Abstract
Japan’s Advanced Medical Care Program (AMCP) seeks to facilitate patient access to promising healthcare technologies through National Health Insurance (NHI) coverage. This study aimed to examine AMCP’s contribution to the accelerated introduction of new technologies through NHI coverage. AMCP‐type B technologies registered May 2006–March 2019 were examined. To investigate the use of AMCP for NHI coverage, data from the AMCP website and from regulatory authority documents were used. Of 127 AMCP‐type B technologies, 38 underwent final review. Fifteen technologies were successfully introduced into NHI coverage. Eight technologies introduced directly through the Advanced Medical Care Conference were related to medical devices. Other technologies, including drugs, required additional accelerated frameworks for market approval. A strategic approach with the careful selection of target therapeutic technologies and accelerated frameworks is key for the rapid introduction of medical technologies through AMCP.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Japan’s Advanced Medical Care Program (AMCP) aims to facilitate patient access to innovative health care through National Health Insurance (NHI) coverage, but little is known regarding the relationship between AMCP data and NHI coverage for innovative technologies.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This study investigated the AMCP system’s contribution to the accelerated introduction of new technologies through NHI coverage in Japan.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ This first comprehensive review of the NHI coverage outcomes of innovative technologies evaluated using AMCP data showed that these data were used for NHI coverage or regulatory approval for only 15 technologies (May 2006–March 2019). AMCP data were used for clinical evaluation in the regulatory approval of medical technologies or, less often, to introduce already‐approved, high‐risk technologies into NHI coverage.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ The findings indicate that facilitating the rapid introduction of medical technologies through the AMCP system will require a strategic approach and careful selection of target therapeutic technologies and accelerated frameworks.
Japan has a well‐established universal health coverage (National Health Insurance (NHI)).1 Different from private insurance, as long as health cares are listed by Ministry of Health, Labour, and Welfare (MHLW), the equity has been achieved under NHI among almost all patients by imposing the same fees for each treatment, (~ 70% are covered by NHI). To be on the list of NHI, a market approval by Japanese regulatory agency; Pharmaceutical Medical Device Agency is needed for any new indications. Reviews in Pharmaceutical Medical Device Agency are principally done for application data from investigational new drug (IND) trials called “Chiken” under the Law on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices 2 (the Pharmaceutical Medical Device Law) and Good Clinical Practice Ministerial Ordinance in Japan.3 Exceptions are drugs/ devices used in off‐label indications or already approved overseas that show a certain amount of evidence can be approved without using new IND trial data, but by referring public domain information, such as publications from non‐IND trials or postmarketing surveys (“Kouchi Shinsei”). On the other hand, regulatory approval is not required for medical technology that is not distributed in the market, such as surgery, but for introduction to NHI, clinical evidence is used every 2 years at the time of NHI medical fee revision based on the opinions from expert society or patient requests. Closely linked to regulatory approvals, this universal health insurance system has made it possible for all Japanese patients to receive quality standard care equally. Given that it is understandable that the introduction of NHI requires a high degree of evidence, and mixed claims are not permitted, there is a strict distinction as to whether NHI is covered. Universal insurance system applies only to treatments that provide clinical evidence equivalent to regulatory approval, but it does not apply to drugs that are delayed for regulatory approvals with any reasons. “Mixed claims are only allowed for “Chiken,” and basically not used for non‐IND tested/unapproved products. As a result, it is difficult for patients to access these drugs in the current situation in Japan. There was a criticism that this could disturb patients from quick access to off‐label or already‐approved medical products even showing promising risk benefits over the risks.4 The Advanced Medical Care Program (AMCP) was established in 2004 through an agreement between the MHLW and the Ministry of State for Special Missions. The precursor to the AMCP, the Highly Advanced Medical Care System, began in 1984 and was later updated and renamed.5 AMCP has started as an exceptional non‐IND clinical trial that allows physicians to apply unapproved technologies under the NHI system using mixed billing. There are two categories in AMPC; type A and B: AMPCs using unapproved or approved but highly invasive new technologies are categorized into AMPC‐type B, whereas approved or unapproved but minimally invasive new technologies are categorized as AMCP‐type A (Figure 1). Although the AMCP does not concern formal IND trials, AMCP data are considered relevant for evaluating the future implementation of technologies in Japan’s NHI scheme. Thus, the AMCP requires highly reliable data obtained from clinical trial settings for future assessment following NHI application.
