Supplemental Digital Content is available in the text.
Keywords: HIV-exposed infants, antiretroviral therapy, infant growth
Background:
There are concerns about the adverse effect of in-utero exposure to antiretroviral therapy (ART) on the growth of HIV exposed-uninfected (HEU) infants. We compared growth of HEU-infants according to the timing and type of ART exposure.
Methods:
A retrospective cohort study was conducted by abstracting clinical data from HIV-infected mothers and HEU-infants in Addis Ababa, Ethiopia between February 2013 and October 2016. Mixed-effects linear models were used to compare changes in weight and length and cox proportional hazard models were used to evaluate stunting (length-for-age z score <−2.0) and underweight (weight-for-age z score <−2.0).
Results:
A total of 624 HEU-infants were included in the analyses. Infants exposed to ART from conception had a lower rate of change in length [β = −0.54, 95% confidence interval (CI): −1.00 to −0.08] the first 3 months of life, as compared with infants exposed from late pregnancy. Risk of stunting was 51.9 per 100 person-years and risk of underweight was 26.7 per 100 person-years. Exposure to ART from conception was associated with a higher rate of stunting as compared with exposure from late pregnancy (adjusted hazard ratio = 1.95, 95% CI: 1.27–2.99). Infants born to mothers with advanced disease had a higher incidence of underweight compared with infants born to mothers with early-stage disease adjusted hazard ratio = 1.99, 95% CI: 1.32–3.03).
Conclusions:
In HEU-infants, exposure to ART from conception was associated with decrease growth during early infancy and higher incidence of stunting compared with treatment exposure later in pregnancy. Close monitoring of HEU-infants’ growth and prompt nutritional intervention is essential.
Antiretroviral therapy (ART) during pregnancy prevent mother-to-child transmission of HIV and improve maternal health.1–4 Currently, most (82%) HIV-infected pregnant women have access to ART.5 The estimated number of HIV exposed-uninfected (HEU) infants reached 14.8 million in 2018, and of these 13.2 million are from sub-Saharan Africa.5
Studies indicate that HEU-infants experience growth restriction,6–11 and excess morbidity and mortality,12,13 compared with HIV-unexposed infants. There are also reports of an association between in-utero ART exposure and growth faltering from resource-limited settings. For instance, a study from Botswana reported that in-utero exposure to ART was associated with both lower length-for-age z-scores (LAZ) and weight-for-age z-scores (WAZ) at 24 months of age.14 Another study showed that infants exposed to ART had a lower WAZ at birth, but a differential and more rapid increase in WAZ and a slower change in LAZ the first 2 months of life than zidovudine (ZDV) monotherapy exposed infants but the 2 groups experienced similar rate of growth from 3 to 6 months.15 Furthermore, exposure to any type of antiretroviral drugs was associated with lower WAZ and LAZ versus no ART in South Africa.16 Studies evaluating timing of in-utero ART exposure also reported inconsistent findings.17,18 Data from developed countries mostly showed no association between in-utero ART exposure and growth of HEU infants.19–22 Other factors associated with HEU-infants’ growth include maternal disease severity,23 infant feeding practice,24 and sociodemographic factors.25
Given the inconsistency of the current evidence, additional data clarifying the role of timing and the potential differential impact types of ART exposure has on the growth of HEU-infants is essential. Evidence from resource-limited settings is particularly important, as a substantial number of these children have suboptimal growth, and malnutrition being a major cause of morbidity and mortality in these settings.26,27 Ethiopia is a low-income country with a high prevalence of child malnutrition. An estimated 38% of under-5 children are stunted and 24% underweight.28 The country is also home to a large number of HIV-infected women (approximately 410,000 women in 2017, and 67% of HIV-infected pregnant women were on ART in 2017).29 Therefore, the aim of this study is to compare postnatal growth up to 12 months of HEU-infants according to type and timing of in-utero ART exposure.
