Abstract
Introduction
Several radiolabeled somatostatin analogues have been developed for molecular imaging of neuroendocrine tumors (NETs) with single-photon emission computed tomography (SPECT) and positron-emission tomography (PET). The aim of the present study was to report our first results using 64Cu-DOTATOC in patients with NETs.
Methods
Thirty-three patients with NETs (15 female, 18 male; mean age 64 ± 13 years) were included in this retrospective study. 64Cu-DOTATOC PET–CT scans were performed on all patients.
Results
Five out of 33 patients with a history of NET after surgical removal of the primary lesion showed no pathological lesions on PET–CT imaging and 8/33 patients had enhanced uptake in the area of recurrent meningioma at the skull base. The remaining 20/33 patients had a history of neuroendocrine tumor in the gastrointestinal tract (GEP-NET) and were presented with at least one pathological lesion.
Conclusion
The high detection rate of suspected lesions in patients with NETs and the high target-to-background contrast found in this study hold promise for the safe application of 64Cu-DOTATOC in patients with NET.
Keywords: 64Cu-DOTATOC, NEN, NET, PET–CT, PRRT
Key Summary Points
| Why carry out this study? |
| Several radiolabeled somatostatin analogues have been developed for molecular imaging of neuroendocrine tumors (NETs) with single-photon emission computed tomography (SPECT) and positron-emission tomography (PET) |
| The aim of the present study was to report our first results using 64Cu-DOTATOC in patients with NETs |
| What was learned from the study? |
| 28/33 patients presented in PET–CT at least one pathological lesion with focal enhanced uptake of 64Cu-DOTATOC |
| The high detection rate of suspected lesions in patients with NETs and the high target-to-background contrast found in this study hold promise for the safe application of 64Cu-DOTATOC PET–CT in patients with NET |
Introduction
The diagnosis of neuroendocrine tumors (NETs) needs sophisticated imaging modalities since the tumors originate in different parts of the body and thus have diverse clinical symptoms. NETs are found most commonly in the lungs and gastrointestinal tract [1, 2]. The symptoms typically precede the diagnosis by approximately 5–7 years [3]. Delayed diagnosis may increase the probability of metastases, which are present in 20–50% of cases at the time of diagnosis [4, 5]. As in other malignancies, the degree of metastases will impact the prognosis of the patient. Timely diagnosis of disease progression will also affect therapy options.
NETs are characterized by high expression of somatostatin receptors on the surface of the tumor cells, which enables functional molecular imaging with radiolabeled somatostatin analogues to be applied in the diagnostic process [3, 5]. Of the five known subtypes of somatostatin receptors (SSRs), subtype 2 is most commonly overexpressed in NETs, followed by subtype 5 [6, 7].
Several radiolabeled somatostatin analogues have been developed for molecular imaging of NETs with single-photon emission computed tomography (SPECT) and positron-emission tomography (PET) [5]. 111In-DOTA-octreotide has been used in conventional imaging for many years [8]. However, PET-based radiopharmaceuticals have major advantages over SPECT tracers owing to higher affinity for the SSRs and the fact that PET is superior to SPECT in terms of spatial resolution and sensitivity [9, 10].
At present, the most widely used molecular imaging technique for NET imaging is SSR PET–computed tomography (CT) using 68Ga-DOTATATE, 68Ga-DOTATOC, and 68Ga-DOTANOC [11–13]. In recent years, somatostatin analogues labeled with 64Cu have been introduced, and a head-to-head analysis comparing 64Cu-DOTATATE and 68Ga-DOTATOC demonstrated advantages of the former in the detection of lesions in patients with NET [14].
The lower positron range of 64Cu than of 68Ga theoretically leads to a better spatial resolution, and the physical half-life of 12.7 h makes 64Cu attractive for routine use in a clinical imaging setting. The aim of the present retrospective study was to report our first results using 64Cu-DOTATOC in patients with NETs.
Methods
Thirty-three patients (15 female, 18 male; mean age 62 ± 13 years) were included in this retrospective study. PET–CT scans (Siemens Healthineers, Biograph mCT 20 Flow, Erlangen, Germany) were performed in all patients 1 h after intravenous injection of 3.5 MBq/kg 64Cu-DOTATOC (64Cu produced by ACOM, Italy; labeled peptide 64Cu-DOTATOC distributed by DSD Pharma GmbH, Austria). CT parameters for low dose (LD) CT were 120 kV and 30 mAs. The PET acquisition was performed using the flow motion method from the base of the skull to the upper legs. Afterwards the data sets were reconstructed using a three-dimensional ordered-subsets iterative time-of-flight algorithm, which corrected for scatter and photon attenuation. A lesion was considered somatostatin receptor positive if DOTATOC uptake was higher than background uptake on PET. A lesion was defined as somatostatin receptor negative if it showed no uptake on PET. The reference levels of uptake were defined by measuring the mean standardized uptake value (SUVmean) in the right liver lobe with normal physiological appearance. The SUVmax values for lesions with an uptake above surrounding tissue were registered (Siemens Healthineers, Syngo.Via). One patient with diffuse liver metastases was excluded for the measurement of the SUVmean in the right liver lobe.
