Abstract
Background
The optimal timing of catheter ablation for atrial fibrillation (AF) in reference to the time of diagnosis is unknown. We sought to assess the impact of the duration between first diagnosis of AF and ablation, or diagnosis-to-ablation time (DAT), on AF recurrence following catheter ablation.
Methods
We conducted a systematic electronic search for observational studies reporting the outcomes associated with catheter ablation for atrial fibrillation stratified by diagnosis-to-ablation time. The primary meta-analysis using a random effects model assessed AF recurrence stratified by DAT ≤ 1 year versus > 1 year. A secondary analysis assessed outcomes stratified by DAT ≤ 3 years versus > 3 years.
Results
Of the 632 screened studies, 6 studies met inclusion criteria for a total of 4,950 participants undergoing AF ablation for symptomatic AF. A shorter DAT ≤ 1 year was associated with a lower relative risk (RR) of AF recurrence compared to DAT > 1 year (RR 0.73; 95% confidence interval (CI) 0.65 to 0.82, p<0.001). Heterogeneity was moderate (I2=51%). When excluding the one study consisting of only persistent AF patients, the heterogeneity improved substantially (I2=0%, Cochran’s Q p=0.55) with a similar estimate of effect (RR 0.78; 95% CI 0.71 to 0.85, p<0.001).
Conclusions
Duration between time of first AF diagnosis and AF ablation is associated with an increased likelihood of ablation procedural success. Additional study is required to confirm these results and to explore implementation of earlier catheter AF ablation and patient outcomes within the current AF care pathway.
Keywords: atrial fibrillation, catheter ablation, systematic review, outcome, effectiveness, diagnosis-to-ablation time
Journal Subject Terms: Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Atrial Fibrillation
Graphical Abstract
Introduction
Catheter ablation is an effective strategy to reduce atrial fibrillation (AF) recurrence and improve quality of life in patients with symptomatic AF compared to antiarrhythmic therapy.1 Despite innovations in ablation technology, such as the development of contact-force catheters and mapping systems, the long-term success of AF ablation remains suboptimal with AF recurrence rates ranging from 20 to 50%.2, 3
The heterogeneity in patient response is thought to reflect, in part, variability in the underlying degree of atrial myopathy. That is, atrial fibrosis is an important determinant of stabilizing re-entrant drivers required to maintain AF, and has been linked to AF recurrence and resistance to therapy.4 AF is a progressive disease, characterized by atrial dilatation, inflammation, atrial myocyte injury and altered collagen turnover, which contributes to scarring and fibrosis.5 The likelihood of restoring and maintaining sinus rhythm diminishes as these structural and electrical remodeling processes accumulate in long-standing AF.6 Given the dynamic process of atrial remodeling, timely catheter ablation may improve procedural success by intervening earlier in the disease process. Furthermore, earlier AF ablation may slow the progression of AF in its progression from paroxysmal to persistent forms.7
There is emerging evidence that the time between first diagnosis of AF and ablation, or diagnosis-to-ablation time (DAT), may predict long-term ablation durability. Several observational cohort studies demonstrated an inverse relationship between increasing DAT and AF recurrence rate following ablation.8, 9 Additionally, in a cohort of patients with persistent AF undergoing ablation, a longer DAT was associated with elevated biomarkers of atrial remodeling, including higher plasma B-type natriuretic peptide levels, larger left atrial volumes, and increased C-reactive protein levels.9
To synthesize the limited data regarding the relationship between time to ablation and subsequent outcomes, we performed a systematic review and meta-analysis to better understand the association between diagnosis-to-ablation time and AF recurrence.
Methods
The authors declare that all supporting data are available within the article and the online supplementary files. The study protocol was designed a priori and reporting was based on best practice guidelines from the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement10 and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE).11
Search Strategy
A systematic electronic search was performed using MEDLINE (1946-), EMBASE (Excerpta Medica Database 1974-) and the Cochrane Library databases for observational studies or randomized control trials that met inclusion criteria. The electronic search was followed by a manual search that included the references cited in all of the relevant articles. The search strategy included both controlled vocabulary (medical subject heading terms (MeSH) terms) as well as key words that were identified during the scoping review. The main search concepts and terms included: (a) atrial fibrillation, (b) catheter ablation, and (c) timing between diagnosis and catheter ablation. All searches were conducted without date limitations, and included manuscripts published up to September 5, 2019. The detailed search strategy is included in Supplemental Table 1.