Figure 1.
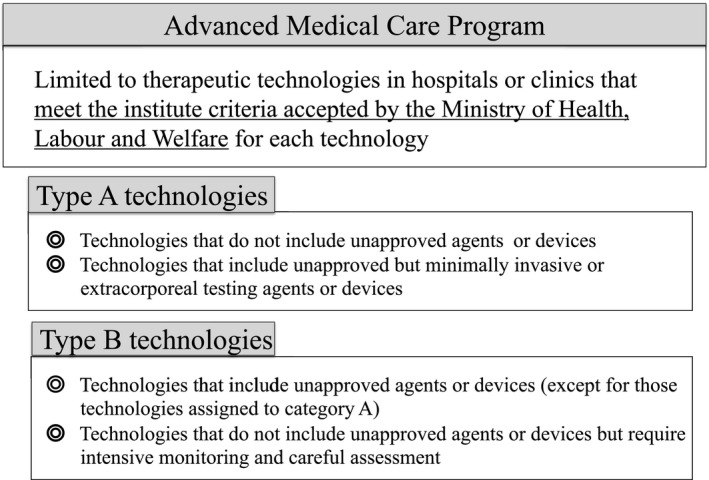
Brief overview of the Advanced Medical Care Program. The categories include (i) type A technologies, which do not include unapproved agents or devices or do include unapproved items but are minimally invasive or extracorporeal testing agents/devices and (ii) type B technologies, which include unapproved agents or devices or do not include unapproved agents or devices but require intensive monitoring and careful assessment.
However, although one aim of the AMCP is to contribute to the NHI coverage of promising technologies through the AMCP‐type B classification, the route to NHI coverage is more obscure with the AMCP than with IND and other associated clinical trials conducted under programs facilitating new drug and device development. Furthermore, few publications have discussed the relationship between AMCP‐type B results and regulatory approvals or NHI coverage, including the perspectives how to consider AMCP for currently initiated rapid drug development frameworks, such as “Sakigake package strategy” which is similar to the US “breakthrough therapy designation” or “compassionate use program.”2, 6, 7
Therefore, this study aimed to review the features of AMCP data, especially those using unapproved drugs or medical devices (AMCP‐type B) and to investigate the relationship between the AMCP results and NHI coverage to shed light on potential research and development strategies for innovative drugs and medical devices.
MATERIALS AND METHODS
AMCP assessments listed on MHLW webpages from May 2006 to March 2019 were examined. The documents and meeting minutes of the Review Board and its predecessor, the Advanced Medical Technology Evaluation Conference, and of the AMC Conference and its predecessor, the Advanced Medical Care Expert Conference, were obtained from the MHLW’s website (http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/iryouhoken/sensiniryo/). Technologies defined as AMCP‐type B were identified in the documents as technologies approved by the Review Board and the AMC Conference during the study period.
AMCP‐type B technologies contain an unapproved drug or medical device as well as advanced medical technology and have been called Highly Advanced Medical Care (2010–2012), Section 3 Advanced Medical Care (2012–2016), and finally AMCP‐type B technologies (2016–). Similarly, AMCP‐type A technologies have been called Advanced Medical Care (2010–2012) and Section 2 Advanced Medical Care (2012–2016), in addition to the current designation of AMCP‐type A technologies (2016–) (Figure 1).
Data collected included the technology’s name and status. The status of clinical trials for AMCP‐type B technologies was “on‐going” or “study terminated.” “Study terminated” technologies included “evaluation completed,” “pending evaluation,” and “study discontinued.” A “pending evaluation” status indicated that a clinical study had been completed but did not meet the criteria for “evaluation completed.” The technologies classified as “study discontinued” had been marked for discontinuation of AMCP‐type B before completion of the clinical trial plan. For technologies with this status, the reasons for discontinuation were classified as being because of reasons related to physicians, industry, or both.
