Abstract
Background
Travellers infected with Schistosoma spp. might be pauci- or even asymptomatic on first presentation. Therefore, schistosomiasis may remain undiagnosed in this population. Active infection, as evidenced by the presence of the tissue-dwelling worm, can be demonstrated via the detection of adult worm-derived circulating anodic antigen (CAA) utilising a robust well-described lateral flow-(LF) based test applying background-free up-converting reporter particles (UCP). In this prospective study, we assessed the diagnostic value of serum and urine UCP-LF CAA test in comparison with two Schistosoma-specific serological assays detecting antibodies against adult worm antigen-immuno fluorescence assay (AWA-IFA) and against soluble egg antigen–enzyme-linked immunosorbent assay (SEA-ELISA) antigens in travellers.
Methods
Samples were collected from 106 Dutch travellers who reported freshwater contact in sub-Saharan Africa and who were recruited up to 2 years after return. Subjects were asked to complete a detailed questionnaire on travel history, water contact, signs and symptoms compatible with schistosomiasis.
Results
Two travellers were positive by serum CAA and an additional one by urine CAA. A total of 22/106 (21%) samples were antibody positive by AWA-IFA and 9/106 (9%) by SEA-ELISA. At follow-up 6 weeks and 6 months after praziquantel treatment, all seropositives remained antibody positive whereas CAA was cleared. Seropositivity could not be predicted by the type of fresh water-related activity, country visited or symptoms reported.
Conclusion
The low number of UCP-LF CAA positives suggests that in travellers, active infections often do not establish or have very low worm burden. Based on our high seroconversion rates, we conclude that the AWA-IFA assay is the most sensitive test to detect schistosome exposure. Given the lack of predictive symptoms or risk factors, we recommend schistosomiasis screening at least by serology in all travellers with reported freshwater contact in high-endemic areas.
Keywords: Schistosomiasis, circulating anodic antigen (CAA), serology, praziquantel, freshwater contact, questionnaire
Introduction
Schistosomiasis is an infectious tropical disease caused by parasites of the genus Schistosoma, affecting ~260 million people worldwide.1 The endemicity of schistosomiasis, mainly Schistosoma mansoni and Schistosoma haematobium, is focal and depends on the presence of the freshwater snail intermediate hosts. With increasing tourism to sub-Saharan Africa, schistosomiasis occurs more frequently in travellers after swimming in freshwater bodies.2,3 Schistosomiasis in travellers differs from what is seen in endemic populations and may present as an acute or chronic disease, with or without stage-specific typical signs and symptoms.4–8 Because many physicians are unfamiliar with the disease and eosinophilia in early infection is infrequent, schistosomiasis in travellers remains underdiagnosed.9 Incidence studies for schistosomiasis in travellers are rare, making it difficult to develop screening algorithms.6 If left untreated, chronic schistosomiasis can cause late genitourinary, gastrointestinal or neurological morbidity.5
The classic diagnosis of schistosomiasis is based on microscopic detection of ova in stool or urine, although microscopy lacks sensitivity and requires multiple consecutive samples particularly when the worm burden is low.10 Consequently, the detection of schistosome-specific antibodies (Abs) plays a prominent role in the diagnosis of acute schistosomiasis in travellers. However, serological titres are not related to worm intensity and remain positive over years, making serology an inadequate tool to discern an active infection from a past infection or to monitor cure.11,12
Alternatively, active infections, as defined by the presence of viable worms, can be visualized by the quantification of genus-specific antigens originating from the gut of the adult worm in the host circulatory system.13 One of these antigens, the circulating anodic antigen (CAA), can be detected in serum and urine through a highly specific and ultra-sensitive lateral flow (LF) test that applies up-converting reporter particles (UCP).14,15 By utilizing this UCP-LF CAA assay, active Schistosoma infections can be demonstrated within weeks after exposure.16,17 In addition, CAA concentrations decline rapidly following successful treatment, making the UCP-LF CAA assay highly suitable to monitor cure.17
Establishing a reliable test, which confirms cure, remains a therapeutic necessity in the treatment of travellers.18,19 Treatment relies on the use of a single oral drug, praziquantel (PZQ). At the recommended single dose of 40 mg/kg, failures are frequent.20 The single dose is recommended based on its use for mass administration in controlled programmes; however, required doses to achieve 100% cure of individual patients has not been well established.21
In order to address the current knowledge gaps in the screening, diagnosis and treatment of travellers for schistosomiasis, we have prospectively followed travellers with reported freshwater contact in sub-Saharan Africa. This unique data set allows us to (i) identify risk factors for schistosomiasis in travellers, (ii) explore the use of the UCP-LF CAA assay in the context of established serological assays for travellers and (iii) determine the cure rates after conventional PZQ treatment.
