Abstract
Interleukin-1 (IL-1) has long been known to be a key mediator of immunity and inflammation. Its dysregulation has been implicated in recent years in tumorigenesis and tumor progression, and its upregulation is thought to be associated with many tumors. Overexpression of the IL-1 agonists IL-1α and IL-1β has been shown to promote tumor invasiveness and metastasis by inducing the expression of angiogenic genes and growth factors. IL-1 blockers such as anakinra and canakinumab are already approved and widely used for the treatment of some autoimmune and autoinflammatory diseases and are currently being tested in preclinical and human clinical trials for cancer therapy. In this paper we review the most recent discoveries regarding the association between IL-1 dysregulation and cancer and present the novel IL-1 blockers currently being tested in cancer therapy and their corresponding clinical trials.
Keywords: Anakinra, Biologics, Canakinumab, Cancer, Can-04, Inflammation, Interleukin-1, MABp1, Nidanilimab, Xilonix
Introduction
In the late nineteenth century, the German pathologist and founder of “Zellularpathologie”, Rudolf Wirchow, postulated that inflammation is a predisposing factor for cancer development—an idea that was since cast aside for almost a century [1]. Recent findings, such as that chronic infections and inflammation contribute to about quarter of all cancer cases worldwide, have sparked a renewed interest of researchers in the concept of an association between inflammation and cancer [2].
The microenvironment of most, if not all, tumors consists of different inflammatory cells and mediators. Some cytokines, alongside different chemokines and growth factors, are thought to contribute to tumor-related inflammation by altering the adaptive immunity, responses to hormones, angiogenesis, tumor growth and progression, invasion, and metastasis [3]. Interleukin-1 (IL-1) is one of the prominent tumorigenic inflammatory cytokines, and as such has been in recent years a target of research of both basic and translational nature, seeking to develop and employ novel IL-1 blockers in cancer therapy.
In this paper we review the role of IL-1 in the pathogenesis of certain cancers and present an up-to-date summary of the medications currently under development and ongoing clinical trials using these medications in cancer treatments.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Association Between Inflammation and Cancer
Under normal conditions, acute inflammation is a desirable, strictly regulated response to infection and tissue damage [4]. It is initiated by macrophages and other sentinel cells in tissues which recognize either pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) during infection or tissue injury, respectively [5]. Following initiation, these cells secret proinflammatory mediators (i.e., amines and cytokines), which act to dilate blood vessels and increase their permeability, and to recruit leukocytes and plasma proteins (i.e., complement, kinins) from the circulation to the site of the offending agent. Once activated, these key components of the inflammatory response work together to eliminate the risk, terminate the inflammatory cascade, repair the tissue, and regain homeostasis [6].
However, when the initiating stimuli cannot be fully cleared or when the inflammation-resolving mechanisms fail, a detrimental, non-resolving inflammatory process occurs, termed chronic or sterile inflammation [7]. This persistent inflammatory environment is considered today to be a risk factor for cancer development providing tumor-supporting molecules from cells infiltrating the tumor microenvironment such as cytokines and growth factors, cell survival signals, angiogenic factors, and other carcinogenesis mediators [8].
Chronic inflammatory conditions are thought to be associated with cancer, regardless of their inflammatory stimuli [9]; examples are inflammatory bowel diseases stimulated by both genetic and environmental factors associated with colorectal cancer [10], purely environmental-related inflammation caused by asbestos, infections, smoking, and silica associated with lung cancer [11], chronic gastritis caused by bacterial stimulus (H. pylori) associated with gastric cancer [12] or E. coli infection of the prostate associated with prostate cancer [13], a viral infection with hepatitis virus B/C associated with hepatocellular carcinoma [14], or human papilloma virus (HPV) associated with many different cancers such as cervical and anogenital cancers, head and neck squamous cell carcinoma, esophageal carcinoma, and even ophthalmologic and breast cancers [15].
Proinflammatory Cytokines and IL-1 Family Members
Cytokines are synthesized by a variety of immune and stromal cells to communicate with each other in order to regulate coordinated responses such as proliferation, cell survival or death, differentiation, migration, and immune cell activation. During chronic inflammation, however, or when activated by tumor cells, cytokines can induce or expedite cell transformation and migration, two mandatory processes of tumor evolution and formation of metastasis [9]. Hypoxia, one of the hallmarks of progressive cancers, induces cytokines which include vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF), IL-1, and IL-6 [16].
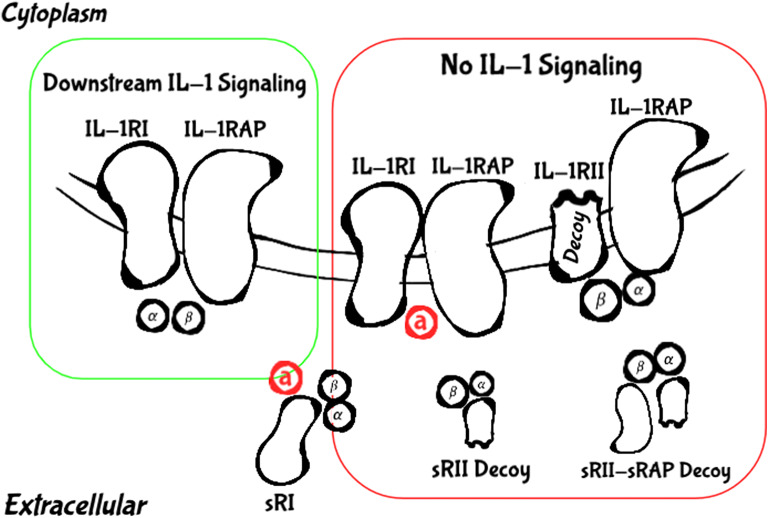
IL-1 is a key mediator of immunity and inflammation [17, 18]. It is known to be upregulated in many tumors and is thought to contribute to tumor invasiveness and metastasis by inducing the expression of angiogenic genes and growth factors [16, 17]. The IL-1 family comprises 11 ligands, specifically seven agonists, three receptor antagonists, and one anti-inflammatory cytokine. The IL-1 receptor (IL-1R) family includes 11 molecules [19]. In this review we focus on the key players of the IL-1/IL-1R families which are being excessively researched for cancer therapeutic purposes, namely two agonists, IL-1α and IL-1β; the main antagonist, IL-1 receptor antagonist (IL-1Ra); and the two main receptors, type 1 IL-1R (IL-1RI) and type 2 IL-1R (IL-1RII). We also discuss the crucial role of IL-1R accessory protein (Il-1RAP), also known as IL-1R3, in regulating the IL-1-dependent responses (summarized in Fig. 1).
Fig. 1.
Key mediators of the IL-1 family. A productive IL-1 signaling (green frame) requires the binding of the IL-1 agonists IL-1α or IL-1β to the transmembrane IL-1RI, following the approximation of IL-1RAP and the formation of the signal-transducing heterotrimeric complex. Binding of IL-1Ra to IL-1RI prevents proper formation of the signaling complex and blocks signal transduction. IL-1RII competes with IL-1RI for the binding of the IL-1 agonists but is not able to transduce a signal because of a truncated cytosolic domain. Finally, sIL-1RI is able to bind both agonists and antagonist and therefore plays a double regulatory role, and sIL-1RII is able to bind free agonists and sequester them from proper binding, either on its own or with greater affinities while connected to sIL-1RAP (red frame)
Role of IL-1 Agonists IL-1α and IL-1β and Antagonist IL-1Ra in Signal Transduction
IL-1α and IL-1β are the main agonists of the IL-1 family [20]. They share some similarities and some differences which give them their own unique functions. Both IL-1α and IL-1β genes are located adjacently on the long arm of chromosome 2 [21]. They are both translated into a precursor form lacking a signal peptide sequence, which can be further processed to create a shorter mature form [22]. Functionally, both agonists bind the membrane-bound IL-1RI, thus recruiting IL-1RAP to form the ternary signaling complex and initiate downstream signaling. The uncleaved pro-IL-1α precursor (pro-IL-1α) can also bind IL-1RI with lesser affinity and is considered biologically active, while precursor pro-IL-1β (pro-IL-1β) is not able to bind IL-1R at all [23]. The main and perhaps the most important difference between the two agonists is that while IL-1α and its bioactive precursor pro-IL-1α are constitutively expressed in non-immune cell types, mainly as a cytosolic or membrane-bound forms [24], IL-1β in its mature bioactive form is produced in response to inflammatory signals, primarily by myeloid cells, most notably monocytes, macrophages, and neutrophils [25]. This grants IL-1α its unique character of an alarmin or a damage-associated molecular pattern (DAMP) and the ability to initiate sterile inflammation as it is secreted from necrotic, but not apoptotic, cells [26–30]. IL-1β, on the other hand, mainly amplifies and exacerbates inflammation and its role in autoimmune and autoinflammatory diseases is well described [25, 31–34].
