Supplemental Digital Content is available in the text.
Keywords: baloxavir marboxil, children, granules, influenza, Japan
Background:
A granule formulation of baloxavir marboxil, a selective inhibitor of influenza cap-dependent endonuclease, was newly developed for children with difficulty swallowing tablets.
Methods:
A multicenter open-label study was conducted during the 2017–2018 influenza season to assess the safety, pharmacokinetics and clinical/virologic outcomes of single, oral, weight-based doses of baloxavir granules in Japanese children infected with influenza virus. The primary clinical endpoint was the time to illness alleviation of influenza.
Results:
All 33 enrolled children completed the study and received baloxavir (1 mg/kg for 12 children weighing <10 kg, 10 mg for 21 children weighing 10 to <20 kg). Detected viruses were influenza B (36.4%), A(H1N1)pdm09 (33.3%) and A(H3N2) (27.3%). Adverse events (AEs) were reported in 54.5% of children. No deaths, serious AEs or AEs leading to discontinuation were reported. The mean (SD) plasma concentrations of baloxavir acid at 24 hours post-dose were 72.8 (24.0) and 51.3 (19.3) ng/mL in the 1-mg/kg and 10-mg dose groups, respectively. The median time to illness alleviation (95% confidence interval) was 45.3 (28.5–64.1) hours. A >4-log decrease in infectious viral titer occurred on day 2 and a temporary 2-log increase on day 4. Polymerase acidic protein/I38T/M-substituted viruses were detected in 5 children infected with influenza A, but none with influenza B.
Conclusions:
Baloxavir granules and the weight-based dose regimen were considered to be well tolerated in children, with rapid influenza virus reduction and associated symptom alleviation. Evidence of baloxavir activity against influenza B was observed, but further data are required for confirmation.
Influenza virus infection is an acute respiratory disease that is common in children. The number of influenza-infected individuals in the 2017–2018 season in Japan was approximately 22.5 million; 42% of these were <15 years old and 10% were ≤4 years old.1 Young children are at high risk of complications such as bronchitis, otitis media and bacterial pneumonia,2–4 or non-respiratory complications such as febrile seizures, abnormal behavior, myositis and influenza encephalopathy.2,5
Neuraminidase inhibitors (NAIs) are used to reduce the duration and severity of illness caused by influenza. NAIs approved in Japan include oral oseltamivir, inhaled zanamivir, inhaled laninamivir and intravenous peramivir. However, nonadherence to therapy affects the outcome, and the route of administration may influence compliance with influenza therapies.6 For example, oseltamivir must be administered for 5 days, potentially resulting in poor adherence; the recommended dose of inhaled zanamivir or laninamivir may not be delivered in young children and those with respiratory symptoms,7 and peramivir must be administered intravenously. To improve adherence, there is a medical need for anti-influenza drugs that are safe, well tolerated, and can be easily administered as a single dose to children.
Baloxavir marboxil (baloxavir) is an oral antiviral prodrug that is metabolized rapidly to the active form, baloxavir acid, which selectively inhibits cap-dependent endonuclease activity of the viral polymerase acidic protein (PA) of influenza types A and B.8,9 In a double-blind randomized controlled study in adults and adolescents with uncomplicated influenza, single-dose baloxavir significantly improved time to alleviation of influenza symptoms compared with placebo and significantly reduced infectious virus compared with placebo and oseltamivir.10 In an open-label study in Japanese children 6 months to <12 years of age with influenza, oral single-dose baloxavir with a dosage based on the body weight of children using a tablet formulation was well tolerated and appeared to be clinically effective compared with prior studies of NAIs in children with influenza.11 The plasma concentrations of baloxavir acid in the pediatric study were similar to those in the double-blind randomized controlled studies in adults and adolescents.12,13 However, no children <1 year of age and only 2 children weighing <10 kg were assessed in the previous study.
A granule formulation of baloxavir has been newly developed for patients with difficulty swallowing tablets, especially younger children. This study was designed to assess the safety, pharmacokinetics (PK), and clinical and virologic outcomes of a granule formulation of baloxavir in influenza-infected children weighing <20 kg.
MATERIALS AND METHODS
Study Design
This was a multicenter, open-label, noncontrolled study in Japanese children during the 2017–2018 season conducted in 20 study centers.
The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines and approved by the institutional review board or ethics committee at each study center. Parents/legal guardians of participating children provided written informed consent; participating children gave written or verbal informed consent according to their capabilities. The study was registered at the Japan Pharmaceutical Information Center Clinical Trials Information (JapicCTI-173811).
