Abstract
Breast cancer is the second-leading cause of metastatic disease in the central nervous system (CNS). Recent advances in the biological understanding of breast cancer have facilitated an unprecedented increase of survival in a subset of patients presenting with metastatic breast cancer. Patients with HER2 positive (HER2+) or triple negative breast cancer are at highest risk of developing CNS metastasis, and typically experience a poor prognosis despite treatment with local and systemic therapies. Among the obstacles ahead in the realm of developmental therapeutics for breast cancer CNS metastasis is the improvement of our knowledge on its biological nuances and on the interaction of the blood–brain barrier with new compounds. This article reviews recent discoveries related to the underlying biology of breast cancer brain metastases, clinical progress to date and suggests rational approaches for investigational therapies.
Key words: breast cancer, brain, targeted therapy, HER2, anti-angiogenesis, immunotherapy
Introduction
In 2016, approximately 246 660 women will be diagnosed with breast cancer within the USA [1]. A population based study of 51, 898 women with breast cancer observed a metastatic incidence of 5.1% involving the central nervous system (CNS) irrespective of initial stage at diagnosis. However, patients presenting with metastatic breast cancer (MBC) have an estimated incidence proportion of 14% of CNS involvement [2]. MBC to the CNS is associated with both clinical decline, as well as increased cost of patient care [3, 4]. Prior to the advent of modern radiation and targeted therapies, patients presenting with MBC in the CNS had a median overall survival ranging from 4 to 5.5 months [5, 6]. Recent advances in radiation therapy techniques and targeted therapies led to some improvement in outcomes (Supplement File 1, available at Annals of Oncology online).
Supplementary Data.

Patients with metastatic HER2+ primary cancer have a risk of 35–50% to develop future metastatic brain disease and the risk CNS as first site of disease recurrence among patients receiving adjuvant trastuzumab treatment is 2.5% [7., 8., 9., 10.]. Similarly, metastatic triple negative breast cancer (TNBC) also harbors an increased probability of CNS involvement with an estimated risk as high as 46% [11]. Other features associated with increased risk of CNS involvement include the expression of cytokeratins 5/6 and 14, epidermal growth factor receptor (EGFR) and p53 [5, 12., 13., 14., 15.].
The efficacy of treatment for patients with CNS metastasis has not advanced at the same rate as for patients without CNS involvement. Those patients remain an underrepresented population in early phase clinical trials as a function of concomitant poor performance status and presumed lack of efficacy in the treatment of intracranial lesions with therapies primarily directed towards extracranial disease [16]. Clinical trial design has also been challenged by the need for concomitant incorporation of measures of effect and endpoints for both intracranial and extracranial metastatic disease in trials for MBC with CNS metastasis. Furthermore, biomarker based drug development has been hampered by the obvious challenge of breast cancer heterogeneity and poorly understood biological pressures imposed by the CNS cellular landscape (Supplement File 2, available at Annals of Oncology online).
The blood–brain barrier
Chemotherapy was originally considered to possess minimal effects in patients with CNS metastatic tumor given the understanding that hydrophilic drugs and/or large molecules do not cross the blood-brain barrier (BBB). This was based on the expression of P-glycoprotein, which is present on the endothelial cells of the BBB and contribute to transporting chemotherapeutic agents out of the CNS [17]. While the role of P-glycoprotein in blocking entry into the CNS remains unclear, the expression in the neovasculature of metastatic brain tumors is much decreased when compared to primary brain tumors that have higher P-glycoprotein expression in their newly formed vessels [18]. Furthermore, metastatic CNS tumors possess an abnormal vascular supply that is dependent, in part, on the fenestrated neovasculature [19, 20]. Other drug efflux transporters in the CNS have been described specifically the multidrug resistance transporter, and various organic anion transporters, such as multidrug resistance-associated proteins, organic anion transporter polypeptides, and organic anion transporters [21]. These drug efflux transporters have also been implicated in the transport of chemotherapy agents [22, 23]. Also crossing of the BBB is not homogenous throughout the MBC brain tumor as in one prospective study metastatic brain tumors had differential paclitaxel concentrations in the tumor center and periphery [24].
Taken together, the evidence suggests that drug BBB permeability is most likely a function of not only P-glycoprotein expression but also the interplay of molecule size, charge, lipophilicity, tumor neovasculature anatomy and plasma protein binding [25]. Furthermore accurate model of not only BBB penetrance but also tumor tissue penetrance is necessary as prospective study of four patients taking lapatinib pre-operatively showed a high variability (0.19–9.8 μM) of lapatinib MBC CNS metastasis-to-serum ratio [26].
