Supplemental Digital Content is available in the text.
Keywords: coronavirus, COVID-19, prognosis, propensity score, survivors
Abstract
Recent case-series of small size implied a pathophysiological association between coronavirus disease 2019 (COVID-19) and severe large-vessel acute ischemic stroke. Given that severe strokes are typically associated with poor prognosis and can be very efficiently treated with recanalization techniques, confirmation of this putative association is urgently warranted in a large representative patient cohort to alert stroke clinicians, and inform pre- and in-hospital acute stroke patient pathways. We pooled all consecutive patients hospitalized with laboratory-confirmed COVID-19 and acute ischemic stroke in 28 sites from 16 countries. To assess whether stroke severity and outcomes (assessed at discharge or at the latest assessment for those patients still hospitalized) in patients with acute ischemic stroke are different between patients with COVID-19 and non-COVID-19, we performed 1:1 propensity score matching analyses of our COVID-19 patients with non-COVID-19 patients registered in the Acute Stroke Registry and Analysis of Lausanne Registry between 2003 and 2019. Between January 27, 2020, and May 19, 2020, 174 patients (median age 71.2 years; 37.9% females) with COVID-19 and acute ischemic stroke were hospitalized (median of 12 patients per site). The median National Institute of Health Stroke Scale was 10 (interquartile range [IQR], 4–18). In the 1:1 matched sample of 336 patients with COVID-19 and non-COVID-19, the median National Institute of Health Stroke Scale was higher in patients with COVID-19 (10 [IQR, 4–18] versus 6 [IQR, 3–14]), P=0.03; (odds ratio, 1.69 [95% CI, 1.08–2.65] for higher National Institute of Health Stroke Scale score). There were 48 (27.6%) deaths, of which 22 were attributed to COVID-19 and 26 to stroke. Among 96 survivors with available information about disability status, 49 (51%) had severe disability at discharge. In the propensity score-matched population (n=330), patients with COVID-19 had higher risk for severe disability (median mRS 4 [IQR, 2–6] versus 2 [IQR, 1–4], P<0.001) and death (odds ratio, 4.3 [95% CI, 2.22–8.30]) compared with patients without COVID-19. Our findings suggest that COVID-19 associated ischemic strokes are more severe with worse functional outcome and higher mortality than non-COVID-19 ischemic strokes.
Coronavirus disease 2019 (COVID-19), a viral disease caused by the severe acute respiratory syndrome coronavirus 2, may predispose patients to arterial thrombotic complications mediated by inflammation, endothelial dysfunction, thrombin generation, and platelet activation.f Three recent case-series of small size implied a pathophysiological association between COVID-19 and severe large-vessel acute ischemic stroke (AIS).2–4 Given that severe strokes are typically associated with poor prognosis and can be very efficiently treated with recanalization techniques, confirmation of this putative association is urgently warranted in a large representative patient cohort to alert stroke clinicians and inform pre- and in-hospital acute stroke patient pathways.
We pooled all consecutive patients hospitalized with laboratory-confirmed COVID-19 and AIS in 28 sites from 16 countries. We excluded patients who were infected after the onset of stroke. Nineteen (70.4%) sites were reference hospitals for patients with COVID-19. A prespecified form was used to register anonymized patient data. To assess whether stroke severity (estimated by the National Institute of Health Stroke Scale) and outcomes (assessed by the modified Rankin Scale, at discharge or at the latest assessment for those patients still hospitalized) in patients with AIS are different between patients with and without COVID-19, we performed 1:1 propensity score matching analyses of our COVID-19 patients with non-COVID-19 patients registered in the Acute Stroke Registry and Analysis of Lausanne5 between 2003 and 2019. For the propensity score matching analysis of stroke severity, patients were matched without replacement on a set of prespecified covariates, including demographics (age, sex), stroke risk factors, and comorbidities (hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, heart failure, cancer, previous stroke, smoking, obesity, dyslipidemia). For the propensity score matching analysis of outcomes, the type of intervention and main stroke symptoms (motor symptoms, sensory symptoms, dysarthria, and aphasia) were additionally matched (further details on statistical methods are available in the supplementary material). The Global COVID-19 Stroke registry was approved by the Institutional Review Board of the co-ordinating site (Larissa University Hospital). Informed consent was waived as this was an observational study on pseudonymized data. Requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the first author.
Between January 27, 2020, and May 19, 2020, 174 patients (median age 71.2 years; 37.9% females) with COVID-19 and AIS were hospitalized (median of 12 patients per site). There were 45 patients aged >80 years and 41 aged <64 years.
In 96% of the cases, COVID-19 was confirmed with PCR and in the others by serology. The most prevalent stroke risk factors and comorbidities were hypertension (68.4%), obesity (37.4%), and diabetes mellitus (31.03%). Previous stroke was reported in 20 (11.5%) patients. The median delay between the initiation of COVID-19 symptoms and stroke onset was 7 days (interquartile range [IQR]: 2–15). The most prevalent COVID symptoms were fever (55.2%,), cough (53.5%), and dyspnoea (43.7%).
