Abstract
Background: Natural killer (NK) cells are essential to innate immunity and participate in cancer immune surveillance. Heterophilic interactions between carcinoembryonic antigen (CEA) on tumor cells and carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) on NK cells inhibit NK cell cytotoxicity against tumor cells. NEO-201 is a humanized IgG1 monoclonal antibody that recognizes members of CEACAM family, expressed specifically on a variety of human carcinoma cell lines and tumor tissues. This investigation was designed to determine whether the binding of NEO-201 with CEACAM5 on tumor cells can block the CEACAM5/CEACAM1 interaction to restore antitumor cytotoxicity of NK cells.
Materials and Methods: In vitro functional assays, using various human tumor cell lines as target cells and NK-92 cells as effectors, were conducted to assess the ability of NEO-201 to block the interaction between CEACAM5 on tumor cells and CEACAM1 on NK cells to enhance the in vitro killing of tumor cells by NK-92. NK-92 cells were used as a model of direct NK killing of tumor cells because they lack antibody-dependent cellular cytotoxicity activity.
Results: Expression profiling revealed that various human carcinoma cell lines expressed different levels of CEACAM5+ and NEO-201+ cells. Addition of NEO-201 significantly enhanced NK-92 cell cytotoxicity against highly CEACAM5+/NEO-201+ expressing tumor cells, suggesting that its activity is correlated with the level of CEACAM5+/NEO-201+ expression.
Conclusions: These findings demonstrate that NEO-201 can block the interaction between CEACAM5 on tumor cells and CEACAM1 on NK cells to reverse CEACAM1-dependent inhibition of NK cytotoxicity.
Keywords: CEACAM5, immune checkpoint pathway, natural killer cells, NEO-201, NK cell cytotoxicity, monoclonal antibody
Introduction
During the last few decades, cancer immunotherapy has become a promising treatment option for cancer patients, as well as a valid alternative to the conventional cancer therapies, such as chemotherapy and radiotherapy. The aim of cancer immunotherapy is to generate or enhance the host immune system antitumor responses to kill cancer cells and prolong survival of cancer patients.1 A major limitation of all immunotherapies is the negative effect of the tumor microenvironment on the function and survival of effector cells that could potentially eliminate the tumor.2
The immune system is regulated by both stimulatory and inhibitory components. One of the mechanisms used by tumor cells to inhibit the effect of immunotherapy is the activation of immune checkpoint pathways that lead to the suppression of antitumor immune responses leading to uncontrolled tumor growth.3
Identification of key inhibitory checkpoints of immune regulation and development of therapies to target those checkpoints are essential for successful cancer treatment. Immunotherapy using checkpoint blockade antibodies that target effector cell inhibitory receptors, such as PD-1 and CTLA-4, has elicited some dramatic and durable responses in several tumor types.4–6
The carcinoembryonic antigen-related cell adhesion molecule (CEACAM) family is a group of 12 independent genes that belong to the immunoglobulin (Ig) supergene family. All CEACAM proteins (other than CEACAM16) are composed on an N domain followed by zero to six constant C2-like Ig domains termed A or B domains. These proteins are highly glycosylated, and they can be secreted or are bound to the plasma membrane through a transmembrane domain (CEACAM1, 3, 4, 18, 19, 20, and 21) or a glycophosphatidyl-inositol anchor (CEACAM5, 6, 7, and 8).7–9 X-ray crystal structures of CEACAM homodimers have been shown, but whether CEACAMs form heterodimers or remain monomers is still poorly understood.8 Among the CEACAM family members, CEACAM5 (CEA, CD66e) and CEACAM6 (NCA, CD66c) are known to play significant roles in tumor biology and are overexpressed in several types of carcinomas.10–13 The detection of CEACAM5 in patient serum is an accepted tumor biomarker and is the basis for commercial CEA tumor marker assays that are used clinically to evaluate recurrence in colorectal cancer.14
CEACAM1 is a cell surface protein expressed by immune cells and tumor cells, and it can inhibit T cell function similar to PD-1 and CTLA-4.15
The level of soluble CEACAM1 in the serum of cancer patients could be also used as tumor biomarker. In this regard, osteosarcoma patients with larger tumors, later-tumor stages, low tumor grades, and distant metastases showed a higher level of soluble CEACAM1 in the serum compared to those with smaller tumors, earlier tumor stages, high tumor grades, and nondistant metastases, suggesting that soluble CEACAM1 is a marker associated with diagnosis and prognosis of osteosarcoma.16
Binding between CEACAM1 on natural killer (NK) cells and CEACAM1 or CEACAM5 on tumor cells inhibits NK activation signaling by NKG2D, which prevents NK cell cytolysis and permits tumor cells to evade NK killing.17,18
NEO-201 is a humanized IgG1 monoclonal antibody (mAb) that was derived from an immunogenic preparation of tumor-associated antigens (TAAs) from pooled allogeneic colon tumor tissue extracts.
As previously reported, NEO-201 reacts against variants of CEACAM5 and CEACAM6 expressed on many different human carcinoma cell lines and tumor tissues, while is largely nonreactive against normal tissues. In addition, NEO-201 does not react with CEACAM1 or CEACAM8.19–21
NEO-201 can kill tumor cells by activating innate immune mechanisms such as antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).19–22
This investigation was designed to test the hypothesis that NEO-201 additionally augments antitumor immunity by blocking the interaction between CEACAM5 on tumor cells and CEACAM1 on NK cells to restore antitumor functionality to NK cells.