Notwithstanding to challenge imposed by the crossing of the BBB new strategies continue to evolve. For instance the new agent ANG1005 contains the chemotherapy agent paclitaxel, which stabilizes microtubule polymer formation. This blocks the progression of mitosis due to a prolonged activation of the microtubule in the mitotic checkpoint, resulting in cell apoptosis or reversion to the GO phase [27]. However, ANG1005 effectively transports across the BBB with much higher transport rate compared to a free paclitaxel as observed in breast cancer pre-clinical model [28]. ANG1005 crosses the BBB via receptor-mediated endocytosis of the low-density lipoprotein receptor-related protein (LRP1), which is upregulated in some cancers [29]. Ester hydrolyzing enzymes then catalyze a highly stereospecific reaction that results in hydrolysis of the ANG1005 ester to carboxylic acids. This results in the intracellular release of paclitaxel and subsequent action on tubulin.
In a recent phase II trial patients (n = 72 safety population; n = 57 efficacy population) with measurable recurrent CNS involvement from breast cancer were treated with ANG1005 at 600 mg/m2 every 3 weeks intravenously. HER2+ patients were allowed to continue trastuzumab±pertuzumab [30]. Clinical benefit (best intracranial partial response + stable disease) was seen in 70% of the patients. Best intracranial response in the efficacy population included 8/57 (14%) patients with partial responses [3 (5%) confirmed PRs] and 32/57 (56%) with stable disease.
2B3-101 is a doxorubicin liposomal formulation that uses glutathione transporters on the BBB to penetrate the brain in xenografts [31]. In an open label study of single agent 2B3-101 25 patients MBC and CNS metastasis were included and received 2B3-101 at a starting dose of either 40 (n = 3) or 50 (n = 22) mg/m2 intravenously every 3 weeks, until disease progression or unacceptable toxicity [32]. Patients with HER2+ MBC were treated with concurrent trastuzumab. A 12-week progression free survival (PFS) rate of 56% was observed among in HER2+ indicating preliminary efficacy of this new compound. Notable Grade 3–4 adverse events were neutropenia (35%) and palmar plantar (13%) erythrodysesthesia.
In the realm of targeted therapies modification of small molecule inhibitors leading to increased BBB penetration could be a stepping stone for future drug development as well. Winkler et al. recently reported results of xenograft study with intact BBB in which GNE-317, a potent dual PI3K/mTOR inhibitor, designed to bypass the two main exclusion transporters (P-glycoprotein and Breast Cancer Resistance Protein) showed significant CNS metastasis inhibitory activity [33]. This was in contrast with results observed with GDC-0980, a closely related dual PI3K/mTOR inhibitor, but a substrate of P-glycoprotein and Breast Cancer Resistance Protein [34].
Potential targets
Human epidermal growth factor receptors
This family of transmembrane receptors includes four members including EGFR/HER1, HER2, HER3 and HER4, that collectively stimulate a multitude of growth factor signaling pathways [35]. Activation of receptor kinase occurs predominantly via ligand-mediated hetero- or homo-dimerization. In the case of HER2, activation is also thought to occur in a ligand-independent manner. [36]. These tyrosine kinase receptors play a key role in the regulation of cell-proliferative growth, survival, and differentiation [37]. In breast cancer HER2, the preferred dimerization partner for HER3 and EGFR, amplifies the signal generated through the dimer receptor complex [38]. HER2–HER3 heterodimers potently activate PI3K oncogenic signaling [39]. HER3 overexpression is also associated with resistance to HER2-targeted therapies in preclinical and clinical [40, 41]. The role HER4 receptors remains unclear in breast cancer biology but it has recently been associated with acquired resistance to HER2 targeted therapy [42]. Targeted strategies against the HER family have been developed in the realm of breast cancer treatment. For instance the humanized monoclonal antibodies (e.g. trastuzumab) prevent the dimerization of HER2 with other HER receptors. Pertuzumab inhibits the pairing of the most potent signaling heterodimer, HER2/HER3, thereby providing a potent strategy for dual HER2 inhibition [43]. Furthermore small molecule tyrosine kinase inhibitors such as lapatinib and neratinib have the ability to inhibit the kinase activity of these HER receptors opposing further cancer cell survival and proliferation [44, 45].
Small molecule anti-HER2
While lapatinib is considered an option for treatment of patients with HER2+ MBC with CNS involvement when combined with capecitabine [46], it has also been tested in combination with temozolomide in a phase I trial [47]. Sixteen patients with HER2+, progressive brain metastases were enrolled with 14 that had previously been treated with WBRT. For the 15 assessable patients, stable disease was achieved in 10 patients (67%) and progression of disease in five patients (33%). The most common adverse events (AEs) included fatigue, diarrhea and constipation.
Separately, a phase II trial in patients with HER2+ MBC in the brain evaluated lapatinib as a monotherapy after WBRT [48]. Thirty-nine patients were enrolled and tumor response was assessed by MRI every 8 weeks and all patients had developed brain metastases while receiving trastuzumab; 37 progressed after prior radiation while one patient achieved a partial response (PR) (objective response rate 2.6%). Taken together, these results indicate that the response rates are low after WBRT. Furthermore, in the CEREBEL trial patients with HER2+ MBC without CNS involvement were randomized to treatment with capecitabine combined with lapatinib or trastuzumab [49]. The study was underpowered and inconclusive for its primary end point (incidence of CNS as site of first relapse); the 3% and 5% rates of CNS as first site of relapse with lapatinib-capecitabine and trastuzumab-capecitabine, respectively, were far lower than the expected rates of 12% and 20%, respectively. PFS and OS were longer with trastuzumab-capecitabine versus lapatinib-capecitabine.