Study Population
Children enrolled were <12 years of age with a body weight of <20 kg (≥2500 g at birth for children <1 year of age), diagnosed with influenza virus illness confirmed by fever ≥38°C, and had a nasal or throat swab sample that was positive for influenza virus by a rapid influenza diagnostic test. The time between onset of symptoms (when body temperature first exceeded 37.5°C) and screening was ≤48 hours.
Treatment Protocol
On day 1, children received a single oral dose of baloxavir 2% granules (Shionogi & Co., Ltd., Osaka, Japan) without regard to food intake. The dose-depended on body weight at screening: children with a body weight <10 kg received 1 mg/kg of baloxavir (50 mg/kg of 2% granules); children with a body weight of 10 to <20 kg received 10 mg of baloxavir (500 mg of 2% granules). This weight-based dose regimen was determined according to a population PK analysis using PK data collected in the phase 3 study of baloxavir tablets in Japanese children.12 Other anti-influenza drugs were prohibited within 30 days before screening.
Safety Assessment
Safety measures included incidence and severity of adverse events (AEs), treatment-related AEs, serious AEs, vital signs and clinical laboratory tests. AEs were classified by system organ class and preferred term using the Medical Dictionary for Regulatory Activities Version 19.1. Severity was categorized using the Common Terminology Criteria for Adverse Events Version 4.0.
Pharmacokinetic Assessments
Blood samples were collected for the measurement of plasma concentrations of baloxavir marboxil (in children <2 years of age only) and baloxavir acid, once between 0.5 and 2 hours post-dose on days 1, 2 and 3 (optional), and once between days 6 and 22 (Table, Supplemental Digital Content 3, http://links.lww.com/INF/D970). Plasma concentrations were analyzed by a validated liquid chromatography-tandem mass spectrometry method and were summarized at 24, 48 and 96 hours post-dose (C24, C48 and C96) with acceptable time windows of 20–28 hours, 44–52 hours and 92–100 hours, respectively.
Clinical and Virologic Assessments
The following outcome measures were assessed by the child or parent/guardian11,14,15 and recorded in an electronic patient diary from pre-dose day 1 to post-dose day 14: body temperature (axillary); assessment of severity of 2 influenza symptoms (cough and nasal discharge/nasal congestion) on a 4-point rating scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe); and assessment of ability to perform daily activities on an 11-point scale (Methods, Supplemental Digital Content 1, http://links.lww.com/INF/D968).
For virology tests, nasopharyngeal swabs (or throat swabs if nasopharyngeal swabbing was not feasible) were collected by investigators on days 1, 2, 3 and/or 4, 6 and 9 (Table, Supplemental Digital Content 3, http://links.lww.com/INF/D970). Virologic testing facilities assessed influenza virus type/subtype, viral RNA load (Viroclinics Biosciences BV, Rotterdam, Netherlands), and infectious influenza virus titer (LSI Medience Corporation, Tokyo, Japan) in accordance with the previous pediatric study.11
The primary clinical endpoint was the time to illness alleviation (TTIA) of influenza defined as the time from baloxavir administration until both the following criteria were reached and sustained for at least 21.5 hours: cough and nasal discharge/nasal congestion both assessed as 0 (absent) or 1 (mild) and body temperature <37.5°C.
The secondary endpoints included the time to resolution of fever (axillary temperature <37.5°C); the time to resumption of normal activity; the incidence of influenza-related complications (death, hospitalization, radiologically confirmed pneumonia, bronchitis, sinusitis, otitis media); the incidence of influenza-related complications particularly seen in children (influenza-associated encephalitis or encephalopathy, febrile seizures, myositis); and the change from baseline in infectious virus titer. The observed value of the virus titer was evaluated as a post-hoc endpoint.
Treatment-emergent influenza virus substitutions in PA/I38 were defined as amino acid changes in PA/I38 occurring between day 1 and the last time point with ≥4 log10 virus particles/mL, using an influenza virus quantitative reverse transcription-polymerase chain reaction (qRT-PCR) result. The baseline characteristics of children with emergence of PA/I38T/M substitutions were presented.