Materials and Methods
Cell lines and culture
The following human carcinoma cell lines were obtained from the American Type Culture Collection (Manassas, VA): pancreas (ASPC-1, BxPC-3, CFPAC-1), lung (H520, HCC827), and colon (LS174T). NK-92 cells were derived from peripheral blood mononuclear cells (PBMCs) from a male with rapidly progressive non-Hodgkin's lymphoma. NK-92 cells were also obtained from American Type Culture Collection. All human carcinoma cell lines were maintained in RPMI 1640, DMEM, or IMDM culture medium (Corning Life Science, Manassas, VA) as designated by the provider for propagation and maintenance. Culture medium was supplemented with 10% U.S.-sourced and heat-inactivated HyClone fetal bovine serum defined (GE Healthcare Life Sciences, Issaquah, WA), 100 U/mL penicillin, and 100 μg/mL streptomycin (Corning Life Science).
NK-92 cells are an immortalized IL-2-dependent human NK cell line that lacks ADCC activity.23 ADCC occurs when the constant region (fragment crystallizable, Fc) of an antibody binds to the Fc gamma receptor IIIa (FcyRIIIa or CD16) expressed on macrophages and NK cells.24,25
NK-92 cells were cultured in X-VIVO™ 10, with L-glutamine, gentamicin, and phenol red (Lonza, Walkersville, MD) supplemented with 100 U/mL recombinant IL-2 (PeproTech, Inc., Rocky Hill, NJ) and 10% U.S.-sourced and heat-inactivated HyClone fetal bovine serum defined (GE Healthcare Life Sciences). All cell cultures were free of mycoplasma.
PBMCs from healthy volunteer donors were obtained from the National Institutes of Health Clinical Center Blood Bank (NCT00001846) under the appropriate Institutional Review Board approval and informed consent.
Reagents
NEO-201 is a humanized IgG1 mAb that was generated from TAAs derived from tumor membrane fractions pooled from surgically resected specimens from patients with colon cancer.21 Briefly, the TAA preparation was used to immunize BALB/c mice. After immunization, mice that had potent antibody responses against immunizing antigens were used to generate immortalized hybridoma cells producing mouse immunoglobulins (IgGs). From these IgGs, the murine 16C3 clone (m16C3) showed a strong reactivity in ELISA with colon tumor cell membrane extract derived from LS174T or HT-29 cells. The murine 16C3 protein sequence was humanized as human 16C3 and designated as NEO-201. Humanization was performed in silico as previously described.21
The DNA for heavy and light chain of NEO-201 was then synthesized chemically, cloned into mammalian expression plasmids, and transfected into mammalian cell lines (HEK293T and CHO). Purified recombinant NEO-201 derived from CHO cells was used in a previous study to test its antitumor activity in vitro and in vivo,21 and then used in all experiments performed in this study.
Human IgG1 isotype control antibody was obtained from Thermo Fisher Scientific, Waltham, MA.
Flow cytometry
Analysis of the expression of cell surface proteins in PBMCs, NK-92 cells, and human carcinoma cell lines was performed by flow cytometry.
Cells (1.0 × 106) were harvested and first incubated with 1 μL per test of LIVE/DEAD Fixable Aqua (Thermo Fisher Scientific) in 1 × phosphate buffered saline (PBS) for 30 min at 4°C to accomplish live versus dead cell discrimination. Cells were then centrifuged, washed twice with cold PBS, and then stained in 1 × PBS +1% BSA (Teknova, Hollister, CA) for 30 min at 4°C with 2–4 μL/sample of the following anti-human mAbs: pacific Blue-conjugated NEO-201 antibody (BioLegend, San Diego, CA), CEACAM1-APC (LSBio; LifeSpan BioScience, Inc., Seattle, WA), CEACAM5-FITC (clone C365D3), and CEACAM6-PE (clone KOR-SA3544) (ThermoFisher Scientific, Waltham, MA). To detect the NK markers, NK-92 cells were labeled with the following antibodies: CD56-PE (clone 5.1H11), CD16-PerCP-Cy5.5 (clone 3G8), Tim-3-PE-Cy7 (clone F38–2E2), NKG2D-BV421 (clone 1D11), CD107a-APC-Cy7 (clone H4A3), and NKp30-APC (clone P30-15) (BioLegend). To evaluate the reactivity of NEO-201 to human hematopoietic cells, PBMCs were labeled with the following antibodies: CD19-APC-Cy7 (clone HIB19), CD4-FITC (clone OKT4), CD8-APC-Cy7 (clone HIT8a), CD56-PE (clone 5.1H11), CD14-PerCP-Cy5.5 (clone 63D3), and CD15-FITC (clone HI98) (BioLegend). After staining, cells were washed twice with cold PBS and examined using a FACSVerse flow cytometer (BD Biosciences, San Jose, CA). Analysis of cellular fluorescence was performed using BD FACSuite software (BD Biosciences). Positivity was determined by using fluorescence-minus-one controls.
