Abstract
Rivaroxaban is a direct‐acting oral anticoagulant approved to prevent strokes in patients with atrial fibrillation. Dosage recommendations are approved for all adult patients to receive either 15 mg or 20 mg once daily depending upon renal function. There are a number of reasons to believe rivaroxaban dosing could be more effective and/or safer for more patients if increased dosing precision is available. Because real‐world patients are more diverse than those studied in phase III clinical trials, we evaluated the extremes of creatinine clearance (CrCl) on rivaroxaban clearance using a published population pharmacokinetic model and applying exposure variation limits (±20%) based on published literature. The proposed dosing recommendations are 10 mg once daily (CrCl 15–29 ml/min), 15 mg once daily (CrCl 30–69 ml/min), 10 mg twice daily (CrCl 70–159 ml/min), and 15 mg twice daily (CrCl 160–250 ml/min). These new dosing recommendations should be prospectively tested for predictive accuracy and to assess the impact on AF patient efficacy and safety.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Rivaroxaban is a relatively safe and effective treatment for atrial fibrillation in preventing strokes, but may be relatively inferior to the other direct‐acting oral anticoagulants for efficacy or safety. There is evidence that rivaroxaban efficacy and safety could be increased with more precise dosing.
WHAT QUESTION DID THIS STUDY ADDRESS?
More precise rivaroxaban dosing recommendations are provided for a wider patient range of creatinine clearance than was studied in the phase III trial, but which exist in real‐world patients.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Dosage recommendations have been provided for a broader range of patient renal function than was previously available.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The methods used in this study could be applied to other drugs where the label provides dosing that may not be suitable for all patients who will use the drug.
Drug dosing recommendations in the US Food and Drug Administration (FDA)‐approved label are influenced by the science‐based strategy behind the key phase III clinical trials as well as the marketing strategy to support postapproval sales goals. FDA guidances regarding special patient characteristics that may influence dosing in the real‐world population also shape drug dosing. The real‐world patient population is often much more diverse than in the sample studied in the phase III trials, which can result in efficacy, safety, and dosing gaps between these trials and real‐world patients. 1 , 2 This is a particularly concerning problem for drugs in which either under‐ or overdosing could result in death or severe morbidity. 3 , 4
We believe that the relatively new direct‐acting oral anticoagulants (DOACs; dabigatran, rivaroxaban, apixaban, edoxaban) used to prevent stroke in patients with atrial fibrillation (AF) represent an opportunity to improve efficacy (by reducing stroke incidence) and safety (by reducing incidence and severity of major bleeding episodes) by more precise dosing than is currently available. We have chosen rivaroxaban to begin developing precision dosing for AF, as it has been one of the most frequently prescribed DOACs in the United States. 5 , 6 There is evidence rivaroxaban may be less effective or safe than the other DOACs with the approved dosing strategies. 7 , 8 , 9 We believe more precise dosing could improve rivaroxaban’s efficacy and safety.
We are only focusing on AF, although rivaroxaban is also approved for the prevention and treatment of venous thromboembolism and acute coronary syndrome. 10 AF was chosen because it is the most commonly prescribed rivaroxaban indication and patients may continue taking it for life. Rivaroxaban inhibits Factor Xa in a concentration‐dependent manner so that the degree of anticoagulation (i.e., prothrombin time (PT)) directly mirrors rivaroxaban plasma concentrations as they rise and fall after oral administration. 11 , 12 , 13 The dosage regimen approved by both the FDA and the European Medicines Agency is 20 mg once daily in patients with creatinine clearance (CrCl) >50 ml/min and 15 mg once daily in patients with CrCl between 15 and 50 ml/min. 10 , 14 Like all DOACs, rivaroxaban has the advantage of dosage simplicity over warfarin because there is no need for international normalized ratio laboratory testing and feedback‐based dosing. 15 One potential dosing advantage rivaroxaban has over other DOACs is that it is only dosed once daily for AF. 16
We have evaluated the degree to which dosing as specified in the rivaroxaban label would result in drug exposure likely to be consistent with drug plasma concentrations close to the levels for the average patient in the pivotal efficacy‒safety trial. Although real‐world patients who need anticoagulation do have both renal function and size extremes well beyond that experienced in phase III trials or accounted for in the label, our recommendation is to use a CrCl‐based method over a weight‐based method because renal function is expected to contribute more to overall rivaroxaban exposure than body weight. This premise is supported by publications suggesting the effect of body weight on drug exposure is minimal and certainly less than the effects seen with CrCl. 17 , 18 Although there is evidence that either PT or drug concentration feedback‐based dosing may have additional advantages, 19 , 20 , 21 we have not addressed this potential.