MATERIALS AND METHODS
Study Population
A retrospective cohort study was conducted in 5 health centers in Addis Ababa, Ethiopia. Information about HEU-infants born to HIV-infected women on ART between February 2013 and October 2016 was abstracted from the Infant Follow-up Charts. We were able to obtain information from 683 singleton infant and mother pairs. To be included in the current study, the children needed to have information on maternal ART use, be HIV negative and have at least one anthropometric measurement available. The information gathered included sex, age, HIV status, breast-feeding status, and anthropometric measurements (weight and length) from birth to 12 months of age. The Ethiopian HIV treatment guideline at that time recommended that HEU-infants should be followed for the first 18 months of life. The follow-up was scheduled monthly for the first 6 months and every 3 months afterwards if the child is not sick. HIV-testing for HEU-infants is performed twice between 6 weeks and 18 months,30 all infants included in this study were HIV-negative at the time of their first test. Anthropometric measurements such as weight and length were performed by nurses who had in-service training on HIV-exposed infant management. Information about maternal demographic characteristics, clinical and obstetric history, and ART regimen during pregnancy was abstracted from the mothers’ clinical charts and the ART databases. The study was approved by the Norwegian Regional Committees of Medical and Health Research Ethics of South/East Norway, Jimma University Ethical Review Board, and Addis Ababa City Administration Health Bureau. This clinical chart review was regarded as clinical practice and outcome assessment, and did not require written informed consent.
Growth Outcomes
Measures of weight (g) and length (cm) were taken at birth (no length measurement), 6 weeks, 10 weeks, 3 months, 4 months, 5 months, 6 months 7 months, 8 months, 9 months, and 12 months. LAZ and WAZ scores were calculated based on age- and sex-specific reference values using the 2006 World Health Organization (WHO) reference values.31 LAZ or WAZ values less than −6 or greater than 6 were defined as implausible values and set to missing. Stunting (defined as LAZ <−2) and underweight (defined as WAZ <−2),32 were evaluated as secondary outcomes.
Exposure Variables
The exposure variables were timing and type of in-utero ART exposure and maternal disease progression. Timing of ART exposure was categorized as: exposed to ART from conception (mother started ART before pregnancy), early pregnancy (started ART before 14 completed weeks of pregnancy), and late pregnancy (started ART between 14 weeks of pregnancy and delivery). Types of ART regimens were categorized as a combination of tenofovir, lamivudine and efavirenz/nevirapine (TDF-3TC-EFV/NVP), a combination of ZDV, lamivudine and efavirenz/nevirapine (ZDV-3TC-EFV/NVP) or protease inhibitor based ARTs. Maternal disease progression was categorized as early stage (CD4 count during pregnancy ≥200 cells/mm3 or WHO stage 1–2) or advanced stage (CD4 count during pregnancy <200 cells/mm3 or WHO stage 3–4).
Covariates
Additional information on maternal and infant characteristics likely to be associated with exposures and infant growth outcomes were collected. These include infant sex, and breast-feeding status which was categorized as “breast-fed” and “not breast-fed/formula-fed” as recorded in the clinical chart. Moreover, maternal characteristics during pregnancy, such as age in years, parity, level of education (no education, primary, secondary or college), and body mass index before pregnancy (kg/m2) were considered as potential confounders.
Statistical Analysis
We compared maternal and infant characteristics by timing and type of ART exposure using χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables. We examined differences in weight and length during the first year of life according to type and timing of ART exposure and maternal disease progression using mixed-effects linear regression. The models included linear splines for age (knot point at 3 months of age), a random intercept and slope, and an unstructured covariance matrix. We examined differences in growth between the exposure groups by including interaction terms between the exposures and the linear splines reflecting different age periods. The models were adjusted for the maternal and offspring characteristics described above. The findings are presented as mean differences in growth velocities with 95% confidence intervals (CIs). Differences in risk of stunting (LAZ <−2.0) and underweight (WAZ <−2) according to timing and type of ART exposure and maternal disease progression were calculated using Cox proportional hazard models, reporting hazard ratios (HRs) with corresponding 95% CIs. Children were followed from birth for the analysis of underweight, and from 6 weeks for the analysis of stunting, until they were first registered with the outcome of interest or until the end of follow-up (12 months of age). The multivariate analyses were adjusted for the same covariates as the mixed-effects linear regression. In addition, we run logistic regression models to assess differences in stunting and underweight at 6 months and 12 months of age, according to timing and type of ART exposure and maternal disease progression, reporting odds ratios with 95% CIs. We used STATA version 14 for all analyses (Stata Corp., College Station, Texas).