Non-ADCC NK cell cytotoxicity assay
On the day of the assay, human carcinoma cell lines (target cells) were labeled with 10 μM Calcein AM cell-permeant dye (Thermo Fisher Scientific) for 30 min and then seeded in triplicate at 3.0 × 103 cells/well into black-walled flat-bottom 96-well culture plates (655090; Greiner Bio-One, Kremsmünster, Austria). Target cells were then treated with 10 μg/mL of human IgG1 isotype control antibody or NEO-201. At the same time, NK-92 cells were added at the following effector-to-target (E:T) ratios: 1.56:1, 3.12:1, 6.25:1, and 12.5:1.
After 16 h of incubation at 37°C, 10 μg/mL of propidium iodide (Thermo Fisher Scientific) was added to each well and the plate was imaged and analyzed using the Celigo Imaging Cytometer (Nexcelom Bioscience LLC, Lawrence, MA). Specific NK lysis was calculated using the following formula:

Statistical analysis
Data were analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA). Comparisons between two groups were conducted by T-test, and p < 0.05 was considered statistically significant.
Results
Characterization of NK-92 cells used in the non-ADCC NK cell cytotoxicity assay
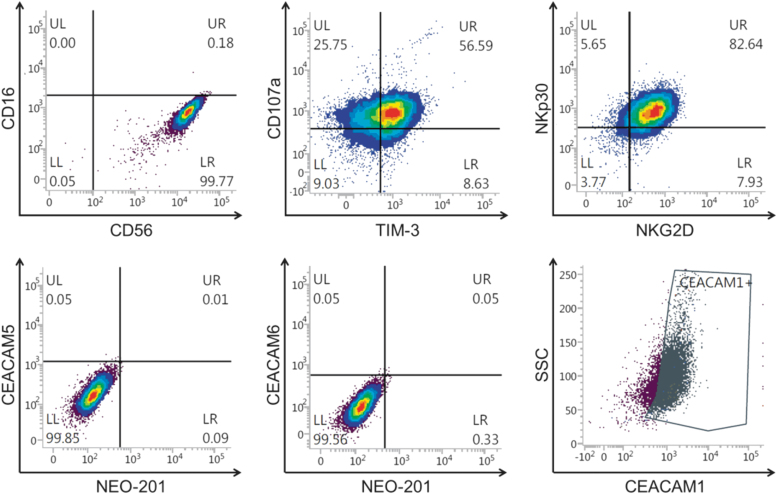
Phenotypic analysis using flow cytometry revealed that NK-92 cells are an optimal model to measure non-ADCC NK cytotoxicity. As shown in Figure 1, NK-92 cells express typical NK cell markers and are CD16 negative. The authors observed that 99.77% of NK-92 cells analyzed had the CD56+/CD16− phenotype. NK-92 cells were also found to have a high expression of activation NK markers such as TIM-3, NKG2D, NKp30, and CD107a.
FIG. 1.
Phenotypic analysis of NK-92 cells. NK-92 cells were analyzed by flow cytometry for the expression of NK markers and CEACAM1, CEACAM5, CEACAM6, and NEO-201 antigen. Data are presented as percentage of viable cells expressing the cell surface proteins. Positivity was determined by using fluorescence-minus-one controls. CEACAM, carcinoembryonic antigen-related cell adhesion molecule; NK, natural killer.
In addition, 82.57% of NK-92 cells expressed CEACAM1, but were negative in the expression of CEACAM5, CEACAM6, or NEO-201 antigen when stained with anti-CEACAM5, anti-CEACAM6, and NEO-201 mAbs (Fig. 1).
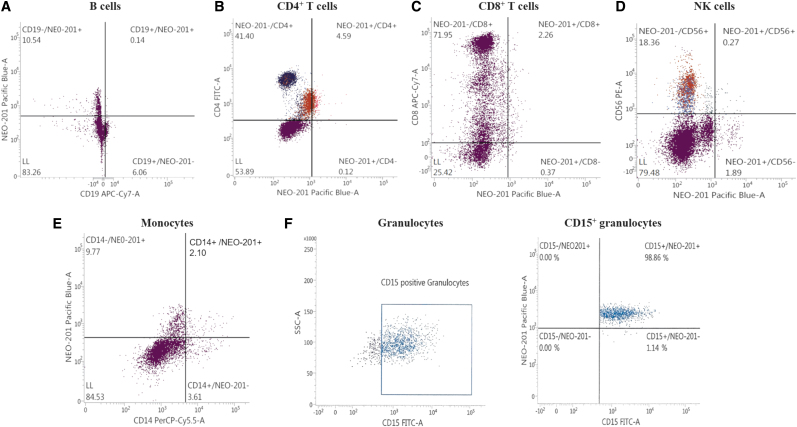
NEO-201 binds only to granulocytes in human hematopoietic cells
Flow cytometry analysis was used to profile a panel of human hematopoietic cells for NEO-201 binding. Assessment of the binding activity of NEO-201 revealed that only human CD15+ granulocytes were highly positive for NEO-201 staining (98.86%) (Fig. 2F). In contrast, no reaction was observed with NEO-201 in the other hematopoietic subsets (B cells, CD4+ T cells, CD8+ T cells, NK cells, and monocytes) (Fig. 2A–E). These data confirmed the authors' observation in a previous study in non-human primates, in which a transient decrease in neutrophils was the only adverse effect observed.21
FIG. 2.