Methods
The fundamental assumption used in estimating more precise dosing for a wider range of renal function than was experienced in the phase III clinical trial is to match drug exposure (i.e., average rivaroxaban 24‐hour steady‐state area under the plasma concentration‐vs.‐time curve (AUC)) from the average patient in the clinical trial to patients with varying CrCl without exceeding a 20% change in AUC for reasons specified in what follows. It is assumed that exposure matching will result in similar benefit and risk regardless of dosing frequency. The underlying model that was used for all pharmacokinetic (PK) calculations, including scaling of AUC and maximum drug concentration was described by by Willmann et al. along with a supplementary file containing the final model code. 22 This model is based on 4,918 clinical study patients with PK data from phase II and III clinical studies for multiple indications. The model holds that apparent rivaroxaban clearance is significantly affected by actual body weight, CrCl, study/indication, and comedications. Apparent volume of distribution is affected by actual body weight, age, and sex. Bioavailability in the model decreases as dose increases, which is congruent with dedicated PK studies of rivaroxaban. 23 The model structure, parameters, and covariate relationships presented in the model were utilized to describe the expected PK of rivaroxaban. Parameter values were fixed to the published final estimates. The value of the “STUDY” covariate that impacted predicted clearance was fixed at 0.849, which was the final estimate for the AF indication. The value of the “COMED” covariate was fixed at 1.0, which reflected the scenario where no comedications were present. Although comedication effects on clearance were omitted, it is worth noting that the magnitude of comedication effects on rivaroxaban exposure in the study by Willmann et al. was much smaller than that observed in clinical drug‒drug interaction studies, possibly due to the small proportion of patients receiving relevant comedications in the Willmann et al. dataset. 24
The covariates of CrCl, weight, and age were centered around the average values from the Willmann et al. dataset (93 ml/min, 81 kg, and 61 years old, respectively), because Willmann et al.’s final parameter estimates for covariate effects were utilized. To focus on average predictions, all between‐subject variability, within‐subject variability, and residual variability data were removed from the original model. This meant that our strategy was more akin to a calculation rather than a true population PK simulation. The final equations for apparent clearance (Eq. 1), apparent volume of distribution (Eq. 2), and relative bioavailability (Eq. 3) were recoded from the supplemental NONMEM code file from Willmann et al. 22 as functions into R software. Supplementary File 1 contains the code for these functions in R along with comments to facilitate use of these functions according to our methods.
| (1) |
| (2) |
| (3) |
where CL denotes drug clearance, CrCL denotes creatinine clearance, WT denotes body weight, AGE denotes biologic age (in years), F denotes relative oral bioavailability, F min and F max are estimated parameters, the TV subscript denotes the typical (population) value, DOSE denotes the administered dose of rivaroxaban (in milligrams), STUDY denotes effect of the type of study, and COMED denotes effect of drug‒drug interactions between rivaroxaban and certain comedications.
The ROCKET‐AF data utilized in the Willmann model had a mean (SD) of 81.76 (32.06) ml/min for CrCl. Although the range was not reported for CrCl, it was likely considerably narrower than those reflective of real‐world patients and the values used in our simulations. The underlying structural covariate models were based on data that were narrower in range compared with real‐world patients, yet were assumed to extend to the wider real‐world patient ranges, and thus we suggest the proposed doses and regimens be prospectively tested.
To determine the values of CrCl and body weight to use to define a target reference patient for scaling purposes, we utilized the average measurements from the ROCKET‐AF patient sample to create a virtual patient who was 73 years old, weighed 73 kg, and had a CrCl of 68 ml/min. 25 , 26 This virtual patient was administered 20 mg once daily. Rivaroxaban currently does not have a defined therapeutic window, but clinical response depends on exposure. 20 , 21 , 27 Other DOACs, such as dabigatran, have more clearly characterized exposure/response relationships, which suggest there may be an optimal plasma drug concentration range. 28 , 29 The mechanisms behind the exposure/response relationship are likely similar in all DOACs, and higher exposures of rivaroxaban were assumed to increase the risk of bleeding, whereas lower exposures increased the risk of stroke. It was also assumed that patients taking rivaroxaban can achieve a balance where efficacy is maximized and adverse events are minimized. Note that, due to potential differences in the exposure/response relationship for the different DOACs, the actual target exposure ranges will differ for the different drugs.
To determine the optimal dose and regimen, the PK profiles and AUCs of several once‐or‐twice‐daily dosing regimens (with a total daily dose ranging from 5 to 60 mg/d) were calculated in virtual patients. Currently‐approved rivaroxaban doses for AF are given once daily, but twice‐daily dosing regimens were also considered. This was due to the dose‐dependent bioavailability of rivaroxaban, which can result in different 24‐hour exposures for the same total daily dose if it is given once or twice per day, as well as the potential risk of increased adverse events with extremely high peak concentrations and decreased efficacy with extremely low trough concentrations.
Every CrCl value between 15 and 250 ml/min was evaluated for a 73‐kg virtual patient for each dosing regimen. This range of CrCl was determined to be physiologically relevant for adults, and rivaroxaban is not recommended in patients with a CrCl <15 ml/min. All virtual patients were male and 73 years old. The predicted PK profiles and AUCs were evaluated to identify dosing regimens, which yielded predicted AUCs within ±20% of the target AUC at each value of CrCl. It was assumed that the outcome would be optimal as long as the AUC is within 20% of the target AUC.