Unlike the two agonists, IL-1Ra contains a signal peptide enabling it to be easily secreted [35]. It binds IL-1RI with similar affinity as the two other agonists, but as a result of only partial binding, the conformational change of IL-1R is not completed, resulting in its incompetence to bind IL-1RAP and thus blocking further intracellular response [36]. The IL-1Ra gene is also located on the long arm of chromosome 2 just by the genes of the two agonists, and as a negative regulator of IL-1α and IL-1β its levels of expression are normally correlated with the levels of the agonists [37]. IL-1Ra is an important natural anti-inflammatory protein [38, 39], and its dysregulation may lead to either sepsis [40] or autoinflammatory diseases [41, 42] (Fig. 1).
Type 1 and Type 2 IL-1 Receptors and IL-1 Receptor Accessory Protein
The genes for IL-1R1 and IL-1R2 are also located on the long arm of chromosome 2, adjacent to each other but distant from the genes of the IL-1 ligands discussed earlier [43]. IL-1RI is the main signal-transducing, proinflammatory receptor of the IL-1R family. It is a transmembrane protein, consisting of an extracellular ligand binding chain of three Ig-like segments and an intracellular toll-IL-1-receptor (TIR) domain [44]. Upon binding of either IL-1α or IL-1β, the IL-1RAP, which contains a cytoplasmic TIR domain as well, approximates to the IL-1RI–agonist complex, and the two cytoplasmic TIR domains react together to initiate the signal [29].
IL-1RII, however, while sharing a highly similar ligand binding domain with IL-1RI, lacks the cytoplasmic TIR domain. Upon binding of IL-1RII to the agonists, IL-1RAP joins to form the ternary complex just as with IL-1RI, but as a result of its truncated cytoplasmic region and lack of TIR domain, no signal is transduced. This makes IL-1RII a prototypic decoy, sequestering both the agonists and IL-RAP from combining with the productive IL-1RI, and attenuating the inflammatory response [44]. IL-1RII, however, binds IL-1β preferably with greater affinity, and IL-1α and IL-1Ra to a lesser extent [45] (Fig. 1).
While IL-1RI is expressed extensively in many cell types, IL-1RII is more distinctly expressed, especially in monocytes, macrophages, neutrophils, and B cells, and known to be upregulated rapidly by regulatory T cells in response to TCR stimulation [46]. Interestingly, out of all the IL-1 members discussed so far, the gene of IL-1RAP is the only one located on a different chromosome (chromosome 3) [23].
All three receptors discussed above exist in a soluble form in addition to the transmembrane one, further fine-tuning IL-1 regulation. Soluble IL-1RI (sIL-1RI) plays both anti- and proinflammatory roles; it can bind IL-1 agonists in solution, preventing them from binding to membrane IL-1RI, but also binds IL-1Ra and thus inhibits the inhibitor, all with the same affinities as the membrane-bound IL-1RI form [44]. sIL-1RII binds the IL-1 agonists with the same affinities as its membrane predecessor with a preference for IL-1β over IL-1α [47] but demonstrates a drastic fall in its affinity to IL-1Ra [45], stressing the importance of both IL-1RII and IL-1Ra as natural key regulators of the IL-1 signaling system, working in synergy. Smith et al. found that sIL-1RAP is able to bind sIL-1RII and increase its affinity to both IL-1 agonists, but not to IL-1Ra, thus making sIL-1RII an effective inhibitor of both IL-1α and IL-1β [48]. Finally, sIL-1RII, but not transmembrane IL-1RII, is able to bind pro-IL-1β intracellularly, controlling its secretion from necrotic cells and preventing its cleavage to the mature form upon stimulation [45, 46] (Fig. 1).
IL-1 Dysregulation and Its Contribution to Cancer and Tumorigenesis
In 2008, Mantovani et al. suggested that inflammation plays a role in the pathogenesis of cancer in two different pathways, an intrinsic pathway, where genetic mutations activate oncogenes and cause neoplasia, and an extrinsic pathway, where an inflammatory environment increases the susceptibility to cancer [49]. Since then, many basic and clinical research studies have been performed to validate this association and IL-1 had been a major target of investigation. The involvement of IL-1 in tumorigenesis, cancer progression, metastasis, and even in the response to cancer treatment (i.e., chemotherapy, surgery, or radiation) has been studied extensively in the last few years [50]. IL-1 dysregulation has been shown to be associated with almost all types of human malignancies. We herein provide a concise review of the recent findings on IL-1 involvement in a variety of cancers (summarized in Table 1).
Table 1.
IL-1 involvement in a variety of human malignancies
| Cancer type | Mechanism | References |
|---|---|---|
| Bladder cancer | Upregulation of ER-β/Il-1/c-MET by infiltrating T cells | [77] |
| Breast cancer ± bone metastasis | Overexpression of IL-1β mediated by TNF-β in monocytes and DCs | [68–70] |
| Colon cancer | IL-1β causes the overexpression of mitogenic, proto-oncogene HGF-cMET | [56] |
| Endometrial cancer | Overexpression of IRAK1 | [74] |
| Esophageal cancer | Overexpression of IL-1β in malignant tissues | [63] |
| Glioma | Activation of IL-1β by NLRP3-triggered caspase-1 | [51–53] |
| Head and neck squamous cell carcinoma | Overexpression of IL-1α and IL-1β, and decreased expression of IL-1Ra in ER-HNSCC cells | [67] |
| Hepatic cancer | Elevated serum levels of IL-1β cause overexpression of oncoprotein gankyrin via IL-1β/IRAK1 signaling | [62] |
| Multiple myeloma and lymphoma | Overexpression of IL-1β in malignant tissues | [72, 73] |
| Non-small cell lung cancer | Increased levels of IL-1Ra correlated with worse prognosis. Overexpression of IL-1β via Tap73/caspase-1 activation | [64, 65] |
| Oropharyngeal cancer | HPV-negative OPC cells show increased expression of IL-1β | [66] |
| Ovarian cancer | Overexpression of IL-1β/IL1-I1 represses p53 tumor suppressor | [75] |
| Pancreatic cancer | Overexpression and secretion of IL-1β by CD133+ tumor-initiating cancer stem cells and TAMs | [57, 58] |
| Prostate cancer | Activation of TR4/Oct4/IL-1Ra axis confers chemotherapy resistance | [78] |
In the brain, IL-1β was shown to be overexpressed in human malignant gliomas [51] and is associated with cell proliferation, migration, and invasion [52] through caspase-1 activation of IL-1β, triggered by Nod-like receptor family pyrin domain containing 3 (NLRP3) inflammasome. Treatment with either NLRP3 silencing or IL-1β suppressed cell proliferation and invasion [53].