Flow cytometry of NEO-201 binding to human hematopoietic cells. PBMCs were analyzed by flow cytometry for the expression of B cells, T cells, NK cells, monocytes, and granulocytes markers. Hematopoietic subsets were also tested for the expression of the NEO-201 antigen. (A) B lymphocyte antigen CD19 was used as marker to phenotype B cells, (B) CD4 was used as marker to phenotype CD4+ T cells, (C) CD8 was used as marker to phenotype CD8+ T cells, and (D) CD56 was used as marker to phenotype NK cells. (E) CD14 was used as marker to phenotype monocytes and (F) CD15 was used as marker to phenotype granulocytes. Data are presented as percentage of viable cells expressing the cell surface proteins. NEO-201 positivity was defined as % positive ≥10%. Positivity was determined by using fluorescence-minus-one controls. PBMC, peripheral blood mononuclear cell.
NEO-201 enhances NK-92 cell cytotoxicity against CEACAM5+/NEO-201+ tumor cells
The authors first analyzed a panel of human carcinoma cells lines using flow cytometry to determine the level of expression of CEACAM1, and the co-staining for CEACAM5 and NEO-201 antibodies (Table 1). ASPC-1 cell line had a high expression of CEACAM1 (61.15%), but a very low expression of CEACAM5+/NEO-201+ cells (9.26%). The percentage of CEACAM5+/NEO-201+ and CEACAM1+ cells was low (26.95% and 18.67%, respectively) in CFPAC-1 cell line. A low percentage of CEACAM5+/NEO-201+ cells (26.84%) was also observed in LS174T cell line. LS174T resulted also negative for CEACAM1 expression (Table 1). Conversely, BxPC-3 cell line showed the highest percentage of CEACAM5+/NEO-201+ cells (58.52%) compared to all other cell lines, while no reaction was observed with the anti-CEACAM1 mAb.
Table 1.
Flow Cytometry Analysis of Reactivity of Anti-CEACAM1, Anti-CEACAM5, and NEO-201 mAbs Against Cultured Tumor Cell Lines Derived from Various Types of Solid Tumors
| Cell line |
CEACAM1+ |
CEACAM5+/NEO-201+ |
|---|---|---|
| % positive (MFI) | ||
| ASPC-1 | 61.15 (707) | 9.26 (869/9078) |
| BxPC-3 | 2.45 (1471) | 58.52 (1447/6420) |
| CFPAC-1 | 18.67 (1938) | 26.95 (1108/1728) |
| LS174T | 2.43 (3287) | 26.84 (1030/858) |
ASPC-1, BxPC-3, and CFPAC-1 are human pancreatic carcinoma cell lines. LS174T is a human colorectal carcinoma cell line.
The percentage of positive cells and median fluorescence intensity (MFI) values are detailed for each cell line. Positive marker expression appears in bold text, where positivity was defined as % positive >10%.
CEACAM, carcinoembryonic antigen-related cell adhesion molecule; mAb, monoclonal antibody.
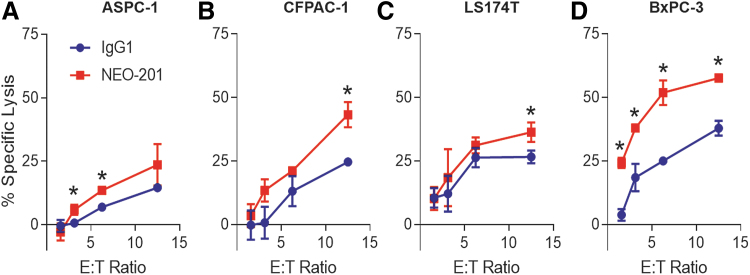
These four cell lines were used as target cells in the NK-92 cytotoxicity assay with or without NEO-201 as blocking antibody to investigate the ability of NEO-201 to block the binding between CEACAM5 on tumor cells and CEACAM1 on NK cells. As shown in Figure 3A, the addition of NEO-201 significantly enhanced the NK-92 killing against ASPC-1 cell line (highly CEACAM1+/very low CEACAM5+/NEO-201+) at 3.125 and 6.25 E:T ratios, with 8.9-fold increase and 1.97-fold increase respectively, compared to IgG1 isotype control antibody. Similar results were also obtained using CFPAC-1 (low CEACAM1/low CEACAM5+/NEO-201+) and LS174T (low CEACAM5+/NEO-201+/CEACAM1−) cell lines as targets. In both cell lines, the addition of NEO-201 enhanced NK-92 killing only at the highest E:T ratio (12.5:1) compared to IgG1 isotype control antibody (CFPAC-1, 1.75-fold increase and LS174T 1.36-fold increase) (Fig. 3B, C).
FIG. 3.