Another threshold that was considered was ±50% of the target AUC, which was inferred from the FDA Clinical Pharmacology Review of rivaroxaban. 24 A dedicated phase I renal function study showed that moderate renal dysfunction (CrCl 30–49 ml/min) was associated with a 52% increase in average AUC compared with healthy controls with CrCl ≥80 ml/min. 30 The FDA and the sponsor decided that this 52% increase in AUC was unacceptable and created a dose adjustment for patients with CrCl 30–49 ml/min. On the other hand, there was no dose adjustment necessitated for patients with mild renal impairment (CrCl 50–79 ml/min), even though they had a 44% increase in AUC compared with healthy controls. This implied that a ≥50% increase in AUC was unacceptable, although there have been concerns that this is too large for acceptable variability in exposure for a drug like rivaroxaban where safety must be balanced with efficacy to avoid serious consequences. Thus, the ±20% change in AUC was selected as the threshold of acceptability.
Acceptable doses were then defined for practical ranges of CrCl based on exposure‐matching. This method was designed after the exposure‐matching process that is part of the typical dose‐determination and drug‐approval process. Groups of acceptable dosing regimens were then combined into strategies that kept the predicted AUC close to the target AUC along the entire tested covariate range. Some considerations were also taken for convenience and practicality. For example, a 20‐mg once‐daily dose would be favored over a 25‐mg once‐daily dose even if the AUC associated with the 20‐mg dose was slightly outside the ±20% target AUC range; this is because rivaroxaban is currently available in 20‐mg tablets, but not 25‐mg tablets. In addition, only two to four doses were selected for the strategy and narrow windows for dosing regimens (such as a dose that was optimal only for 30–35 ml/min) were avoided because they would be hard to measure or enact in practice. Once‐daily dosing was generally preferred over twice‐daily because it is easier to adhere to. However, some once‐daily regimens were associated with significantly higher peaks and lower troughs despite closely matching the target AUC; in these cases, twice‐daily regimens were preferred to optimize exposure.
Results
The average 24‐hour steady‐state AUC is 3,325.6 μg/h per liter for a 20‐mg once‐daily dose administered to the average 73‐year‐old male patient weighing 73 kg with a CrCl of 68 ml/min, as calculated using equations from the Willmann et al. model and patient characteristics from the phase III atrial fibrillation trial. 22 , 25 , 26 The calculated average peak and trough concentrations for this virtual average patient are 255.2 μg/L and 46.6 μg/L, respectively. The ±20% AUC lower and upper bounds are 2,660.5 and 3,990.7 μg/h per liter. The calculated rivaroxaban reference AUC of 3,325.6 μg/h per liter served as the target AUC.
To determine the impact of renal function on rivaroxaban steady‐state exposure, the predicted AUC was calculated for the average AF patient with a CrCl that increased in 1‐ml/min increments from 15 to 250 ml/min. The predicted AUCs were then compared with the reference AUC for the average patient with a CrCl of 68 ml/min to match the median CrCl in atrial fibrillation patients, 26 as shown in Figure 1 . With the currently approved rivaroxaban dosing, the threshold CrCl values that crossed the upper and lower 20% reference bounds were 26 and 117 ml/min, respectively. Acceptable dose amounts based on renal function were selected for patients above and below the 20% reference values by matching AUC and then creating a change in dosing interval if needed to approximate the reference rivaroxaban peak and trough. Acceptable dosing regimens were then grouped into a strategy based on renal function (Table 1 ), which kept the predicted AUC close to the target AUC along the entire tested CrCl range. The model‐predicted 24‐hour steady‐state rivaroxaban AUCs according to this novel strategy is presented in Figure 2 . The model‐predicted 24‐hour steady‐state rivaroxaban plasma concentration‐vs.‐time profiles for the current label and our new dosing strategy are presented according to renal function in Figure 3 .
Figure 1.

Current rivaroxaban dosing recommendations are predicted to yield clinically significant deviations in the average 24‐hour steady‐state area under the plasma concentration‐vs.‐time curve (AUC) for patients outside a certain range of creatinine clearance (CrCl). The solid gray horizontal line demarcates where the predicted AUC is equal to 3,325.6 μg/h per liter. The dotted gray reference lines represent a 20% increase and 20% decrease in AUC from 3,325.6 μg/h per liter. The predicted AUC vs. CrCl from 15 ml/min to 250 ml/min for virtual 73‐kg, 73‐year‐old male subjects is displayed. The solid black line represents the recommended dose of 20 mg once daily for patients with CrCl >50 ml/min. The dashed black line represents the recommended dose of 15 mg once daily for patients with CrCl between 15 and 50 ml/min. The predicted AUC exceeds the ±20% threshold from target AUC when CrCl is <26 ml/min or >117 ml/min.
Table 1.