Co-infection with other respiratory viruses during the observation period was monitored using nasal or throat swab samples assayed by singleplex qRT-PCR for 20 respiratory viruses (including influenza) and bacteria (Methods, Supplemental Digital Content 2, http://links.lww.com/INF/D969 and Table, Supplemental Digital Content 4, http://links.lww.com/INF/D971).16
Statistical Analysis
Sample size (N = 30) was set to provide sufficient numbers for a safety assessment of the granule formulation of baloxavir, taking into consideration the number of enrolled children weighing <20 kg in the previous phase 3 study,11 and was not based on a power calculation.
The safety population was all children who received study drug. The clinical and virologic analysis population was the intention-to-treat infected population, defined as children who received study drug with a confirmed diagnosis of influenza virus infection based on the qRT-PCR results.
A survival curve of TTIA was estimated using the Kaplan-Meier method, and the median TTIA and its 95% confidence interval (CI) were calculated. Unless otherwise noted, continuous variables were summarized using the number of non-missing observations, mean, SD, median, minimum and maximum values; categorical variables were summarized using the frequency count and the percentage of children in each category. No formal hypothesis testing was performed. The data are presented by weight group (<10 kg, 10 to <20 kg, and overall). All analyses were performed using SAS Version 9.2 or higher (SAS Institute, Cary, North Carolina) and WinNonlin Version 6.2.1 (Certara L.P., Princeton, New Jersey).
RESULTS
Demographic and Baseline Clinical Characteristics
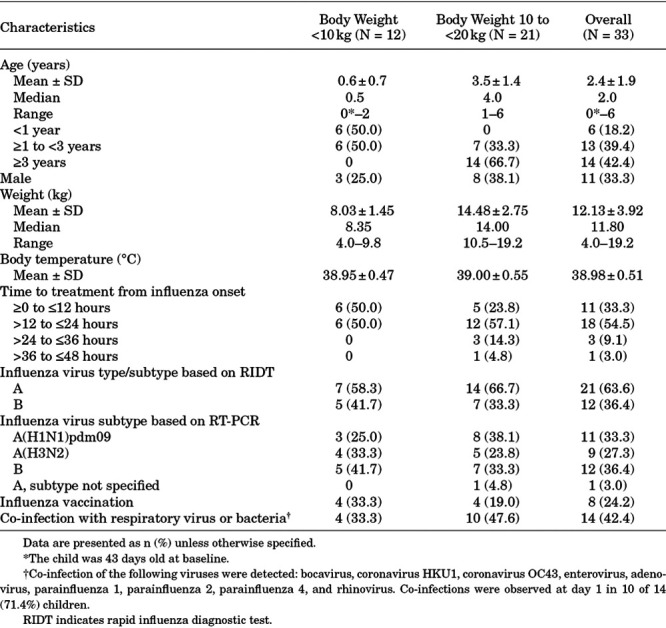
All 33 children who received baloxavir granules as per the dose regimen (safety population) were included in the ITTI population. Thirty-two children were included in the PK analyses. One child weighing 14.0 kg and administered a 10-mg dose was excluded because the child did not take the entire dose. In the ITTI population (Table 1), 6 children were <1 year of age, 13 children were 1 to <3 years of age, and 14 children were ≥3 years of age, and the age range from 43 days to 6 years; 12 children weighed <10 kg and 21 children weighed 10 to <20 kg. Virus subtyping based on qRT-PCR showed that the most common influenza virus strain was B subtype (36.4%; n = 12), followed by A(H1N1)pdm09 (33.3%; n = 11) and A(H3N2) (27.3%; n = 9). Co-infection was observed in 14 children.
TABLE 1.
Demographics and Baseline Characteristics in the ITTI Population
Safety Outcomes
No serious AEs or AEs leading to study discontinuation were reported. AEs were reported in 18 (54.5%) of 33 children (Table 2). Most AEs (10/19) occurred on day 7 or earlier. All AEs were of mild or moderate (grade 1 or 2) severity.
TABLE 2.