Colon cancer is a one of the most metastatic cancers, and overexpression of mitogen hepatocyte growth factor (HGF) and its proto-oncogene receptor c-MET was shown to be associated with enhanced metastatic properties of cancer cells [54, 55]. Ma et al. found that IL-1α produced by colon carcinoma cells increases HGF secretion from fibroblasts which promoted proliferation, migration, and tube formation of human umbilical vein endothelial cells (HUVECs), and that the use of IL-1Ra significantly inhibited those functions via the inhibition of IL-1/PI3K/NF-кB pathway [56].
Pancreatic cancer is a deadly cancer. Maker et al. found a positive correlation between the levels of IL-1β in the fluids collected from pancreatic cysts and the grade of dysplasia and were highly predictive of high risk lesion of intraductal papillary mucinous neoplasms [57]. The overexpression of CD133+ tumor-initiating cancer stem cells promotes pancreatic cancer through the increased expression and secretion of IL-1β, and upregulation of NF-кB signaling, epithelial–mesenchymal transition, and invasiveness. Tumor associating macrophages (TAMs) were also found to secrete IL-1β, further activating and enhancing this pathway [58].
IL-1β is known to be a key player in chronic liver inflammation [59] and the connection between hepatic inflammation and cancer is already relatively well established [60]. IL-1β has recently been implicated in hepatocellular carcinoma (HCC). Its deficiency in obese mice prevented HCC development compared to wild type [61]. Later on, it was found that IL-1β but not IL-1α serum levels were elevated in patients with hepatitis, liver fibrosis/cirrhosis, and primary HCC, causing the overexpression of gankyrin, an oncoprotein commonly overexpressed in hepatic cancer responsible for cell growth, invasiveness, and metastasis through IL-1β/IRAK-1 signaling [62].
Chen et al. found increased levels of IL-1β in both mRNA and protein levels in 147 esophageal squamous cell carcinoma (SCC) samples compared with nonmalignant tissues, which correlated with clinical stage, response rate to treatment, and recurrence. Blocking the signaling with a recombinant IL-1β antibody attenuated tumor growth, invasiveness, and treatment resistance [63].
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death today [64]. In 2017 Yigit et al. found that elevated levels of IL-1Ra in NSCLC patients were correlated with worse progression-free and overall survival [64]. Overexpression of the transcription factor TAp73 promotes upregulation of caspase-1, which is in charge of pro-IL-β cleavage to its mature form. Patients with lung and breast cancer who had strong p73/Il-1β interactions exhibited significantly worse survival than patients who showed no interaction [65].
Human papilloma virus (HPV)-negative oropharyngeal cancer (OPC) is known to have worse prognosis than HPV-positive OPC. It was shown that HPV-negative, but not HPV-positive, OPC cells expressed IL-1β and together with a constitutive expression of IL-1R1 on normal tonsillar fibroblasts caused robust chemokine secretion. The use of either anakinra, a recombinant IL-1Ra, or siRNA of IL-1RI significantly decreased chemokine secretion in these cell lines. In contrast, IL-1α was found to be expressed by both HPV-negative and HPV-positive lines, though slightly but significantly higher in the former [66]. Overexpression of IL-1α and IL-1β was found to be associated with poor response to erlotinib (an epidermal growth factor inhibitor) treatment in head and neck squamous cell carcinoma (HNSCC) [67]. In animal models the addition of IL-1 antagonist (anakinra) improved response to the treatment. The addition of anakinra alone showed some benefit [67].
Wu et al. analyzed 149 primary breast cancer tissues and found a correlation between overexpression of IL-1β, but not IL-1α, and breast cancer staging. IL-1β expression was mediated by malignant cell-membrane-associated TGF-β in dendritic cells (DC) and monocytes, and neutralizing TGF-β and IL-1β prevented cancer progression in humanized model mice [68, 69]. Moreover, both IL-1β and IL-1RI were found to be upregulated in a cancer mouse model with bone metastases, and their blockage with anakinra decreased tumor size and metastases [70].
Both IL-1 agonists are known to play important roles in many benign and malignant skin diseases, as review by Bou-Dargham et al. They suggest focusing on IL-1α as the main target of IL-1 immunotherapy in skin cancer because of the unique expression of IL-1α and its bioactive pre-IL-1α in different skin cell types [71]. Elevated levels of IL-1β were found in hematologic cancers, such as multiple myeloma (MM) and some types of lymphoma compared with healthy controls [72, 73].
IL-1 involvement is also implicated in some gynecologic cancers; in endometrial cancer (EC) IL-1R-associated kinase 1 (IRAK1) expression was shown to be elevated in EC compared with normal tissues, correlating with worse staging, metastasis, invasiveness, and low survival rate [74]. IL-1β/IL-1RI expression was found to be elevated in both cancer epithelial and stromal cells in samples from ovarian cancer patients, leading to repression of p53 tumor suppressor and correlated with reduced overall survival [75].
Both IL-1 agonists are expressed under normal conditions in a variety of testicular cells and thought to play a role in normal testicular homeostasis—IL-1α regulating Sertoli cell proliferation and IL-1β regulating testosterone production in Leydig cells [76]. On the other hand, IL-1 overexpression was found in certain types of genitourinary cancers; Tao et al. found in an in vitro/murine model that infiltrating T cells in bladder cancer cause the upregulation of IL-1 through estrogen receptor beta (ER-β)/IL-1/receptor tyrosine kinase (c-MET) pathway, and that the use of IL-1 antagonist could partially reverse cell invasion and proliferation [77]. Furthermore, Yang et al. found that overexpression of IL-1Ra in prostate cancer via the testicular nuclear receptor 4 (TR4)-Oct4-IL-1Ra axis in CD133+ prostate cancer progenitor cells increases resistance to chemotherapy, which may be reversed by downregulation of this axis and inhibition of IL-1Ra [78].
Lastly, intrinsic mutations and polymorphisms in IL-1 genes have been implicated as well in many cancer types as reviewed by Khazim et al. [79], though results from studies are sometimes conflicting, and the association is not always clear. Single and IL-1 gene-cluster polymorphisms have been suggested to be associated with cancers such as cervical [80] and ovarian cancer [81], MM [82], esophageal [83], colorectal cancer (CRC) [84], NSCLC [85], pancreatic cancer [86], prostate cancer [87], and gastric cancer [88–90].
Development of IL-1 Blockers and Their Use in Clinical Trials
Since the discovery of IL-1 and until the beginning of the 1990s, IL-1 agonists and not antagonists were paradoxically used to treat cancer patients but resulted in toxicity and severe adverse events related to uncontrolled inflammation [91]. The first IL-1-blocking drugs were designed to treat autoinflammatory and autoimmune diseases, and the only approved IL-1 blockers so far are indicated for diseases of this kind. In the rest of this paper we will review the IL-1 products being currently developed and tested as cancer therapies, and both ongoing and completed clinical trials using these products in cancer indications (summarized in Table 2 and illustrated in Fig. 2).
Table 2.