RESULTS
From 683 mother and infant pairs, we excluded infants for whom information about maternal ART during pregnancy was not available (n = 11), infants who only had one anthropometric measurement (n = 43), and infants who were HIV-positive (n = 5). This left a total of 624 infant and mother pairs for analyses (Figure, Supplemental Digital Content 1, http://links.lww.com/INF/D911). Among these, 239 (38.3%) infants were exposed to ART from conception (ART initiated before pregnancy), 95 (15.2%) were exposed to ART from early pregnancy, and 290 (46.5%) were exposed to ART from late pregnancy. Mothers of 531 (85%) children were on TDF-3TC-EFV/NVP during pregnancy and the type of ART differed according to duration of ART exposure. The median age of mothers during pregnancy was 28 years (interquartile range 25–30). Mothers of infants exposed to ART since conception were older, as compared with mothers of infants exposed from early or late pregnancy onwards. Mothers of children exposed to ART since conception also had a more advanced WHO disease stage, but their CD4 count was higher during pregnancy. Maternal education and body mass index did not differ significantly according to duration of ART exposure. There was no strong evidence that infant sex or gestational age at birth differed by duration of ART exposure (Table, Supplemental Digital Content 2, http://links.lww.com/INF/D912). The distribution of maternal and infant characteristics by type of ART and maternal disease progression are presented in Tables, Supplemental Digital Content 3, http://links.lww.com/INF/D913 and Supplemental Digital Content 4, http://links.lww.com/INF/D914.
Observed Weight and Length
The average number of measurements per child was 7.9 for weight (range 2–11) and 6.1 for length (range 2–10). Average weight and length at each visit by infant sex and timing of in-utero ART are presented in Figure 1. Mean birth weight was 2.89 kg (SD = 0.54), in infants exposed to ART since conception; 2.94 kg (0.48) in infants exposed to ART since early pregnancy, and 2.84 kg (0.47) in infants exposed to ART since late pregnancy (P = 0.13). Mean weight increased from 2.97 kg (SD = 0.49) at birth to 9.78 kg (SD = 1.11) at 12 months of age among male infants, and from 2.81 kg (SD = 0.47) to 9.03 kg (SD = 1.21) among female infants. Mean length at 6 weeks was 54.5 cm (SD = 3.93) in infants exposed to ART since conception; 54.1 cm (SD = 4.48) in infants exposed since early pregnancy, and 54.6 cm (SD = 3.98) in infants exposed since late pregnancy (P = 0.24). Average increase in length among male infants was from 54.9 cm (SD = 4.4) at 6 weeks of age to 71.7 cm (SD = 4.3) at 12 months of age, while the average increase in length was from 53.8 cm (SD = 3.9) at birth to 71.3 cm (SD = 4.0) at 12 months of age among female infants (Fig. 1).
FIGURE 1.
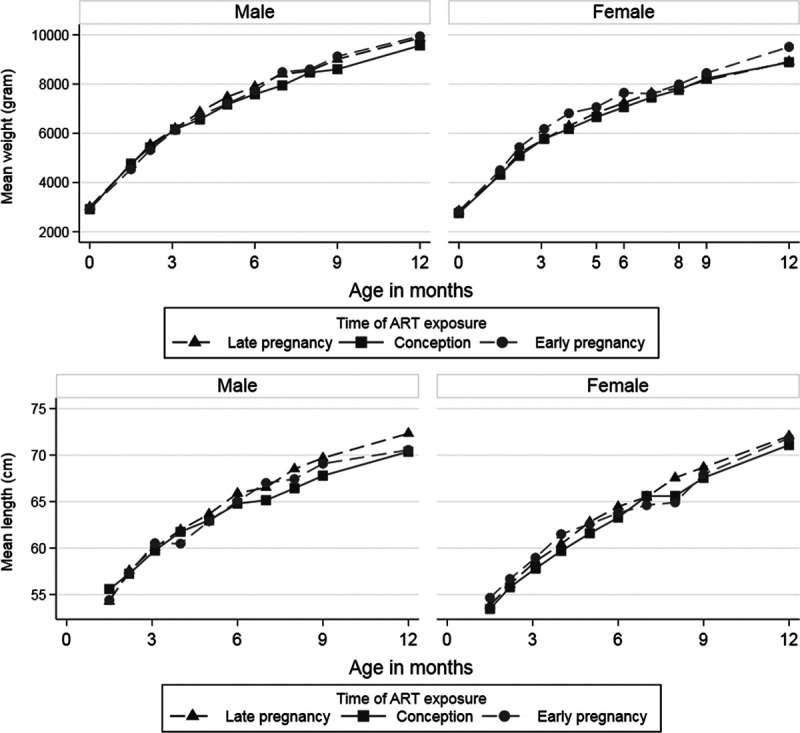
Mean weight and length by age of male and female HIV-exposed uninfected infants by time of in-utero ART exposure. The figure is based on observed data.