Formal consent was obtained from all patients prior to examination. All patients were examined with PET–CT in compliance with the 1964 Declaration of Helsinki, and the responsible regulatory bodies in Austria. As this is a retrospective analysis of routine exams, ethical approval was not required.
Results
Five out of 33 patients with a history of NET after surgical removal of the primary lesion showed no pathological lesions on PET–CT imaging, and in the follow-up until preparation of this manuscript (approx. 1 year), there was no clinical evidence of recurrent disease. Eight out of 33 patients had a recurrent meningioma at the skull base and were sent for better delineation of the meningiomas prior to radiation with protons (not published data). One patient had a history of multiple endocrine neoplasia type 1 (MEN1) and the remaining 19/33 patients had a history of neuroendocrine tumor in the gastrointestinal tract (GEP-NET) and presented with at least one pathological lesion on imaging. In this latter group (20/33 patients) the median SUVmax was 10.8 with a median size of 2 cm. The further patient characteristics are given in Table 1. One patient with GEP-NET, who was imaged prior to surgery, showed focal uptake in the ileocecal region (Fig. 1), which was confirmed surgically as a NET. Another patient in this group showed multiple focal areas of intense uptake throughout the whole body, which were in good correlation with the 177Lu- somatostatin analogue post-therapy images (Fig. 2). In another patient with genetically and histologically proved MEN1, we observed lesions in the pancreas after surgical removal of parathyroid adenomas (Fig. 3).
Table 1.
Characteristics of patients with NET and at least one lesion on 64Cu-DOTATOC PET–CT
| Age (years) | Sex | SUVmean liver | SUVmax lesion | Number of lesions | Lesion size (cm) |
|---|---|---|---|---|---|
| 37 | M | 8.5 | 5.7 | 1 | 0.9 |
| 54 | M | 23 | 6.3 | 1 | 1 |
| 53 | M | 5 | 3.4 | 2 | 1 |
| 62 | M | 3.8 | 5.6 | 2 | 1.2 |
| 82 | M | 24 | 15.7 | 1 | 1.3 |
| 69 | F | 3.2 | 2.8 | 1 | 1.5 |
| 65 | F | 3.2 | 7.9 | 1 | 2 |
| 54 | F | 18.3 | 6 | 1 | 2 |
| 67 | F | 14.7 | 16.6 | 3 | 2 |
| 62 | F | 4.3 | 22 | 1 | 2 |
| 84 | F | 21 | 7 | 1 | 2.7 |
| 54 | F | 7.5 | 134 | 2 | 4 |
| 78 | F | 22 | 8 | 1 | 4.5 |
| 51 | F | 6.7 | 41 | Multiple | 0.5–2.5 |
| 31 | F | 3.4 | 22 | Multiple | 0.5–2.5 |
| 61 | M | 10.5 | 35 | Multiple | 0.5–3 |
| 63 | F | 3.3 | 35 | Multiple | 0.7–2.7 |
| 71 | M | 10.8 | 19.2 | Multiple | 0.9–2 |
| 60 | M | 17 | 28.5 | 2 | 1–2 |
| 42 | F | 4.9 | 80 | 4 | 1–2 |
Fig. 1.
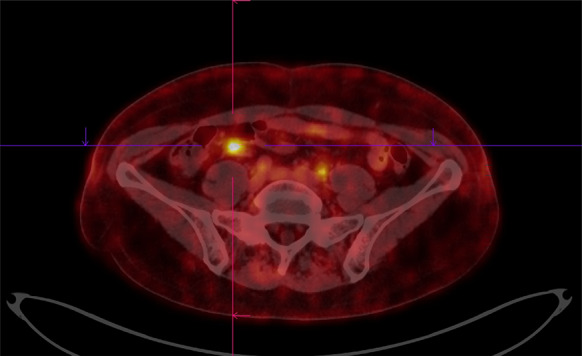
64Cu-DOTATOC PET–CT image of a 65-year-old female with histologically proven NET in the ileocoecal region (stage G2)
Fig. 2.