Materials and methods
Study design and participants
Travellers were recruited through advertisements at the travel clinics and infectious diseases outpatient departments of the Leiden University Medical Centre, the Amsterdam University Medical Centres and the Harbour Hospital in Rotterdam between November 2014 and April 2019. Travellers with reported freshwater contact in sub-Saharan Africa were requested to participate in the study and were offered free diagnostic evaluation. Travellers were asked to complete a detailed questionnaire about their exposure history and about their previous and current related signs and symptoms, as well as to donate blood, urine and stool for diagnosis between 12 weeks and 2 years after the last self-reported freshwater contact. Inclusion criteria were: (i) travellers with self-reported freshwater contact in the past 2 years at a site known for schistosomiasis endemicity in sub-Saharan Africa, regardless of signs or symptoms, (ii) agreement to undergo study procedures (serology) and (iii) willingness to provide a maximum of three additional blood samples. Travellers were excluded from participation when (i) previously treated for schistosomiasis, (ii) known to be positive for schistosomiasis serology before last water contact at endemic site or (iii) using immunosuppressive or immunomodulatory drugs at presentation, as this could compromise the interpretation of schistosomiasis serological findings.
All travellers with positive Schistosoma serology were treated with 40 mg/kg of PZQ at least once and were asked to donate another sample of serum and urine at 6 weeks and 6 months after treatment (Table 1, overview of samples taken). At 6 weeks after treatment, some travellers received another dose of 40 mg/kg of PZQ as per discretion of the treating physician.
Table 1.
Summary of sample collection and diagnostic tests performed over time
| Baseline | Treatment | T+ 6 weeks | T+ 6 months | |
|---|---|---|---|---|
| Ab diagnostics | X | |||
| Serum/urine CAA | X | |||
| Stool/urine microscopy/PCR | X | |||
| Questionnaire | X | |||
| In case of Ab positivity | ||||
| PZQ (40 mg/kg) | X | |||
| Ab diagnostics | X | X | ||
| Serum/Urine CAA | X | X | ||
All participants provided informed consent. The study was approved by the LUMC Institutional Medical Ethical Research Committee (P14.239, clinicaltrials.gov NCT02194712) and endorsed by the respective institutional boards of the other partaking centres.
Serological assays
An immunofluorescence assay (IFA) was used for the detection of Schistosoma-specific Immunoglobulin M (IgM Abs) against carbohydrate epitopes of gut-associated adult worm antigen (AWA) as previously described.17,22 In brief, Rossman’s fixed adult S. mansoni worm sections were incubated with 2-fold dilution series of serum starting at a 1:8 dilution. Following incubation with goat anti-human IgM (μ-chain specific)–fluorescein isothiocyanate (FITC) antibody Sigma-Aldrich; F5384), slides were examined by fluorescence microscopy. The titre was determined as the dilution of the sample at which the fluorescence of adult worm gut epithelium was still visible. A negative control and a positive reference serum were run in parallel at each slide. Samples were considered to be positive at a titre > 1:8.
An enzyme-linked immunosorbent assay (ELISA) was used for the detection of Schistosoma-specific IgG Abs directed against S. mansoni soluble egg antigen (SEA) as previously described.17,23 In brief, crude SEA was prepared from S. mansoni eggs recovered from the livers of infected hamsters, and diluted to a concentration of 5 μg protein/ml for overnight coating of 96 well plates (Polysorb Nunc) and stored until use.24,25 After thawing, plates were washed and blocked in 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) after which they were incubated with a dilution series of serum samples (1:16 until 1:2048 in PBS/Tween 0.05/5% foetal calf serum). After washing and staining with secondary antibody (mouse anti-human IgG alkaline phosphatase 1:10.000, Sigma-Aldrich; A2064), para-nitrophenylphosphate substrate (Sigma-Aldrich; P5994) was added for Ab detection. A negative control and a positive reference serum were run in parallel on each plate. The titre was determined as the dilution of the sample at which the extinction is higher or equal to the reference standard. Samples were considered positive showing a titre > 1:16.
Both the AWA-IFA and the SEA-ELISA are in-house tests, which have been implemented at the LUMC as routine diagnostic tests for the serological diagnosis of schistosomiasis several decades ago. They fulfil all laboratory quality assessment requirements (ISO 15189:2012 certified), including successful participation in an external quality assessment scheme (UK NEQAS).
Antigen detection assay
CAA concentrations were determined in serum (0.5 ml; SCAA500 format) and urine (2 ml; UCAA2000 format) using the dry format of the UCP-LF CAA assay as described before.15 In brief, samples were pre-treated with trichloroacetic acid (TCA) in order to remove interfering proteins and to dissociate potential immune complexes. Following a centrifuge step and concentrating the supernatant, the samples were left to incubate 1 h after which a LF strip was placed in each well of a microtitre plate. The strips were scanned for UCP reporter signals with a dedicated Packard FluoroCount strip reader.14
Results were reported quantitatively expressed as CAA levels in pg/ml serum. A TCA-soluble fraction with known CAA concentration was used as a reference standard for the quantification of the antigen.14,15 Serum CAA concentrations above 3 pg/ml and urine CAA concentration above 0.3 pg/ml were used as cut-off values indicating active infection.15
Microscopy and DNA detection
Only a single stool sample and a single urine sample collected before treatment were included in the analysis. Stool and urine samples were examined for the presence of Schistosoma eggs via microscopy performed according to the procedure of the centre where the study participant was recruited. To detect the presence of Schistosoma DNA in stool or urine, a real-time polymerase chain reaction (PCR) analysis targeting the internal transcribed spacer (ITS-2) gene was performed. Sample handling, DNA extraction and PCR analysis were performed as previously described.26,27
Sample size calculation
Based on a previously reported incidence study, we estimated a positive serology of ~50% after freshwater contact.9 We aimed to include 155 travellers, which allowed us to determine sensitivity and specific of the UCP-LF CAA assay as compared to serology at an estimated 95% with 5% confidence interval. Because of slow recruitment rates, we were only able to include 106 travellers within the pre-specified time period.