Study Eligibility and Data Extraction
Publications were selected based on the following inclusion criteria: (a) observational studies or randomized control trials that included patients with symptomatic atrial fibrillation (paroxysmal, persistent or long-standing persistent) who underwent catheter ablation of AF consisting of at least pulmonary vein isolation, and (b) studies that reported the timing between AF diagnosis and the ablation procedure. All publications were limited to those involving adult (age 18 years or older) human participants. Studies of surgical or hybrid ablation procedures, AV node ablation with pacemaker implantation for AF and catheter ablation for arrhythmias other than AF were excluded from the analysis.
Two reviewers (DC and EBM) independently screened the study titles and abstracts to exclude irrelevant studies. Disagreements were resolved through consensus, and consultation of a third reviewer (JPP) if necessary. A structured data collection form was used to abstract the baseline characteristics of the study populations, study design, the time duration between AF diagnosis and ablation, and outcomes of interest. Abstracted outcomes were pre-specified and included AF recurrence, repeat AF ablation procedures, and all-cause mortality. The risk of bias was assessed using the Newcastle-Ottawa scale for non-randomized clinical trials 12.
Statistical Analysis
A random effects model according to the Dersimonian and Laird method was chosen a priori as the primary analysis on the basis of the anticipated heterogeneity among study baseline characteristics. The measurement of treatment effect was reported as an aggregated risk ratio with 95% confidence interval (CI). The primary outcome of AF recurrence and secondary outcomes were assessed with respect to DAT pre-specified as a dichotomous variable (i.e. DAT ≤ 1 year, versus DAT > 1 year). A secondary analysis using a different DAT cut-point (≤ 3 years versus > 3 years) was also performed.
Heterogeneity across the studies was tested with the Cochran Q and I2 statistics.13, 14 We considered an I2 statistic of >25% as a low degree of heterogeneity, >50% as moderate heterogeneity, and >75% as high heterogeneity.15 In order to ascertain potential meta-bias, a funnel plot was qualitatively assessed for asymmetry. Additionally, contoured-enhanced funnel plot was created to specifically explore the possibility of publication bias.16 To assess the contribution of each study to the pooled estimate, we performed a series of sensitivity analyses by excluded individual studies one at a time and recalculating the RR for remaining studies. Statistical testing was two-sided with p values less than 0.05 considered significant. All analyses were performed using Stata IC 15.1 (Stata Corp, Texas).
Results
Search Results
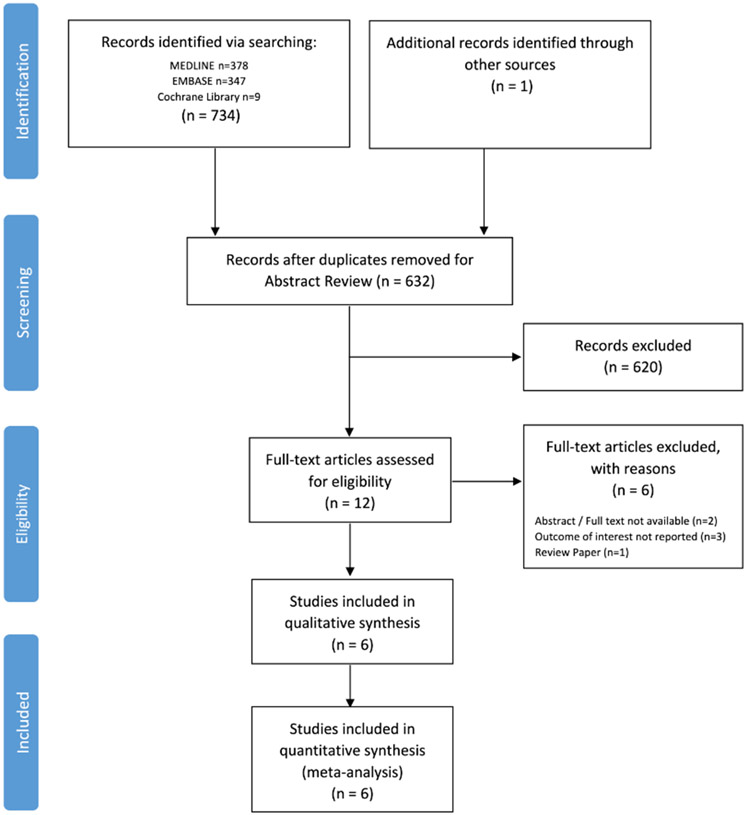
Among 632 unique citations identified in the literature, 12 studies were retrieved for full text review (Figure 1). Following full-text review, six studies met inclusion criteria.8, 9, 17-20 There were no randomized control trials identified in the systematic review and all included studies were retrospective observational cohorts in design. Of the six studies that were excluded, three studies did not report the outcomes of interest,21-23 two studies were only available in abstract form without publication of the full-text manuscript,24, 25 and one study was a review paper.26
Figure 1.