Difference in Weight and Length Growth Rate
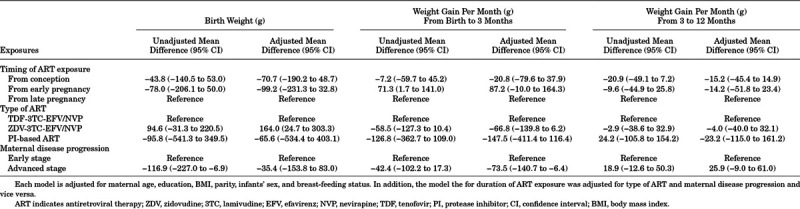
The mixed-effects linear regression model, comparing infants exposed to ART from late pregnancy with infants exposed to ART from conception or early pregnancy indicated no strong evidence of a difference in birth weight or in the rate of weight gain up to 12 months of age (Table 1). Moreover, birth weight and rate of weight gain during the first 12 months of life did not differ by type of ART. Weight gain was lower among infants born to mothers with advanced disease compared with early stage of disease, from birth to 3 months of age (β = −73.5, 95% CI: −140.7 to −6.4) (Table 1).
TABLE 1.
Linear-mixed Effects Model Evaluating Differences in the Rate of Weight Gain Among HIV-exposed Uninfected Infants According to Duration and Type of ART Exposure and Maternal Disease Progression
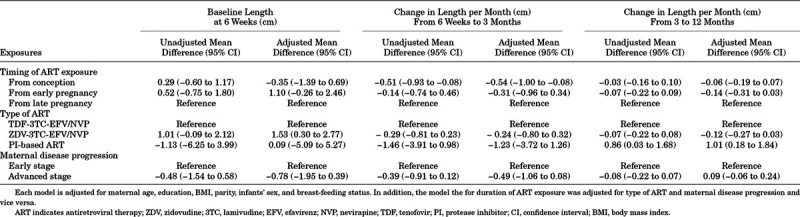
Infants exposed to ART from conception had lower rate of change in length in the first 3 months, as compared with infants exposed to ART from late pregnancy onwards (β = −0.54, 95% CI: −1.00 to −0.08). No strong evidence of a difference in the rate of change in length was observed between 3 and 12 months (β = −0.14, 95% CI: −0.31 to 0.03) (Table 2). We observed no difference in the rate of change in length between infants exposed from early pregnancy as compared with infants exposed from late pregnancy. There was also no strong evidence of a difference in the rate of change in length according to type of ART or maternal disease progression (Table 2). We also found a lower rate of length change associated with preconception ART compared with ART initiated during pregnancy (Table, Supplemental Digital Content 5, http://links.lww.com/INF/D915). Evaluating the interaction terms between sex and the timing of ART exposure showed no evidence of any sex difference (P-value 0.15 for weight and 0.92 for length).
TABLE 2.
Linear-mixed Effects Model Evaluating Differences in the Rate of Change in Length Among HIV-exposed Uninfected Infants According to Duration and Type of ART Exposure and Maternal Disease Progression
Z-score Comparison With the WHO Reference
The age and sex standardized weight of the children was below the WHO reference at birth, with a mean WAZ of −0.94 (SD = 1.12). However, WAZ progressively improved with age and reached of 0.03 (SD = 1.10) at 12 months (Table, Supplemental Digital Content 6, http://links.lww.com/INF/D916). Similarly, LAZ were below the WHO reference at 6 weeks, with a mean of −0.90 (SD = 2.10). Notably, LAZ progressively declined and reached −1.37 (SD = 1.74) at 12 months of age (Table, Supplemental Digital Content 6, http://links.lww.com/INF/D916). Findings from mixed-effects linear regression analyses using WAZ and LAZ as outcomes were consistent with what was observed using weight and length (Tables, Supplemental Digital Content 7, http://links.lww.com/INF/D917 and Supplemental Digital Content 8, http://links.lww.com/INF/D918).