Data analysis
Data were collected using Castor EDC.28 Analyses were performed using IBM SPSS statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). P values were considered significant when P < 0.05. Proportions, means or where appropriate medians and interquartile range (IQR) were calculated. Significance was calculated by Chi-square test, t-test or non-parametric as appropriate.
Results
Baseline data
A total of 106 Dutch travellers of which 41 (39%) were male and 65 (61%) were female, with a median age of 32.5 years (range, 20–70 years) were enrolled into the study (Figure 1, Table 2). All the study participants reported predefined freshwater contact at least once within 2 years before inclusion in the study, as per study protocol.
Figure 1.
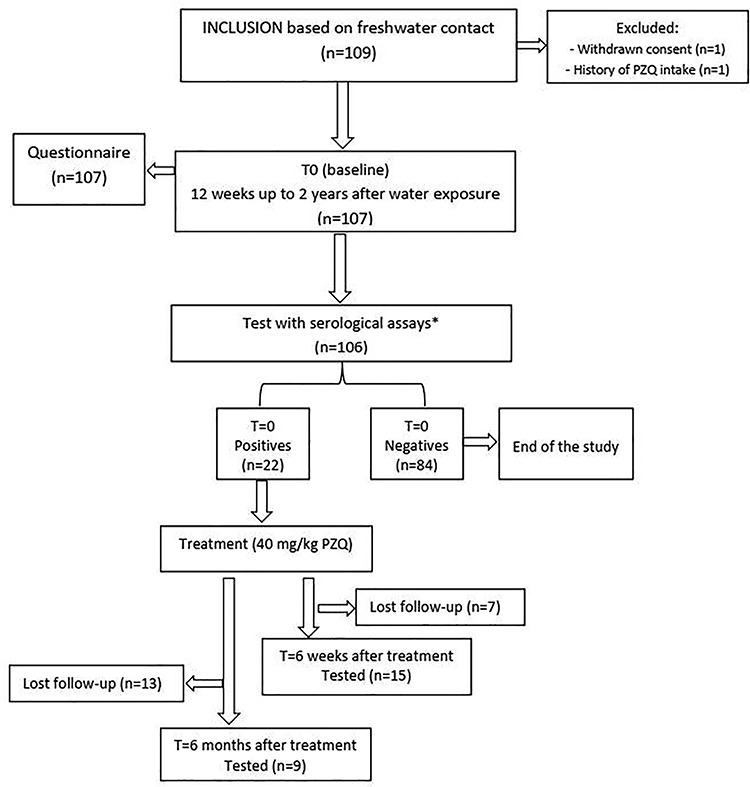
Study flowchart detailing study participation of Dutch travellers for submitting serum and urine samples for the diagnosis of schistosomiasis before and after administration of PZQ during 2014–2019. Asterisk represents schistosomiasis Ab diagnostics via AWA-IFA and SEA-ELISA
Table 2.