PRISMA Diagram of Study Selection and Inclusion Process
Study Characteristics
The characteristics of the included studies are summarized in Table 1. Six studies comprising of 4,950 patients were included in the analysis. The range of the mean age was 57 to 65 years and the proportion of women included in the studies was between 22 to 39%. The studies mainly included patients with symptomatic drug refractory paroxysmal AF (i.e. greater than 60% of each study cohort), although one study enrolled only patients with persistent AF.9 The overall cohort of patients had mainly preserved left ventricular (LV) systolic function with a range of mean LV ejection fractions from 51 to 63%, and a relatively low risk of stroke. AF ablation consisted primarily of pulmonary vein isolation with radiofrequency ablation, and additional lesion sets were performed at the discretion of the operator. Lunati et al. enrolled a cohort that exclusively underwent cryoballoon ablation.20 The duration of mean follow-up after AF ablation varied substantially from 14 to 43 months.
Table 1.
Baseline Cohort Characteristics of Included Studies
| Study | Bunch et al. (2013)18 |
De Greef et al. (2018)8 |
Hussein et al. (2016)9 |
Kawaji et al. (2019)19 |
Lunati et al. (2018)20 |
Bisbal et al. (2019)17 |
|---|---|---|---|---|---|---|
| Sample, N | 684 | 1000 | 1241 | 1206 | 510 | 309 |
| Mean Age, years | 65 | 60 | 61 | 64 | 59 | 57 |
| Female, % | 39 | 28 | 22 | 29 | 33 | 29 |
| BMI, kg/m2 | nr | 28 | nr | nr | 27 | 28 |
| Paroxysmal AF, % | 58 | 59 | 0 | 71 | nr | 67 |
| Prior Stroke, % | 6 | 7 | nr | 10 | 5 | 3 |
| Diabetes mellitus, % | 23 | 9 | 9 | 16 | 5 | 8 |
| Hypertension, % | 75 | 42 | 39 | 59 | 47 | 45 |
| Heart Failure, % | 30 | 42 | nr | 9 | nr | Nr |
| LA diameter, mm | nr | nr | nr | 41 | 41 | 42 |
| LVEF, % | 53 | nr | 52 | 63 | 60 | 61 |
| CHADS2 Score | 1.6 | nr | nr | 1.2 | nr | nr |
| CHADS2 VASc | nr | 1 | nr | 2.0 | nr | 1 |
| Prior Ineffective AAD, no. | nr | 2.1 | nr | nr | 1.7 | nr |
| Blanking Period | 3 months | 1 month | 3 months | 1.5 months | 1.5 months | None |
| Mean Follow Up | 39 months | 43 months | 24 months | 60 months | 16 months | 14 months |
Abbreviations: BMI, body mass index; LA, left atrial; LVEF, left ventricular ejection fraction; nr, not reported
Study Quality
The Newcastle-Ottawa Scale was used to assess risk of bias in each included studies (Supplemental Table S2); the six measures that were graded were: (a) cohort representativeness, (b) selection of non-exposed cohort, (c) exposure ascertainment, (d) outcome absence at baseline, (e) cohort comparability, (f) assessment of outcome, (g) adequacy of observation duration and (h) completeness of cohort follow-up. Except for incomplete reporting of the proportion of the study cohort lost to follow up, the majority of studies had the highest quality-level indicators all measures. One study reported a highly-selected study population undergoing only cryoballoon ablation, which is unlikely representative of overall AF ablation population.20
AF Recurrence and Diagnosis-to-Ablation Time
All included studies defined AF recurrence as any documented AF or flutter lasting more than 30 seconds during the follow up period, with variable blanking periods (range 0 to 3 months).