NEO-201 enhances NK-92 cell cytotoxicity against CEACAM5+/NEO-201+ tumor cells. Representation of the non-ADCC NK cell cytotoxicity assays using human pancreatic carcinoma cell lines ASPC-1 (A), CFPAC-1 (B), and BxPC-3 (D), and the human colorectal carcinoma cell line LS174T (C) as target cells. NEO-201 and human IgG1 (negative control) were used at a concentration of 10 μg/mL. NK-92 cells were used as effector cells at the following E:T ratios: 1.56:1, 3.12:1, 6.25:1, and 12.5:1. Results are presented as mean ± SEM from 3 replicate wells. Asterisks denote statistical significance of NK-92+NEO-201 versus NK-92+IgG1 (T-test). *p < 0.05. ADCC, antibody-dependent cellular cytotoxicity; E:T, effector-to-target; SEM, standard error of the mean.
On the other hand, the addition of NEO-201 resulted in a greater enhancement of NK-92 killing against BxPC-3 cell line (high CEACAM5+/NEO-201+/CEACAM1−) at all E:T ratios compared to IgG1 isotype control antibody (Fig. 3D). These data demonstrate that NEO-201 can significantly enhance NK-92 killing only in carcinoma cell lines that express high levels of CEACAM5+/NEO-201+ cells and indirectly provided evidence that NEO-201 can block the binding between CEACAM5 on tumor cells and CEACAM1 on NK92 cells.
NEO-201-mediated enhancement of NK-92 killing is directly correlated with the level of CEACAM5+/NEO-201+ cells and is independent from the level of expression of CEACAM6 on tumor cells
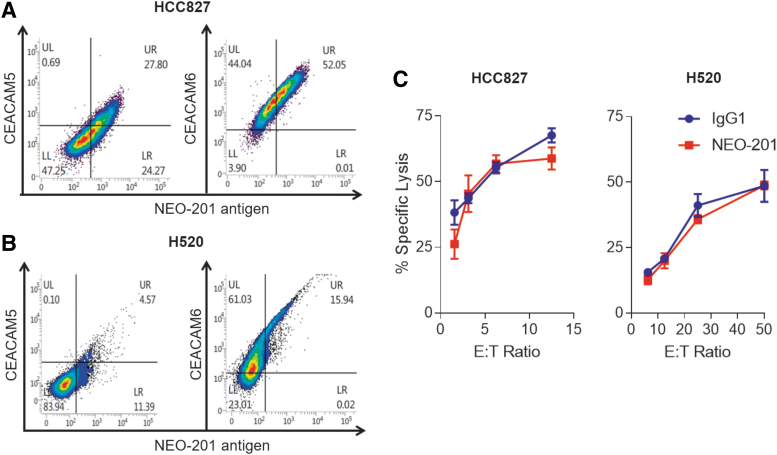
To ascertain that NEO-201-mediated enhancement of NK-92 killing is directly correlated with levels of CEACAM5+/NEO-201+ cells and that this phenomenon is independent from the expression of the tumor variant of CEACAM6 recognized by NEO-201 (CEACAM6+/NEO-201+ cells), two additional human lung carcinoma cell lines (HCC827 and H520) were used as targets in NK-92 cytotoxicity assay.
As shown in Figure 4A, HCC827 cell line expresses a low level of CEACAM5+/NEO-201+ cells (27.80%) and a high level of CEACAM6+/NEO-201+ cells (52.05%). Low level of CEACAM6+/NEO-201+ cells (15.94%) and no CEACAM5+/NEO-201+ cells were found in the H520 cell line (Fig. 4B). The authors also observed that the addition of NEO-201 does not enhance NK-92 killing against these two carcinoma cell lines and that there is no correlation between the levels of CEACAM6+/NEO-201+ cells and NEO-201-mediated enhancement of NK-92 killing (Fig. 4C).
FIG. 4.
Blocking with NEO-201 enhances NK-92 killing and is directly correlated with the level of CEACAM5+/NEO-201+ cells. Figure represents additional flow cytometry analysis and non-ADCC NK cell cytotoxicity assays performed on two human lung carcinoma cell lines. HCC827 (A) and H520 (B) cells were analyzed by flow cytometry for the expression of CEACAM5, CEACAM6, and NEO-201 antigen. Data are presented as percentage of viable cells expressing the cell surface proteins. Positivity was determined by using fluorescence-minus-one controls. Non-ADCC NK cell cytotoxicity assays (C) were performed using human lung carcinoma cell lines HCC827 and H520 as target cells. NEO-201 and human IgG1 (negative control) were used at a concentration of 10 μg/mL. NK-92 cells were used as effector cells and added at the following E:T ratios: 1.56:1, 3.12:1, 6.25:1, and12.5:1. Results are presented as mean ± SEM from three replicate wells.
These data confirmed results achieved with ASPC-1, CFPAC-1, and LS174T cancer cell lines, suggesting that NEO-201 mAb-mediated enhancement of NK-92 killing is directly correlated with the level of CEACAM5+/NEO-201+ cells.