Stunting and Underweight
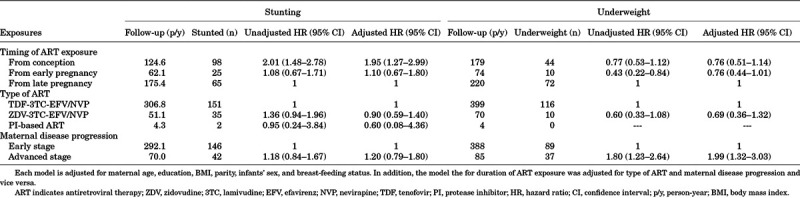
The rate of stunting among the children was 51.9 per 100 person-years, while the rate of underweight was 26.7 per 100 person-years. Kaplan-Meier curves of the probability of stunting and underweight according to timing of ART exposure are presented in Figures, Supplemental Digital Content 9, http://links.lww.com/INF/D919 and Supplemental Digital Content 10, http://links.lww.com/INF/D920. Infants exposed to ART from conception had higher risk of stunting as compared with infants exposed to ART from late pregnancy (adjusted HR = 1.95, 95% CI: 1.27–2.99). There was, however, no notable difference in the risk of stunting between infants exposed to ART from early compared with late pregnancy (adjusted HR = 1.10, 95% CI: 0.67–1.80) (Table 3). Infants born to mothers with advanced disease exhibited a significantly higher risk of underweight (adjusted HR = 1.99, 95% CI: 1.32–3.03) (Table 3). Using logistic regression, we found no difference in the prevalence of stunting and underweight at 6 or 12 months of age according to timing and type of ART exposure or maternal disease progression (Table, Supplemental Digital Content 11, http://links.lww.com/INF/D921).
TABLE 3.
Risk of Stunting and Underweight Among HIV-exposed Uninfected Infants According to Duration and Type of ART Exposure and Maternal Disease Progression
DISCUSSION
The number of HEU-infants is increasing in resource-limited settings as more and more HIV-infected women have access to ART. Clarifying the role of type and timing of in-utero ART exposure on growth of HEU-infants is therefore imperative. In the current study, we found no difference in birth weight or length at 6 weeks according to duration of ART exposure. However, infants exposed to ART from conception had a lower rate of change in length up to 3 months compared with infants exposed to ART from late pregnancy. The observed difference seems temporary, since we found no difference in the rate of change in length from 3 to 12 months. Our analysis of the risk of stunting and underweight showed that infants exposed to ART from conception were at increased risk of stunting when compared with infants exposed to ART from late pregnancy. Maternal disease progression was positively associated with risk of underweight, but not stunting.
Prior studies evaluating the role of timing of ART exposure on HEU-infants growth report inconsistent findings. A study from Brazil described a difference in rate of length change comparing infants exposed to ART from early versus late pregnancy.17 However, this study included ZDV mono-therapy and dual therapy in addition to triple ART, and their analysis restricted to children exposed to triple ART showed no significant association. A South African study did not find an association between duration of exposure to TDF-based ART and change in length through 12 months.18
In our study, comparing TDF-3TC-EFV/NVP versus ZDV-3TC-EFV/NVP indicated no significant association with rate of change in weight or length through 12 months of age. The finding is consistent with previous reports comparing TDF-based and non-TDF based ARTs.17,18,21,33–42 However, others report that infants exposed to TDF-based ART had significantly lower LAZ at 1 year of age43; a higher risk of under-weight (WAZ <5%) at age 6 months,35 and lower weight and length growth as compared with those without TDF.44 A systematic review and meta-analysis on this topic is forthcoming.45
We found a high probability of being stunted and underweight at least once, during the follow-up period. Our finding is consistent with reports of high risk of growth faltering among HEU-infants.6,11,24,46–49 However, the finding may be a reflection of high burden of childhood malnutrition in resource-limited settings.28 Stunting is associated with impaired cognitive development, low level of school attainment and other health consequences.50
In our study, a number of factors could explain the increased risk of stunting among infants exposed to ART from conception; mothers initiating ART before pregnancy could be sicker, since we have only adjusted for CD4 count and WHO disease stage during pregnancy, but not for CD4 level at the time of HIV diagnosis. Moreover, micronutrient deficiency is common among women with advanced stage of disease, which could impact breast-feeding. The underlying biologic mechanism explaining the effect of in-utero ART exposure on infant growth is not clear. However, some studies theorized that ART, specifically nucleoside reverse transcriptase inhibitors, could damage mitochondrial DNA,51,52 resulting in restricted growth.