For “evaluation completed” technologies, either a clinical research report had been submitted to and reviewed by the Review Board or an evaluation of the study results was identified in the documents. In brief, a technology receiving a Review Board score of “A” from more than two of three or all three reviewers, or a score of “B” with positive comments on the acceleration of regulatory approval or NHI introduction were considered to be evaluated as “high.” Those scored “B” by more than two reviewers or all three reviewers scored “C” with positive comments were considered to be evaluated as “intermediate.” Technologies receiving a score of “C” to “D” from more than two reviewers or all three reviewers and those with negative comments for the next steps considered to be evaluated as “low.”
Further evaluation was conducted for “evaluation completed” technologies. For these technologies, we examined the clinical research evaluation results, the Review Board’s meeting minutes, and the published literature referenced in these documents. On the basis of this information, we selected technologies identified for NHI coverage or for regulatory approval. In selecting these technologies, we checked for medical fee revisions for NHI coverage of advanced medical technologies, and we confirmed regulatory approval (including the review process) using published Pharmaceuticals and Medical Devices Agency documents (reports and application dossiers). For the review process, we confirmed the possibility of using AMCP‐type B data in the review report, the presence or absence of relevant clinical trials or previous research, related systems including the fast‐track scheme for unapproved drugs of the Conference on Unapproved Drugs or Off‐label Prescription Drugs for Unmet Medical Needs (“Conference on Unapproved Drugs”) and the Orphan Drug Designation System, and the early introduction of medical devices judged to meet unmet medical needs. Finally, we also confirmed whether there were technologies treated by special notification from the MHLW where NHI introduction was not confirmed despite a “high” or “intermediate” final evaluation. The clinical trials design and sample size for AMPC‐type B that are associated to the NHI coverage or for regulatory approval were also examined.
The AMCP technologies in each category were counted and reported as percentages. Each technology was descriptively evaluated on how the AMCP review result impacted NHI coverage or regulatory approval.
This retrospective review did not enroll human subjects.
RESULTS
We identified 127 AMCP‐type B technologies during the study period. Figure 2 shows the clinical trial status for these technologies. A total of 66 technologies (52.0%) were categorized as “study terminated,” 32 (25.2%) as “evaluation completed,” 16 (12.6%) as “pending evaluation,” and 18 (14.2%) as “study discontinued” (Figure 2). From the 32 studies with the status of “evaluation completed,” 15 (11.8%) technologies obtained regulatory or NHI coverage approval (Table 1).
Figure 2.
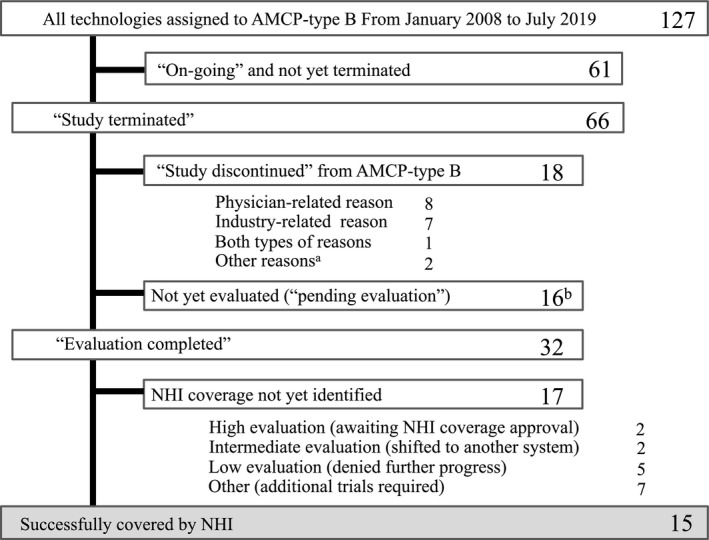
Flowchart for all technologies designated as Advanced Medical Care Program (AMCP)‐type B from January 2008 to March 2019. Of 66 studies that were conformed to terminate, evaluations were completed in 32. The technologies in 15 of these studies were introduced into National Health Insurance (NHI) coverage, whereas 17 were not. The details of the 15 covered technologies are shown in Table 1. Seventeen were not yet identified for NHI coverage, but two of these were evaluated as “high” and were awaiting pharmaceutical approval. Another two of these technologies, evaluated as “intermediate,” were confirmed and introduced through a different system (a Japanese system of companionate use: patients dispensation care (n = 1) or a special insurance framework (n = 1)). Five technologies that were evaluated as “low” were not promoted for regulatory approval or introduced into NHI coverage. Seven technologies were to undergo additional study, including investigator‐initiated clinical trials or another AMCP‐type B in the next phase (n = 7). One study was judged as inappropriate for fast‐track approval (n = 1), and 18 were discontinued for development via AMCP‐type B because of physician‐related reasons (n = 8), industry‐related reasons (n = 7), or both types of reasons (n = 1). Two technologies were introduced into NHI coverage by medical insurance revision before the technologies were evaluated through AMCP‐type B. aThese technologies were introduced into NHI coverage through medical fee revision in 2018 before completing AMCP‐type B clinical trials. bThis includes cases where the process of evaluation was unclear.