Discussion
NK cells play a crucial role in recognizing and suppressing the proliferation and dissemination of malignant cancer cells through the mechanism of immunoediting.26
Several studies have shown the potential value of NK cells as effectors to counteract the growth of tumor cells that have become resistant to classical immune checkpoint therapy, such as the blockage of immunological checkpoint axis PD-1/PD-L1.27–29
The cytotoxic activity of NK cells against tumor cells is modulated by the binding between activating receptors expressed on the membrane of NK cells and ligands present on tumor cells. The activating receptors include the cytotoxicity receptors (NCRs) (NKp46, NKp30, and NKp44), C-type lectin receptors (CD94/NKG2C, NKG2D, NKG2E/H, and NKG2F), and killer cell immunoglobulin-like receptors (KIRs) (KIR-2DS and KIR-3DS).30
One of the pathways that leads to the activation of NK cells against target cells is the NKG2D/NKG2D ligand (NKG2D-L) pathway.31,32 NKG2D is an activating receptor that binds several stress-induced NKG2D-Ls widely expressed on tumor cells, such as MICA/B, and UL16-binding proteins 1–6 (ULBP1–6).33 This interaction induces NK cell activation and allows NK cells to directly kill tumor cells.31,32
Unfortunately, cancer cells have developed several mechanisms to inhibit NK activation. One of those mechanisms is the binding between ligands, expressed on cancer cells, and NK inhibitory receptors, such as C-type lectin receptors (CD94/NKG2A/B) and KIRs (KIR-2DL and KIR-3DL), expressed on NK cells.30
For example, the interaction between NKG2A-CD94, expressed on NK cells, and HLA-E, the noncanonical MHC class I molecule expressed on cancer cells, suppresses the NK cell activation signaling pathway and allows to cancer cells to evade from NK-mediated killing.29,30
Another mechanism that tumor uses to suppress NK cytotoxicity is the interaction between CEACAM1 expressed on NK cells and CEACAM1 or CEACAM5 expressed on tumor cells.
In a study performed on human melanoma cells, Markel et al. observed that a direct interaction between the CEACAM1 (CD66a) proteins expressed on both NK cells and tumor cells lead to inhibition of NK cell cytotoxicity.34 In addition, both NK cells and melanoma cells derived from lymph nodes of melanoma patients were found to have a dramatic increase of CEACAM1 expression and this phenomenon correlated with the poor prognosis of the melanoma patients. It is important to note that no increase of CEACAM1 was detected on NK cells derived from healthy donors or from melanoma patients with no detectable disease. These data suggested that tumor evades NK killing, promoting CEACAM1-CEACAM1 interactions between NK cells and cancer cells either through the increase of CEACAM1 expression on their surface or through the enhancement of CEACAM1 expression on NK cells.34 Other studies confirmed this hypothesis, showing that the increased expression of CEACAM1 on primary human NK cells inhibits the ability of NKG2D to stimulate cytolysis of CEACAM1-bearing cells,17 and that the overexpression of CEACAM1 on tumor cells results in the intracellular retention of various NKG2D ligands in human tumor cells.35 These mechanisms allow tumor cells to escape from immune surveillance and increase the risk for cancer patients to develop metastasis and have a poor prognosis.36–39
The binding between CEACAM1 on NK cells and CEACAM5 on cancer cells was also found to play a role in the suppression of cancer cell killing by NK cells. CEACAM5 is overexpressed in a wide range of human carcinomas, including colorectal, gastric, pancreatic, non-small cell lung, and breast carcinomas and plays a role in several biological mechanisms involved in cancer progression such as cell adhesion, intracellular and intercellular signaling, angiogenesis, and metastasis.12,40
Stern et al. proved that the heterophilic interaction between CEACAM5 and CEACAM1 inhibits the killing of NK cells that express the CEACAM1 protein, suggesting for the first time a role of CEACAM5 in enabling the escape of tumor cells from NK-mediated killing.41
Several mAbs against CEACAM5 developed in the last decades have been found to be effective in diagnosis and therapy of multiple types of cancers,42–45 but the availability of anti-CEACAM5 mAbs able to interfere with the CEACAM1-CEACAM5 interaction is very poor. In this regard, Zheng et al. reported for the first time the antitumor activity of a anti-CEACAM5 mAb, namely CC4.46 This mAb significantly suppresses cell proliferation, migration, and aggregation of colorectal cancer cells in vitro and in vivo not only through ADCC but also through the enhancement of NK cytotoxicity by blocking intercellular interaction between CEACAM5 on cancer cells and CEACAM1 on NK cells.46
Recently, the authors have demonstrated that NEO-201 reacts specifically to TAAs expressed on a wide range of human cancer cells and tumor tissues, but not with healthy normal tissues.21 In addition, NEO-201 has been proved to have both ADCC and CDC activity against human carcinoma cells in vitro, and to have the capacity to counteract the growth of human pancreatic xenograft tumors in vivo.21,22
Preliminary studies indicated also that NEO-201 may recognize tumor-associated variants of CEACAM5 and CEACAM6 on tumor cells, although further detailed studies are still ongoing.19,20
To confirm that NEO-201 recognizes specifically cancer cells, in this study, the authors showed that NEO-201 binds only to CD15+ granulocytes, but not to the other hematopoietic subsets (Fig. 2). These data suggest that the only adverse effect in a clinical administration of NEO-201 could be neutropenia.
These findings confirm what the authors observed in a previous study on non-human primates, in which the main laboratory change in blood counts was a decrease in neutrophil counts. However, the neutropenia was transient and neutrophil counts recovered nearly totally or partially 15 days after NEO-201 administration in the majority of animals.21 No infusion reactions were observed in non-human primates in conjunction with the transient neutropenia.