Type and Duration of AEs
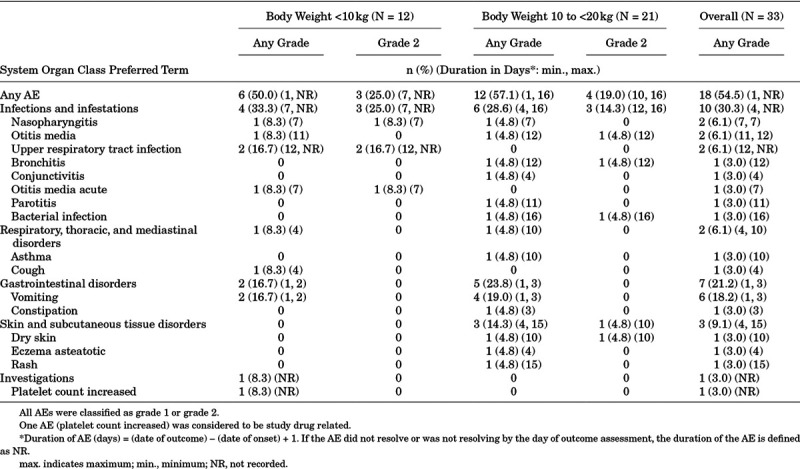
The most common AE, vomiting (all mild), was reported in 6 children (18.2%) and was considered not related to study drug. One child had an increase in platelet count on day 12 that was considered a mild treatment-related AE. There was no apparent difference in the incidence of AEs between children weighing <10 kg and 10 to <20 kg. No clinically meaningful findings were observed in clinical laboratory tests and vital signs.
Pharmacokinetic Measures
Plasma baloxavir marboxil concentrations were below the lower limit of quantification (0.100 ng/mL) in 5 of 12 children at 0.5–2 hours post-dose and for all 12 children at all other sampling points. Plasma concentrations of baloxavir acid were generally within the range of the concentrations observed in adults/adolescents, or in children in the previous studies using the tablet formulation (Fig. 1).12,13 The arithmetic mean (SD) plasma concentration of baloxavir acid was 72.8 (24.0) ng/mL for the 1-mg/kg dose (n = 6) and 51.3 (19.3) ng/mL for the 10-mg dose (n = 14) at 24 hours (C24), 34.2 (5.85) ng/mL for the 1-mg/kg dose (n = 3) and 35.0 (13.3) ng/mL for the 10-mg dose (n = 3) at 48 hours (C48), and 11.9 (5.19) for the 10-mg dose (n = 10) at 96 hours (C96). No clear relationship was found between C24 and efficacy endpoints.
FIGURE 1.
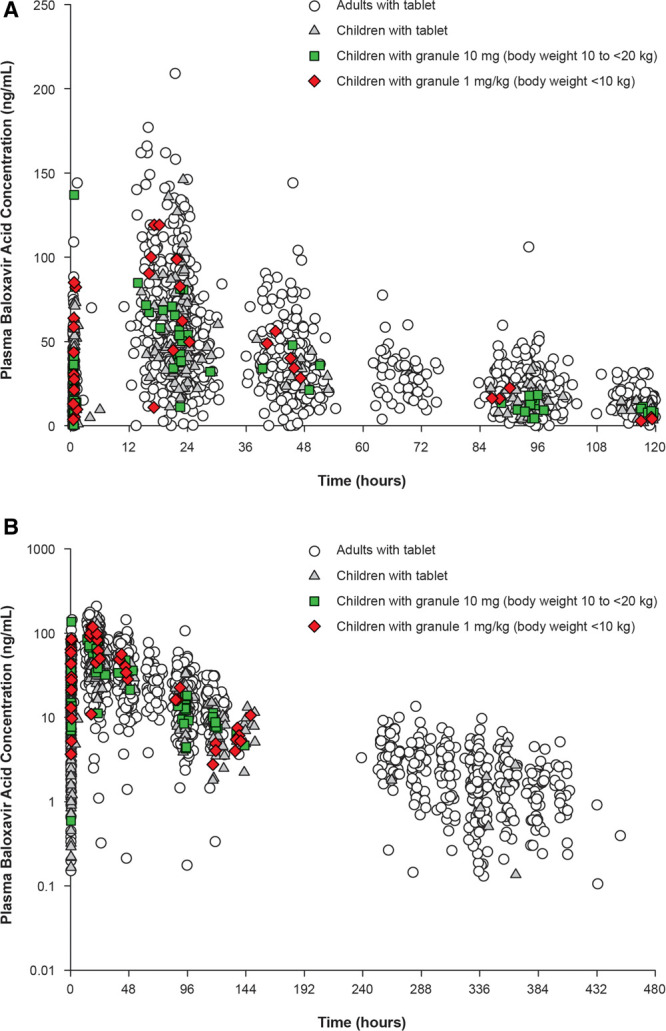
Plasma concentrations of baloxavir acid (active form of baloxavir marboxil) from (A) 0 to 120 hours and (B) 0 to 480 hours. Data from the adult phase 3 study and previous pediatric study using a tablet formulation were included.9,10
Clinical Measures
The median (95% CI) TTIA was 45.3 (28.5–64.1) hours (Table 3), with approximately 80% of children being alleviated 120 hours after treatment (Figure, Supplemental Digital Content 6, http://links.lww.com/INF/D973). No obvious difference in the median TTIA between children weighing <10 kg and 10 to <20 kg was observed. The median (95% CI) TTIA was 58.9 (17.5–154.3) hours, 26.8 (12.3–199.3) hours and 41.7 (19.3–86.9) hours in children infected with A(H1N1)pdm09, A(H3N2) and B virus, respectively.