Table 1.
Advanced Medical Care Program‐type B technologies ultimately covered by the National Health Insurancea
| Name of technology | Category | n d | Path to NHI coverage | Special track |
|---|---|---|---|---|
| Designated for NHI overage via AMC Conference recommendation | ||||
| Da Vinci robotic‐assisted surgery for partial nephrectomy | Device | 100 | AMCP‐type B validation | |
| Da Vinci robotic‐assisted surgery for prostatectomy | Device | 176 | AMCP‐type B validation | |
| Da Vinci robotic‐assisted surgery for gastrectomy | Device | 300 | AMCP‐type B validation | |
| Endoscopic neck lumpectomy for benign tumor | Technology | ‐ | AMCP‐type A validation after AMCP‐type Be | |
| Endoscopic neck lumpectomy for malignancy | Technology | ‐ | AMCP‐type A validation after AMCP‐type Be | |
| Laparoscopic hepatectomy | Technology | ‐ | AMCP‐type A validation after AMCP‐type Be | |
| Radio wave hepatectomyb | Technology | 80 | AMCP‐type B validation | |
| Genetic diagnosis of malignant lymphoma in the sentinel lymph node | Technology (diagnostic) | 95 | AMCP‐type B validation | |
| Designated for NHI coverage via conventional pharmaceutical approval | ||||
| Cochlear implant | Device | 24 | Reference for conventional approval | Needed device |
| Leptin for lipodystrophy | Drug | 12 | Reference for conventional approval | Orphan |
| Fetal ventriculoperitoneal shunt | Device | 20 | Clinical evaluation of medical device for conventional approval | Orphan |
| Sentinel lymph node identification for malignant lymphoma | Drug | 6 | Equivalent to application based on public knowledge | Unmet need (public domain) |
| Sentinel lymph node identification for breast cancer | Drug | 516 | Equivalent to application based on public knowledge | Unmet need (public domain) |
| Da Vinci transoral robotic surgery for laryngopharyngeal cancerc | Device | 16 | Reference for conventional approval | Needed device |
| Angiogenesis by HGF gene therapy | Regenerative medicine | 6 | Reference for conditional time‐limited approval | Time‐limited conditional |
AMC Conference, Advanced Medical Care Conference; AMCP, Advanced Medical Care Program; HGF, hepatocyte growth factor; NHI, National Health Insurance.
All clinical trials in this table are done with single arm design.
Radio wave technology was approved in 2005, and the technology for hepatectomy was introduced into NHI coverage with AMCP‐type B data.
Da Vinci transoral robotic surgery is approved for laryngopharyngeal cancer but is not yet covered by the NHI.
Sample size for a clinical trial under the AMCP‐type B.
AMPC type A do not require a form of clinical trial, but a practice with registries, sample sizes for clinical trials were not found in the public documents from Ministry of Health, Labour, and Welfare.