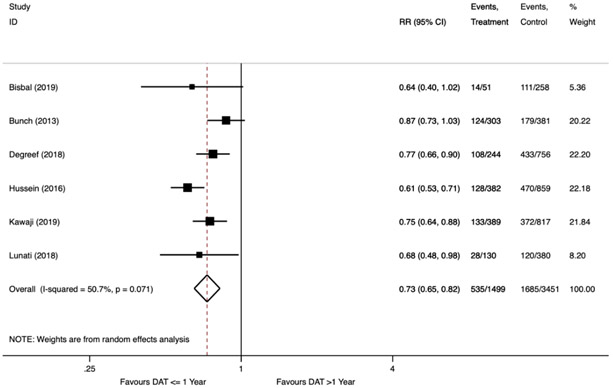
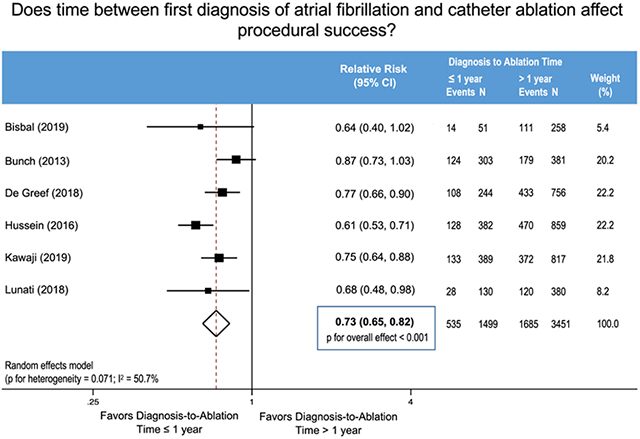
In the primary analysis, 535 (36%) AF recurrences were documented among 1,499 patients with DAT one year or less, whereas there were 1,685 (49%) AF recurrences among 3,451 patients with DAT greater than one year (Figure 2). A diagnosis-to-ablation time one year or less was associated with a reduced relative risk of AF recurrence following AF ablation (RR 0.73; 95% confidence interval (CI) 0.65 to 0.82, p<0.001). There was a moderate degree of heterogeneity (I2=51%, Cochran’s Q p=0.07). In our pre-specified sensitivity analyses, stepwise exclusion of the studies preserved the statistical benefit with similar estimated effect size in all cases (Table S3). When excluding Hussein et al., which studied a patient cohort consisting of only persistent AF, the heterogeneity improved substantially (I2=0%, Cochran’s Q p=0.55) with a similar estimate of effect (RR 0.78; 95% CI 0.71 to 0.85, p<0.001).
Figure 2.
Forest Plot Showing Recurrence of Atrial Fibrillation after Catheter Ablation Stratified by Diagnosis-to-Ablation Time ≤ 1 Year versus > 1 Year.
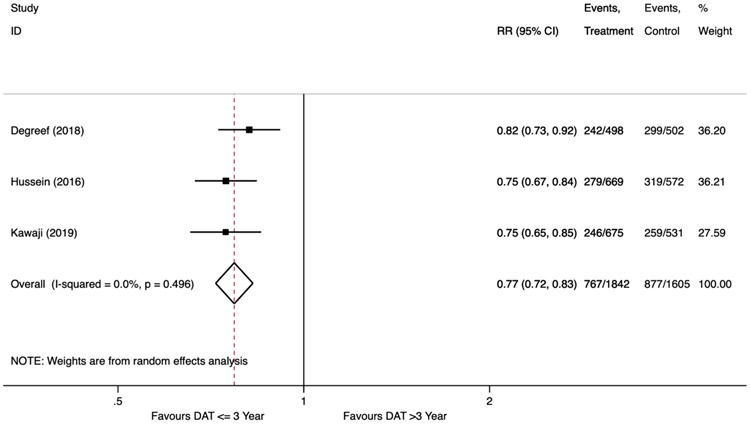
In our secondary analysis, only three studies reported AF recurrence rates stratified by DAT at three years.8, 9, 19 During the follow up period, there were 767 (42%) AF recurrences among 1,842 patients with a DAT three years or less, whereas there were 877 (55%) AF recurrences among the 1,605 patients with a DAT greater than three years. Similar to the primary analysis, a shorter diagnosis-to-ablation time of three years or less was associated with a reduced relative risk of AF recurrence (RR 0.77; 95% CI 0.72 to 0.83, p<0.001). There was no heterogeneity observed in the secondary analysis (I2=0%, Cochran’s Q p=0.5) (Figure 3).