IL-1 inhibitors and their corresponding clinical trials
| Name | Company | Target molecular mechanism | Cancer indication | Most advanced phase | Publication | Status | Trial number |
|---|---|---|---|---|---|---|---|
| Other/previous names | |||||||
| Xilonix | Xbiotech | IL-1α | Pancreatic cancer with cachexia | Phase I | Recruiting | NCT03207724 | |
|
Advanced cancers NSCLC (subgroup) |
Phase I |
Hong et al. 2014 [92] Hong et al. 2015 [93] |
Completed | NCT01021072 | |||
| MABp1, CV-18C3, T2-18C3, RA-18C3, CA-18C3, bermekimab, hutruo | IgG1 True Human mAb | ||||||
| CRC |
Phase III Phase III |
Hickish et al. 2017 [95] Fisher 2015 [94] |
Completed Completed |
||||
| Hematologic malignancies | Phase I | Completed | NCT01260545 | ||||
| Canakinumab | Novartis | IL-1β | NSCLC |
Phase III Phase III Phase III |
Not yet recruiting Not yet recruiting Recruiting |
||
| Melanoma | Phase II | Not yet recruiting | NCT03484923 | ||||
| Ilaris, ACZ885 | Human IgG1/k mAb | CRC, TNBC, NSCLC—adenocarcinoma | Phase IB | Recruiting | NCT02900664 | ||
| Atherosclerosis → lung cancer | Ridker et al. 2017 [98] | Active, not recruiting | NCT01327846 | ||||
| P2D7KK | A*STAR | IL-1β mAb | Multiple myeloma | Goh et al. 2014 [99] | Preclinical phase | ||
| Anakinra | Swedish Orphan Biovitrum AB | A recombinant, nonglycosylated Il-1Ra | Multiple myeloma |
Phase II Phase II |
Recruiting | NCT03233776 | |
|
Lust et al. 2016 [102] Lust et al. 2009 [101] |
Completed | NCT00635154 | |||||
| Early multiple myeloma | Phase I/II | Suspended (09/2018) | NCT02492750 | ||||
| Pancreatic cancer |
Phase I Phase I |
Recruiting Active, not recruiting |
|||||
| Metastatic colorectal cancer | Phase II | Completed | NCT02090101 | ||||
| Kineret | |||||||
| Metastatic breast cancer | Phase I | Unknown | NCT01802970 | ||||
| Advanced cancers | Phase I | Active, not recruiting | NCT01624766 | ||||
| Metastatic adult solid tumors | Phase I | Completed | NCT00072111 | ||||
| IP-1510 | Itis Pharmaceuticals | A synthetic IL-1Ra | Cancer-related cachexia | Phase I/II | Paspaliaris et al. 2011 [103] | ||
| IP-1510D | |||||||
| Nidanilimab | Cantargia | IL-1RAP | NSCLC, PDAC, TNBC, CRC | Combined phase I/IIA | Recruiting | NCT03267316 | |
| Can-04 | IgG1 True Human and ADCC enhanced mAb | Leukemia | Preclinical development | ||||
| CSC012-ADC | Cellerant Therapeutics | IL-1RAP | Acute myeloid leukemia | Preclinical development | |||
| CLT012 | Ab–drug conjugate | ||||||
mAb monoclonal antibody, NSCLC non-small cell lung cancer, CRC colorectal cancer, TNBC triple-negative breast cancer, PDAC pancreatic ductal adenocarcinoma
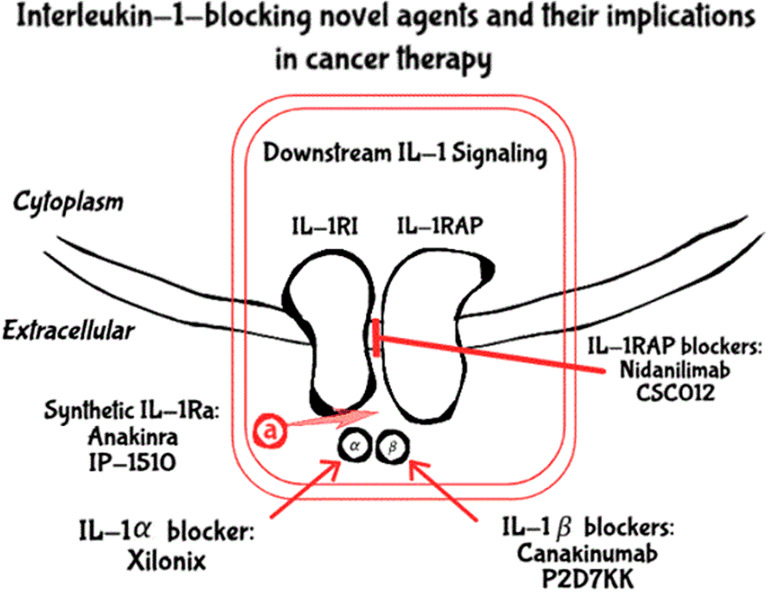
Fig. 2.
Novel IL-1 blockers used in clinical trials for cancer therapy. Xilonix, an IL-1α True Human monoclonal antibody, and canakinumab and P2D7KK, IL-1β human monoclonal antibodies, bind to specific regions on their targets, preventing the agonists’ binding to IL-1RI, thus inhibiting downstream signaling. Nidanilimab and CSC012, antibodies specific for IL-1RAP, prevent it from forming the heterotrimeric signaling complex. Lastly, anakinra and IP-1050 are synthetic IL-1Ra agents that compete with the agonists for binding to IL-1RI, thus attenuating the IL-1 signaling system
IL-1α Neutralizing Antibody
Xilonix, also known as MABp1 or bermekimab, is an IL-1α True Human™ monoclonal antibody (mAb) cloned from in vivo human naturally occurring immune response and was developed by Xbiotech. To our knowledge it is currently the only drug targeting IL-1α being tested in cancer treatment. The first phase 1 clinical trial of Xilonix (NCT01021072) included 52 patients with metastatic cancer of 18 tumor types. It was designed primarily to assess safety, tolerability, pharmacokinetic profile, and recommended dose for phase 2. The results were encouraging, and the drug was found to be well tolerated and with a good pharmacokinetic profile. Assessable patients showed a decrease in proinflammatory IL-6 normally regulated by IL-1/IL-1Ra, increase in lean body mass (LBM), and no serious treatment-related adverse events (AEs). The most common AEs were proteinuria, nausea, and fatigue [92]. A subpopulation of 16 NSCLC patients was separately analyzed and though not statistically significant (possibly due to small sampling number), trends were found in IL-1, platelet counts, C-reactive protein (CRP), and lean body mass (LBM), and improvement in self-reported pain, fatigue, and appetite [93]. Following this trial, a phase 3 trial (NCT01767857) was performed on 40 refractory CRC patients with cachexia who failed standard chemotherapy, using the dose decided upon in the phase 1 trial (3.75 mg/kg IV every 2 weeks). This 1:1 randomized Xilonix/placebo trial resulted in longer overall survival for the Xilonix group (2.8 months vs. 2.0 months), with better physical and functional status and no discontinuations due to AEs. Platelets are known to contribute to cancer growth and metastasis, with increased counts during cancer progression, and reduced platelets counts were observed in the treatment group [94]. The last completed trial with published results for Xilonix was performed between 2014 and 2015 (NCT02138422). A total of 333 metastatic or unresectable CRC patients received an increased dose of Xilonix (7.5 mg/kg IV every 2 weeks for 8 weeks) in a 2:1 Xilonix/placebo phase 3 trial. More Xilonix patients reached the primary endpoint compared with the placebo group with stable/improved LBM and stable/improved two of three fatigue, pain, and anorexia. IL-6 levels and platelets count decreased significantly in the treatment group. This trial design, however, did not allow one to compare overall survival between the groups. Again, no significant difference in serious AEs was observed [95].
A new phase 1 clinical trial using Xilonix in combination with Onivyde and 5-FU in advanced pancreatic cancer patients with cachexia is currently underway and recruiting (NCT03207724). Primary endpoint is the determination of maximum tolerated dose (MTD) and secondary endpoints include weight stability, LBM, overall survival (OS), progression-free survival (PFS), changes in quality of life (QOL), and patient-reported response to therapy.
At first sight the overall results, including significant improvement in LBM and different QOL parameters, and the great safety profile with seemingly almost no serious AEs seem promising. However, we must take into consideration that no antitumor effect or improved OS was detected in those up-to-date trials, and that no long-term AEs were assessed. Are these findings a strong enough reason to use Xilonix widely in cancer treatment? More research is probably needed to provide the missing information.