Demographic, travel-related characteristics, signs, symptoms and eosinophilia of 106 Dutch travellers
| Serology + (n = 22) | Serology − (n = 84) | Risk (%)a | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Demographic | Males (%) | 7 | 31.8 | 34 | 40.5 | 17.1 |
| Females (%) | 15 | 68.2 | 50 | 59.5 | 23.1 | |
| Age (med) | 29 | 33.5 | ||||
| Contact Place | Malawi | 6 | 27.3 | 45 | 53.6 | 11.8 |
| Lake Victoria | 3 | 13.6 | 8 | 9.5 | 27.3 | |
| Nile River | 8 | 36.4 | 17 | 20.2 | 32.0 | |
| Omo Rivers, Ethiopia | 2 | 9.1 | 0 | 0 | 100 | |
| Lake Tanganyika | 2 | 9.1 | 2 | 2.4 | 50.0 | |
| Dogon, Mali | 0 | 0 | 1 | 1.2 | 0 | |
| Zambezi River | 0 | 0 | 5 | 6.0 | 0 | |
| Volta River, Ghana | 1 | 4.5 | 3 | 3.6 | 25.0 | |
| Okavango Delta, Botswana | 0 | 0 | 3 | 3.6 | 0 | |
| Other | 5 | 22.7 | 24 | 28.6 | 17.2 | |
| Water contact | Swimming | 21 | 95.5 | 55 | 65.5 | 27.6 |
| Surfing | 0 | 0 | 3 | 3.6 | 0 | |
| Diving | 1 | 4.5 | 1 | 1.2 | 50.0 | |
| Wading | 4 | 18.2 | 24 | 28.6 | 14.3 | |
| Showering | 5 | 22.7 | 52 | 61.9 | 8.8 | |
| Drinking | 3 | 13.6 | 5 | 6.0 | 37.5 | |
| Signs or Symptoms | Signs or Symptoms (Y/N) | 7 | 31.8 | 17 | 20.2 | 29.2 |
| Rash | 3 | 13.6 | 8 | 9.5 | 27.3 | |
| Fever | 2 | 9.1 | 1 | 1.2 | 66.7 | |
| Tiredness | 4 | 18.2 | 12 | 14.3 | 25.0 | |
| Joint pain | 1 | 4.5 | 7 | 8.3 | 12.5 | |
| Headache | 2 | 9.1 | 5 | 6.0 | 28.6 | |
| Swelling | 0 | 0 | 0 | 0 | ||
| Coughing | 3 | 13.6 | 2 | 2.4 | 60.0 | |
| Abdominal pain | 4 | 18.2 | 7 | 8.3 | 36.4 | |
| Haematuria | 0 | 0 | 1 | 1.2 | 0 | |
| Diarrhoea | 2 | 9.1 | 8 | 9.5 | 20.0 | |
| None of the above | 0 | 0 | 1 | 1.2 | 0 | |
| Eosinophils | Eosinophils tested (%) | 17 | 77.3 | 77 | 91.7 | |
| Positives (%) | 6 | 35.3 | 3 | 3.9 | ||
| Median (positives) | 0.79 | 0.53 | ||||
| Range | 0.5–0.88 | 0.51–0.86 | ||||
| IFA titres (range) | 512–>1024 | Neg. | ||||
| ELISA titres (range) | Neg.–1024 | Neg. | ||||
| Urine CAA (%) | 1/6 (16.6) | 0/3 | ||||
| Serum CAA (%) | 0/6 | 0/3 | ||||
aRisk defined as number of new cases of schistosomiasis confirmed by serology (IFA-AWA and ELISA-SEA) in a population at risk, divided by number of persons in that population at risk.
Participants filled out their travel history and freshwater exposures in a detailed questionnaire. The most frequently reported freshwater source was Lake Malawi (51/106; 48%) followed by Nile River (25/106; 24%) and Lake Victoria (11/106; 10%). The most frequently reported freshwater activities were swimming 76/106 (72%), showering 57/106 (54%) and wading 28/106 (27%). Travellers stayed at their travel destination for a median of 28 days [range 7 days–8 years; (IQR) = 14–79 days]. Seropositives stayed longer (median 75 days) at their travel destination than seronegatives (median 22 days) (P < 0.05). No differences were seen in self-reported length for freshwater contact between seronegatives [median 4 days; (IQR) = 2–29] and seropositives [median 17 days; (IQR) = 2–137].
A total of 24/106 (23%) travellers reported signs and symptoms that could indicate schistosomiasis, of which 7 had detectable anti-Schistosoma antibodies. Eleven participants reported rash after freshwater contact and three out of those were antibody positive. Fatigue (16, 15%), abdominal pain (11, 10%), diarrhoea (10, 9%), myalgia (8, 8%) and headache (7, 7%) were the most frequently reported symptoms (Table 2).
Serological data
At baseline, 21 of the 106 sera (20%) tested Ab positive in the AWA-IFA, whereas 9 (9%) tested positive in the SEA-ELISA (Table 3). There was one sample positive in SEA-ELISA, which was negative in the AWA-IFA, making a total of 22 samples (21%) positive in either antibody test at baseline.
Table 3.
Comparison between number of positives over time based on serology or CAA detection
| n = 22 positives | n = 22 positives | |||||
|---|---|---|---|---|---|---|
| Baseline | T+ 6 weeks | T+ 6 months | ||||
| Diagnostic Test | Positives/tested | % | Positives/tested | % | Positives/tested | % |
| AWA-IFA | 21/106 | 19.8 | 15/15 | 100 | 8/8 | 100 |
| SEA-ELISA | 9/106 | 8.5 | 12/15 | 80 | 6/9 | 67 |
| Serum CAA | 2/105 | 1.9 | 0/14 | 0 | 0/8 | 0 |
| Urine CAA | 1/106 | 0.9 | 0/14 | 0 | 0/7 | 0 |
| Cumulative positive (%)a | 22 | 20.8 | 15 | 14.2 | 8 | 7.5 |
aOut of 106 tested.