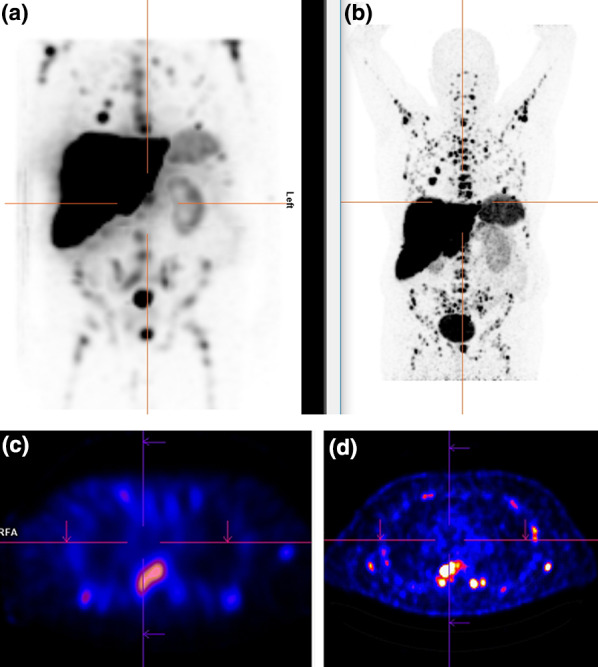
a177Lu-somatostatin analogue–maximum intensity projection (MIP) image of a 61-year-old male patient and b 3 months later follow-up with 64Cu-DOTATOC PET–CT MIP images showing metastasized NET (G1). Transaxial images with 177Lu-somatostatin analogue (c) and 3 months later follow-up with 64Cu-DOTATOC PET–CT (d)
Fig. 3.
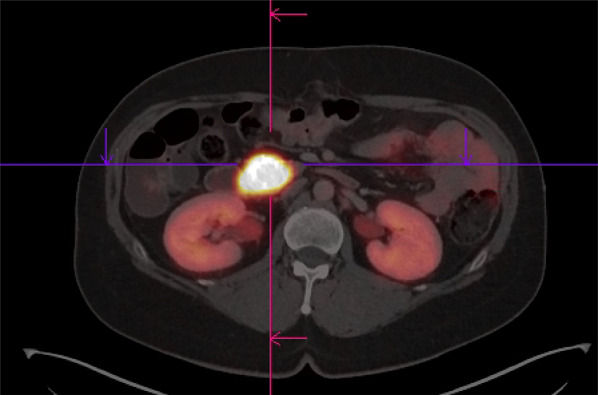
64Cu-DOTATOC PET–CT image of a 54-year-old female patient with histologically proven MEN1 showing intense tracer uptake in the pancreatic lesion (note the intense uptake with SUVmax 134, SUVmean of the liver 7.5)
The median SUVmax of the lesions was 10.8 (range 3–134) and as shown in Figs. 1, 2 and 3 there was a very high tumor-to-background uptake providing high contrast imaging.
Discussion
In this retrospective study of a small heterogeneous group of 33 patients, we observed high target-to-background contrast in the suspected lesions. 64Cu-DOTATOC was safely used in the workup of patients with NETs. It is to our best knowledge the first-in-human use of this radiopharmaceutical.
64Cu ligands have logistical advantages for centers without 68Ge/68Ga generators, and with a half-life of 12.7 h 64Cu can be easily transported from other production facilities [15]. Moreover, 64Cu has a lower positron range than68Ga resulting in a better spatial resolution that could improve the detection of small lesions [14]. Since the smallest lesion in this study was 5 mm, one could presume, as in other studies with64Cu-somatostatin analogues, that PET–CT with 64Cu-DOTATOC is more sensitive than conventional imaging with 111In-octreotide and comparable to other PET somatostatin ligands [16].
Furthermore, since we perform therapy with 177Lu-DOTATOC [17], the use of 64Cu-DOTATOC with somatostatin analogues would more precisely enable us to predict therapy response, as demonstrated in the case illustrated in Fig. 2, which is a good example for the so-called theranostic twins [18]. In contrast to a previous study, where we described more lesions in the post-therapeutic scan [19], we observed more lesions in this patient on PET imaging compared to SPECT imaging, as a result of the characteristics of the chelator and radionuclide used as PET radiopharmaceuticals and as a result of the known superior spatial resolution of PET compared to SPECT [16, 20]. As a result, lesions of size 6 mm or less were detected only by PET. In a similar study using 64Cu-DOTATATE, the authors demonstrated the superiority of 64Cu-DOTATATE PET both in radiation dose and lesion detection compared to 111In-DTPA-octreotide [16]. The authors concluded that 64Cu-DOTATATE should be preferred whenever possible over 111In-DTPA-octreotide.