Creatinine clearance (CrCl)‐based strategy for rivaroxaban dosing in adults with atrial fibrillation
| CrCl a (ml/min) | Dose |
|---|---|
| <15 | Not recommended |
| 15–29 | 10 mg once daily |
| 30–69 | 15 mg once daily |
| 70–159 | 10 mg twice daily (20 mg/d) |
| 160‒250 | 15 mg twice daily (30 mg/d) |
CrCl calculated using Tietz‐truncated Cockcroft‒Gault equation with actual body weight.
Figure 2.
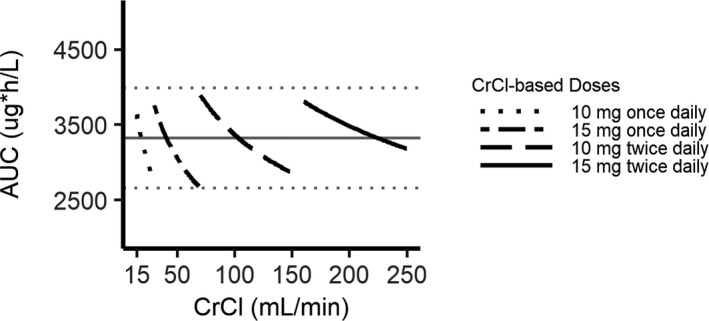
The proposed precision dosing strategies are predicted to result in rivaroxaban average 24‐hour steady‐state area under the plasma concentration‐vs.‐time curve (AUC) values within ±20% of the target AUC for physiologically relevant ranges of creatinine clearance (CrCl) (15–250 ml/min) in adults. The target AUC (3,325.6 μg/h per liter) is represented by the solid gray horizontal line. The ±20% thresholds from the target AUC are demarcated by the dotted gray horizontal lines. The predicted AUC according to CrCl is displayed for the CrCl‐based dosing strategy. Predictions are for a 73‐kg, 73‐year‐old male subject.
Figure 3.

(a) Average predicted pharmacokinetic profiles for atrial fibrillation patients with creatinine clearance (CrCl) between 15 and 250 ml/min have significant differences when current rivaroxaban dose recommendations are used. (b) Proposed CrCl‐based precision dosing strategy is predicted to yield comparable exposure across the broad range of physiologically relevant CrCl values while reducing large fluctuations in peak and trough concentrations. All plasma concentration‐vs.‐time profiles were predicted for a 73‐kg, 73‐year‐old male virtual patient according to various levels of renal function. Line color corresponds to CrCl and is consistent across panels (a) and (b). BID, twice daily; CrCl, creatinine clearance; QD, once daily.
Discussion
It is informative to review the evidence comparing adjusted‐dose warfarin to placebo for stroke along with the various warfarin‒DOAC noninferiority results. Feedback‐based dosing of warfarin for AF patients produces a 64% reduction in stroke and a 26% decrease in all‐cause mortality compared with placebo according to a published meta‐analysis. 31 A separate meta‐analysis showed that fixed doses of DOACs compared with warfarin feedback‐based dosing in AF produced a 19% reduction in total strokes or systemic embolic events, a 51% decrease in hemorrhagic stroke, a 10% decrease in all‐cause mortality, and a 52% decrease in intracranial hemorrhage. 8 However, it also showed a 25% increase in gastrointestinal bleeding compared with warfarin. The individual DOAC measures of efficacy are striking; e.g., dabigatran produces a 34% reduction in stroke risk vs. only a nonsignificant 12% reduction for rivaroxaban and edoxaban. 8 For major bleeding events, apixaban reduces risk of major bleeding by 29%, whereas neither rivaroxaban nor dabigatran significantly reduced risk when compared with warfarin‐adjusted dosing. 8 Could it be that the observed differences in outcomes between DOACs are related to how well the currently marketed individual DOAC dosing schemes (dosage, interval) perform rather than differences in the chemical moieties? By using more precise rivaroxaban fixed dosing, could rivaroxaban overall efficacy come closer to dabigatran and safety closer to apixaban? Could a feedback‐based rivaroxaban dosing scheme outperform the other DOACs with fixed dosing and warfarin‐adjusted dosing for efficacy and safety? Historically, both oral and parenteral anticoagulants have been dose‐adjusted based on the individual pharmacodynamic end point of the patient. 32 , 33 The introduction of DOACs about 10 years ago has been notable in that these drugs apply relatively fixed dosing with no need for individual patient dose adjustment. 25 , 34 , 35 , 36 This is remarkable in that DOAC changes in drug plasma concentration produce an instantaneous change in PT or partial thromboplastin time. 11 , 12 , 13 The DOACs all gained market approval for stroke prevention in AF patients with dosing based on phenotype compared with warfarin doses, which must be adjusted to an international normalized ratio in the therapeutic range of 2‒3. 19 Each DOAC was found to be at least noninferior to warfarin for efficacy and safety even though there is evidence for individual drug differences (i.e., superiority for dabigatran 150 mg for stroke reduction, apixaban superiority for less major bleeding). 25 , 34 , 35 , 36 Rivaroxaban was approved by the FDA to prevent stoke in AF based on a noninferiority study comparing either 15 or 20 mg dosed once daily based on CrCl. 25 Although the mean DOAC elimination half‐lives are similar (e.g., 7‒17 hours 37 ), only rivaroxaban is administered once daily for patients at higher renal function (i.e., CrCl >95 ml/min). 16