The main stroke symptoms were motor (67.8%), dysarthria (46%), and sensory (42%). The median National Institute of Health Stroke Scale was 10 (IQR: 4–18). In the 1:1 matched sample of 336 patients with and without COVID-19, the median National Institute of Health Stroke Scale was higher in patients with COVID-19 (10 [IQR: 4–18] versus 6 [IQR: 3–14], P=0.03; odds ratio, 1.69 [95% CI, 1.08–2.65] for higher National Institute of Health Stroke Scale score; Figure).
Figure.
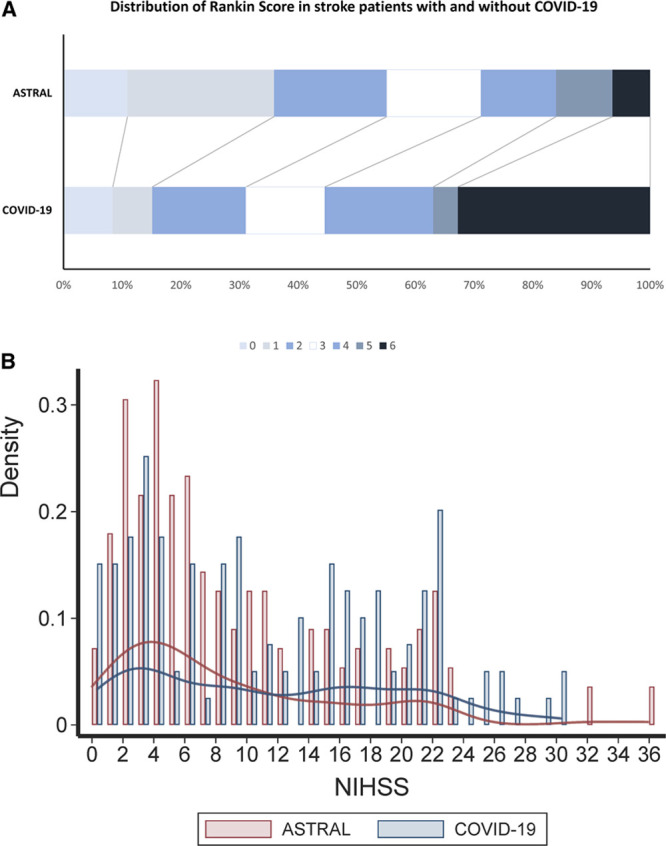
Matched populations of patients with coronavirus disease 2019 (COVID-19) from the Global COVID-19 Stroke Registry and patients without COVID-19 from the Acute Stroke Registry and Analysis of Lausanne (ASTRAL) registry. The upper panel presents the distribution of the modified Rankin Scale scores. The lower panel presents the histogram and Kernel Density Estimates of the National Institute of Health Stroke Scale (NIHSS) scores.
The vascular territory most frequently affected was the middle cerebral artery (in 93 out of 163 patients with available information). In the matched database (n=366), 132 of 168 patients with COVID-19 had a complete diagnostic workup towards stroke cause. Among those 132 patients, 30 (22.7%) and 10 (7.6%) had a large-artery or a lacunar stroke, respectively. In the non-COVID-19 matched cohort, we recorded 22 (13.1%) patients with large-artery atherosclerosis and 17 (10.2%) with lacunar stroke. Overall, we did not find a difference in the prevalence of large-artery and lacunar stroke between the 2 populations (nonparametric χ2 test P=0.082).
In the overall population of the patients with COVID-19, alteplase was administered in 34 (19.7%) of patients and endovascular thrombectomy was performed in 21 (12.1%) patients (33 and 20 among the patients with COVID-19who were included in the matched analysis, respectively). The outcomes per intervention in the matched cohort of patients with stroke with and without COVID-19 are presented in the Data Supplement. Any hemorrhagic transformation of the infarct was diagnosed in 22 patients, of whom 5 had been treated with intravenous alteplase. Malignant brain edema was present in 4 (5.1%) patients and 3 of these patients were treated with hemicraniectomy.
Among 112 patients who had pulmonary imaging with chest CT, 89.3% had lung opacities. Twenty-seven patients (15.5%) were intubated, 40 (23%) were transferred to an intensive care unit, and 110 (63.2%) were discharged from the hospital. There were 48 (27.6%) deaths, of which 22 were attributed to COVID-19 and 26 to stroke. Among 96 survivors with available information about disability status, 49 (51%) had severe disability at discharge. In the propensity score matched population (n=330), patients with COVID-19 had higher risk for severe disability (median modified Rankin Scale 4 [IQR: 2–6] versus 2 [IQR: 1–4], P<0.001) and death (odds ratio, 4.3 [95% CI, 2.22–8.30]) compared with patients with non-COVID-19 (Figure).
The strengths of this analysis are the large patient cohort, the multicentre international design, the inclusion of all consecutive known COVID-19 AIS patients treated in each site, and the propensity score matching comparison with a non-COVID-19 AIS cohort. A limitation of the study is that the propensity score match cohort was from a single site; however, as described in the supplemental material, our sensitivity analysis for potential hidden bias in the matched database confirmed the robustness of the main findings. Even if this extra, unobserved indicator (ie, site of recruitment) could affect the odds of a patient’s conditional classification to the case or control group by up to 130% and in parallel was totally unrelated to all of the variables taken into consideration (demographic characteristics, risk factors, comorbidities, stroke symptoms, and treatment), still our results of increased mortality and disability in patients with COVID-19 would not substantially change.