The discontinued studies included technologies terminated because of industry‐related reasons (n = 10), physician‐related reasons (n = 7), or both types of reasons (n = 1; Table 2). Studies terminated for industry‐related reasons included four studies that received market approval before completing the AMCP‐type B clinical trial. Three studies were discontinued because of strategic changes in the drug/device development. Competitive product approval (n = 2) and manufacturing reasons (n = 1) were also reported. Physician‐related reasons for discontinuation were poor recruitment (n = 2), deviation from the AMCP rules (n = 2), safety concern (n = 1), ineffectiveness stop (n = 1), and technical difficulty (n = 1).
Table 2.
Discontinuation of AMCP‐type B technologies before study completion
| Discontinuation of AMCP‐type B | 18 |
|---|---|
| Industry‐related reasons | 10 |
| Approval outside of AMCP‐type B | 4a |
| Market approval of a similar product | 2 |
| Other changes in the research and development strategy | 3 |
| Manufacturing‐related reason | 1 |
| Physician‐related reasons | 7 |
| Recruitment difficulty | 2 |
| Technical difficulty | 1 |
| Safety | 1 |
| Lack of effectiveness | 1 |
| Deviation from the AMCP rules | 2 |
| Both types of reasons | 2 |
AMCP, Advanced Medical Care Program.
This includes two technologies that were introduced into National Health Insurance coverage through medical fee revision in 2018 before completing AMCP‐type B clinical trials.
Regarding technologies whose evaluation had been completed but for which NHI coverage was not determined, two received “high” evaluations and were considered to deserve fast‐track designation but were not confirmed in the subsequent process (preparation for application based on data from the public domain). Two other studies received “intermediate” evaluations and were confirmed after being shifted to another system, such as the patient‐proposed health service or treatment by the special notification from the MHLW regarding the additional information required to assign a medical fee. Another eight of these studies received “intermediate” evaluations but were confirmed to have changed in design, a new AMCP‐type B was implemented for the next phase (n = 3), and two technologies were introduced into the fast‐track approval process (the Conference on Unapproved Drugs or early introduction of medical devices serving unmet needs; Table 3).
Table 3.
AMCP‐type B technologies for which NHI coverage was not identified after study completion
| No NHI or regulatory coverage after AMCP‐type B | 17 |
|---|---|
| High evaluation | 2 |
| Market approval in process | 2 |
| Intermediate evaluation | 10 |
| Shift to clinical trial framework driven by patient demand | 1 |
| “Special notification” | 1 |
| Move to another clinical trial: AMCP‐type B | 3 |
| Move to another clinical trial: IND trial | 2 |
| Move to another clinical trial: Other | 3 |
| Low evaluation | 5 |
AMCP, Advanced Medical Care Program; IND, investigational new drug; NHI, National Health Insurance.
The technologies with confirmed NHI coverage or regulatory approval were classified as those whose NHI coverage was determined by the Advanced Medical Care Conference (“AMC Conference”) associated with medical fee revision in 2 years and those covered by the NHI after regulatory approval (Table 1). Technologies whose coverage was determined by the AMC Conference included (i) surgical technologies for endoscopic neck lumpectomy and laparoscopic hepatic resection, which were judged as appropriate for NHI coverage by the AMC Conference after being changed from the originally designated as AMCP‐type B category to AMCP‐type A; (ii) robotic partial nephrectomy, prostatectomy, and gastrectomy, for which the medical device (da Vinci robotic‐assisted surgery) already had regulatory approval but needed AMCP‐type B data for NHI coverage; and (iii) other surgical technologies and diagnostics, including laparoscopic hepatectomy with radiofrequency ablation and genetic diagnosis of malignant lymphoma in the sentinel lymph node.