Figure 3.
Forest Plot Showing Recurrence of Atrial Fibrillation after Catheter Ablation Stratified by Diagnosis-to-Ablation Time ≤ 3 Years versus > 3 Years.
To assess for the potential impact of confounders, we pooled the hazard ratios of studies that adjusted for covariates when reporting the primary outcome. Two studies reported the multivariable models of AF recurrence after adjusting for age, comorbidities, and DAT as a dichotomous variable (i.e. cut-point of 1 year).17, 20 DAT of one year or less was an independent predictor of post-ablation AF recurrence (pooled adjusted HR 0.41, 95% CI 0.18 to 0.93, p=0.03).
Only two studies reported the rates of mortality and HF hospitalization.18, 19 We were unable to perform a pooled analysis since the reported DAT time was not consistent across the studies for these outcomes.
Meta-bias
To evaluate for the presence of potential meta-bias, we plotted the standard error of the log risk ratio against the log risk ratio for the treatment effect (AF recurrence) (Supplemental Figure 1). The funnel plot appears asymmetric with the relative absence of small studies that favor DAT > 1 year. To explore for potential causes in this asymmetry, a contoured-enhanced funnel plot was created to explore for possible publication bias (Supplemental Figure 2). The majority of studies were either very statistically significant (p<0.01) or statistically significant (p<0.05). The relative paucity of studies with non-statistically significant findings raises the concern of publication bias, which would exaggerate the pooled effect estimate.
Discussion
In this meta-analysis, we found that a shorter duration between first diagnosis of AF and AF ablation is associated with lower rates of AF recurrence post-procedure. That is, diagnosis-to-ablation times of one year or less were associated with a 27% less risk of AF recurrence compared to DAT > 1 year. Our secondary analysis that explored a DAT threshold of 3 years also demonstrated lower rates of AF recurrence among those with shorter duration of DAT. To our knowledge, this is the first systematic review and meta-analysis exploring the impact of DAT on AF ablation outcomes.
Diagnosis-to-Ablation Time as a Marker of Atrial Remodeling
Diagnosis-to-ablation time is a likely surrogate for the status of atrial substrate and remodeling that occurs during the natural history of AF. That is, a more prolonged DAT and longer duration of ongoing AF reflect more progressive atrial remodeling, greater resistance to successful AF ablation, and higher AF recurrence rates.
Prior studies have explored the dynamic changes in structural, contractile and electrical remodeling associated with the progression of atrial fibrillation. Using voltage mapping to identify areas of scar, early studies demonstrated the association between LA fibrosis and AF recurrence following ablation.27 The advent of cardiac magnetic resonance imaging has provided further insight into the process of atrial remodeling and its impact on AF ablation success. The prospective multicenter DECAAF (Delayed-Enhancement MRI Determinant of Successful Radiofrequency Catheter Ablation of Atrial Fibrillation) study found a linear relationship between the degree of atrial fibrosis as identified by late gadolinium enhancement cardiac magnetic resonance (CMR) imaging and AF recurrence rates post-ablation.28 Similarly, in a retrospective cohort of 305 patients undergoing first AF ablation with pre-procedure CMR, Chelu et al. found that a higher degree of atrial fibrosis was associated with three-fold increased risk of AF recurrence and repeated ablation compared to patients with minimal fibrosis. Furthermore, higher degrees of left atrial fibrosis were associated with a longer history of preceding AF.29
Nevertheless, compared to imaging predictors of AF ablation outcome, diagnosis-to-ablation is a modifiable and actionable risk marker. That is, optimizing patient care processes to reduce the time to first AF ablation may increase the likelihood of procedural success and improvement in patient quality of life. These data have implications not only for improving outcomes, but also for cost-effectiveness. Nationwide data have shown that healthcare costs are significantly higher for patients undergoing repeat ablation, even after excluding the cost of the repeat ablation procedure itself.30 Thus, reductions in DAT may also lead to improvements in cost-effectiveness, a key priority given the current healthcare environment.