There are several potential explanations for the relation between COVID-19 associated ischemic strokes and increased stroke severity, which may co-exist. It was proposed that viral infections may cause a direct vasculopathic effect (endotheliopathy) or potentiate the prothrombotic milieu via several mechanisms including immune-mediated platelet activation, dehydration, and infection-induced cardiac arrhythmias.6 It is unclear whether the finding of increased severity in COVID-19 associated ischemic strokes applies to all ischemic stroke types or is mainly driven by an increase in large-vessel occlusion strokes, as implied by recent findings.2 Finally, it may be possible that the stay-at-home recommendations/orders and the general fear of the hospital during the COVID-19 pandemic may have prevented patients with acute stroke with smaller deficits from presenting to the hospital, although a recent study in Barcelona reported that initial stroke severity was not different between patients with stroke admitted in March 2019 and March 2020.7
The increased stroke severity at admission in COVID-19 associated stroke patients compared with the non-COVID-19 cohort may explain the worse outcomes. The broad multi-system complications of COVID-19 including acute respiratory distress syndrome, cardiac arrhythmias, acute cardiac injury, shock, pulmonary embolism, cytokine release syndrome and secondary infection,8 probably contribute further to the worse outcomes including higher mortality in these patients.
Our findings suggest that COVID-19 associated ischemic strokes are more severe with worse functional outcome and higher mortality than non-COVID-19 ischemic strokes. The association between COVID-19 and severe stroke highlights the urgent need for studies aiming to uncover the underlying mechanisms and is relevant for prehospital stroke awareness and in-hospital acute stroke pathways during the current and future pandemics, since severe strokes have typically poor prognosis and can potentially be treated with recanalization techniques.
Sources of Funding
None.
Disclosures
Dr Michel reports grants from Swiss National Science Foundation, grants from Swiss Heart Foundation, during the conduct of the study; grants from European Thrombosis Investigator-Initiated Research Program (Bristol-Myers Squibb/Pfizer), personal fees from Medtronic (used for research), outside the submitted work. Dr Gonçalves reports grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil outside the submitted work. Dr Dan reports personal fees from Boehringer Ingelheim, personal fees from Bayer, and personal fees from Pfizer outside the submitted work. Dr Strambo reports grants from Swiss Heart Foundation and grants from University of Lausanne outside the submitted work. Dr Werring reports personal fees from Bayer, personal fees from Alnylam, and personal fees from Portola outside the submitted work. Dr Simister is part funded by the UCLH/UCL Biomedical Research Centre. Dr Benjamin reports grants from GlaxoSmithKline outside the submitted work. Dr Jabbour reports grants from Medtronic, other from Microvention, and other from Balt outside the submitted work. Dr Korompoki reports personal fees from Pfizer, nonfinancial support from Bayer, and personal fees from Amgen outside the submitted work. Dr Lip reports other from Consultant for Bayer/Janssen, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi-Sankyo. Speaker for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. The other authors report no conflicts.
Supplemental Materials
Supplementary Methods.
Supplementary Tables.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AIS
- acute ischemic stroke
- COVID-19
- coronavirus disease 2019
- IQR
- interquartile range
For Sources of Funding and Disclosures, see page xxx.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.120.031208.
References
- 1.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, et al. ; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020; 75:2950–2973. doi: 10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020; 382:e60.doi: 10.1056/NEJMc2009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, Humphries F, Jager HR, Losseff NA, Perry RJ, et al. Characteristics of ischaemic stroke associated with covid-19. J Neurol Neurosurg Psychiatry. 2020doi: 10.1136/jnnp-2020-323586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020; 51:2002–2011. doi: 10.1161/STROKEAHA.120.030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michel P, Odier C, Rutgers M, Reichhart M, Maeder P, Meuli R, Wintermark M, Maghraoui A, Faouzi M, Croquelois A, et al. The Acute STroke Registry and Analysis of Lausanne (ASTRAL): design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke. 2010; 41:2491–2498. doi: 10.1161/STROKEAHA.110.596189 [DOI] [PubMed] [Google Scholar]
- 6.Miller EC, Elkind MS. Infection and stroke: an update on recent progress. Curr Neurol Neurosci Rep. 2016; 16:2.doi: 10.1007/s11910-015-0602-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudilosso S, Laredo C, Vera V, Vargas M, Renu A, Llull L, Obach V, Amaro S, Urra X, Torres F, et al. Acute stroke care is at risk in the era of covid-19: experience at a comprehensive stroke center in barcelona. Stroke.. 202051:1991–1995.doi: 10.1161/STROKEAHA.120.030329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntosh K. Coronavirus disease 2019 (covid-19): Clinical features. Available at: https://www.Uptodate.Com/contents/coronavirus-disease-2019-covid-19-clinical-features. Accessed June 18, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