Lapatinib (750 mg twice on day one followed by 1000, 1250, or 1500 mg once daily) has also been investigated as a radiosensitizer in a phase I trial of 27 patients with HER2+ breast cancer and ≥1 brain metastasis [50]. WBRT (37.5 Gy, 15 fractions) was administered on days 1–8 after beginning lapatinib treatment. Overall, 7/27 patients treated with 1250 mg (maximum tolerated dose) were associated with dose limiting toxicities including grade III rash (n = 2), diarrhea (n = 2), hypoxia (n = 1) and a grade IV pulmonary embolus (n = 2). Among 28 evaluable patients, the CNS objective response rate was 79% by pre-specified volumetric criteria and 46% remained progression-free (CNS or nonCNS) at 6 months. However the study did not meet pre-defined criteria for feasibility due to the high level of toxicity.
In summary, when considering lapatinib for CNS metastasis for patients with low burden CNS disease, data suggest it is most effective when combined with capecitabine, and in patients who are WBRT naïve.
Neratinib is an irreversible inhibitor of EGFR, HER2, and HER4 with promising activity in HER2+ breast cancer with preclinical data suggesting that it crosses the BBB. Also, secondary endpoint analysis of a phase III trial assessing the efficacy paclitaxel combined with neratinib compared with paclitaxel-trastuzumab combination showed delayed time to CNS metastases (HR, 0.45; 95% CI, 0.26–0.78) favoring paclitaxel-neratinib combination [51].
Neratinib has also been evaluated in patients with HER2+ MBC with brain involvement in a phase II trial and was given at a dose of 280 mg by mouth once daily on 28-day cycles to 40 patients with 85% patients having previously received lapatinib and 75% WBRT [52]. Three women experienced a response (CNS ORR = 7.5%; 95% CI 2–27%) with the most common grade 3 or higher event associated with diarrhea (25%), indicating a low anti-tumor efficacy and increased toxicity. Other small molecule HER2-directed tyrosine kinase inhibitors are under development (Table 1).
Table 1.
On going targeted therapy clinical trials for breast cancer with brain metastasis
| Agent | Phase of study | Target | Clinicaltrial.gov Identification number |
|---|---|---|---|
| Lapatinib/WBRT | II | HER2 | NCT01622868 |
| ARRY-380/trastuzumab | I | HER2 | NCT01921335 |
| Bevacizumab/etoposide/cisplatin followed by WBRT | II | VEGF | NCT02185352 |
| ITC trastuzumab/ITC pertuzumab | I | HER2 | NCT02598427 |
| Cabozantinib/trastuzumab | II | HER2 | NCT02260531 |
| Lapatinib/everolimus/capecitabine | I/II | HER2/EGFR/mTOR | NCT01783756 |
| Pertuzumab/trastuzumab | II | HER2/EGFR | NCT02536339 |
| Intermittent lapatinib tandem capecitabine | I | HER2/EGFR | NCT02650752 |
| GRN1005/Trastuzumab | II | HER2 | NCT01480583 |
| Lapatinib/WBRT followed by trastuzumab | I | HER2/EGFR | NCT00470847 |
| Bevacizumab plus cisplatin/etoposide | II | VEGF-A | NCT01281696 |
| Iniparib | II | PARP | NCT01173497 |
| Sorafenib/WBRT | II | VEGFR/PDGFR/Raf | NCT01724606 |
| Lapatinib/temozolomide | I | HER2 | NCT00614978 |
| Capecitabine and buparlisib | II | PI3K | NCT02000882 |
| Tremelimumab WBRT or SRS | II | Immune checkpoint blockade | NCT02563925 |
| Neratinib and capecitabine | II | HER2/EGFR | NCT01494662 |
| Bevacizumab plus carboplatin | II | VEGF | NCT01004172 |
| Afatinib plus venorelbine | II | HER2/EGFR | NCT01441596 |
| Palbociclib | II | CDK4/6 | NCT02774681 |
| Abemacicliba | II | CDK4 | NCT02308020 |
| Durvalumaba | II | Immune checkpoint blockade | NCT02669914 |
| Vorinostata | I | Histone deacetylase | NCT00838929 |
| Bevacizumaba | II | VEGF | NCT01898130 |
Accessed on 5 July 2016 at www.clinicaltrials.gov.
WBRT, whole brain radiation therapy; SRS, stereotactic radiosurgery, ITC, intrathecal.
Allows for of other histological types with brain involvement other than breast cancer.