Our findings should be understood in light of the following limitations. The study was conducted in health centers located in urban areas of Ethiopia. The findings may therefore not be generalizable to rural settings. Despite adjusting the analyses for a number of known confounders, the influence of unmeasured/residual confounding could not be excluded. For example, we could not adjust for family income, which is a predictor of infant growth. However, we were able to adjust for educational level, as a proxy for income. We analyzed anthropometric measurements taken as part of routine health care services for children. This might affect the findings due to observer and instrument variability. There were missing anthropometric measurements (32% missing values for weight and 37% missing values for length at 12 months). We accounted for the differential number of anthropometric measurements available by using mixed-effects linear regression. However, the missing measurements could have influenced our estimate of the effects on underweight and stunting. Infants’ HIV-status was determined from 6 weeks and any HIV infection that is first detectable later in the postnatal period is not known. However, this is unlikely to bias our findings as the rate of vertical transmission due to breast-feeding is minimal (<1%).53 Even though we were able to adjust for maternal CD4 count and WHO disease stage, we cannot exclude the possibility of residual confounding due to our inability to adjust for maternal viral load.
In conclusion, in this study, the HEU-infants exposed to ART from conception had a modest decrease in rate of change in length during the first 3 months of life and an increased risk of stunting, as compared with infants exposed to ART later in pregnancy. We also observed a greater risk of underweight among children of mothers with an advanced disease stage. The health and growth of HEU-infants should be closely monitored and appropriate nutritional interventions considered where necessary. Further research in resource-limited settings, evaluating the long-term growth of HEU-infants is warranted.
Supplementary Material
Footnotes
This publication was supported by NORAD (Norwegian Agency for Development Cooperation) under the NORHED-Programme, agreement no. ETH-13/0024. M.C.M. works at the MRC Integrative Epidemiology Unit which receives infrastructure funding from the UK Medical Research Council (MRC) (MC_UU_12013/5). M.C.M. is funded by a UK MRC fellowship (MR/M009351/1). This work was also partly supported by the Research Council of Norway through the Centers of Excellence funding scheme (project number 262700).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
References
- 1.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–1180. [DOI] [PubMed] [Google Scholar]
- 2.Birth outcomes following zidovudine therapy in pregnant women. MMWR Morbidity and mortality weekly report. 1994;43:409, 415–406. [PubMed] [Google Scholar]
- 3.Anglemyer AT, Rutherford G, Horvath H, et al. Antiretroviral therapy for asymptomatic adults and adolescents with HIV-1 infection and CD4+ T-cell counts≥ 500 cells/μL: a meta-analysis. 2018.
- 4.Song A, Liu X, Huang X, et al. From CD4-Based initiation to treating All HIV-infected adults immediately: an evidence-based meta-analysis. Frontiers immunol. 2018;9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. UNAIDS DATA 2018. 2018.