Technologies covered by the NHI after regulatory approval included (i) fetal thoracoamniotic shunts and da Vinci transoral robotic surgery for laryngopharyngeal cancer, for which AMCP‐type B data were used as part of the devices’ clinical evaluations; (ii) those for which the reference material of medical devices and drug applications were used (leptin replacement therapy for lipodystrophy, acoustic amplification cochlear implantation, and hepatocyte growth factor (HGF) gene therapy); and (iii) those used as domain data for “Kouchi Shinsei” (sentinel lymph node identification for breast cancer and for malignant lymphoma). Technologies covered by the NHI after regulatory approval used AMCP data as reference data for the application dossier, combined with investigator‐led clinical trial data. For example, AMCP‐type B data were used as a reference for the long‐term safety for leptin replacement therapy and for Japanese data on HGF gene therapy. AMCP results for cochlear implantation, combined with data from a multicenter study, were used as clinical evaluation materials; and the efficacy and safety of fetal shunts were evaluated using an AMCP‐type B study, combined with other studies, because of the difficulty of conducting an IND trial given the vulnerable target population.
Overall, two technologies were given orphan designation (fetal shunts and leptin therapy); one was designated as a medical device meeting unmet medical needs (cochlear implantation); and two (sentinel lymph node identification for breast cancer and for malignant lymphoma) were identified as appropriate for other systems, including evaluation by the Conference on Unapproved Drugs, especially the fast‐track scheme or application based on public domain data. HGF gene therapy was approved through a conditional, time‐limited approval for regenerative medicine.
Regarding the Review Board’s evaluation, final reports were rarely found for technologies that were successfully covered by the NHI or that obtained market approval because the clinical trials of most of these technologies began before 2006, and the evaluation system has changed over time. However, one drug (leptin) in this category was evaluated as “high” and two others (HGF gene therapy and da Vinci robotic‐assisted surgery for laryngopharyngeal cancer) were evaluated as “intermediate.”
DISCUSSION
The present study was the first attempt to comprehensively review the information regarding AMCPs submitted to the Advanced Medical Technology Review Board (“Review Board”) and their NHI coverage outcomes. AMCP‐type B data were used for NHI coverage or regulatory approval for only 15 technologies during the study period. This is not surprising considering the difficulty of assessing AMCP‐type B technologies. Whereas several AMCP‐type A technologies can be introduced into the NHI system with every medical fee revision, unapproved AMCP‐type B technologies are seldom incorporated directly through NHI revisions without regulatory approval. Similar to Medicare Clinical Trial Policy system in the United States, 8 AMPC‐type B is a partial implementation of the NHI payment to the clinical research. A mixed billing for unapproved marketing products can diminish patient’s financial burdens as well as facilitate data collection for market approvals. However, our study showed that AMPC‐type B data were rarely used for regulatory application.
Looking at the 15 technologies for which AMCP‐type B data were used for NHI coverage and regulatory approval, several patterns in AMCP data use can be identified. First, some AMCP‐type B technology data are used for clinical evaluation in the regulatory approval of medical devices. Here, “clinical evaluation” refers to the procedure of collecting, appraising, and analyzing clinical data pertaining to a medical device without conducting an IND trial. This includes clinical research pertaining to the examined medical device or similar devices, irrespective of whether these data have been published, while also taking into account case reports, complaints, and failure reports. Our findings indicated that the use of AMCP data for the clinical evaluation of a device is usually combined with other rapid approval frameworks. Notably, one of the examined devices was given orphan designation because of its indication for fetal treatment, which is an ethically complicated situation, making conducting an industry‐led clinical trial for this device difficult. In another case, a device was judged to meet unmet medical needs. The potential users of this device were too few for significant industry resources to be devoted toward developing the device, but there was a willingness to work with researchers if they had reliable data on the device’s efficacy and safety. AMCP‐type B study protocols are subject to a certain level of expert review, and these studies can thus be considered sufficient for use as clinical evidence. Therefore, AMCP‐type B trials conducted by physicians could be an initial step in involving industry in clinical development in challenging fields.
A second pattern was found for three technologies in applications for reimbursement coverage for already‐approved medical devices. This is a rare case because AMCP‐type B assessment is generally for unapproved technologies, but it can also apply to approved technologies considered to be relatively high risk. Several technologies fall into this category (e.g., robotic surgery), and the targets for market approval and NHI coverage are not always the same (e.g., in the case of the da Vinci Surgical System); therefore, the MHLW required reliable clinical data on this technology, including AMCP data, to evaluate the technology for reimbursement. This case suggests a possibility for formulating a strategic approach using AMCP‐type B assessment.
