journal, 458(3), 481–489. doi: 10.1042/BJ20131432 [DOI] [PubMed] [Google Scholar]
- Vega BA, Schober LT, Kim T, Belinka BA, & Kachlany SC (2019). Aggregatibacter actinomycetemcomitans leukotoxin (LtxA) requires death receptor Fas, in addition to LFA-1, to trigger cell death in T lymphocytes. Infection and Immunity, 87(8), e00309–00319. doi: 10.1128/iai.00309-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma NK, & Kelleher D (2017). Not just an adhesion molecule: LFA-1 contact tunes the T lymphocyte program. Journal of Immunology, 199(4), 1213. doi: 10.4049/jimmunol.1700495 [DOI] [PubMed] [Google Scholar]
- Virtanen JA, Cheng KH, & Somerharju P (1998). Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model. Proceedings of the National Academy of Sciences of the United States of America, 95(9), 4964–4969. doi: 10.1073/pnas.95.9.4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai SN, Takade A, & Amako K (1995). The release of outer membrane vesicles from the strains of enterotoxigenic Escherichia coli. Microbiology and Immunology, 39(7), 451–456. doi: 10.1111/j.1348-0421.1995.tb02228.x [DOI] [PubMed] [Google Scholar]
- Walker CB (1996). The acquisition of antibiotic resistance in the periodontal microflora. Periodontology 2000, 10(1), 79–88. doi: 10.1111/j.1600-0757.1996.tb00069.x [DOI] [PubMed] [Google Scholar]
- Walling BL, & Kim M (2018). LFA-1 in T cell migration and differentiation. Frontiers in Immunology, 9, 952. doi: 10.3389/fimmu.2018.00952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MJ, Brown AC, Edrington TC, Baranwal S, Du Y, Lally ET, & Boesze-Battaglia K (2013). Membrane association and destabilization by Aggregatibacter actinomycetemcomitans leukotoxin requires changes in secondary structures. Molecular Oral Microbiology, 28(5), 342–353. doi: 10.1111/omi.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JN, Koufos E, & Brown AC (2016). Inhibition of bacterial toxin activity by the nuclear stain, DRAQ5™. Journal of Membrane Biology, 249(4), 503–511. doi: 10.1007/s00232-016-9892-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber KS, York MR, Springer TA, & Klickstein LB (1997). Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: The αL and β2 subunits are interdependent for cell surface expression. Journal of Immunology, 158(1), 273–279. [PubMed] [Google Scholar]
- Yamagata K, Tagami M, & Yamori Y (2015). Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition, 31(1), 28–37. doi: 10.1016/j.nut.2014.04.011 [DOI] [PubMed] [Google Scholar]
- Young J, & Holland IB (1999). ABC transporters: Bacterial exporters-revisited five years on. Biochimica et Biophysica Acta, 1461, 177–200. doi: 10.1016/s0005-2736(99)00158-3 [DOI] [PubMed] [Google Scholar]
- Zambon JJ (1985). Actinobacillus actinomycetemcomitans in human periodontal disease. Journal of Clinical Periodontology, 12, 1–20. doi: 10.1111/j.1600-051X.1985.tb01348.x [DOI] [PubMed] [Google Scholar]
- Zambon JJ, DeLuca C, Slots J, & Genco RJ (1983). Studies of leukotoxin from Actinobacillus actinomycetemcomitans using the promyelocytic HL-60 cell line. Infection and Immunity, 40(1), 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon JJ, Haraszthy VI, Hariharan G, Lally ET, & Demuth DR (1996). The microbiology of early-onset periodontitis: Association of highly toxic Actinobacillus actinomycetemcomitans strains with localized juvenile periodontitis. Journal of Periodontology, 67(3), 282–290. doi: 10.1902/jop.1996.67.3s.282 [DOI] [PubMed] [Google Scholar]
- Zambon JJ, Slots J, & Genco RJ (1983). Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infection and Immunity, 41(1), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavan L, Bitto NJ, Johnston EL, Greening DW, & Kaparakis‐Liaskos M (2019). Helicobacter pylori growth stage determines the size, protein composition, and preferential cargo packaging of outer membrane vesicles. Proteomics, 19, 1800209. doi: 10.1002/pmic.201800209 [DOI] [PubMed] [Google Scholar]
- Zitzer A, Bittman R, Verbicky CA, Erukulla RK, Bhakdi S, Weis S, … Palmer M (2001). Coupling of cholesterol and cone-shaped lipids in bilayers augments membrane permeabilization by the cholesterol-specific toxins streptolysin o and Vibrio cholerae cytolysin. Journal of Biological Chemistry, 276, 14628–14633. doi: 10.1074/jbc.M100241200 [DOI] [PubMed] [Google Scholar]