There are a number of reasons to believe relatively small difference in either drug dose or drug plasma concentration could lead to significant difference in efficacy and safety. Each DOAC has an instantaneous impact on blood coagulation indices, as shown in phase II dose‐range studies, 13 and thus the DOAC drug anticoagulant effect (e.g., prothrombin time), leading to both efficacy and safety changes as the drug concentration rises and falls with drug absorption and elimination. Dabigatran was compared in two different doses in the phase III study and the dose‒response relationship was related to efficacy and safety. 34 The dabigatran and edoxaban trials were adequately designed phase III trials that showed a relationship between drug plasma concentration and both efficacy and safety. 38 , 39 Although these drugs were found to be efficacious and safe, many patients had drug exposures beyond those reported to be safe and effective. Dabigatran clinical trial simulations based on the phase III Re‐Ly study showed dosing titrated to be within an optimal drug trough plasma concentration range (90–140 ng/ml) may result in a statistically significant reduction in major bleeding compared with warfarin (relative risk = 0.60) or fixed‐dose dabigatran (i.e., 150 mg twice daily) (relative risk = 0.80). 40 A DOAC registry study showed that lower‐than‐recommended doses of DOACs were associated with a greater risk of cardiovascular hospitalization, whereas higher‐than‐recommended doses had an increased risk of all‐cause mortality. 41 For rivaroxaban in the pivotal AF trial (ROCKET‐AF), a ≥20% reduction in CrCl during the trial was associated with a significant decrease in stroke risk. 25 , 26 Presumably, decreasing renal function with the same rivaroxaban dose would increase drug plasma concentrations, and the resulting increase in exposure was responsible for the increased efficacy. A review of DOACs in AF patients with kidney disease indicated that the rivaroxaban 15‐mg/d dose was probably too high for some patients based on exposure‐matching to patients with normal renal function. 42 In summary, the aforementioned aggregate findings are the basis for our decision to choose the ±20% threshold deviation from the calculated AUC for the average patient to represent a clinically significant change in pharmacokinetics. Had the ROCKET‐AF study provided exposure information similar to that provided for dabigatran or edoxaban, a more precise dosage recommendation could be made.
Selecting a preferred oral anticoagulant in AF patients with advanced chronic kidney disease (CrCl <30 ml/min) is difficult for several reasons. 42 Patients with CrCl <25 ml/min were excluded from the phase III DOAC clinical trials. We have shown the rivaroxaban dose for patients with a CrCl between 15 and 29 ml/min should be decreased from 15 mg/d to 10 mg/d. Although warfarin is often recommended in these patients, there is a four‐ to fivefold higher bleeding risk when using warfarin in patients with advanced chronic kidney disease compared with patients with normal renal function. 42 We believe patients with chronic kidney disease need more precise DOAC dosing and objective evidence for efficacy and safety.
We have proposed a rivaroxaban dosing scheme that should produce more uniform drug plasma concentrations across a wider range of CrCl than was studied in the phase III clinical trial. It should be noted that most of the doses in our strategy do not exceed the labeled daily dosage, with the exceptions of 15 mg twice daily (30 mg/d) in patients with CrCl ≥160 ml/min. When dosing obese patients using renal function, CrCl should be calculated based on lean body mass. 43 Care must be taken in selecting dosing beyond the label (i.e., 30 mg/d) for AF. Rivaroxaban daily doses up to 60 mg/d (30 mg twice daily) have been studied in healthy volunteers and patients with deep vein thrombosis and pulmonary embolus. 11 , 23 Although the rivaroxaban maximum labeled dose for AF is 20 mg/d, 15 mg twice daily with food (30 mg/d) is indicated for the first 21 days treatment of deep vein thrombosis and pulmonary embolus before reducing the dose to 20 mg once daily. It may be worth confirming that the patient has a peak or trough rivaroxaban plasma concentration close to the reference standard used in this study (i.e., peak 255.2 μg/L and trough 46.6 μg/L). Another alternative is to consider adjusted‐dose warfarin. Rivaroxaban AUC intersubject variability at steady state has a 29% coefficient of variation as compared with 24% for apixaban. 44 This degree of variability is moderate, is not unusual, and does not influence our dosing recommendations.