TABLE 3.
Efficacy Time-to-Event Outcomes by Influenza Virus Type or Subtype Based on RT-PCR in the ITTI Population (N = 33)
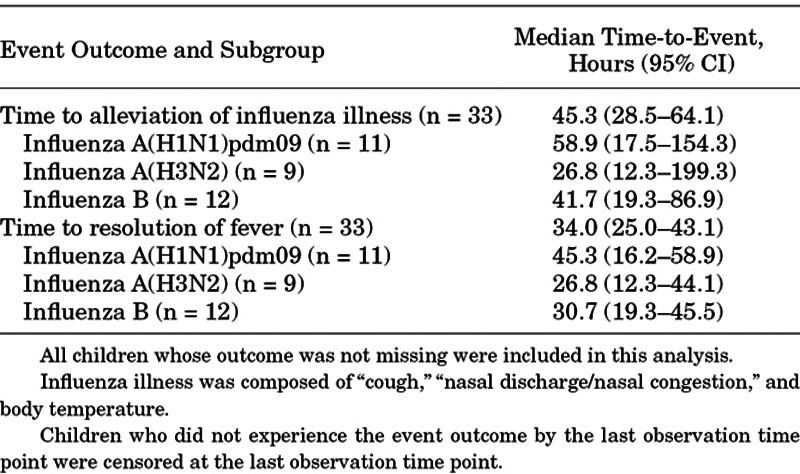
The median (95% CI) time to resolution of fever for all children was 34.0 (25.0–43.1) with 45.3 (16.2–58.9) hours, 26.8 (12.3–44.1) hours and 30.7 (19.3–45.5) hours in children infected with A(H1N1)pdm09, A(H3N2) and B virus, respectively (Table 3). At 48 hours, 93.9% (31/33) of children had a normal temperature. The median time to resumption of normal activity was 80.3 (95% CI: 51.5–131.4) hours. Three children developed influenza-related complications (bronchitis = 1, otitis media = 2) as diagnosed by the study investigator. All were classified as mild or moderate in severity, resolved, and considered not related to study drug.
Virologic Measures
A greater than 4-log (log10 50% tissue culture infective dose per mL) reduction in mean infectious influenza virus titer was observed 1 day after baloxavir treatment (Fig. 2). An increase in infectious virus titer was observed in children with influenza B at day 4 and in children with A(H3N2) at day 6. For most children, infectious virus titer reached the lower limit of detection by day 9.
FIGURE 2.
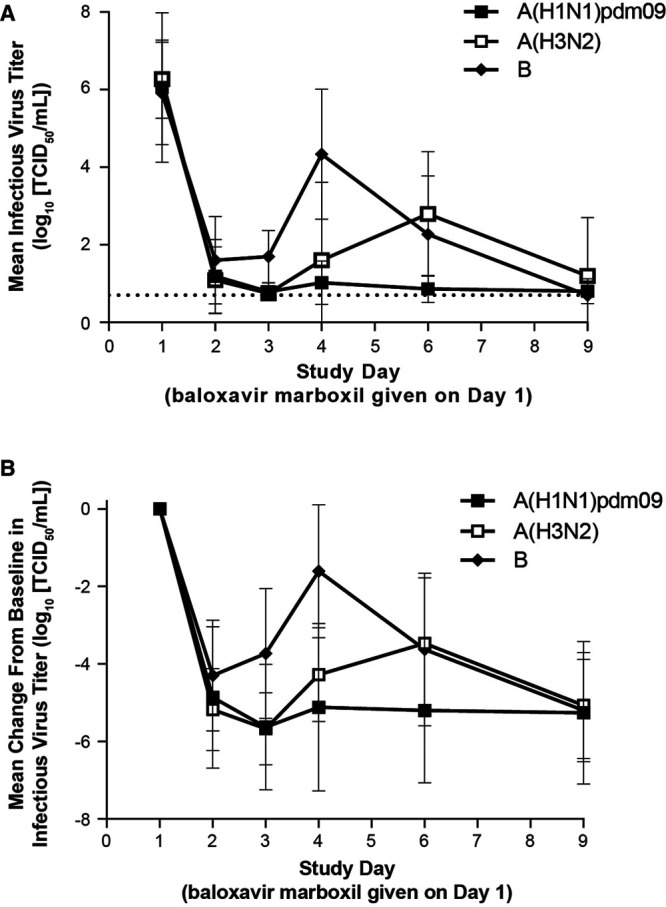
(A) Mean infectious virus titer and (B) change from baseline in mean infectious virus titer by virus type/subtype. Data are presented as mean ± SD. The dotted line in panel A indicates the lower limit of detection (0.7 log10 TCID50). The subset of children who were positive for influenza virus titer at baseline was included in this analysis. Child numbers for A(H1N1)pdm09, A(H3N2) and B, respectively, were: n = 10, 9 and 12 on day 1, day 2, day 6 and day 9; n = 7, 6 and 4 on day 3; and n = 5, 5 and 9 on day 4. TCID50 indicates 50% tissue culture infective dose.