Timing of Pulmonary Vein Isolation
In the present American Heart Association / American College of Cardiology / Heart Rhythm Society guidelines for management of patients with AF, anti-arrhythmia drugs (AADs) are considered first-line treatment for symptomatic AF. As a first-line therapy, PVI is recommended in a limited subset of patients: (a) those with paroxysmal AF who are low risk for procedure-associated complications and patient preference for invasive management, and (b) possibly HF patients with severely reduced left ventricular ejection fraction. Often multiple AADs are trialed before consideration of ablation, despite suboptimal success at maintaining sinus rhythm compare to ablation.31, 32 A stepwise approach to rhythm management of AF likely delays referral and timely access to catheter ablation.33 Interestingly, there is emerging evidence that earlier ablation may alter the natural history of AF, and slow the progression from paroxysmal to persistent phenotypes.7 Furthermore, catheter ablation may be more effective compared to AAD at delaying progression to persistent AF.34 Additional study is required to understand this interaction between catheter ablation and AF progression, and whether the benefit is conferred due to the degree of rhythm control achieved. Nevertheless, efforts to improve wait list times following a decision to undergo AF ablation may be undertaken given the potential impact on patient prognosis.
Given the potential publication bias identified by our meta-analysis, additional studies are required to confirm the prognostic finding of DAT. Further research is also required to explore the impact of DAT on hard endpoints such as heart failure hospitalization and mortality. Only two of the six included studies reported these outcomes, albeit with conflicting results. Bunch et al. found that delays in ablation were associated with an increased risk of all-cause mortality and heart failure hospitalization at 1 year; however, Kawaji et al. found no significant difference in 5-year clinical outcomes between the short (≤3 years) and long (>3 years) DAT groups including ischemic stroke, HF hospitalization, and death. 9, 19
Limitations
The results of this meta-analysis should be interpreted in the context of several limitations. First, all included studies were retrospective cohort studies that varied in patient population, and details of AF ablation procedure, which likely contributed to the moderate heterogeneity of the pooled effect size in the primary analysis. Interestingly, upon exclusion of the study by Hussein et al. in our sensitivity analyses, study heterogeneity improved (I2=0). The inclusion of a cohort consisting of only persistent AF likely contributed to the increased heterogeneity, since the other study cohorts were mainly compromised of patients with paroxysmal AF. Second, the duration of follow up and definitions of AF recurrence varied between studies. Specifically, the post-ablation blanking period varied from zero to three months. Additionally, post-ablation monitoring varied substantially between the studies, ranging from 12-lead ECGs performed at AF clinic follow up to insertion of an implantable loop recorder. Nevertheless, in our sensitivity analyses where we excluded individual studies one at a time, the association between shorter DAT and lower AF recurrence remained constant. Finally, given the inclusion of a relatively small number of observational studies in the meta-analysis, our findings raise the concern of publication bias, which would exaggerate the pooled effect estimate. Several studies were excluded from the meta-analysis as they did not report post-ablation outcomes stratified by DAT despite reporting pre-ablation AF duration. Thus, the findings of this meta-analysis could potentially be strengthened by the open availability of primary data.
Conclusions
Duration between time of first AF diagnosis and AF ablation is associated with lower ablation procedural success and higher AF recurrence rates. A diagnosis-to-ablation time of one year or less is associated with a 27% lower risk in AF recurrence post-ablation. Further study is required to explore implementation of earlier catheter AF ablation and patient outcomes within the current AF care pathway.
Supplementary Material
What is known?
Procedural success is variable among patients undergoing catheter ablation for symptomatic atrial fibrillation.
What the study adds
The time between atrial fibrillation diagnosis and first AF ablation, or diagnosis-to-ablation time, is an emerging marker of clinical response to catheter ablation.
In this meta-analysis of observational studies, a diagnosis-to-ablation time of one year or less is associated with a 27% lower risk in AF recurrence post-ablation.