- 6.Sudfeld CR, Lei Q, Chinyanga Y, et al. Linear growth faltering among HIV-exposed uninfected children. J Acquir Immune Defic Syndr. 2016;73:182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson L, Chisenga M, Siame J, et al. Growth and health outcomes at school age in HIV-exposed, uninfected Zambian children: follow-up of two cohorts studied in infancy. BMC Pediatr. 2015;15:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muhangi L, Lule SA, Mpairwe H, et al. Maternal HIV infection and other factors associated with growth outcomes of HIV-uninfected infants in Entebbe, Uganda. Public Health Nutr. 2013;16:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jumare J, Datong P, Osawe S, et al. INFANT Study Team. Compromised growth among HIV-exposed uninfected compared with unexposed children in Nigeria. Pediatr Infect Dis J. 2019;38:280–286. [DOI] [PubMed] [Google Scholar]
- 10.Omoni AO, Ntozini R, Evans C, et al. Child growth according to maternal and child HIV Status in Zimbabwe. Pediatr Infect Dis J. 2017;36:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosala-Hallas A, Bartlett JW, Filteau S.Growth of HIV-exposed uninfected, compared with HIV-unexposed, Zambian children: a longitudinal analysis from infancy to school age. BMC Pediatr. 2017;17:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pierre RB, Fulford TA, Lewis K, et al. Infectious disease morbidity and growth among young HIV-exposed uninfected children in Jamaica. Rev Panam Salud Publica. 2016;40:401–409. [PubMed] [Google Scholar]
- 13.Locks LM, Manji KP, Kupka R, et al. High burden of morbidity and mortality but not growth failure in infants exposed to but uninfected with human immunodeficiency virus in Tanzania. J Pediatr. 2017;180:191–199.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powis KM, Smeaton L, Hughes MD, et al. In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. AIDS. 2016;30:211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powis KM, Smeaton L, Ogwu A, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr. 2011;56:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morden E, Technau KG, Giddy J, et al. Growth of HIV-exposed uninfected infants in the first 6 months of life in South Africa: the IeDEA-SA collaboration. PLoS One. 2016;11:e0151762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hofer CB, Keiser O, Zwahlen M, et al. In utero exposure to antiretroviral drugs: effect on birth weight and growth among HIV-exposed uninfected children in Brazil. Pediatr Infect Dis J. 2016;35:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Roux SM, Jao J, Brittain K, et al. Tenofovir exposure in utero and linear growth in HIV exposed, uninfected infants: a prospective study. AIDS (London, England). 2017;31:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hankin C, Thorne C, Newell MLEuropean Collaborative Study. Does exposure to antiretroviral therapy affect growth in the first 18 months of life in uninfected children born to HIV-infected women? J Acquir Immune Defic Syndr. 2005;40:364–370. [DOI] [PubMed] [Google Scholar]
- 20.Kakkar FW, Samson L, Vaudry W, et al. Safety of combination antiretroviral prophylaxis in high-risk HIV-exposed newborns: a retrospective review of the Canadian experience. J Int AIDS Soc. 2016;19:20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson DL, Patel K, Williams PL, et al. Pediatric HIVAIDS Cohort Study. Growth at 2 Years of Age in HIV-exposed uninfected children in the United States by trimester of maternal antiretroviral initiation. Pediatr Infect Dis J. 2017;36:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moseholm E, Helleberg M, Sandholdt H, et al. Children exposed or unexposed to HIV: weight, height and BMI during the first five years of life. A Danish Nationwide Cohort Study. Clin Infect Dis. 2019;ciz605. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41:1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bork KA, Cames C, Newell ML, et al. Kesho Bora Study Group. Formula-feeding of HIV-Exposed uninfected African Children is associated with faster growth in length during the first 6 months of life in the Kesho Bora Study. J Nutr. 2017;147:453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans C, Jones CE, Prendergast AJ.HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis. 2016;16:e92–e107. [DOI] [PubMed] [Google Scholar]
- 26.Pelletier DL, Frongillo EA, Jr, Schroeder DG, et al. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ. 1995;73:443–448. [PMC free article] [PubMed] [Google Scholar]
- 27.Olofin I, McDonald CM, Ezzati M, et al. Nutrition Impact Model Study (anthropometry cohort pooling). Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One. 2013;8:e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Central Statistical Agency (CSA) Ethiopia and ICF. Ethiopia Demographic and Health Survey 2016. 2016Addis Ababa, Ethiopia, and Rockville, Maryland, USA: CSA and ICF; [Google Scholar]
- 29.UNAIDS. UNAIDS data 2017. 2018.
- 30.Federal Ministry of Health. Guidelines for Comprehencive HIV Prevention, Care and Treatment 2014, 2014Addis Ababa, Ethiopia. [Google Scholar]
- 31.WHO. World Health Organization Child Growth Standards. 2006.
- 32.WHO. Nutrition Landscape Information System (NLIS) Country Profile Indicators: Interpretation Guide. 2010.