Although there is ample evidence to believe more precise DOAC dosing will improve patient outcomes, creating the evidence to support dosing improvements is unlikely to come from prospective clinical trials. The rivaroxaban phase III studies for various indications have been reported to cost nearly $3 billion due to the large number of patients studied over several years needed to detect treatment differences in relatively infrequent outcomes (stroke, major bleeding events, death). 45 Drug dosing in the FDA‐approved label is either supported by clinical trials or by matching either a drug biomarker (e.g., PK and pharmacodynamics) associated with efficacy and/or safety or, second, by simply matching drug plasma concentration to the average patient in the phase III trial. For example, this “bridging” strategy is often used to adjust dosing for children when the disease progression, response to the intervention, and exposure‒response relationship are expected to be similar in children and adults. 46
One limitation for our proposed rivaroxaban dosing scheme is there is no provision to provide dosing estimates for patients with multiple characteristics influencing either PK or pharmacodynamics. Our simulations are based entirely on the population PK model that does not account for pharmacodynamic interactions and underpredicts metabolic induction and inhibition. Dosage adjustments for these interactions will need to be made either by avoiding interacting drugs, as indicated in the label, or making informed adjustments. In the longer term, we believe feedback‐based dosing for rivaroxaban and the other DOACs will be the ultimate dosing solution.
Regarding the more precise rivaroxaban dosing strategy presented in this study, we plan to prospectively measure the prediction accuracy for our dosing recommendations in a diverse real‐world patient sample. In addition, a feedback‐based dosing scheme for rivaroxaban based on a target drug concentration range will be developed and outcome studied. Because only 161 of several thousand patients in the ROCKET‐AF clinical trial had rivaroxaban plasma concentration measured, it was not possible to identify a relationship between rivaroxaban plasma concentration and outcomes (i.e., stroke, bleeding). 47 However, we believe a rivaroxaban‐adjusted dosing scheme can be built using strategies such as exposure‐matching to the average Rocket AF study patient exposures, as we have used in this report, or response‐matching to prothrombin time, as suggested by other researchers. 48
The proposed precision dosing scheme will be evaluated to determine the impact on efficacy/safety outcome by using a hybrid electronic health record‒insurance claims database. Several investigators have replicated the ROCKET‐AF rivaroxaban clinical trial findings using insurance claims. 7 , 49 Dabigatran was compared with rivaroxaban using labeled doses in AF patients that reported dabigatran to be superior in terms of safety defined by major bleeding incidence. 7 Assuming this difference is real, the choices made in dosing regimen could contribute to efficacy outcomes.
The recent FDA precision dosing public meeting highlighted the potential importance of developing more precise dosing for drug‐disease targets where the potential outcome from under‐ or overdosing could result in serious morbidity or even death. 50 One senior FDA physician indicated this could be the third major milestone (the age of dosing individualization) in drug development and regulation after the ages of safety in 1938 and efficacy in 1962. 51 Our investigation is meant to initiate this scientifically‐based drug dosing approach for rivaroxaban, which may supplant marketing‐driven drug dosing.
Funding
This study was supported by the Eshelman Institute for Innovation at the UNC Eshelman School of Pharmacy. R.K. receives a stipend for a pharmacokinetics/pharmacodynamics postdoctoral fellowship from the UNC/IQVIA.
Conflict of interest
J.H.P declares consulting and research support from Novartis and research support from Amgen, Merck, and Boehringer Ingelheim. D.G. received a travel grant through University of North Carolina at Chapel Hill to give a presentation at Boehringer Ingelheim. A.K.G. has received grant funding from Bristol Myers Squibb; consulting income from Biosense‐Webster; and speaker honoraria from Zoll Medical, Boston Scientific, and Abbott. All other authors declared no competing interests for this work.
Author contributions
J.R.P., R.K., D.W., D.G., Y.C.C., P.W., A.K., and A.G. wrote the manuscript; D.W., J.R.P., and R.K. designed and performed the research; and R.K. and D.W. analyzed the data.
Supporting information
Supinfo
Acknowledgments
The authors acknowledge Ned McWilliams, JD, of Levin Papantonio, PA, for his early contributions and unrestricted financial support, and Farah Al Qaraghuli, MS, for an invaluable review of the equations and code that were utilized.
References
- 1. Spong, C.Y. & Bianchi, D.W. Improving public health requires inclusion of underrepresented populations in research. JAMA 319, 337–338 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eichler, H.‐G. et al. Bridging the efficacy–effectiveness gap: a regulator’s perspective on addressing variability of drug response. Nat. Rev. Drug. Discov. 10, 495–506 (2011). [DOI] [PubMed] [Google Scholar]
- 3. Powell, J.R. Are new oral anticoagulant dosing recommendations optimal for all patients? JAMA 313, 1013–1014 (2015). [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez, D. et al. Precision dosing: public health need, proposed framework, and anticipated impact. Clin. Transl. Sci. 10, 443–454 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta, K. et al. Real‐world comparative effectiveness, safety, and health care costs of oral anticoagulants in nonvalvular atrial fibrillation patients in the U.S. Department of Defense Population. J. Manag. Care Spec. Pharm. 24, 1116–1127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnes, G.D. , Lucas, E. , Alexander, G.C. & Goldberger, Z.D. National trends in ambulatory oral anticoagulant use. Am. J. Med. 128, 1300–1305.e2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham, D.J. et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern. Med. 176, 1662–1671 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Ruff, C.T. et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomized trials. Lancet 383, 955–962 (2014). [DOI] [PubMed] [Google Scholar]
- 9. FDA Cardiovascular and Renal Drugs Advisory Committee . Transcript for the September 8, 2011 Meeting of the Cardiovascular and Renal Drugs Advisory Committee [Archived]. 2011 Meeting Materials, Cardiovascular and Renal Drugs Advisory Committee <https://wayback.archive‐it.org/7993/20170403223812/https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/ucm250287.htm> (2015). Accessed September 23, 2019.