Of 33 children, 31 had baseline samples available for virus sequencing; no PA/I38X-substituted viruses were detected. Twenty-six children had baseline and posttreatment samples available; PA/I38T/M-substituted viruses were detected in 5 children at day 6 (n = 4) and day 9 (n = 1). Variants included 3 PA/I38T/I mixture (2 with A(H3N2) and 1 with A(H1N1)pdm09) and PA/I38M (2 with A(H3N2) virus). No PA/I38-substituted viruses were observed in children infected with influenza B. An increase in infectious virus around day 4 or 6 was observed in children with influenza B and in children with PA/I38T/M-substituted viruses, respectively (Figure, Supplemental Digital Content 7A, http://links.lww.com/INF/D974). Fever recurrence after day 4 was seen in 3 of 5 children with PA/I38T/M-substituted influenza A, 1 of 10 children with unsubstituted influenza A and 7 of 12 children with influenza B (Figure, Supplemental Digital Content 7B, http://links.lww.com/INF/D974).
DISCUSSION
This is the first study to examine the safety, PK and clinical/virologic outcomes of a single oral dose of baloxavir 2% granules in young children with influenza virus infection. The granule formulation of baloxavir, which younger children can easily swallow, was administered using a weight-based dose regimen. Although the number of children investigated was small, this study provided complementary data for children weighing <10 kg and <2 years old, only a few of whom were included in the previous study using a tablet formulation.11 Overall, the single-dose regimen was well tolerated, and no significant safety concerns related to the granule formulation were identified. The PK of baloxavir granules in children <20 kg (arithmetic mean C24 51.3 and 72.8 ng/mL in the 10-mg and 1-mg/kg dose groups, respectively) was not different from that of the tablet formulation in adults (59.7 ng/mL) and children (86.4, 59.0 and 45.5 ng/mL in the 40-, 20-, and 10-mg dose groups, respectively), especially when compared with the same dose (10 mg).12,13 Although the influenza seasons and target population, including body weight and age, differed from the previous studies, the median TTIA (45.3 hours) in this study were similar to those in the previous pediatric study (44.6 hours).11 Compared with non-controlled studies for NAI in Japan, the median TTIA was longer than that in children treated with an intravenous single-dose peramivir (29.1 hours) but shorter than that in inhaled laninamivir treated children.14,15 Also, the median time to resolution of fever (34.0 hours) was shorter than those in oseltamivir treated children (41–48 hours) in placebo-controlled studies.17–19 Moreover, the recently completed miniSTONE-2 study showed comparable time to resolution of fever in children treated with baloxavir (41.2 hours) tablets or oseltamivir (46.8 hours).20 Taken together, these findings support the use of the granule formulation as an alternative to tablets for administration to younger children with influenza infection.
This study was conducted in the 2017–2018 season when influenza B virus infection was relatively common. Reflecting this, more children were infected with influenza B (12 children, 36%) than A(H3N2) (9 children, 27%) or A(H1N1)pdm09 (11 children, 33%). The median TTIA in children infected with A(H3N2) or B virus was 26.8 or 41.7 hours, respectively, similar to those in the previous pediatric study using the tablet formulation (A(H3N2): 45.2 hours, n = 86; B: 44.7 hours, n = 8).11 Although increases in infectious virus at Day 3 or later were observed in some children, mostly those with influenza B, >4-log reduction in infectious virus was seen in all children at say day, that may result in symptoms alleviation and fever resolution around day 2.