Of the samples positive at baseline by AWA-IFA, two participants (10%) had low antibody titres (1:16–1:64) whereas 12 (60%) had moderate (1:128–1:512) and six (29%) had high (>1:512) IgM titres (Figure 2). For the SEA-ELISA, three samples in each group had low, moderate and high antibody titres, respectively. Samples with higher AWA-IFA titres generally also had higher titres in the SEA-ELISA. The sample, which was negative by AWA-IFA but positive by SEA-ELISA, had a titre of 1:64. This particular traveller had been to Nile River and reported water contact by swimming, showering and drinking during 3 years without any symptoms. There was no follow-up sample from this participant available.
Figure 2.
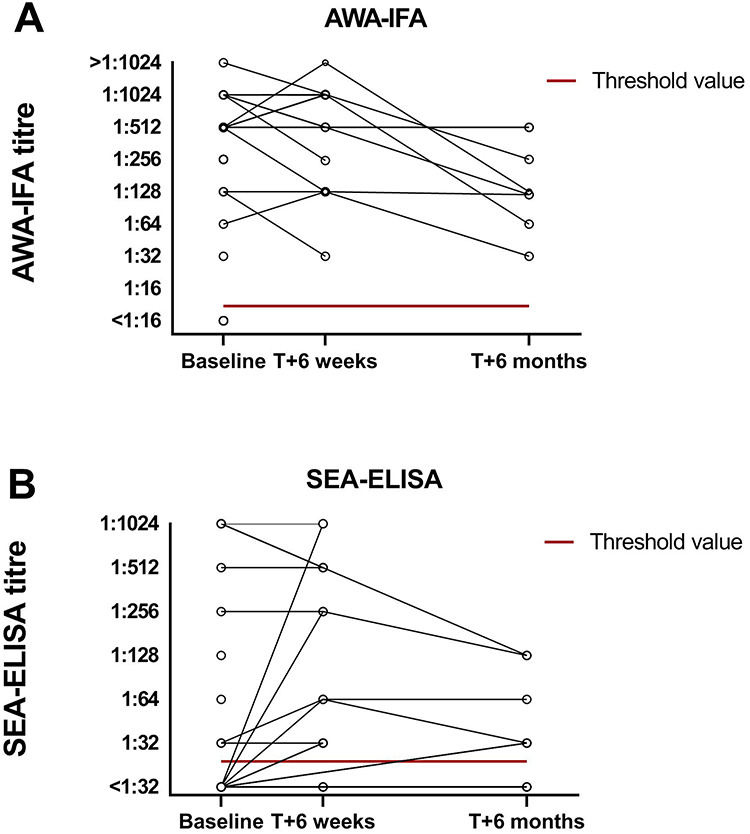
Antibody titres as measures by AWA-IFA (a) and SEA-ELISA (b) at baseline, 6 weeks after PZQ treatment and 6 months after PZQ treatment. The red line indicates threshold value for the test (AWA-IFA > 1:8 titre, SEA-ELISA > 1:16 titre)
Antigen detection
Only a small number of samples were found positive in antigen detection assays; 2 positive sample (of 105 tested) in the UCP-LF CAA serum assay and 1 positive sample (of 106 tested) in the urine assay (Table 3). The two participants positive for serum CAA (6.51 and 5.22 pg/ml) were also positive for AWA-IFA (moderate and high antibody titres, respectively) and for SEA-ELISA (moderate and low titres) but not for urine CAA (0.05 and 0.06 pg/ml). The participant that was positive for urine CAA (0.47 pg/ml) had serum CAA levels just below the threshold (2.45 pg/ml) but was positive for both AWA-IFA (high antibody titres) and SEA-ELISA (high antibody titres). In conclusion, none of the three (serum or urine) positive samples by CAA were negative in serological assays confirming the high specificity of this test.
Risk factors for schistosomiasis in travellers
We investigated whether gender, age, geographical place, water activity, signs or symptoms were risk factors for schistosomiasis in travellers. There was no difference in gender or age between those travellers who were seropositive either by AWA-IFA or by SEA-ELISA (seropositives) and those who were seronegative. Seropositives had more frequently been to the Nile River (Uganda, 36%) and Lake Victoria (14%), whereas those that were seronegative had most frequently visited Lake Malawi (56%) (Table 2). The risk of seroconversion at these most frequently visited sites culminated to 12–32% (Table 2). In addition, seropositives were more frequently exposed to freshwater by swimming (96 vs 66% in seronegatives), and less so by showering (23 vs 62%) or wading (18 vs 29%). Roughly 32% of seropositives had experienced signs and symptoms compatible with schistosomiasis, whereas 20% in the seronegative group (P > 0.05) also reported symptoms. Rash was reported by equal numbers in the seropositive (14%) and seronegative group (10%). In conclusion, no relationship was found between signs and symptoms and seropositivity.
Eosinophilia was more often present in seropositive participants (35%) as compared to seronegatives (3.9%, P = 0.01), although eosinophil elevations were generally not high [seropositives median 0.79 × 109/L; (IQR) = 0.66–0.82 vs seronegatives median 0.53 × 109/L; (IQR) = 0.51–0.53]. The risk of serconversion when having eosinophilia was 67%.