IL-1β Antagonists
Canakinumab, previously known as ACZ885, is a human IgG1/κ mAb very specific for IL-1β and was developed by Novartis Pharma. It is already authorized in both the USA and Europe as a treatment of cryopyrin-associated periodic syndrome (CAPS), systemic juvenile idiopathic arthritis (SJIA), and for gouty arthritis in Europe alone. Canakinumab neutralizes IL-1β by blocking its IL-1RI binding surface, thus preventing the agonist–receptor association [96]. The first and only report so far describing the beneficial effects of canakinumab in cancer was derived from the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS, NCT02327846). In this large-scale trial, 10,061 patients with atherosclerosis who suffered from a myocardial infection, with no previous diagnosis of cancer, and with high levels of high-sensitivity CRP (hsCRP) were randomized into a placebo group and three escalating canakinumab dose groups (50 mg, 150 mg, and 300 mg), administered subcutaneously (SC) every 3 months. The original primary endpoints were cardiovascular death or non-fatal myocardial infarction and stroke [97]. The combination of increased hsCRP concentrations and high incidence of smokers among atherosclerotic patients put the CANTOS population at higher risk of developing lung cancer and led the researchers to assess the effect of canakinumab on incident lung cancer among these patients after the termination of the trial. The results were intriguing. During follow-up of median 3.7 years, the incidence of lung cancer was significantly lower in the 150 mg and 300 mg treatment groups compared with placebo and the incidence of fatal lung cancer and incidence of fatal cancers of all types were significantly reduced in the 300 mg group. On the contrary, no significant change was found in the incidence of non-lung cancers and in overall cancer incidence, pointing to specificity for this type of solid cancer. On the other side of the immunity spectrum, however, blocking IL-1β with canakinumab was associated with a significant increase in fatal infections and sepsis in all three treatment groups compared with placebo. However, one must consider that all of the patients who participated in the trial were unhealthy to begin with, many of them old, obese, and/or diabetic, and indeed the patients who died from infections tended to be older and diabetic. Additionally, the success in reducing the incidence of lung cancer balanced the increase in sepsis and fatal infections so the overall mortality rate did not change significantly [98]. These results must be put into the context of being extracted from a trial designed for a completely different purpose, and although the potential of canakinumab in lung cancer therapy may seem promising, it has to be validated in designated clinical trials, with an emphasis on dosing and safety.
Indeed, a variety of new trials are on their way with specific cancer indications for canakinumab; a phase IB clinical trial using canakinumab in combination with checkpoint inhibitor PDR001 in patients with CRC, triple-negative breast cancer, and NSCLC (NCT02900664) aiming to assess the safety, tolerability, and pharmacological and clinical activity of the canakinumab–PDR001 combination, and a phase 3 trial in patients with NSCLC aiming to compare the efficacy and safety of canakinumab versus placebo (NCT03447769) are currently recruiting patients. A phase 2 trial evaluating the efficacy and safety of canakinumab in combination with PDR001 in previously treated unresectable or metastatic melanoma is planned to start recruiting patients (NCT03484923). A phase 3 trial evaluating efficacy and safety of the anti-PD-1 pembrolizumab (Keytruda) plus platinum-based doublet chemotherapy with or without canakinumab in previously untreated locally advanced or metastatic non-squamous and squamous NSCLC patients (CANOPY-1, NCT03631199) and the efficacy and safety of canakinumab in combination with docetaxel in NSCLC patients as a second- or third-line therapy (CANOPY-2, NCT03626545) are future planned clinical trials. The results from these trials could properly validate or refute the intriguing data extracted from the CANTOS trial. P2D7KK, developed by A*STAR, is another IL-1β mAb, which has only been tested in mice so far. The preclinical study showed that P2D7KK binds to IL-1R1 using the same mechanism as canakinumab, but with affinity 11 times higher. In an MM mice model, the survival in the P2D7KK group was 70% compared with 20% in the control group [99].
Synthetic IL-1Ra
Anakinra (Kineret) is a recombinant, nonglycosylated IL-1Ra developed by Swedish Orphan Biovitrum AB. It was approved by the FDA as treatment for rheumatoid arthritis, CAPS, and neonatal-onset multisystem inflammatory disease (NOMID). Xiong et al. distinguished two groups of patients with smoldering multiple myeloma (SMM), depending on IL-6 production by their bone marrow stromal cells as a surrogate biomarker of IL-1β activity; IL-1β production is known to be dysregulated in MM patients and is an inducer of IL-6 [100]. They found that some SMM patients were higher producers of IL-6, with levels similar to active MM patients, and some SMM patients were lower IL-6 producers, with IL-6 levels comparable to monoclonal gammopathy of undetermined significance (MGUS) patients, suggesting a possible method of predicting SMM patients’ progression to active MM [100].
In a phase 2 clinical trial (NCT00635154) conducted by Lust et al. between 2002 and 2007, 47 patients with a diagnosis of smoldering/indolent MM not requiring immediate therapy and at high risk of progressing to active myeloma received anakinra for 6 months, in an attempt to inhibit the IL-1/IL-6 axis and to prolong their PFS period [101]. Patients who showed clinical improvement continued receiving anakinra alone, and patients who were stable or showed progression after 6 months received low dose dexamethasone in addition to anakinra. Short-term and long-term follow-up results were highly encouraging. Treatment with anakinra alone decreased the high-sensitivity C-reactive protein (hs-CRP) levels, a surrogate marker for plasma IL-6 which reached undetectable concentrations and slowed down the myeloma cell growth rate. Dexamethasone, however, was able to cause apoptosis of the nonproliferative myeloma fraction, thereby improving MM parameters and reducing the production of IL-1β. Patients who received the combined therapy had better OS and PFS, suggesting a synergistic effect for the two drugs. The most common AE was injection site reactions occurring in almost all patients (86%) but resulting in the withdrawal of only two patients in the beginning of the study as they typically resolved within the first month of treatment. Asymptomatic neutropenia occurred in 21 patients, and infections occurred in 9 patients [101]. A long-term follow-up (median 7.7 years for the entire group) strengthened the results achieved at the end of the original study period, and proved good long-term toxicity profile, with only four patients out of the 47 ending active treatment as a result of AEs (including the two patients described above) [102]. In addition, and very importantly, patients who achieved more than 40% decrease in their hs-CRP levels compared to the baseline value at 6 months exhibited significantly better PFS and OS than patients who did not achieve such a decrease [102].
More trials using anakinra for treating different cancers followed. Phase 1 trials for treating advanced cancers (NCT01624766), metastatic tumors (NCT00072111), metastatic breast (NCT01802970), pancreatic cancer (NCT02550327, NCT02021422) and a phase 2 CRC trial (NCT02090101) have been registered, and another phase 1/2 MM trial is currently suspended (NCT02492750), but none has yet published results. Somewhat surprisingly, we could not find any phase 3 anakinra trials for cancer indication.
IP-1510 is another synthetic IL-1Ra agent that was developed by Itis Pharmaceuticals. It was evaluated in a phase 1/2 trial in patients with cancer-related cachexia after showing encouraging results in rats. Subcutaneous injections twice daily were well tolerated with no serious AEs, with improvement in appetite, weight gain, and depression scores [103]. Out of 26 enrolled patients only 20 completed the course of treatment [103]. We could not find newer information regarding this agent in cancer treatment and larger trials must be performed for a significant validation of these single trial results.