Anti-HER2 monoclonal antibodies
HER2 targeted monoclonal antibodies such as trastuzumab were once thought not to cross the BBB. Recent data support that metastatic brain lesions can be visualized by Cu-DOTA-trastuzumab indicating that trastuzumab passes through the BBB [53]. The prospective observational registHER trial demonstrated continuation of trastuzumab after intracranial metastases to be associated with improved survival. The median survival for patients who received trastuzumab subsequent to CNS diagnosis was 17.5 months compared with 3.7 months for patients who did not [8].
Results from the CLEOPATRA trial in HER2+, first-line treatment of MBC demonstrated significant improvement in overall survival with pertuzumab over placebo in addition to trastuzumab-docetaxel treatment [54]. An exploratory analyses of the incidence and time to development of CNS metastases in patients from CLEOPATRA suggest that pertuzumab added to trastuzumab and docetaxel delays the onset of CNS disease compared with the control arm, as well as an OS trend in favor of pertuzumab combined with trastuzumab and docetaxel in patients who developed CNS metastases as the first site of disease progression [55].
Chargari et al. recently reported results of a prospective trial with 31 patients presenting HER2+ metastatic breast cancer in the brain and treated with WBRT and trastuzumab [56]. The patients received trastuzumab [2 mg/kg weekly (n = 17) or 6 mg/kg every 21 days n= 14)]. In 26 patients, concurrent WBRT delivered 30 Gy over 10 daily fractions. In 6 patients, alternative fractionation schedules were chosen due to poor performance or patient convenience. After WBRT, radiologic responses were observed in 23 patients (74.2%), including 6 (19.4%) with a complete radiologic response and 17 (54.8%) with a partial radiologic response. No grade 2 or greater toxicity was observed indicating that trastuzumab can be safely combined with WBRT.
In an ongoing phase II trial (PATRICIA), patients with HER+ MBC and CNS metastases, receive pertuzumab in combination with high-dose trastuzumab. Pertuzumab is given as 840 mg during the first intravenous infusion, followed every 3 weeks thereafter by a standard dose of 420 mg, while trastuzumab is given as 6 mg/kg once weekly intravenously [57].
HER2-directed antibody drug conjugates
Animal models suggest that trastuzumab emtansine (T-DM1) is active against HER2+ metastatic breast cancer in the brain [58]. Jacot et al. recently reported results of a retrospective study of 17 patients with HER2+ MBC with brain involvement and treated with T-DM1 at a standard dose of 3.6 mg/kg every 21 days with five PR (29.4%) and 35.3% disease stabilization for a total 64.7% of patients with clinical benefit (median PFS 5.5 months) [59]. Similarly, Ferrario et al. reported results of phase Ib trial of ONT380, an oral HER2-specific inhibitor in combination with T-DM1 where eight patients had evaluable CNS metastatic disease and 5/8 (63%) showed clinical benefit from the combination therapy [60]. In light of the high risk for CNS involvement among patients with HER2+ MBC, there are many trials being developed that target MBC with CNS metastases (Table 1).
Epidermal growth factor receptor
EGFR expression in brain metastases specimens from 30 patients has been evaluated by immunohistochemistry (IHC) [61]. The prevalence of EGFR expression on MBC brain tumor is as high as 40% with similar results replicated by others [62]. In TNBC cell lines overexpressing the EGFR receptor, cetuximab is effective [63]. To follow-up, 181 patients with triple negative breast cancer randomized in a phase II trial and receiving no more than six cycles of cisplatin plus cetuximab or cisplatin alone, the extracranial ORR was 20% and 10%, respectively. Cisplatin plus cetuximab also resulted in a longer PFS when compared with cisplatin alone (median, 3.7 versus1.5 months; hazard ratio, 0.67; 95% CI, 0.47–0.97; P = 0.032) [64]. This study provides evidence suggesting that EGFR could be a valid target in a subset of patients with TNBC. Baird et al. recently reported results of a phase I expansion trial of S-222611 at 800 mg daily, a reversible inhibitor of EGFR and HER2 in solid tumors overexpressing HER2 and or EGFR, including HER2+ MBC to the brain [65]. Six patients had HER2+ MBC with CNS disease and two prolonged stable diseases (≥6 months).
Human epidermal growth factor receptor 3 (HER3)
Da Silva et al. reported the results from 39 matched pairs of primary breast cancers and brain metastases, 22 unmatched brain metastases of breast cancer, 11 nonbreast brain metastases and 6 autopsy cases of patients with breast cancer metastases to multiple sites for select gene mutations and RNA expression [66]. HER3 expression was positive by IHC in 22/37 (59%) of the matched brain metastases patient specimens and 13/21 (62%) of the unmatched breast cancer brain, in contrast to the primary breast tumor, which showed HER3 expression in 11/37 (29.7%). In addition, HER3-associated targets were ubiquitously phosphorylated in matched brain metastases [(HER3 64%), (AKT 86%), (ERK1/2 97%), (JNK1/2 91%), (ERK5 97%)] and unmatched brain metastases from breast cancer [(HER3 85%), (AKT71%), (ERK1/2 95%), (JNK1/2 90%), (ERK5 95%)]. HER3 mRNA levels showed an increase ranging from 1.1- to 5.8-fold change in the matched brain metastases compared to primary breast cancer tissue. Interestingly, heregulin induced breast cancer cell transmigration across a tight barrier of primary human brain microvascular endothelia that was dependent on HER3 activity was abrogated by combinatorial HER2–HER3 blockade with trastuzumab and EV20 [67].