Non-ADCC NK cell cytotoxicity assays, employing NK-92 cells, showed also that NEO-201 enhances NK-92 cell cytotoxicity against CEACAM5+/NEO-201+ carcinoma cells. The authors observed that NEO-201 significantly enhanced the NK-92 cell cytotoxicity at all E:T ratio only against BxPC-3 cell line, which is the cell line that expresses the highest percentage of CEACAM5+/NEO-201+ cells and is CEACAM1 negative. NEO-201 activity decreased in carcinoma cell lines with a lower level of CEACAM5+/NEO-201+ cells, suggesting that NEO-201-mediated enhancement of NK-92 killing is directly correlated with the level of CEACAM5+/NEO-201+ cells.
Additional non-ADCC NK cell cytotoxicity assays, using human lung carcinoma cells as targets, confirmed this observation and showed also that CEACAM6 is not involved in this mechanism.
Conclusion
This study provides evidence that NEO-201-mediated enhancement of NK-92 killing is directly correlated with the level of CEACAM5+/NEO-201+ cells. Indeed, the binding between NEO-201 and the tumor variant of CEACAM5 can block the interaction between CEACAM5 on tumor cells and CEACAM1 on NK cells to reverse CEACAM1-dependent inhibition of NK cytotoxicity. These results suggest that NEO-201 may potentially reverse CEACAM1-dependent immunosuppression of NK cells in patients whose tumors express high levels of the NEO-201-reactive variant of CEACAM5.
It is important to note that the tumor in situ could express different levels of CEACAM5 compared to carcinoma cells lines in vitro. This variation due to heterogeneity of tumor and the role of the soluble CEACAM5 in affecting the passive immunotherapy with NEO-201 in cancer patients will be evaluated in correlative studies from the ongoing open-label, first-in-human, phase 1, dose escalation study of the mAb NEO-201 in adults with solid tumors, which have not responded to standard treatments.47
Authors' Contributions
Conception and design: M.F., J.M.D., P.M.A., and K.Y.T.; Development of methodology: M.F., J.M.D., and K.Y.T.; Acquisition of data: M.F. and J.M.D.; Analysis and interpretation of data: M.F., J.M.D., C.M.A., M.P.M., P.M.A., and K.Y.T.; Writing, review, and revision of article: M.F., J.M.D., C.M.A., M.P.M., P.M.A., and K.Y.T.; Administrative, technical, and material support: P.M.A. and K.Y.T.; Study supervision: P.M.A. and K.Y.T.
Disclosure Statement
M.F., J.M.D., P.M.A., and K.Y.T. are employees of Precision Biologics, Inc., P.M.A. has ownership interest in Precision Biologics, Inc. C.M.A. and M.P.M. have no conflicts of interest to declare.
Funding Information
This research was funded by Precision Biologics, Inc.
References
- 1. Yang Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J Clin Invest 2015;125:3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 2011;7:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campoli M, Ferris R, Ferrone S, et al. Immunotherapy of malignant disease with tumor antigen-specific monoclonal antibodies. Clin Cancer Res 2010;16:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Day SJ, Maio M, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann Oncol 2010;21:1712. [DOI] [PubMed] [Google Scholar]
- 6. Kline J, Gajewski TF. Clinical development of mAbs to block the PD1 pathway as an immunotherapy for cancer. Curr Opin Investig Drugs 2010;11:1354. [PubMed] [Google Scholar]
- 7. Kuespert K, Pils S, Hauck CR. CEACAMs: Their role in physiology and pathophysiology. Curr Opin Cell Biol 2006;18:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonsor DA, Günther S, Beadenkopf R, et al. Diverse oligomeric states of CEACAM IgV domains. Proc Natl Acad Sci U S A 2015;112:13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tchoupa AK, Schuhmacher T, Hauck CR. Signaling by epithelial members of the CEACAM family—Mucosal docking sites for pathogenic bacteria. Cell Commun Signal 2014;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blumenthal RD, Hansen HJ, Goldenberg DM. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res 2005;65:8809. [DOI] [PubMed] [Google Scholar]
- 11. Blumenthal RD, Leon E, Hansen HJ, et al. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer 2007;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turriziani M, Fantini M, Benvenuto M, et al. Carcinoembryonic antigen (CEA)-based cancer vaccines: Recent patents and antitumor effects from experimental models to clinical trials. Recent Pat Anticancer Drug Discov 2012;7:265. [DOI] [PubMed] [Google Scholar]
- 13. Johnson B, Mahadevan D. Emerging role and targeting of carcinoembryonic antigen-related cell adhesion molecule 6 (CEACAM6) in human malignancies. Clin Cancer Drugs 2015;2:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao Y, Wang J, Zhou Y, et al. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep 2018;8:2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Cai P, Li L, et al. Co-expression of TIM-3 and CEACAM1 promotes T cell exhaustion in colorectal cancer patients. Int Immunopharmacol 2017;43:210. [DOI] [PubMed] [Google Scholar]
- 16. Yu H, Yu J, Ren Y, et al. Serum CEACAM1 level is associated with diagnosis and prognosis in patients with osteosarcoma. PLoS One 2016;11:e0153601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosomi S, Chen Z, Baker K, et al. CEACAM1 on activated NK cells inhibits NKG2D-mediated cytolytic function and signaling. Eur J Immunol 2013;43:2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helfrich I, Singer BB. Size matters: The functional role of the CEACAM1 isoform signature and its impact for NK cell-mediated killing in melanoma. Cancers (Basel) 2019;11:E356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeligs K, Arlen PM, Tsang K, et al. Abstract 3025: Preclinical characterization of a novel monoclonal antibody targeting a neo-antigen expressed in ovarian and GI malignancies. Cancer Res 2017;77:3025 [Google Scholar]