In contrast, it may be difficult to use AMCP data for drug approvals. The present study found that four drugs used AMCP‐type B data for their approvals, including two that had been used as off‐label drugs for a long time and were approved through “Kouchi Shinsei” using public domain information. Another drug, which treated an orphan disease, making it difficult to conduct an industry‐led clinical trial, was approved for marketing on the basis of an investigator‐led clinical trial conducted concurrently with an AMCP‐type B assessment. HGF gene therapy that once had not been approved but finally been approved with the regulation of time‐limited conditional approval after the revision of the Pharmaceutical Device Law.9 AMCP‐type B was reviewed as a Japanese data accompanying with US clinical trials for regulatory approvals for HGF gene therapy approvals. These cases suggest that favorable AMCP‐type B results can consist of an application package for market authorization, even for drugs, as part of the assessment of efficacy/safety, as a complement to public domain data, or as references for the conventional approval process. Interestingly, AMCP‐type B “pre‐agreements” with the regulatory agency, regarding Japanese data for HGF gene therapy and clinical evaluation for da Vinci robotic‐assisted surgery, for example, were associated with successful regulatory approval, despite these technologies being evaluated as “intermediate.” This observation suggests the importance of having a strategy before initiating the AMCP process.
Our data suggested that the background of drug lags or long‐term off‐label uses are associated with success cases of AMCP‐type B. Such attempts to compensating drag lags with a framework of Expanded access/Compassionate Use (EAP/CU) is being implemented in many countries.10, 11 EAP/CU is exceptionally applied for a patient in a life‐threatening condition where investigational drugs are usually on the market but difficult to obtain the regulatory authorization. However, there is a controversy over the use of EAP/CU data for market approvals. Bannik et al. point out that EAP/CU should not be a replacement for a well‐planned clinical trial due to a various type of bias.12 The aim of AMPC‐type B is not similar to that of EAP/CU, but it has an aspect to make patients access the promising regulatory unapproved/NHI uncovered products. Therefore, AMPC has similar difficulty regarding data collection from the clinical trial for off‐label/lagged drug application, which has been strongly demanded. More complexity, the scope of AMPC‐ type B has been expanded these days for not only examining an off‐label use, but also promoting an innovative drug. Our findings on the success case of AMPC‐type B indicated that the purpose of AMPC‐type B to eliminate delayed approvals/off‐label use of drugs should be emphasized. On the other hand, using AMPC‐type B data for new drug approval should be carefully discussed considering a differentiation from the various other frameworks for accelerating regulatory approvals for innovative medicine.7, 13, 14
This study had several methodological limitations. First, we could not include discussions regarding NHI coverage outside the AMCP system, including the “1980 MHLW Notification” and the subcommittee for Health Technical Assessment in Central Social Insurance Medical Council requested by the medical academic society, such as Social insurance Union of Societies related to Internal medicine/surgery. The “1980 MHLW Notification” from MHLW tells Health Insurance Claim Review and Reimbursement Services that unapproved/uncovered drug uses can be considered similarly to the covered medications based on its similar pharmacological action. Our search strategy did not systematically identify cases using this information. Second, there were also cases where AMCP data were utilized as reference material in application dossiers for similar products rather than for unapproved drugs or medical devices designated as AMCP‐type B, but systematic investigation of this indirect use of AMCP data is difficult. A further limitation involves problems associated with several revisions to the AMCP system, where the names of some technologies were slightly changed, leading to confusion in identifying NHI coverage when data from several assessments with similar names may be used, whether those names indicate one technology or not. Future research should address the above limitations.
This first review of NHI coverage outcomes for AMCP technologies found that only 15 AMCP‐type B technologies obtained regulatory or NHI coverage approval during the study period. Especially when conducting an industry‐led clinical trial would be problematic, AMCP data are sometimes used to evaluate medical devices, usually in combination with other rapid approval frameworks. AMCP data are also used in NHI coverage applications for approved devices considered high risk. Using AMCP results to introduce NHI coverage will require a strategic approach with careful selection of targeted therapeutic technologies throughout the product development process.