Poly(adenosine diphosphate–ribose) polymerase (PARP)
In TNBC, deregulation of BRCA1, a protein with critical roles in the homologous recombination-dependent DNA-repair pathway, has been attributed to a number of mechanisms, including BRCA1-promoter methylation and overexpression of the negative regulators ID4 and HMG [68., 69., 70.]. Other defects in homologous-recombination pathways have been implicated in the tumorigenesis of TNBC (including aberrations in MRE11–RAD50–NBS1, ATM, p53, and PALB2) [71., 72., 73.]. Tumors with a compromised ability to repair double-stranded DNA breaks by homologous recombination are sensitive to blockade of DNA single-strand breaks by PARP inhibition, providing the basis for a synthetic lethal approach to cancer treatment [74].
McMullin et al. reported pre-clinical data whereby BRCA1 deficient like (BD-L) signature is enriched in HER2+ breast cancer brain [75]. Also, BD-L tumors were found across all breast cancer subtypes in addition to HER2+ tumors. In the same study evaluation of pharmacological sensitivity in CNS breast cancer cell lines representing all breast cancer subtypes suggested that the BD-L signature may serve as a biomarker to identify breast cancer patients who benefit from a therapeutic combination of PARP inhibition with olaparib and temozolomide; the latter being a small lipophilic alkylating agent capable of crossing the BBB [76]. Olaparib has also shown synergy in inducing BRCA-deficient TNBC cell death and reducing tumor volume in xenografts when combined with PI3K inhibitor (PI-103) as a radiosensitizer [77]. Olaparib is currently being tested in patients with recurrent glioblastoma with the primary objective to determine whether olaparib crosses the BBB and achieves tumor penetration [78].
Veliparib, a potent oral PARP inhibitor is known to cross the BBB and has been tested in 25 patients with MBC and brain involvement in a phase I trial [79]. Escalating doses (10–300 mg, orally BID) were administered in combination with e (30.0 or 37.5 Gy in 10 or 15 fractions) and were well-tolerated. The 6-month survival rate was 61% (39–78%) for breast cancer patients and a 41% intracranial ORR was observed. Breast cancer subtypes were not reported and further exploration of PARP inhibitors is needed among patients with MBC with CNS involvement.
Insulin growth factor 1 (IGF1) and insulin growth factor receptor 1 (IGF1-R)
IGF-1R is a transmembrane cellular receptor with a heterotetrameric structure characterized by two ‘half receptors’. Each half is comprised of an extracelluar α-chain that is involved in ligand binding and an intracellular β-chain that includes the tyrosine kinase domain [80]. Activation of the IGF-1R by its ligands, insulin-like growth factor 1 and 2 (IGF1 and IGF2, respectively), results in activation of the PI3K/AKT/mTOR cascade and the RAS-MAPK pathway leading to stimulation of protein translation and cell proliferation (Figure 1) [81].
Figure 1.
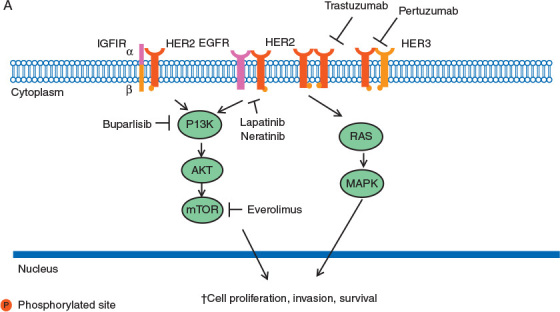
HER2 signal transduction. Activation of the receptor tyrosine kinase occurs by homodimerization or heterodimerization with other HER family members; i.e., epidermal growth factor receptor (EGFR) and HER3 and insulin growth factor receptor 1 (IGFIR). Activated HER2 initiates downstream signaling through the PI3K-AKT-mTOR and RAS-MAPK pathways, promoting cell proliferation and survival.
Thomson et al. reported results for 41 patients, 12 of which had IGF1 expression in brain metastatic breast cancer tissue [82]. Notably, primary breast tumor expression of IGF1 was seen in three matched samples suggesting that an IGF pathway aberration could play an important role in a subset of breast tumor metastasis. Breast cancer brain metastases showed significantly higher activation of the c-HER2/IGFR-AKT pathway networks compared to lung cancer metastasis; in contrast to lung cancer metastases that have higher levels of members associated with EGFR-ERK activity [83]. In addition, the crosstalk between HER2 and IGF-1R with IGF-1 leads to phosphorylation of HER2 and activation of PI3K [35], while inhibition of IGF-1R signaling blocks HER2 phosphorylation and restores sensitivity to trastuzumab in select preclinical models [84]. In pre-clinical models knockdown of IGF-1R signaling decreased migration and invasion of MDA-MB-231Br brain-seeking cells [85]. Early phase clinical trials are ongoing for patients with metastatic breast cancer including patients with CNS metastasis (e.g. NCT00684983, NCT01013506, and NCT02045368).