The three travellers who had detectable CAA in serum (n = 2) or urine (n = 1) were all women, symptomatic and swam in freshwater bodies. Two of these three travellers had been to Lake Malawi; the other had been to both Lake Victoria and Nile River and had the highest eosinophil counts (0.88 × 109/L).
Microscopy and PCR
Stool and urine samples were examined at baseline for intestinal and urinary schistosomiasis, respectively, by microscopy and PCR. No eggs were detected out of 9 stool specimen or in 77 urine samples tested. PCR was performed in 11 stools and 67 urines, with detectable Schistosoma DNA in one stool sample. This traveller had been exposed for 8 years in the Nile River and Ethiopia (Omo rivers) and showed high IFA titres and high ELISA titres but negative CAA antigen.
Post-treatment follow-up
All travellers who were seropositive were treated with 40 mg/kg PZQ and checked for antibody and antigen CAA detection 6 weeks after treatment. At that time, all samples were negative by antigen detection assays (Supplementary Figure 1) and remained so for 6 months. However, antibody levels persisted whereby none of the seropositives or seronegatives seroconverted by AWA-IFA after 6 weeks (Figure 2). Quantitatively, 4/15 had increasing AWA-IFA titres, 5/15 had equal titres and 6/15 titres had declined (Figure 2). SEA-ELISA titres were more variable, five seronegative travellers seroconverted and were seropositive (3 low, 1 moderate and 1 high titres) at follow-up (Figure 2). All of these were moderate (n = 4) to high (n = 1) positive in AWA-IFA at baseline. Of those travellers who were persistent seropositive SEA-ELISA between baseline and 6 weeks after treatment, one increased in titre, four persisted at the same level and two declined.
Six months after the treatment, titres in the AWA-IFA decreased with low (n = 2) and moderate (n = 6) titres, but none converted to seronegative. IgG titres by SEA-ELISA showed six out of 9 (67%) samples still positive at low (n = 4) and moderate (n = 2) titres (Supplementary Table 1).
Discussion
This study is the first of its kind to prospectively assess the value of circulating antigen detection in addition to antibody detection as diagnostic tool for schistosomiasis in travellers with freshwater contact in sub-Saharan Africa. Because of its prospective nature, we were able to analyse risk factors for schistosomiasis in travellers and explore the practical use of UCP-LF CAA dry assay format for travellers before and after treatment.
In the current study, we found AWA-IFA to be the most sensitive test for detecting exposure to schistosomes, detecting 21 positives out of 106 travellers. The seroconversion of SEA-ELISA typically occurs later.16 As previously reported, serological assays are known to be the most sensitive and specific methods for testing of travellers for schistosome exposure.10,13 Our study furthermore confirmed that AWA-IFA titres remain at high levels over a longer period over time and are even detectable 6 months after treatment despite the fact that IgM Ab are usually short lived.17 The serological data also confirmed that seroconversion can be confidently assessed from 12 weeks after exposure. Earlier studies from LUMC (Parasitology Department, The Netherlands) demonstrate that seroconversion in the AWA-IFA assay can even occur as early as 4–6 weeks after exposure.17 This contrasted with the kinetics of the IgG Ab against SEA, which were more variable over time and seroconversion was assessed in some cases even after treatment.12,13,16,17 Taken together, our data validate the excellent application of serological assays and the superior performance of the in-house IFA-AWA as compared to the SEA-ELISA for screening purposes, but the inevitable shortcoming of these serological assays in discerning active from past infections.11,12,17
With the serum and urine antigen tests (UCP-LF CAA), we found only 3 out of 22 seropositive patients to have active infections. Possibly, worm burden in this population of travellers is typically low or self-cleared. In animal models the percentage of cercariae developing into adult worms is generally low (11–39%), highlighting the difference between exposure and active infection.29 The UCP-LF CAA assay has proven better sensitivity than traditional methods to detect active infection and is applicable to all schistosome species.15 Of note, the use of serum CAA and urine CAA dry reagent format, as deployed here, facilitates implementation into routine diagnostic laboratories. Better sensitivity can be achieved when using the wet reagent format or higher samples volumes.15,17 The main advantage of CAA detection as opposed to antibody detection is the potential to follow up after treatment those who are antibody positive in order to monitor cure. In doing so, we found all travellers to have cleared CAA after a single dose of 40 mg/kg PZQ.
These data reignite the discussion around the PZQ dose regimens. The World Health Organization (WHO) recommended treatment schedule is 40 mg/kg PZQ twice with a 6-week interval. However, the low CAA levels from our study population suggest that treatment with 40 mg/kg PZQ only once may be sufficient for most travellers. Alternatively, one could consider testing for CAA after the first PZQ dose. If CAA levels at that time are still detectable, a second round of PZQ treatment can be provided.