IL-1RAP Inhibitors
Two anti-IL-1RAP antibodies are currently being tested in cancer. CSC012 is an antibody–drug conjugate currently in the preclinical phase and developed by Cellerant therapeutics as a targeted treatment for acute myeloid leukemia. Cantargia’s Can-04 (or nidanilimab) is another True Human mAb, with a special characteristic—according to preclinical trials results reported by the company it elicits a strong antibody-dependent cell-mediated cytotoxicity (ADCC) response, recruiting the immune killer cells to eradicate the target cells. A planned phase 1/2A study CANFOUR is designed to evaluate the safety and tolerability at escalating doses and to assess antitumor profile as monotherapy and in combination with the best standard of care in NSCLC, pancreatic ductal adenocarcinoma, triple-negative breast cancer, and CRC (NCT03267316). CSC012 is also being tested in the preclinical phase for treating leukemia.
Concluding Remarks
Dysregulation of the IL-1 proinflammatory cascade has been shown to play an important role in cancer initiation, progression, and invasiveness, and it seems that the clinical relevance of IL-1 in cancer therapy is just beginning to be unveiled. Blocking the IL-1 system has been shown to be beneficial in a variety of cancers in both basic research and early clinical trials, and indeed new IL-1 blocking agents are continuously emerging. The safety profile of these agents was assessed in the short term and they were found to be relatively very safe. Long-term follow-ups of adverse events will help to establish these findings. In most of the clinical trials, blocking the IL-1 system improved cancer-related symptoms, especially when the novel drugs were added to other chemotherapy drugs. However, there is currently no robust data regarding survival improvement and antitumor activity, which may be argued to be of higher relevance.
IL-1 blockers have already shown great efficacy and safety in the treatment of several autoimmune diseases. These conditions, however, are systemic in nature, and thus a systemic approach is logical in that case. In cancer treatment, however, a more localized approach may fit better, minimizing systemic adverse events influencing other healthy systems, and enhancing the therapeutic effect on the affected one.
Several clinical trials are currently ongoing or planned to start recruiting patients. These trials may elucidate many open questions regarding the use of IL-1 blockers in the treatment of several tumor types. In addition, new IL-1 drugs are currently being developed and tested for non-cancer indications, which could be considered for cancer therapy as well; examples are the already FDA-approved rilonacept (Regeneron Pharmaceuticals), an IL-1 trap indicated for treating CAPS, and IL-1β mABs gevokizumab (XOMA Corporation) tested in many autoimmune diseases and LY2189102 (Eli Lilly and Co) tested in diabetic patients.
The pleotropic nature of IL-1 and its role under homeostatic conditions, together with its crucial role in defense against infections, make it difficult to predict the systemic and long-term effects of such blocking. More data must be obtained from these human clinical trials, with emphasis on antitumor effect, survival properties, and toxicity. However, owing to a great safety profile and intriguing preliminary results in caner trials together with already established results in other indications, we believe that IL-1 could be a major player in oncology therapy in the future.
Acknowledgements
Funding
Work in our lab was supported by the Israeli Society of Nephrology and Hypertension (ISNH) and the Sir Dr. Naim Eliahou Dangoor Foundation for Personalized Medicine. Dr. Idan Cohen was funded by the Ministry of Aliya and Immigrant Absorption. No article processing charges were received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Adi Litmanovich, Khaled Khazim, and Idan Cohen have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7334435.
References
- 1.Schmidt A, Weber OF. Memoriam of Rudolf Virchow: a historical retrospective including aspects of inflammation, infection and neoplasia. In: Dittmar T, Zanker KS, Schmidt A, editors. Infection and inflammation: impacts on oncogenesis. Basel: Karger; 2006. pp. 1–15. [DOI] [PubMed] [Google Scholar]
- 2.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 3.Germano G, Allavena P, Mantovani A. Cytokines as a key component of cancer-related inflammation. Cytokine. 2008;43:374–379. doi: 10.1016/j.cyto.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 5.Escamilla-Tilch M, Filio-Rodriguez G, Garcia-Rocha R, et al. The interplay between pathogen-associated and danger-associated molecular patterns: an inflammatory code in cancer? Immunol Cell Biol. 2013;91:601–610. doi: 10.1038/icb.2013.58. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, Abbas AK, Aster JC, editors. Inflammation and repair. In: Robbins and Cotran pathologic basis of disease, 9th edition. Philadelphia, Elsevier Saunders; 2015. p. 69–112.
- 7.Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. doi: 10.1038/s41698-018-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;4:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jess T, Loftus EV, Velayos FS, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from Olmsted county, Minnesota. Gastroenterology. 2006;130:1039–1046. doi: 10.1053/j.gastro.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Vainio H, Boffetta P. Mechanisms of the combined effect of asbestos and smoking in the etiology of lung cancer. Scand J Work Environ Health. 1994;20(4):235–242. doi: 10.5271/sjweh.1402. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Kato J, Inoue I, et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014;134:1445–1457. doi: 10.1002/ijc.28470. [DOI] [PubMed] [Google Scholar]
- 13.Kriger JN, Riley DE, Vessela RL, Miner DC, Ross SO, Lange PH. Bacterial DNA sequences in prostate tissue from patients with prostate cancer and chronic prostatitis. J Urol. 2000;164(4):1221–1228. [PubMed] [Google Scholar]
- 14.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araldi RP, Sant’Ana TA, Modolo DG, et al. The human papillomavirus (HPV)-related cancer biology: an overview. Biomed Pharmacother. 2018;106:1537–1556. doi: 10.1016/j.biopha.2018.06.149. [DOI] [PubMed] [Google Scholar]
- 16.Lewis AM, Varghese S, Xu H, Alexander HR. Interleukin-1 and cancer progression: the emerging role of interleukin-1 receptor antagonist as a novel therapeutic agent in cancer treatment. J Transl Med. 2006;4:48. doi: 10.1186/1479-5876-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voronov E, Shouval DS, Krelin Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100(5):2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen I. DNA damage talks to inflammation. Cytokine Growth Factor Rev. 2017;33:35–39. doi: 10.1016/j.cytogfr.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krelin Y, Voronov E, Dotan S, et al. Interleukin-1beta-driven inflammation promotes the development and invasiveness of chemical carcinogen-induced tumors. Cancer Res. 2007;67(3):1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 21.Modi WS, Masuda A, Yamada M, Oppenheim JJ, Matsushima K, O’Brien SJ. Chromosomal localization of the human interleukin 1 alpha (IL-1 alpha) gene. Genomics. 1988;2(4):310–314. doi: 10.1016/0888-7543(88)90019-5. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1α. Proc Natl Acad Sci USA. 1990;87(14):5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boraschi D, Italiani P, Weil S, Martin MU. The family of the interleukin-1 receptors. Immunol Rev. 2018;281:197–232. doi: 10.1111/imr.12606. [DOI] [PubMed] [Google Scholar]
- 24.Rider P, Carmi Y, Voronov E, Apte RN. Interleukin-1α. Semin Immunol. 2013;25:430–438. doi: 10.1016/j.smim.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA. An expanding role for interleukin-1 blockade from gout to cancer. Mol Med. 2014;20(Supplement 1):S43–S58. doi: 10.2119/molmed.2014.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen I, Rider P, Vornov E, et al. IL-1α is a DNA damage sensor linking genotoxic stress signaling to sterile inflammation and innate immunity. Sci Rep. 2015;5:14756. doi: 10.1038/srep14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107(6):2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: feel the stress. J Immunol. 2017;198:1395–1402. doi: 10.4049/jimmunol.1601342. [DOI] [PubMed] [Google Scholar]