Antiangiogenesis
Vascular endothelial growth factor (VEGF) is a critical mediator of angiogenesis in breast cancer [86]. VEGF-A is a multifunctional cytokine widely expressed by tumor cells that acts through receptors (VEGFR-1, VEGFR-2, and neuropilin) primarily expressed on vascular endothelium. VEGF increases microvascular permeability, induces endothelial cell migration and division, reprograms gene expression, promotes endothelial cell survival, prevents senescence, and induces angiogenesis [87]. VEGF-A targeted therapy with bevacizumab possesses modest clinical efficacy in patients with metastatic breast cancer, and a survival benefit has never been demonstrated [88, 89]. Patient derived breast cancer CNS lesions do not show increased microvascular density when compared to primary breast tumor tissue [90]. Paradoxically patient-derived xenograft models suggest that metastatic breast cancer is associated with increased angiogenesis in the CNS [91]. Additionally, the inhibition of VEGF receptor decreases the ability of breast cancer cells to form brain metastases [92]. Preliminary data from a phase II clinical trial also shows an intracranial ORR of 45% with the combination of bevacizumab and chemotherapy [93]. Similarly, the combination of trastuzumab or lapatinib with antibodies targeting VEGF receptor-2 is effective in reducing tumor microvascular density in HER2-amplified breast cancer brain metastases using an orthotopic xenograft although studies evaluating this combination in patients have yet to be addressed [94].
Estrogen and progesterone receptors
Targeting steroid hormone receptors was one of the earliest strategies used against CNS metastases. A small study showed that estrogen (73.3%) and progesterone (83%) receptors are expressed in the majority of MBC cases to the brain [61]. Notably, there was no significant difference between the primary and the brain metastatic deposits with respect to hormone receptor expression. Conversely, loss of hormone receptor expression occurred in up to 50% of BM in a retrospective series of 24 patients of matched primary and BM pairs [95]. Agents with high antitumor activity like tamoxifen, a selective estrogen receptor modulator, penetrate the BBB [96]. Their efficacy in the setting of MBC with CNS involvement in hampered by the fact that CNS involvement is a late event in the natural progression of HR positive MBC when most patients have already developed anti-estrogen resistant tumors. As such, only anecdotal case reports have described the activity of agents such as letrozole, anastrozole and exemestane in the MBC with CNS involvement [97., 98., 99., 100.]. Moreover, pre-clinical models suggest that P-glycoprotein at the BBB limits the effects of anastrozole in the CNS, whereas letrozole does cross the BBB [101, 102].
PIK3/AKT/mTOR pathway
The PIK3/AKT/mTOR pathway is one of the most common genomic aberrations in MBC [103]. In the metastatic setting, mTOR inhibition with everolimus has shown significant clinical efficacy in combination with an aromatase inhibitor. Notably, this combination is now a standard of care option in this setting [104]. Adamo et al. reported immunohistochemical analysis of p-AKT and p-S6 and PTEN on brain metastatic tissue of 52 patients with MBC with CNS involvement [105]. A small study of gene copy number in breast cancer brain metastases showed that PIK3CA amplification is common in metastatic brain tissue [106]. Interestingly, the expression of p-AKT and p-S6, but lack of PTEN, was observed in 75%, 69%, and 25% of breast cancer brain metastases, respectively. Concordance between primary BCs and matched CNS metastasis was 67% for p-AKT, 58% for p-S6, and 83% for PTEN. PTEN loss was more common in TNBC when compared with HR+/HER2− and HER2+ [105]. The PI3K/AKT/mTOR pathway was found to be active in ∼70% of brain tumor samples in same study. In another study sequencing 110 primary breast tumors and MBC with CNS involvement, alterations in PTEN were found in a significantly larger fraction of CNS metastatic tumor tissue when compared with samples from primary tumors [62]. Buparlisib is an oral pyrimidine-derived pan-PI3K inhibitor with specific and potent activity against class I PI3Ks. Buparlisib inhibits wild-type and mutant PI3Kα isoforms and PI3K-β, -γ, and -δ isoforms at nanomolar concentrations [107]. Importantly, a pre-clinical human xenograft model has demonstrated that buparlisib penetrates the BBB and inhibits the PI3K/AKT/mTOR pathway [108]. In a phase I dose-escalation study of buparlisib in 31 evaluable patients with advanced solid tumors, there was one PR (TNBC and PIK3CA mutation positive) while 16 patients (52%) had stable disease, including five patients with MBC and one patient showed a 28% reduction in a CNS lesion with MBC [109].