The most used PET tracers for NET imaging are68Ga-DOTATATE,68Ga-DOTATOC, and68Ga-DOTANOC. DOTATOC, DOTATATE, and DOTANOC are collectively referred to as DOTA peptides. There is some variation in the affinity profile of the DOTA peptides towards the SSRs. They have all high affinity to SSR subtype 2, DOTATATE higher than the two others, but DOTATOC and DOTANOC also have affinity to SSR subgroup 5 (DOTANOC also to a lesser extent to SSR subgroup 3). However, even though their affinities to the SSR differ, results from several studies report comparable clinical performance [14, 21–25]. Yang et al. performed a meta-analysis on the diagnostic role of68Ga-DOTATATE and68Ga-DOTATOC and found a high sensitivity and specificity for both tracers [24]. Johnbeck et al. described later that both 64Cu-DOTATATE and 68Ga-DOTATOC on a patient-by-patient basis had no differences in their diagnostic performance [14]; however, more lesions were detected by 64Cu-DOTATATE. Additionally, the scanning window of at least 3 h for 64Cu-DOTATATE was found to be favorable and easy to use in the clinical setting [14]. The difference in lesion detection rate was attributed to use of 64Cu instead of 68Ga rather than differences in peptide. The substantially shorter positron range of 64Cu was anticipated to be the most important factor providing better detection of small lesions [14]. It must be emphasized that regarding peptides used for PET imaging, there are still conflicting reports and no peptide has been concluded to be the optimal peptide for imaging of NETs.
Despite the small number of heterogenous patients, which is a limitation of the study, it has been shown that baseline PET–CT with 68Ga-DOTATATE helps to determine somatostatin receptor expression status and disease stage in patients, but SUV calculations do not necessarily have a role in the prediction of treatment response [26]. However, because of the long half-life of 64Cu of 12.7 h, it is possible to perform images later than 1 h, e.g., either for dosimetric purposes or for any other logistical reasons in routine clinical work. This may be the focus of future studies in order to establish personalized dose determination for peptide receptor radionuclide therapy (PRRT) in NETs.
Conclusion
The high detection rate of possible lesions with high target-to-background contrast showed the possibility of the safe application of 64Cu-DOTATOC in patients with NETs. The good correlation of multiple metastases with lesions detected in a post-therapeutic scan with 177Lu-somatostatin analogue presents another advantage of this radiopharmaceutical to be used in treatment planning of patients with metastatic NETs.
Acknowledgements
Siroos Mirzaei holds the copyright for all the figures in the manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
All authors (Siroos Mirzaei, Mona-Eilsabeth Revheim, William Raynor, Walter Zehetner, Peter Knoll, Shahin Zandieh, Abass Alavi) have nothing to disclose.
Compliance with Ethics Guidelines
Formal consent was obtained from all patients prior to examination. The study has been performed in compliance with the 1964 Declaration of Helsinki, and the responsible regulatory bodies in Austria. As this is a retrospective analysis of routine exams, ethical approval was not required.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.10289603.
References
- 1.Volante M, Gatti G, Papotti M. Classification of lung neuroendocrine tumors: lights and shadows. Endocrine. 2015;50(2):315–319. doi: 10.1007/s12020-015-0578-x. [DOI] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Modlin IM, Oberg K, Chung DCJ, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008;9(1):61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 4.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 5.Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10(14):2259–2277. doi: 10.2217/fon.14.139. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer D, Bell GI, Berelowitz M, et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16(3):86–88. doi: 10.1016/S0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 7.Reubi JC, Waser B, Schaer JC, et al. Somatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med. 2001;28(7):836–846. doi: 10.1007/s002590100541. [DOI] [PubMed] [Google Scholar]