- 30.Martin SJ. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 2016;283(14):2599–2615. doi: 10.1111/febs.13775. [DOI] [PubMed] [Google Scholar]
- 31.Zhao R, Zhou H, Bo SuS. A critical role for interleukin-1β in the progression of autoimmune diseases. Int Immunopharmacol. 2013;17:658–669. doi: 10.1016/j.intimp.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Zhao R, Zhou H, Zhang J, Liu X, Bo SuS. Interleukin-1β promotes the induction of retinal autoimmune disease. Int Immunopharmacol. 2014;22:285–292. doi: 10.1016/j.intimp.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 33.Dinarello CA. A clinical perspective of IL-1b as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 34.Cantarini L, Lopalco G, Cattalini M, Vitale A, Galeazzi M, Rigante D. Interleukin-1: Ariadne’s thread in autoinflammatory and autoimmune disorders. IMAJ. 2015;17:93–97. [PubMed] [Google Scholar]
- 35.Netea MG, Van de Veerdonk FL, Van der Meer JWM, Dinarello CA, Joosten LAB. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 36.Scheuder H, Tardif C, Trump-kallmeyer S, et al. A new cytokine-receptor binding mode revealed by the crystal structure of the IL-1 receptor with an antagonist. Nature. 1997;386:194–200. doi: 10.1038/386194a0. [DOI] [PubMed] [Google Scholar]
- 37.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13(4–5):323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 38.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 39.Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family—balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76:25–37. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Fang XM, Schroder S, Hoeft A, Stuber F. Comparison of two polymorphisms of the interleukin-1 gene family: interleukin-1 receptor antagonist polymorphism contributes to susceptibility to severe sepsis. Crit Care Med. 1999;27(7):1330–1334. doi: 10.1097/00003246-199907000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Aksentijevich I, Masters SL, Ferguson PJ, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360(23):2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddy S, Jia S, Geoffrey R, et al. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N Engl J Med. 2009;360(23):2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters VA, Joesting JJ, Freund GG. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav Immun. 2013;32:1–8. doi: 10.1016/j.bbi.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boraschi D, Tagliabue A. The interleukin-1 receptor family. Semin Immunol. 2013;25:394–407. doi: 10.1016/j.smim.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Symons JA, Young PR, Duff GW. Soluble type II interleukin 1 (IL-1) receptor binds and blocks processing of IL-1β precursor and loses affinity for IL-1 receptor antagonist. Proc Natl Acad Sci USA. 1995;92:1714–1718. doi: 10.1073/pnas.92.5.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molgora M, Supino D, Mantovani A, Garlanda C. Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8. Immunol Rev. 2018;281:233–247. doi: 10.1111/imr.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Symons JA, Eastgate JA, Duff GW. Purification and characterization of a novel soluble receptor for interleukin 1. J Exp Med. 1991;174:1251–1254. doi: 10.1084/jem.174.5.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith DE, Hanna R, Friend D, et al. The soluble form of IL-1 receptor accessory protein enhances the ability of soluble type II IL-1 receptor to inhibit IL-1 action. Immunity. 2003;18:87–96. doi: 10.1016/s1074-7613(02)00514-9. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 50.Mantovani A, Barajon I, Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev. 2018;281:57–61. doi: 10.1111/imr.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarassishin L, Casper D, Lee SC. Aberrant expression of interleukin-1b and inflammasome activation in human malignant gliomas. PLoS One. 2014;9(7):e103432. doi: 10.1371/journal.pone.0103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hurmath KF, Ramaswamy P, Nandakumar DN. IL-1b microenvironment promotes proliferation, migration and invasion of human glioma cells. Cell Biol Int. 2014;38:1415–1422. doi: 10.1002/cbin.10353. [DOI] [PubMed] [Google Scholar]
- 53.Xue L, Lu B, Gao B, et al. NLRP3 promotes glioma cell proliferation and invasion via the interleukin-1β/NF-κB p65 signals. Oncol Res. 2018 doi: 10.3727/096504018x15264647024196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sakai K, Aoki S, Matsumoto K. Hepatocyte growth factor and Met in drug discovery. J Biochem. 2015;157(5):271–284. doi: 10.1093/jb/mvv027. [DOI] [PubMed] [Google Scholar]
- 55.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 56.Ma J, Sun X, Guo T, et al. Interleukin-1 receptor antagonist inhibits angiogenesis via blockage IL-1α/PI3K/NF-κB pathway in human colon cancer cell. Cancer Manag Res. 2017;9:481–493. doi: 10.2147/CMAR.S147699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maker AV, Katabi N, Qin LX, et al. Cyst fluid interleukin-1b (IL1b) levels predict the risk of carcinoma in intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2011;17(6):1502–1508. doi: 10.1158/1078-0432.CCR-10-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nomura A, Gupta VK, Dauer P, et al. NFkB-mediated invasiveness in CD133+ pancreatic TICs is regulated by autocrine and paracrine activation of IL-1 signaling. Mol Cancer Res. 2018;16(1):162–172. doi: 10.1158/1541-7786.MCR-17-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57(3):642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 60.Bishayee A. The role of inflammation in liver cancer. In: Aggarwal BB, Sung B, Gupta SC, editors. Inflammation and cancer. Advances in experimental medicine and biology, vol 816. Basel: Springer; 2014. p. 401–435. [DOI] [PubMed]
- 61.Yoshimoto S, Loo TM, Atarashi K, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 62.Su B, Luo T, Zhu J, et al. Interleukin-1β/interleukin-1 receptor-associated kinase 1 inflammatory signaling contributes to persistent gankyrin activation during hepatocarcinogenesis. Hepatology. 2015;61(2):585–597. doi: 10.1002/hep.27551. [DOI] [PubMed] [Google Scholar]
- 63.Chen MF, Lu MS, Chen PT, Chen WC, Lin PY, Lee KD. Role of interleukin 1 beta in esophageal squamous cell carcinoma. J Mol Med (Berl) 2012;90(1):89–100. doi: 10.1007/s00109-011-0809-4. [DOI] [PubMed] [Google Scholar]
- 64.Yigit M, Değirmencioğlu S, Ugurlu E, Yaren A. Effect of serum interleukin-1 receptor antagonist level on survival of patients with non-small cell lung cancer. Mol Clin Oncol. 2017;6(5):708–712. doi: 10.3892/mco.2017.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vikhreva P, Petrova V, Gokbulut T, et al. TAp73 upregulates IL-1β in cancer cells: potential biomarker in lung and breast cancer? Biochem Biophys Res Commun. 2017;482(3):498–505. doi: 10.1016/j.bbrc.2016.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Sahaf S, Hunter KD, Bolt R, Ottewell PD, Murdoch C. The IL-1/IL-1R axis induces greater fibroblast-derived chemokine release in human papillomavirus-negative compared to positive oropharyngeal cancer. Int J Cancer. 2018;4:4. doi: 10.1002/ijc.31852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanam A, Gibson-Corley KN, Love-Homan L, Ihejirika N, Simons AL. Interleukin-1 blockade overcomes erlotinib resistance in head and neck squamous cell carcinoma. Oncotarget. 2016;7(46):76087–76100. doi: 10.18632/oncotarget.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu TC, Xu K, Martinek J, et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 2018;78(18):5243–5258. doi: 10.1158/0008-5472.CAN-18-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dinarello CA. An interleukin-1 signature in breast cancer treated with interleukin-1 receptor blockade: implications for treating cytokine release syndrome of checkpoint inhibitors. Cancer Res. 2018;78(18):5200–5202. doi: 10.1158/0008-5472.CAN-18-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holen I, Lefley DV, Francis SE, et al. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7(46):75571–75584. doi: 10.18632/oncotarget.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bou-Dargham MJ, Khamis ZI, Cognetta AB, Sang QA. The role of interleukin-1 in inflammatory and malignant human skin diseases and the rationale for targeting interleukin-1 alpha. Med Res Rev. 2017;37(1):180–216. doi: 10.1002/med.21406. [DOI] [PubMed] [Google Scholar]