Treatment-emergent PA/I38X-substituted viruses with reduced susceptibility to baloxavir were detected in the previous adult and pediatric phase 3 studies.10,11 The previous pediatric study reported that children with PA/I38X-substituted viruses showed transient increases and prolongation of infectious virus detection.11 In the current study, 5 children (19.2% of 26 children with paired sequence data available) were identified as having PA/I38X-substituted virus. The PA/I38X-substituted A(H3N2) was 4 of 9 children with paired sequence data available, which was more frequent than that in the previous pediatric study (age <12 years).11 In the previous pediatric study, a higher rate of PA/I38 substitution was associated with low baseline hemagglutination inhibition antibody titer, suggesting that acquired influenza virus immunity plays a beneficial role for suppressing PA/I38 substitution virus emergence.11 The higher rate of PA/I38X-substituted virus emergence in this study appears to be associated, in part, with low hemagglutination inhibition antibody titer in children (Table, Supplemental Digital Content 5, http://links.lww.com/INF/D972). A higher rate of resistant virus emergence in young children was also reported for oseltamivir.21 A diminish or lack of a memory T cell response to control influenza viral replication, might be a common theme in the emergence of drug-resistant viruses in children during antiviral treatment.
Increases in infectious virus were observed in most children with A(H3N2), particularly those with PA/I38X-substituted virus, and B infections. In children with A(H3N2), this increase was more common than in the previous study.11 The differences in virologic endpoints between this study and the previous one may be caused by the younger age of the children, the greater prevalence of influenza B, and the high frequency of co-infections.
Age-based analyses showed that young children with PA/I38X-substituted virus had longer TTIA and some also had co-infections with other viruses (Figure, Supplemental Digital Content 8, http://links.lww.com/INF/D975), which were also observed in the miniSTONE-2 study.20 In children with fever resolution before day 4, fever recurrence was observed in approximately 60% of children with PA/I38X-substituted virus or influenza B after day 4, although fever resolved again during the 14-day observation period (Figure, Supplemental Digital Content 7B, http://links.lww.com/INF/D974). Our analyses suggest a potential association between the immature immune status of children and co-infection, fever recurrence, symptoms, or PA/I38X-substituted virus emergence. No influenza-related complication was observed in those children with PA/I38X-substituted virus, all of whom recovered without any additional influenza medication. However, further studies are required to draw any firm conclusions.
In conclusion, the weight-based dose regimen of baloxavir granules was considered to be well tolerated in children, with a rapid reduction in infectious virus associated with symptom alleviation. The clinical impact of later increases in virus among younger children requires further assessment in a large study.
ACKNOWLEDGMENTS
The authors would like to thank all the study participants. We also thank Satoshi Kojima, Keiko Kawaguchi and Keita Fukao of Shionogi & Co., Ltd. for assistance in preparing the manuscript and for technical support, and LSI Medience Corporation for conducting infectious influenza virus titer assessment.
Supplementary Material
Footnotes
Japan Pharmaceutical Information Center Clinical Trials Information (JapicCTI-173811), initial registration on December 15, 2017.
This study was sponsored by Shionogi & Co., Ltd. (Osaka, Japan), manufacturer/licensee of baloxavir marboxil. Medical writing assistance funded by Shionogi was provided by Hiroko Ebina, BPharm, MBA, CMPP, and Rebecca Lew, PhD, CMPP, of ProScribe—Envision Pharma Group and complied with international guidelines for Good Publication Practice (GPP3).
T.I., T.S., H.S., C.S., K.T. and T.U. are employees of Shionogi & Co., Ltd.; T.I. and K.T. also own stock in the company. P.A.P. has a grant from Shionogi and serves as a scientific advisor for Roche Ltd. T.Y. has no conflicts of interest to declare.
Shionogi & Co., Ltd. was involved in the study design, data collection, data analysis and preparation of the manuscript.