Acknowledgments:
We thank Dr. Felipe Bisbal for providing additional cohort characteristics to facilitate study inclusion in the meta-analysis.
Sources of Funding: DSC is supported by a Canadian Institutes of Health Research Banting Fellowship and an Arthur JE Child Cardiology Fellowship.
Nonstandard Abbreviations and Acronyms
- AAD
anti-arrhythmia drugs
- CI
Confidence Interval
- CMR
cardiac magnetic resonance imaging
- DAT
Diagnosis-to-Ablation Time
- LV
Left Ventricular
- MOOSE
Meta-Analysis of Observational Studies in Epidemiology
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
Footnotes
Disclosures: DVE receives grants for clinical research from Medtronic Inc, GE Healthcare, and St. Jude Medical, and is supported by a Government of Canada Tier 1 Research Chair in Cardiovascular Clinical Trials, the Cardiac Arrhythmia Network of Canada, and a Canadian Institutes of Health Research Operating Grant. JPP receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, NHLBI, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, Boston Scientific, LivaNova, Medtronic, Milestone, Sanofi, Philips, and Up-to-Date. The remaining authors have no relevant disclosures.
References:
- 1.Asad ZUA, Yousif A, Khan MS, Al-Khatib SM, Stavrakis S. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2019;12:e007414. [DOI] [PubMed] [Google Scholar]
- 2.Pallisgaard JL, Gislason GH, Hansen J, Johannessen A, Torp-Pedersen C, Rasmussen PV, Hansen ML. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: a nationwide Danish cohort study. Eur Heart J. 2018;39:442–449. [DOI] [PubMed] [Google Scholar]
- 3.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Trends in atrial fibrillation ablation: have we maximized the current paradigms? J Interv Card Electrophysiol. 2012;34:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nattel S. Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin Electrophysiol. 2017;3:425–435. [DOI] [PubMed] [Google Scholar]
- 5.Goudis CA, Kallergis EM, Vardas PE. Extracellular matrix alterations in the atria: insights into the mechanisms and perpetuation of atrial fibrillation. Europace. 2012;14:623–30. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–74. [DOI] [PubMed] [Google Scholar]
- 7.Proietti R, Hadjis A, AlTurki A, Thanassoulis G, Roux JF, Verma A, Healey JS, Bernier ML, Birnie D, et al. A Systematic Review on the Progression of Paroxysmal to Persistent Atrial Fibrillation: Shedding New Light on the Effects of Catheter Ablation. JACC Clin Electrophysiol. 2015;1:105–115. [DOI] [PubMed] [Google Scholar]
- 8.De Greef Y, Schwagten B, Chierchia GB, de Asmundis C, Stockman D, Buysschaert I. Diagnosis-to-ablation time as a predictor of success: early choice for pulmonary vein isolation and long-term outcome in atrial fibrillation: results from the Middelheim-PVI Registry. Europace. 2018;20:589–595. [DOI] [PubMed] [Google Scholar]
- 9.Hussein AA, Saliba WI, Barakat A, Bassiouny M, Chamsi-Pasha M, Al-Bawardy R, Hakim A, Tarakji K, Baranowski B, Cantillon D, et al. Radiofrequency Ablation of Persistent Atrial Fibrillation: Diagnosis-to-Ablation Time, Markers of Pathways of Atrial Remodeling, and Outcomes. Circ Arrhythm Electrophysiol. 2016;9:e003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
- 13.Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37:256–66. [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61:991–6. [DOI] [PubMed] [Google Scholar]
- 17.Bisbal F, Alarcon F, Ferrero-De-Loma-Osorio A, Gonzalez-Ferrer JJ, Alonso-Martin C, Pachon M, Valles E, Cabanas-Grandio P, Sanchez M, Benito E, et al. Diagnosis-to-ablation time in atrial fibrillation: A modifiable factor relevant to clinical outcome. J Cardiovasc Electrophysiol. 2019;30:1483–1490. [DOI] [PubMed] [Google Scholar]