- 72.Mehtap O, Atesoglu EB, Tarkun P, Hacihanefioglu A, Dolasik I, Musul MM. IL-21 and other serum proinflammatory cytokine levels in patients with multiple myeloma at diagnosis. J Postgrad Med. 2014;60(2):141–144. doi: 10.4103/0022-3859.132319. [DOI] [PubMed] [Google Scholar]
- 73.Miyata-Takata T, Takata K, Toji T, et al. Elevation of serum interleukins 8, 4, and 1β levels in patients with gastrointestinal low-grade B-cell lymphoma. Sci Rep. 2015;5:18434. doi: 10.1038/srep18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Wang Y, Duan X, Wang Y, Zhang Z. Interleukin-1 receptor-associated kinase 1 correlates with metastasis and invasion in endometrial carcinoma. J Cell Biochem. 2018;119(3):2545–2555. doi: 10.1002/jcb.26416. [DOI] [PubMed] [Google Scholar]
- 75.Schauer IG, Zhang J, Xing Z, et al. Interleukin-1β promotes ovarian tumorigenesis through a p53/NF-κB-mediated inflammatory response in stromal fibroblasts. Neoplasia. 2013;15(4):409–420. doi: 10.1593/neo.121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lysiak JJ. The role of tumor necrosis factor-alpha and interleukin-1 in the mammalian testis and their involvement in testicular torsion and autoimmune orchitis. Reprod Biol Endocrinol. 2004;2:9. doi: 10.1186/1477-7827-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tao L, Qiu J, Slavin S, et al. Recruited T cells promote the bladder cancer metastasis via up-regulation of the estrogen receptor β/IL-1/c-MET signals. Cancer Lett. 2018;430:215–223. doi: 10.1016/j.canlet.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 78.Yang DR, Ding XF, Luo J, et al. Increased chemosensitivity via targeting testicular nuclear receptor 4 (TR4)-Oct4-interleukin 1 receptor antagonist (IL1Ra) axis in prostate cancer CD133+ stem/progenitor cells to battle prostate cancer. J Biol Chem. 2013;288(23):16476–16483. doi: 10.1074/jbc.M112.448142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khazim K, Azulay EE, Kristal B, Cohen I. Interleukin 1 gene polymorphism and susceptibility to disease. Immunol Rev. 2018;281(1):40–56. doi: 10.1111/imr.12620. [DOI] [PubMed] [Google Scholar]
- 80.Wu S, Hu G, Chen J, Xie G. Interleukin 1β and interleukin 1 receptor antagonist gene polymorphisms and cervical cancer: a meta-analysis. Int J Gynecol Cancer. 2014;24(6):984–990. doi: 10.1097/IGC.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 81.Ahmed AB, Zidi S, Sghaier I, et al. Common variants in IL-1RN, IL-1β and TNF-α and the risk of ovarian cancer: a case control study. Cent Eur J Immunol. 2017;42(2):150–155. doi: 10.5114/ceji.2017.69356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vangsted AJ, Nielsen KR, Klausen TW, Haukaas E, Tjønneland A, Vogel U. A functional polymorphism in the promoter region of the IL1B gene is associated with risk of multiple myeloma. Br J Haematol. 2012;158(4):515–518. doi: 10.1111/j.1365-2141.2012.09141.x. [DOI] [PubMed] [Google Scholar]
- 83.Upadhyay R, Jain M, Kumar S, Ghoshal UC, Mittal B. Potential influence of interleukin-1 haplotype IL-1 beta-511*T-IL-1RN*1 in conferring low risk to middle third location of esophageal cancer: a case-control study. Hum Immunol. 2008;69(3):179–186. doi: 10.1016/j.humimm.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 84.Fei BY, Lv HX, Cheng YW, Yang JM. Association between the IFN-γ and IL-1 genetic polymorphisms and colorectal cancer in the Chinese Han population. J Genet. 2014;93(1):235–239. doi: 10.1007/s12041-014-0354-3. [DOI] [PubMed] [Google Scholar]
- 85.Bhat IA, Naykoo NA, Qasim I, et al. Association of interleukin 1 beta (IL-1β) polymorphism with mRNA expression and risk of non-small cell lung cancer. Meta Gene. 2014;2:123–133. doi: 10.1016/j.mgene.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamacher R, Diersch S, Scheibel M, et al. Interleukin 1 beta gene promoter SNPs are associated with risk of pancreatic cancer. Cytokine. 2009;46(2):182–186. doi: 10.1016/j.cyto.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 87.Xu H, Ding Q, Jiang HW. Genetic polymorphism of interleukin-1A (IL-1A), IL-1B, and IL-1 receptor antagonist (IL-1RN) and prostate cancer risk. Asian Pac J Cancer Prev. 2014;15(20):8741–8747. doi: 10.7314/apjcp.2014.15.20.8741. [DOI] [PubMed] [Google Scholar]
- 88.Chen X, Xu Y, Cao X, Chen Y, Jiang J, Wang K. Associations of Il-1 family-related polymorphisms with gastric cancer risk and the role of Mir-197 in Il-1f5 expression. Medicine (Baltimore) 2015;94(47):e1982. doi: 10.1097/MD.0000000000001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raza Y, Khan A, Khan AI, et al. Combination of interleukin 1 polymorphism and Helicobacter pylori infection: an increased risk of gastric cancer in Pakistani population. Pathol Oncol Res. 2017;23(4):873–880. doi: 10.1007/s12253-017-0191-9. [DOI] [PubMed] [Google Scholar]
- 90.Sultana Z, Bankura B, Pattanayak AK, et al. Association of Interleukin-1 beta and tumor necrosis factor-alpha genetic polymorphisms with gastric cancer in India. Environ Mol Mutagen. 2018;59(7):653–667. doi: 10.1002/em.22208. [DOI] [PubMed] [Google Scholar]
- 91.Rider P, Carmi Y, Cohen I. Biologics for targeting inflammatory cytokines, clinical uses, and limitations. Int J Cell Biol. 2016;2016:9259646. doi: 10.1155/2016/9259646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong DS, Hui D, Bruera E, et al. MABp1, a first-in-class True Human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15(6):656–666. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 93.Hong DS, Janku F, Naing A, et al. Xilonix, a novel True Human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer. Invest New Drugs. 2015;33(3):621–631. doi: 10.1007/s10637-015-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fisher GA. A phase III study of Xilonix in refractory colorectal cancer patients with weight loss. J Clin Oncol. 2015;33: 3_suppl:685. [Google Scholar]
- 95.Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18(2):192–201. doi: 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- 96.Rondeau JM, Ramage P, Zurini M, Gram H. The molecular mode of action and species specificity of canakinumab, a human monoclonal antibody neutralizing IL-1β. MAbs. 2015;7(6):1151–1160. doi: 10.1080/19420862.2015.1081323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 98.Ridker PM, MacFadyen JG, Thuren T, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 99.Goh AX, Bertin-Maghit S, Ping Yeo S, et al. A novel human anti-interleukin-1β neutralizing monoclonal antibody showing in vivo efficacy. MAbs. 2014;6(3):765–773. doi: 10.4161/mabs.28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiong Y, Donovan KA, Kline MP, et al. Identification of two groups of smoldering multiple myeloma patients who are either high or low producers of interleukin-1. J Interferon Cytokine Res. 2006;26(2):83–95. doi: 10.1089/jir.2006.26.83. [DOI] [PubMed] [Google Scholar]
- 101.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1β-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84(2):114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lust JA, Lacy MQ, Zeldenrust SR, et al. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91(6):571–574. doi: 10.1002/ajh.24352. [DOI] [PubMed] [Google Scholar]
- 103.Paspaliaris V, Langan B, DeAndrea R, Wood J, Tsouvelekas A, Demosthenes B. Phase I/II study of IP-1510 a novel interleukin-1 receptor antagonist in the management of cancer-related cachexia. J Cachexia Sarcopenia Muscle. 2011;2(209–261):261. [Google Scholar]