Our results showed no clear risk factors that predict seropositivity for schistosomiasis based on type of water contact or the location where freshwater contact took place. Although some locations such as Lake Malawi have previously been identified as high-risk spots for travellers, it seems that the overall risk of acquiring schistosomiasis at these ‘hotspots’ is somewhat lower than previously estimated.9 A possible explanation could be the reduced sensitivity of our serological assays for S. haematobium or a differential risk for specific tourist spots around the same water body. In addition, different types of water activities seem to have similar risks. Unfortunately, we did not collect data on the frequency and duration of the water contact, which might increase cumulative risk. Moreover, the symptomology in the questionnaire was asked without a specific time-frame and that could lead to the unspecific nature of symptoms. Also, the use of a towel, previously reported to significantly reduce the risk, had not been recorded in the study.30 However, we did find a longer travel duration for the seropositives as compared to the seronegatives, indicating that the time spend in schistosome-endemic destinations generally increases risks.31
We found similar rates of symptoms as previously reported (36–50%).31,32 Most likely, this is an overestimation due to the fact that symptomatic subjects are more likely to participate in these type of studies and are more likely to report. Surprisingly, we found very little difference in the occurrence of symptoms between seropositive and seronegative individuals. Fatigue and abdominal pain were the most frequent symptoms, which contrasts the general notion that fever is the most frequently reported symptom of travellers, although fatigue has been reported as a hallmark for acute schistosomiasis before.4,31–33 As expected, the frequency of eosinophilia was very low (35% of seropositives). Taken together, these data suggest that there is no clear anamnestic feature to predict seropositivity in travellers with reported water contact and thus we recommend screening in all individuals with fresh water contact in endemic areas from 12 weeks after return.
In conclusion, the AWA-IFA showed the highest sensitivity for use as a screening assay and can be confidently performed from 12 weeks after exposure onwards. CAA detection may be particularly useful to determine the worm burden and to point out the possible need of a second treatment rounds with PZQ. Given the high rates of seropositivity and the lack of anamnestic risk factors (albeit exposure or symptoms), we recommend antibody screening in all travellers with reported freshwater contact in Schistosoma-endemic areas, preferably followed by CAA antigen detection.
Supplementary Material
Acknowledgements
We thank all the participants who contributed to this study.
Author contributions
All authors contributed in the reviewing of the manuscript. M.P.G., P.J.J.v.G., L.v.L. and M.R. completed study design. Writing and data interpretation was carried out by M.C.P., L.v.L., P.L.A.M.C. and M.R. Trial execution performed by J.J.J., K.S., M.A.A.E., J.J.M., M.v.A., M.P.G., P.J.J.v.G. and M.R. Antigen testing and development was carried out by R.v.S., M.C.P., C.d.D., P.L.A.M.C. and G.v.D. Antibody testing was performed by M.A.A.E., J.C.d.C. and L.v.L..
Funding
This study was funded through a research grant from the European Society of Clinical Microbiology and Infectious Diseases (2015).
Conflict of interest
None declared.
References
- 1. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet 2014; 383:2253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wyler N, Green S, Boddington N, Davies C, Friedli K, Lankester T. Travel related illness in short-term volunteers from the UK to developing countries. Travel Med Infect Dis 2012; 10:172–8. [DOI] [PubMed] [Google Scholar]
- 3. Sharafeldin E, Soonawala D, Vandenbroucke JP, Hack E, Visser LG. Health risks encountered by Dutch medical students during an elective in the tropics and the quality and comprehensiveness of pre-and post-travel care. BMC Med Educ 2010; 10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitty CJM, Mabey DC, Armstrong M, Wright SG, Chiodini PL. Presentation and outcome of 1107 cases of schistosomiasis from Africa diagnosed in a non-endemic country. Trans R Soc Trop Med Hyg 2000; 94:531–4. [DOI] [PubMed] [Google Scholar]
- 5. Meltzer E, Schwartz E. Schistosomiasis: current epidemiology and management in travelers. Curr Infect Dis Rep 2013; 15:211–5. [DOI] [PubMed] [Google Scholar]
- 6. Soonawala D, van Lieshout L, den Boer MA et al. Post-travel screening of asymptomatic long-term travelers to the tropics for intestinal parasites using molecular diagnostics. Am J Trop Med Hyg 2014; 90:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agbessi CA, Bourvis N, Fromentin M et al. La bilharziose d'importation chez les voyageurs: enquête en France métropolitaine. Rev Med Interne 2006; 27:595–9. [DOI] [PubMed] [Google Scholar]
- 8. Clerinx J, Van Gompel A. Schistosomiasis in travellers and migrants. Travel Med Infect Dis 2011; 9:6–24. [DOI] [PubMed] [Google Scholar]