All authors participated in the interpretation of the data, and in the drafting, critical revision, and approval of the final version of the manuscript. T.I., T.S., H.S., C.S., K.T. and T.U. contributed to the study design; T.Y. and C.S. also contributed to the data collection; T.I. contributed to the pharmacokinetic analysis; H.S. contributed to the statistical analysis; and P.A.P. contributed to the co-infection analysis; T.Y. was an investigator in the study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
References
- 1.National Institute of Infectious Diseases. Infectious Agents Surveillance Report (IASR). Influenza in the 2017/2018 season (as of November 27, 2018). Available at: https://www.niid.go.jp/niid/en/asr-e/865-iasr/8438-465te.html. Accessed March 19, 2020.
- 2.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the infectious diseases society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis. 2019;68:e1–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarner J, Paddock CD, Shieh WJ, et al. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003-2004 season. Clin Infect Dis. 2006;43:132–140. [DOI] [PubMed] [Google Scholar]
- 4.Finelli L, Fiore A, Dhara R, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. 2008;122:805–811. [DOI] [PubMed] [Google Scholar]
- 5.Morishima T, Togashi T, Yokota S, et al. Collaborative Study Group on Influenza-Associated Encephalopathy in Japan. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35:512–517. [DOI] [PubMed] [Google Scholar]
- 6.Flicoteaux R, Protopopescu C, Tibi A, et al. Factors associated with non-persistence to oral and inhaled antiviral therapies for seasonal influenza: a secondary analysis of a double-blind, multicentre, randomised clinical trial. BMJ Open. 2017;7:e014546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murasaka T, Ikemura K, Enokiya T, et al. Impact of the number of repeated inhalations and patient characteristics on the residual amount of inhaled laninamivir octanoate hydrate dry powder in pediatric patients with influenza. J Pharm Health Care Sci. 2017;3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omoto S, Speranzini V, Hashimoto T, et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep. 2018;8:9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noshi T, Kitano M, Taniguchi K, et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral Res. 2018;160:109–117. [DOI] [PubMed] [Google Scholar]
- 10.Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir Marboxil Investigators Group. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379:913–923. [DOI] [PubMed] [Google Scholar]
- 11.Hirotsu N, Sakaguchi H, Sato C, et al. Baloxavir marboxil in Japanese pediatric patients with influenza: safety and clinical and virologic outcomes. Clin Infect Dis. 2019;pii:ciz908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshimichi H, Ishibashi T, Wajima T.Population pharmacokinetics of baloxavir marboxil in Japanese pediatric influenza patients. J Pharm Sci. 2019;108:3112–3117. [DOI] [PubMed] [Google Scholar]
- 13.Koshimichi H, Tsuda Y, Ishibashi T, et al. Population pharmacokinetic and exposure-response analyses of baloxavir marboxil in adults and adolescents including patients with influenza. J Pharm Sci. 2019;108:1896–1904. [DOI] [PubMed] [Google Scholar]
- 14.Sugaya N, Ohashi Y.Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother. 2010;54:2575–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugaya N, Kohno S, Ishibashi T, et al. Efficacy, safety, and pharmacokinetics of intravenous peramivir in children with 2009 pandemic H1N1 influenza A virus infection. Antimicrob Agents Chemother. 2012;56:369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harada Y, Kinoshita F, Yoshida LM, et al. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J. 2013;32:441–445. [DOI] [PubMed] [Google Scholar]
- 17.Whitley RJ, Hayden FG, Reisinger KS, et al. Oral oseltamivir treatment of influenza in children. Pediatr Infect Dis J. 2001;20:127–133. [DOI] [PubMed] [Google Scholar]
- 18.Heinonen S, Silvennoinen H, Lehtinen P, et al. Early oseltamivir treatment of influenza in children 1-3 years of age: a randomized controlled trial. Clin Infect Dis. 2010;51:887–894. [DOI] [PubMed] [Google Scholar]
- 19.Fry AM, Goswami D, Nahar K, et al. Efficacy of oseltamivir treatment started within 5 days of symptom onset to reduce influenza illness duration and virus shedding in an urban setting in Bangladesh: a randomised placebo-controlled trial. Lancet Infect Dis. 2014;14:109–118. [DOI] [PubMed] [Google Scholar]
- 20.Baker J, Block SL, Matharu B, et al. Baloxavir marboxil single-dose treatment in influenza-infected children: a randomized, double-blind, active controlled phase 3 safety and efficacy trial (miniSTONE-2). Pediatr Infect Dis J. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lina B, Boucher C, Osterhaus A, et al. Five years of monitoring for the emergence of oseltamivir resistance in patients with influenza A infections in the Influenza Resistance Information Study. Influenza Other Respir Viruses. 2018;12:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