- 10. Janssen Pharmaceuticals, Titusville, NJ, USA . Package insert for Xarelto (rivaroxaban). <https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022406s030s032lbledt.pdf> (2019). Accessed April 12, 2019.
- 11. Kubitza, D. , Becka, M. , Wensing, G. , Voith, B. & Zuehlsdorf, M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59–7939—an oral, direct Factor Xa inhibitor—after multiple dosing in healthy male subjects. Eur. J. Clin. Pharmacol. 61, 873–880 (2005). [DOI] [PubMed] [Google Scholar]
- 12. Douxfils, J. et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J. Throm. Haemost. 16, 209–219 (2018). [DOI] [PubMed] [Google Scholar]
- 13. Eikelboom, J.W. , Quinlan, D.J. , Hirsh, J. , Connolly, S.J. & Weitz, J.I. Laboratory monitoring of non‐vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation: a review. JAMA Cardiol. 2, 566–574 (2017). [DOI] [PubMed] [Google Scholar]
- 14. European Medicines Agency . Xarelto‐H‐C‐944‐II‐0012: EPAR―Assessment Report―Variation. <https://www.ema.europa.eu/en/documents/variation‐report/xarelto‐h‐c‐944‐ii‐0012‐epar‐assessment‐report‐variation_en.pdf> (2012). Accessed July 24, 2019.
- 15. Trujillo, T. & Dobesh, P.P. Clinical use of rivaroxaban: pharmacokinetic and pharmacodynamic rationale for dosing regimens in different indications. Drugs 74, 1587–1603 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American College of Cardiology . DOAC dosing for AFib infographic now available. DOAC Dosing for Atrial Fibrillation (AFib). <https://www.acc.org/membership/sections‐and‐councils/electrophysiology‐section/section‐updates/2018/10/12/12/42/doac‐dosing‐for‐afib‐infographic‐now‐available> (2018). Accessed August 29, 2019.
- 17. Barsam, S.J. et al. The impact of body weight on rivaroxaban pharmacokinetics. Res. Pract. Thromb. Haemost. 1, 180–187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubitza, D. , Becka, M. , Zuehlsdorf, M. & Mueck, W. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59–7939) in healthy subjects. J. Clin. Pharmacol. 47, 218–226 (2007). [DOI] [PubMed] [Google Scholar]
- 19. Chan, N. et al. Is there a role for pharmacokinetic/pharmacodynamic‐guided dosing for novel oral anticoagulants? Am. Heart J. 199, 59–67 (2018). [DOI] [PubMed] [Google Scholar]
- 20. Rottenstreich, A. et al. Direct‐acting oral anticoagulant drug level monitoring in clinical patient management. J. Thromb. Thrombolysis 45, 543–549 (2018). [DOI] [PubMed] [Google Scholar]
- 21. Temple, R. NOAC dosing precision dosing―YES! Presented at the Is There a Role for Pharmacokinetic/Pharmacodynamics Guided Dosing for Novel Anticoagulants? Meeting hosted by the Cardiac Safety Research Consortium on December 3, 2015 in Washington, DC. <http://www.cardiac‐safety.org/wp‐content/uploads/2015/12/S2_1a_Temple.pdf> (2015). Accessed August 29, 2019.
- 22. Willmann, S. et al. Integrated population pharmacokinetic analysis of rivaroxaban across multiple patient populations. CPT Pharmacometrics Syst. Pharmacol. 7, 309–320 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mueck, W. , Stampfuss, J. , Kubitza, D. & Becka, M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin. Pharmacokinet. 53, 1–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US FDA Center for Drug Evaluation and Research . Clinical pharmacology and biopharmaceutics review(s): Application No. 022406Orig1s000. <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022406Orig1s000ClinPharmR.pdf> (2011). Accessed June 13, 2019.