- 18.Bunch TJ, May HT, Bair TL, Johnson DL, Weiss JP, Crandall BG, Osborn JS, Anderson JL, Muhlestein JB, Lappe DL, et al. Increasing time between first diagnosis of atrial fibrillation and catheter ablation adversely affects long-term outcomes. Heart Rhythm. 2013;10:1257–62. [DOI] [PubMed] [Google Scholar]
- 19.Kawaji T, Shizuta S, Yamagami S, Aizawa T, Komasa A, Yoshizawa T, Kato M, Yokomatsu T, Miki S, Ono K, et al. Early choice for catheter ablation reduced readmission in management of atrial fibrillation: Impact of diagnosis-to-ablation time. Int J Cardiol. 2019;291:69–76. [DOI] [PubMed] [Google Scholar]
- 20.Lunati M, Arena G, Iacopino S, Verlato R, Tondo C, Curnis A, Porcellini S, Sciarra L, Molon G, Senatore G, et al. Is the time between first diagnosis of paroxysmal atrial fibrillation and cryoballoon ablation a predictor of efficacy? J Cardiovasc Med (Hagerstown). 2018;19:446–452. [DOI] [PubMed] [Google Scholar]
- 21.Yu HT, Kim TH, Uhm JS, Kim JY, Joung B, Lee MH, Pak HN. How long the duration of atrial fibrillation is associated with poor rhythm outcome after catheter ablation? Heart Rhythm. 2018;15:S426. [Google Scholar]
- 22.Nalliah CJ, Lim TW, Kizana E, Qian P, Kovoor P, Thiagalingam A, Ross DL, Thomas SP. Clinical significance of early atrial arrhythmia type and timing after single ring isolation of the pulmonary veins. Europace. 2015;17:1038–44. [DOI] [PubMed] [Google Scholar]
- 23.Gaztanaga L, Frankel DS, Kohari M, Kondapalli L, Zado ES and Marchlinski FE. Time to recurrence of atrial fibrillation influences outcome following catheter ablation. Heart Rhythm. 2013;10:2–9. [DOI] [PubMed] [Google Scholar]
- 24.Jarrett-Smith L, Butcher C, Lysitsas DN, Wong T, Markides V, Jones DG, Hussain W. Does time from diagnosis of atrial fibrillation effect success of catheter ablation regardless of arrhythmic burden? Heart Rhythm. 2015;12:S48. [Google Scholar]
- 25.Barakat AF, Hussein AA, Bassiouny M, Hakim A, Al Halabi S, Tarakji K, Baranowski B, Cantillon D, Dresing T, Tchou P, et al. Radiofrequency ablation for persistent atrial fibrillation: Earlier catheter-based interventions are associated with better outcomes and in direct association with markers of pathways of atrial remodeling. Circulation. 2015;132. [Google Scholar]
- 26.Eickholt C Finding the right timing in cryoballoon ablation for pulmonary vein isolation. Int J Cardiol. 2018;255:99–100. [DOI] [PubMed] [Google Scholar]
- 27.Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, et al. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–92. [DOI] [PubMed] [Google Scholar]
- 28.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. [DOI] [PubMed] [Google Scholar]
- 29.Chelu MG, King JB, Kholmovski EG, Ma J, Gal P, Marashly Q, AlJuaid MA, Kaur G, Silver MA, Johnson KA, et al. Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging and Catheter Ablation of Atrial Fibrillation: 5-Year Follow-Up Data. J Am Heart Assoc. 2018;7:e006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansour M, Karst E, Heist EK, Dalal N, Wasfy JH, Packer DL, Calkins H, Ruskin JN, Mahapatra S. The Impact of First Procedure Success Rate on the Economics of Atrial Fibrillation Ablation. JACC Clin Electrophysiol. 2017;3:129–138. [DOI] [PubMed] [Google Scholar]
- 31.Khan SU, Rahman H, Talluri S, Kaluski E. The Clinical Benefits and Mortality Reduction Associated With Catheter Ablation in Subjects With Atrial Fibrillation: A Systematic Review and Meta-Analysis. JACC Clin Electrophysiol. 2018;4:626–635. [DOI] [PubMed] [Google Scholar]
- 32.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA. 2019;321:1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pappone C, Radinovic A, Manguso F, Vicedomini G, Ciconte G, Sacchi S, Mazzone P, Paglino G, Gulletta S, Sala S, et al. Atrial fibrillation progression and management: a 5-year prospective follow-up study. Heart Rhythm. 2008;5:1501–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.