Cyclin-dependent kinases (CDK)
CDK4 and CDK6 are activated by D-type cyclins that promote cell-cycle entry by phosphorylating retinoblastoma protein (Rb), among other proteins, to initiate transition from the G1 phase to the S phase (Figure 2) [110]. Gojis et al. reported studies on the immunohistochemical landscape of 30 patients with MBC and brain involvement [61]. In 7 cases out of 30 (23.3%) patients, brain metastatic tissue was positive for CDK4/6 expression. Separately, Da Silva et al. [66] reported CDK5/6 expression in up to 34% of patients among 26 patients with MBC with CNS metastasis. Multiple oncogenic signals in hormone receptor-positive breast cancer converge to promote expression of cyclin D1 and activation of CDK4/6 to drive breast-cancer proliferation [111, 112]. In vitro evidence also suggests that breast cancer with resistance to prior endocrine therapy remains dependent on CDK1/4 to promote proliferation [113, 114]. Clinically, phase II and III trials support the significant clinical benefit of treatment with CDK4/6 inhibitor palbociclib among patients with HR positive HER2 negative MBC without CNS involvement either in the first line or progressing after first line aromatase inhibitor therapy [115., 116., 117.]. In the PALOMA3 trial 521 patients with MBC without CNS metastasis were randomized to fulvestrant±palbociclib in the setting of disease progression after treatment with anti-estrogen therapy [115]. This trial met its primary endpoint with a hazard ratio for disease progression or death, of 0.42; 95% CI, 0.32–0.56; P < 0.001. In the PALOMA1/TRIO-18 phase II trial 165 patients with MBC were randomized to treatment with letrozole±palbociclib [116]. In this study patients must not have received prior treatment for advanced disease and primary endpoint analysis showed a hazard ratio disease progression of 0.488, 95% CI, 0.319–0.748; P = 0.0004. All patients accrued to both trials had HR positive and HER2 negative tumors.
Figure 2.
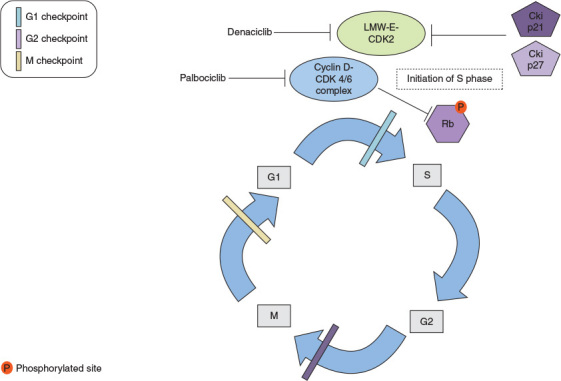
The figure depicts the role of cyclin-dependent kinases 4 and 6 (CDK4/6) in the cell cycle. The normal cell replication processes are represented i.e., G1 first growth period to S DNA replication phase G2 second growth period, and M, which is the mitosis period. Cyclin D1-CDK4/6 inactivates retinoblastoma protein (Rb) through phosphorylation. The latter event allows for cell cycle progression from G1 to S phase. Palbociclib targets cyclin-CDK4/6 complex formation ultimately generating cell cycle arrest. Also low molecular weight cyclin E-cycle dependent kinase 2 (LMW-E-CDK2) complex activation is depicted. When active LMW-E-CDK2 complex is resistant to inhibitory effect of cyclin inhibitors p21 and p27 and promote initiation of S phase. Denaciclib targets CDK2 promoting cell cycles arrest.
Cancer cell lines overexpressing low molecular weight cycle E (LMW-E) have increased genomic instability, increased cyclin E associated activity, prolonged S phase, and accelerated G1 to S phase transition (Figure 2) [118., 119., 120.]. In mice, the activity of LMW-E is mediated through binding and activation of CDK2, which triggers the resistance of CDK2 to inhibitors of p21 and p27 [121, 122]. LMW-E overexpression correlates with poor outcomes in various models of breast cancer regardless of HR and HER2 status [123]. Nanos-Webb et al. reported results of cyclin E and LMW-E overexpressing breast cancer cell lines whereby CDK2 inhibition demonstrated synergistic effects with doxorubicin [124]. CDK pathobiology has also been studied in TNBC. Horiuchi et al. showed the exploitation of increased MYC expression found in TNBCs by using a synthetic-lethal approach dependent on CDK inhibition [125, 126]. The CDK1/2 inhibitor, dinaciclib, effectively induces arrest in both G1-S and G2-M and tumor regression in triple-negative tumor xenografts. Unfortunately, phase I dose escalation with dinaciclib combined with anthracycline in patients with TNBC was stopped after the first dose cohort was found to be too toxic [127].