- 25. Patel, M.R. et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 365, 883–891 (2011). [DOI] [PubMed] [Google Scholar]
- 26. Fordyce, C.B. et al. On‐treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from Rocket AF. Circulation 134, 37–47 (2016). [DOI] [PubMed] [Google Scholar]
- 27. Testa, S. et al. Low drug levels and thrombotic complications in high‐risk atrial fibrillation patients treated with direct oral anticoagulants. J. Thromb. Haemost. 16, 842–848 (2018). [DOI] [PubMed] [Google Scholar]
- 28. Eikelboom, J.W. et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 377, 1319–1330 (2017). [DOI] [PubMed] [Google Scholar]
- 29. Sennesael, A.‐L. et al. Rivaroxaban plasma levels in patients admitted for bleeding events: insights from a prospective study. Thromb. J. 16 (2018). 10.1186/s12959-018-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubitza, D. et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br. J. Clin. Pharmacol. 70, 703–712 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hart, R.G. , Pearce, L.A. & Aguilar, M.I. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 146, 857–867 (2007). [DOI] [PubMed] [Google Scholar]
- 32. Quick, A.J. Clinical interpretation of the one‐stage prothrombin time. Circulation 24, 1422–1428 (1961). [DOI] [PubMed] [Google Scholar]
- 33. Raschke, R.A. , Reilly, B.M. , Guidry, J.R. , Fontana, J.R. & Srinivas, S. The weight‐based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann. Intern. Med. 119, 874–881 (1993). [DOI] [PubMed] [Google Scholar]
- 34. Connolly, S.J. et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 361, 1139–1151 (2009). [DOI] [PubMed] [Google Scholar]
- 35. Granger, C.B. et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 365, 981–992 (2011). [DOI] [PubMed] [Google Scholar]
- 36. Giugliano, R.P. et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 369, 2093–2104 (2013). [DOI] [PubMed] [Google Scholar]
- 37. Ieko, M. , Naitoh, S. , Yoshida, M. & Takahashi, N. Profiles of direct oral anticoagulants and clinical usage—dosage and dose regimen differences. J. Intensive Care 4, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reilly, P.A. et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE‐LY trial (randomized evaluation of long‐term anticoagulation therapy). J. Am. Coll. Cardiol. 63, 321–328 (2014). [DOI] [PubMed] [Google Scholar]
- 39. Ruff, C.T. et al. Association between edoxaban dose, concentration, anti‐Factor Xa activity, and outcomes: an analysis of data from the randomised, double‐blind ENGAGE AF‐TIMI 48 trial. Lancet 385, 2288–2295 (2015). [DOI] [PubMed] [Google Scholar]
- 40. Boehringer Ingelheim . An idea for a mid to long term strategy for Pradaxa (BIPI‐PRA‐0028572360/Kliewer 3204854 REDACTED). <https://journals.bmj.com/sites/default/files/BMJ/dabigatran/titration_presentation.pdf> (2012). Accessed August 29, 2019.
- 41. Steinberg, B.A. et al. Off‐label dosing of non‐vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT‐AF II Registry. J. Am. Coll. Cardiol. 68, 2597–2604 (2016). [DOI] [PubMed] [Google Scholar]
- 42. Chan, K.E. et al. Nonvitamin K anticoagulant agents in patients with advanced chronic kidney disease or on dialysis with AF. J. Am. Coll. Cardiol. 67, 2888–2899 (2016). [DOI] [PubMed] [Google Scholar]
- 43. Chew‐Harris, J.S.C. , Florkowski, C.M. , Elmslie, J.L. , Livesey, J. , Endre, Z.H. & George, P.M. Lean mass modulates glomerular filtration rate in males of normal and extreme body composition. Intern. Med. J. 44, 749–756 (2014). [DOI] [PubMed] [Google Scholar]
- 44. Frost, C. et al. A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin. Pharmacol. 6, 179–187 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roy, A. How the FDA stifles new cures, Part II: 90% of clinical trial costs are incurred in Phase III. Forbes <https://www.forbes.com/sites/theapothecary/2012/04/25/how‐the‐fda‐stifles‐new‐cures‐part‐ii‐90‐of‐clinical‐trial‐costs‐are‐incurred‐in‐phase‐iii/#292d609e7b52> (2012). Accessed August 29, 2019.
- 46. Mehrotra, N. et al. Role of quantitative clinical pharmacology in pediatric approval and labeling. Drug Metab. Dispos. 44, 924–933 (2016). [DOI] [PubMed] [Google Scholar]
- 47. Girgis, I.G. et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non‐valvular atrial fibrillation: results from ROCKET AF. J. Clin. Pharmacol. 54, 917–927 (2014). [DOI] [PubMed] [Google Scholar]
- 48. Solms, A. et al. Enhancing the quality of rivaroxaban exposure estimates using prothrombin time in the absence of pharmacokinetic sampling. CPT Pharmacometrics Syst. Pharmacol. 8, 805–814 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hernandez, I. , Baik, S.H. , Piñera, A. & Zhang, Y. Higher risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern. Med. 175, 18–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Office of Translational Sciences , US Food and Drug Administration. Precision dosing: defining the need and approaches to deliver individualized drug dosing in the real‐world setting. <http://www.fda.gov/drugs/precision‐dosing‐defining‐need‐and‐approaches‐deliver‐individualized‐drug‐dosing‐real‐world‐setting> (2019). Accessed August 29, 2019.
- 51. McCaughan, M. New age thinking: US FDA’s temple sees third era of drug development. Pink sheet. <https://pink.pharmaintelligence.informa.com/PS140700/New‐Age‐Thinking‐US‐FDAs‐Temple‐Sees‐Third‐Era‐Of‐Drug‐Development> (2019). Accessed August 28, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supinfo