Funding
This study was supported by a University Grant from Osaka University and Oita University.
Conflict of Interest
S.S. and N.U. are members of in the Technical Review Board for the Advanced Medical Care Program. There are no other disclosures, including employment, consultancies, honoraria, stock ownership, stock options, expert testimony, grants received or pending, patents received or pending, royalties, or in‐kind contributions. K.U. declared no competing interest for this work. As an Associate Editor for Clinical and Translational Science, Naoto Uemura was not involved in the review or decision process for this paper.
Author Contributions
K.U. wrote the manuscript. K.U. and S.S designed the research. K.U, S.S., and U.N. performed the research. K.U. analyzed the data.
Acknowledgments
H. Hase and S. Toyomaru (Medical Affairs Division, The University of Tokyo Hospital) and A. Katayama A (Center for Innovative Clinical Medicine, Okayama University) provided support for the data collection. We thank Jennifer Barrett, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1. Ikegami, N. , Yoo, B.K. & Hashimoto, H. Japanese universal health coverage: evolution, achievements, and challenges. Lancet 378, 1106–1115 (2011). [DOI] [PubMed] [Google Scholar]
- 2. Law on securing quality, efficacy and safety of pharmaceuticals, medical devices, regenerative and cellular therapy products, gene therapy products, and cosmetics. Law No. 145, August 10, 1960; Final Revisions: Law No. 69, June 13, 2014.
- 3. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) . Harmonised tripartite guideline: guideline for good clinical practice E6 (R2) (Current Step 4 version). (ICH, 2016). [Google Scholar]
- 4. Fujiwara, Y. et al Japanese universal health care daces a crisis in cancer treatment. Lancet Oncol. 16, 251–252 (2015). [DOI] [PubMed] [Google Scholar]
- 5. Fujiwara, Y. III. Advanced Medical Care B and patient‐proposed health services [in Japanese]. Nihon Naika Gakkai Zasshi 105, 2336–2345 (2016). [PubMed] [Google Scholar]
- 6. Ministry of Health, Labour and Welfare . Strategy of SAKIGAKE. Ministry of Health, Labour, and Welfare; <https://www.mhlw.go.jp/english/policy/health-medical/pharmaceuticals/140729-01.html> (2014). Accessed August 18, 2019. [Google Scholar]
- 7. Fujiwara, Y. Evolution of frameworks for expediting access to new drugs in Japan. Nat. Rev. Drug Discov. 15, 236–294 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Medicare Clinical Trial Politics, Center for Medicare & Medicade Service <https://www.cms.gov/Medicare/Coverage/ClinicalTrialPolicies/index> Accessed January 30, 2020.
- 9. Review Reports; Regenerative Medical Products “Collategene® beperminogene perplasmid (March 2019) <https://www.pmda.go.jp/english/reviewservices/reviews/approved-information/0004.html>
- 10. Wileman, H. & Mishra, A. Drug lag and key regulatory barriers in the emerging markets. Perspect. Clin. Res. 1, 51–56 (2010). [PMC free article] [PubMed] [Google Scholar]
- 11. Klopfenstein, M. et al Expanded access programs: ethical and practical considerations for biopharmaceutical sponsors. Ther. Innov. Regul. Sci. 49, 352–358 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bunnik, E.M. , Aarts, S. & van de Vathorst, S. Little to lose and no other options: ethical issues in efforts to facilitate expanded access to investigational drugs. Health Policy 122, 977–983 (2018). [DOI] [PubMed] [Google Scholar]
- 13. Yamanaka, T. & Kano, S. Strategic balance of drug lifecycle management options differs between domestic and foreign companies in Japan. Expert Opin. Ther. Pat. 26, 497–503 (2016). [DOI] [PubMed] [Google Scholar]
- 14. Springer Nature . Stem the tide: Japan has introduced an unproven system to make patients pay for clinical trials. Nature 528, 163–164 (2015). [Google Scholar]