- 9. Cetron MS, Chitsulo L, Sullivan JJ et al. Schistosomiasis in Lake Malawi. Lancet 1996; 348:1274–8. [DOI] [PubMed] [Google Scholar]
- 10. Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S. New diagnostic tools in schistosomiasis. Clin Microbiol Infect 2015; 21:529–42. [DOI] [PubMed] [Google Scholar]
- 11. Tosswill JHC, Ridley DS. An evaluation of the ELISA for schistosomiasis in a hospital population. Trans R Soc Trop Med Hyg 1986; 80:435–8. [DOI] [PubMed] [Google Scholar]
- 12. de Jonge N, Polderman AM, Hilberath GW, Krijger FW, Deelder AM. Immunodiagnosis of schistosomiasis patients in The Netherlands: comparison of antibody and antigen detection before and after chemotherapy. Trop Med Parasitol 1990; 41:257–61. [PubMed] [Google Scholar]
- 13. van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of schistosomiasis by determination of the circulating antigens CAA and CCA, in particular in individuals with recent or light infections. Acta Trop 2000; 77:69–80. [DOI] [PubMed] [Google Scholar]
- 14. van Dam GJ, de Dood CJ, Lewis M et al. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol 2013; 135:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Corstjens PLAM, De Dood CJ, Kornelis D et al. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology 2014; 141:1841–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Grootveld R, van Dam GJ, de Dood C et al. Improved diagnosis of active Schistosoma infection in travellers and migrants using the ultra-sensitive in-house lateral flow test for detection of circulating anodic antigen (CAA) in serum. Eur J Clin Microbiol Infect Dis 2018; 37:1709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Langenberg MCC, Hoogerwerf MA, Koopman JPR et al. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat Med 2020; 26:326–332. [DOI] [PubMed] [Google Scholar]
- 18. Duus LM, Christensen AV, Navntoft D, Tarp B, Nielsen HV, Petersen E. The schistosoma-specific antibody response after treatment in non-immune travellers. Scand J Infect Dis 2009; 41:285–90. [DOI] [PubMed] [Google Scholar]
- 19. Helleberg M, Thybo S. High rate of failure in treatment of imported schistosomiasis. J Travel Med 2010; 17:94–9. [DOI] [PubMed] [Google Scholar]
- 20. Praticò L, Mariani B, Brunetti E et al. Failure of repeated treatment with praziquantel and arthemeter in four patients with acute schistosomiasis. J Travel Med 2014; 21:133–6. [DOI] [PubMed] [Google Scholar]
- 21. Ross AG, Chau TN, Inobaya MT, Olveda RM, Li Y, Harn DA. A new global strategy for the elimination of schistosomiasis. Int J Infect Dis 2017; 54:130–7. [DOI] [PubMed] [Google Scholar]
- 22. Deelder AM, van Zeyl RJM, Fillié YE, Rotmans JP, Duchenne W. Recognition of gut-associated antigens by immunoglobulin M in the indirect fluorescent antibody test for schistosomiasis mansoni. Trans R Soc Trop Med Hyg 1989; 83:364–7. [DOI] [PubMed] [Google Scholar]
- 23. Deelder AM, Kornelis D, Makbin M et al. Applicability of different antigen preparations in the enzyme-linked immunosorbent assay for schistosomiasis mansoni*. Am J Trop Med Hyg 1980; 29:401–10. [DOI] [PubMed] [Google Scholar]
- 24. Dalton JP, Day SR, Drew AC, Brindley PJ. A method for the isolation of schistosome eggs and miracidia free of contaminating host tissues. Parasitology 1997; 115:29–32. [DOI] [PubMed] [Google Scholar]
- 25. Deelder AM. Immunology of experimental infections with Schistosoma mansoni in the Swiss mouse and Fasciola hepatica in the rabbit. Acta Leiden 1973; 39:107. [PubMed] [Google Scholar]
- 26. Meurs L, Brienen E, Mbow M et al. Is PCR the next reference standard for the diagnosis of Schistosoma in stool? A comparison with microscopy in Senegal and Kenya. PLoS Negl Trop Dis 2015; 9:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Obeng BB, Aryeetey YA, de Dood CJ et al. Application of a circulating-cathodic-antigen (CCA) strip test and real-time PCR, in comparison with microscopy, for the detection of Schistosoma haematobiumin urine samples from Ghana. Ann Trop Med Parasitol 2013; 102:625–33. [DOI] [PubMed] [Google Scholar]
- 28. Castor EDC. Castor Electronic Data Capture, 2019. https://www.castoredc.com/ (September 2019, date last accessed).
- 29. Smithers SR, Terry RJ. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 2015; 55:695–700. [DOI] [PubMed] [Google Scholar]
- 30. Outwater AH, Mpangala E. Schistosomiasis and US peace corps volunteers in Tanzania. J Travel Med 2005; 12:265–9. [DOI] [PubMed] [Google Scholar]
- 31. Nicolls DJ, Weld LH, Schwartz E et al. Characteristics of schistosomiasis in travelers reported to the GeoSentinel surveillance network 1997–2008. Am J Trop Med Hyg 2008; 79:729–34. [PubMed] [Google Scholar]
- 32. Lingscheid T, Witzenrath M, Bouchaud O et al. Schistosomiasis in European travelers and migrants: analysis of 14 years TropNet surveillance data. Am J Trop Med Hyg 2017; 97:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bierman WFW, Wetsteyn JCFM, van Gool T. Presentation and diagnosis of imported schistosomiasis: relevance of eosinophilia, microscopy for ova, and serology. J Travel Med 2006; 12:9–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