- 20. David JM, Fantini M, Annunziata CM, et al. Abstract 3821: The neoantigen-targeting antibody NEO-201 enhances NK cell-dependent killing of tumor cells through blockade of the inhibitoryCEACAM5/CEACAM1 immune checkpoint pathway. AACR Annual Meeting. April 14–18, 2018. Chicago, Illinois, USA: Proceedings of the American Association for Cancer Research 2018;59:980 [Google Scholar]
- 21. Fantini M, David JM, Saric O, et al. Preclinical characterization of a novel monoclonal antibody NEO-201 for the treatment of human carcinomas. Front Immunol 2018;8:1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fantini M, David JM, Wong HC, et al. An IL-15 superagonist, ALT-803, enhances antibody-dependent cell-mediated cytotoxicity elicited by the monoclonal antibody NEO-201 against human carcinoma cells. Cancer Biother Radiopharm 2019;34:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tonn T, Becker S, Esser R, et al. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res 2001;10:535. [DOI] [PubMed] [Google Scholar]
- 24. Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol 2013;4:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jochems C, Hodge JW, Fantini M, et al. ADCC employing an NK cell line (haNK) expressing the high affinity CD16 allele with avelumab, an anti-PD-L1 antibody. Int J Cancer 2017;141:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329. [DOI] [PubMed] [Google Scholar]
- 27. Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007;7:329. [DOI] [PubMed] [Google Scholar]
- 28. Berrien-Elliott MM, Romee R, Fehniger TA. Improving natural killer cell cancer immunotherapy. Curr Opin Organ Transplant 2015;20:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z, Yang Y, Liu LL, et al. Strategies to augment natural killer (NK) cell activity against solid tumors. Cancers (Basel) 2019;11:E1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng M, Chen Y, Xiao W, et al. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol 2013;10:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frazao A, Rethacker L, Messaoudene M, et al. NKG2D/NKG2-ligand pathway offers new opportunities in cancer treatment. Front Immunol 2019;10:661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duan S, Guo W, Xu Z, et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer 2019;18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zingoni A, Molfetta R, Fionda C, et al. NKG2D and its ligands: “One for All, All for One.” Front Immunol 2018;9:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Markel G, Lieberman N, Katz G, et al. CD66a interactions between human melanoma and NK cells: A novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol 2002;168:2803. [DOI] [PubMed] [Google Scholar]
- 35. Chen Z, Chen L, Baker K, et al. CEACAM1 dampens antitumor immunity by down-regulating NKG2D ligand expression on tumor cells. J Exp Med 2011;208:2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi JF, Xu SX, He P, et al. Expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) and its correlation with angiogenesis in gastric cancer. Pathol Res Pract 2014;210:473. [DOI] [PubMed] [Google Scholar]
- 37. Ling Y, Wang J, Wang L, et al. Roles of CEACAM1 in cell communication and signaling of lung cancer and other diseases. Cancer Metastasis Rev 2015;34:347. [DOI] [PubMed] [Google Scholar]
- 38. Wang FF, Guan BX, Yang JY, et al. CEACAM1 is overexpressed in oral tumors and related to tumorigenesis. Med Mol Morphol 2017;50:42. [DOI] [PubMed] [Google Scholar]
- 39. Dankner M, Gray-Owen SD, Huang YH, et al. CEACAM1 as a multi-purpose target for cancer immunotherapy. Oncoimmunology 2017;6:e1328336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 2013;32:643. [DOI] [PubMed] [Google Scholar]
- 41. Stern N, Markel G, Arnon TI, et al. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol 2005;174:6692. [DOI] [PubMed] [Google Scholar]
- 42. Govindan SV, Cardillo TM, Moon SJ, et al. CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res 2009;15:6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schoffelen R, Boerman OC, Goldenberg DM, et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: First clinical results. Br J Cancer 2013;109:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dotan E, Cohen SJ, Starodub AN, et al. Phase I/II trial of labetuzumab govitecan (anti-CEACAM5/SN-38 antibody-drug conjugate) in patients with refractory or relapsing metastatic colorectal cancer. J Clin Oncol 2017;35:3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dong W, Shi J, Yuan T, et al. Antibody-drug conjugates of 7-ethyl-10-hydroxycamptothecin: Sacituzumab govitecan and labetuzumab govitecan. Eur J Med Chem 2019;167:583. [DOI] [PubMed] [Google Scholar]
- 46. Zheng C, Feng J, Lu D, et al. A novel anti-CEACAM5 monoclonal antibody, CC4, suppresses colorectal tumor growth and enhances NK cells-mediated tumor immunity. PLoS One 2011;6:e21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. ClinicalTrials.gov (2019. QUILT-3.017: Study of NEO-201 in Solid Tumors. Identifier NCT03476681. Online document at https://clinicaltrials.gov/ct2/show/NCT03476681 Accessed on September12, 2019