- 33.Williams PL, Hazra R, Van Dyke RB, et al. Pediatric HIV/AIDS Cohort Study. Antiretroviral exposure during pregnancy and adverse outcomes in HIV-exposed uninfected infants and children using a trigger-based design. AIDS. 2016;30:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owor M, Mwatha A, Donnell D, et al. Long-term follow-up of children in the HIVNET 012 perinatal HIV prevention trial: five-year growth and survival. J Acquir Immune Defic Syndr. 2013;64:464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ransom CE, Huo Y, Patel K, et al. P1025 Team of the International Maternal Pediatric Adolescent AIDS Clinical Trials Group. Infant growth outcomes after maternal tenofovir disoproxil fumarate use during pregnancy. J Acquir Immune Defic Syndr. 2013;64:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibb DM, Kizito H, Russell EC, et al. DART trial team. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med. 2012;9:e1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viganò A, Mora S, Giacomet V, et al. In utero exposure to tenofovir disoproxil fumarate does not impair growth and bone health in HIV-uninfected children born to HIV-infected mothers. Antivir Ther. 2011;16:1259–1266. [DOI] [PubMed] [Google Scholar]
- 38.Liotta G, Floridia M, Andreotti M, et al. Growth indices in breastfed infants pre and postnatally exposed to tenofovir compared with tenofovir-unexposed infants. AIDS. 2016;30:525–527. [DOI] [PubMed] [Google Scholar]
- 39.Jao J, Agwu A, Mhango G, et al. Growth patterns in the first year of life differ in infants born to perinatally vs. nonperinatally HIV-infected women. AIDS. 2015;29:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nachega JB, Uthman OA, Mofenson LM, et al. Safety of tenofovir disoproxil fumarate-based antiretroviral therapy regimens in pregnancy for HIV-infected women and their infants: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2017;76:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pintye J, Langat A, Singa B, et al. Maternal tenofovir disoproxil fumarate use in pregnancy and growth outcomes among HIV-exposed uninfected infants in Kenya. Infect Dis Obstet Gynecol. 2015;2015:276851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floridia M, Liotta G, Andreotti M, et al. Levels of bone markers in a population of infants exposed in utero and during breastfeeding to tenofovir within an Option B+ programme in Malawi. J Antimicrob Chemother. 2016;71:3206–3211. [DOI] [PubMed] [Google Scholar]
- 43.Siberry GK, Williams PL, Mendez H, et al. Pediatric HIV/AIDS Cohort Study (PHACS). Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26:1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denneman L, Cohen S, Godfried MH, et al. In-utero exposure to tenofovir is associated with impaired fetal and infant growth: need for follow-up studies in combination antiretroviral therapy/HIV-exposed infants. AIDS. 2016;30:2135–2137. [DOI] [PubMed] [Google Scholar]
- 45.Ekali GL, Jesson J, Enok PB, et al. Effect of in utero exposure to HIV and antiretroviral drugs on growth in HIV-exposed uninfected children: a systematic review and meta-analysis protocol. BMJ Open. 2019;9:e023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans C, Humphrey JH, Ntozini R, et al. HIV-exposed uninfected infants in Zimbabwe: insights into health outcomes in the pre-antiretroviral therapy era. Front Immunol. 2016;7:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGrath CJ, Nduati R, Richardson BA, et al. The prevalence of stunting is high in HIV-1-exposed uninfected infants in Kenya. J Nutr. 2012;142:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finkelstein JL, Mehta S, Duggan C, et al. Maternal vitamin D status and child morbidity, anemia, and growth in human immunodeficiency virus-exposed children in Tanzania. Pediatr Infect Dis J. 2012;31:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kupka R, Manji KP, Bosch RJ, et al. Multivitamin supplements have no effect on growth of Tanzanian children born to HIV-infected mothers. J Nutr. 2013;143:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Springer PE, Slogrove AL, Kidd M, et al. Neurodevelopmental and behavioural outcomes of HIV-exposed uninfected and HIV-unexposed children at 2-3 years of age in Cape Town, South Africa. AIDS care. 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 51.Gingelmaier A, Grubert TA, Kost BP, et al. Mitochondrial toxicity in HIV type-1-exposed pregnancies in the era of highly active antiretroviral therapy. Antivir Ther. 2009;14:331–338. [PubMed] [Google Scholar]
- 52.Jao J, Abrams EJ.Metabolic complications of in utero maternal HIV and antiretroviral exposure in HIV-exposed infants. Pediatr Infect Dis J. 2014;33:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coovadia HM, Brown ER, Fowler MG, et al. HPTN 046 protocol team. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet. 2012;379:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