- 8.Bombardieri E, Ambrosini V, Aktolun C, et al. 111In-pentetreotide scintigraphy: procedure guidelines for tumour imaging. Eur J Nucl Med Mol Imaging. 2010;37(7):1441–1448. doi: 10.1007/s00259-010-1473-6. [DOI] [PubMed] [Google Scholar]
- 9.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34(10):1617–1626. doi: 10.1007/s00259-007-0450-1. [DOI] [PubMed] [Google Scholar]
- 10.Pauwels E, Cleeren F, Bormans G, et al. Somatostatin receptor PET ligands—the next generation for clinical practice. Am J Nucl Med Mol Imaging. 2018;8(5):311–331. [PMC free article] [PubMed] [Google Scholar]
- 11.Sandström M, Velikyan I, Garske-Román U, et al. Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med. 2013;54(10):1755–1759. doi: 10.2967/jnumed.113.120600. [DOI] [PubMed] [Google Scholar]
- 12.Pfeifer A, Knigge U, Mortensen J, et al. Clinical PET of neuroendocrine tumors using 64Cu-DOTATATE: first-in-humans study. J Nucl Med. 2012;53(8):1207–1215. doi: 10.2967/jnumed.111.101469. [DOI] [PubMed] [Google Scholar]
- 13.Wild D, Bomanji JB, Benkert P, et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013;54(3):364–372. doi: 10.2967/jnumed.112.111724. [DOI] [PubMed] [Google Scholar]
- 14.Johnbeck CB, Knigge U, Loft A, et al. Head-to-head comparison of (64)Cu-DOTATATE and (68)Ga-DOTATOC PET/CT: a prospective study of 59 patients with neuroendocrine tumors. J Nucl Med. 2017;58(3):451–457. doi: 10.2967/jnumed.116.180430. [DOI] [PubMed] [Google Scholar]
- 15.Sevcenco S, Klingler HC, Eredics K, et al. Application of Cu-64 NODAGA-PSMA PET in prostate cancer. Adv Ther. 2018;35(6):779–784. doi: 10.1007/s12325-018-0711-3. [DOI] [PubMed] [Google Scholar]
- 16.Pfeifer A, Knigge U, Binderup T, et al. 64Cu-DOTATATE PET for neuroendocrine tumors: a prospective head-to-head comparison with 111In-DTPA-octreotide in 112 patients. J Nucl Med. 2015;56(6):847–854. doi: 10.2967/jnumed.115.156539. [DOI] [PubMed] [Google Scholar]
- 17.Bodei L, Mueller-Brand J, Baum RP, et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2013;40(5):800–816. doi: 10.1007/s00259-012-2330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werner RA, Bluemel C, Allen-Auerbach MS, et al. 68Gallium- and 90yttrium-/177lutetium: “theranostic twins” for diagnosis and treatment of NETs. Ann Nucl Med. 2015;29(1):1–7. doi: 10.1007/s12149-014-0898-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirzaei S, Bastati B, Lipp RW, et al. Additional lesions detected in therapeutic scans with 177Lu-DOTATATE reflect higher affinity of 177Lu-DOTATATE for somatostatin receptors. Oncology. 2011;80(5–6):326–329. doi: 10.1159/000329808. [DOI] [PubMed] [Google Scholar]
- 20.Basu S, Kumar R, Rubello D, et al. PET imaging in neuroendocrine tumors: current status and future prospects. Minerva Endocrinol. 2008;33(3):257–275. [PubMed] [Google Scholar]
- 21.Poeppel TD, Binse I, Petersenn S, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011;52(12):1864–1870. doi: 10.2967/jnumed.111.091165. [DOI] [PubMed] [Google Scholar]
- 22.Poeppel TD, Binse I, Petersenn S, et al. Differential uptake of (68)Ga-DOTATOC and (68)Ga-DOTATATE in PET/CT of gastroenteropancreatic neuroendocrine tumors. Recent Results Cancer Res. 2013;194:353–371. doi: 10.1007/978-3-642-27994-2_18. [DOI] [PubMed] [Google Scholar]
- 23.Putzer D, Kroiss A, Waitz D, et al. Somatostatin receptor PET in neuroendocrine tumours: 68Ga-DOTA0, Tyr3-octreotide versus 68Ga-DOTA0-lanreotide. Eur J Nucl Med Mol Imaging. 2013;40(3):364–372. doi: 10.1007/s00259-012-2286-6. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, Kan Y, Ge BH, et al. Diagnostic role of gallium-68 DOTATOC and gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol. 2014;55(4):389–398. doi: 10.1177/0284185113496679. [DOI] [PubMed] [Google Scholar]
- 25.Kabasakal L, Demirci E, Ocak M, et al. Comparison of 68Ga-DOTATATE and 68Ga-DOTANOC PET/CT imaging in the same patient group with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39(8):1271–1277. doi: 10.1007/s00259-012-2123-y. [DOI] [PubMed] [Google Scholar]
- 26.Soydal Ç, Peker A, Özkan E, et al. The role of baseline Ga-68 DOTATATE positron emission tomography/computed tomography in the prediction of response to fixed-dose peptide receptor radionuclide therapy with Lu-177 DOTATATE. Turk J Med Sci. 2016;46(2):409–413. doi: 10.3906/sag-1412-11. [DOI] [PubMed] [Google Scholar]