Palbociclib has been investigated in primary brain tumors. In vivo experiments in glioblastoma multiforme (GBM) has shown that palbociclib can suppress growth of GBM in intracranial xenografts, and prevented tumor-related death of treated mice [128]. In these experiments, palbociclib was not only present in intracranial tissues, but was 25–35× higher in tumor tissue than in normal tissue [128]. Recent studies, however, have shown that certain BBB transports, P-glycoprotein and Breast Cancer Resistance Protein (BCRP/ABCG2), can inhibit CNS uptake of palbociclib, although more studies are needed [129]. Palbociclib has been shown to have cytostatic effects on Retinoblastoma tumor suppressor (Rb) pathway proficient TNBC models [130]. While basal like cancers do not tend to display alterations in cyclin D1 or CDK4/6, the PALOMA-1 study did not find these genomic aberrations to predict response to therapy outside the CNS [116]. While palbociclib inhibits CDK 4/6 primarily, it can have some effect on other CDKs relevant to TNBC (such as low molecular weight CDK-E, and CDK1/2) [131]. HER2+ breast cancers on the other hand, have been demonstrated to require cyclin D1 and CDK4 for tumor progression and maintenance [132, 133]. Palbociclib has been found to have single agent activity in transgenic HER2+ models by causing near complete cessation of tumor proliferation, leading to improved survival of mice [133]. Palbocicib is being investigated currently in TNBC and HER2-positive disease in patients who have brain metastasis (NCT02774681).
In HER2+ breast cancer, Shom et al. reported that in transgenic mouse models, CDK1/4 inhibition mediates resistance to HER2 targeted therapy in breast cancer [134]. In addition, CDK4/6 inhibition suppresses Rb phosphorylation and decreases TSC2 phosphorylation; thereby partially attenuating mTORC1 activity. This relieves the feedback inhibition of upstream EGFR family kinases and resensitizes tumors to EGFR/HER2 blockade. CDK4/6 inhibition has also been shown to sensitize patient-derived xenograft tumors to HER2-targeted therapies and delay tumor recurrence in a transgenic model of HER2+ breast cancer. A phase II clinical trial of CDK 4/6 inhibitor, abemaciclib, in combination with trastuzumab±fulvestrant, is in the process of being developed [135]. Notably, pre-clinical xenografting studies support the idea that abemaciclib crosses the BBB more effectively when compared to palbociclib [136]. Measurable levels of abemaciclib and active metabolites were detected in brain tumor tissue for all 3 patients and breast cancer and brain metastasis treated with at least 5 days of abemaciclib [137]. Unbound concentrations of abemaciclib in the plasma and tumor tissue were comparable and generally consistent with the CSF concentration for each of the patients.
Aurora kinase A/B
Salhia et al. reported the results of a comprehensive analysis of 19 fresh-frozen samples of breast brain metastases [138]. As expected, according to PAM50 gene expression classifier luminal B, HER2+/ER−, and basal-like tumors were identified as the most commonly represented breast cancer subtypes in this brain metastasis cohort. Frequently amplified and overexpressed genes included ATAD2, BRAF, DERL1, DNMTRB and NEK2A. The ATM, CRYAB and HSPB2 genes were also commonly deleted and under-expressed. Data mining revealed enrichment in genes controlling cell cycle and G2/M transition pathways, which contained AURKA, AURKB and FoxM1. Also, both FoxM1 and AURKB were up regulated when compared to primary breast cancer and nonneoplastic controls.
The Aurora kinases (Aurora A/B/C) constitute a family of highly conserved serine/threonine kinases, which play important roles in cell cycle control as they regulate progression through mitosis and cytokinesis [139, 140]. Aurora A and B have been linked to cancer [141, 142]. While Aurora A coordinates centrosome maturation, assembly of the bipolar spindle and chromosome separation, Aurora B regulates chromosome condensation, the spindle checkpoint and cytokinesis [140]. In aromatase inhibitor-resistant breast cancer cell lines, the aurora kinase inhibitors, alisertib and danusertib, blocked cell cycle progression at the G2/M phase, interfered with chromosome alignment and spindle pole formation, as well as resulted in preferential growth inhibition compared with parental MCF-7 cells [143]. Even further growth inhibition was obtained when combining the Aurora kinase inhibitors with the antiestrogen fulvestrant. A phase I clinical trial is planed with an aurora kinase A inhibitor (alisertib) in combination with dual TORC1/2 inhibitor (MLN8237) [144]. The dose expansion cohort will consist of patients with metastatic TNBC with CNS involvement permitted for study accrual.
Immune system
Immune checkpoint inhibitors, predominantly in the form of anti-PD1 antibodies, have demonstrated meaningful antitumor activity in solid tumors in melanoma, lung cancer and renal cell carcinoma [145., 146., 147.]. In addition to a favorable toxicity profile, some patients show sustained antitumor responses that provide compelling rationale for also investigating this therapeutic in the realm of breast cancer. Notably, pre-clinical data suggest that cytolytic CD8+ T cell-mediated immunity can take place against tumors located in the CNS [148], as evidenced in melanoma patients with brain metastases. Margolin et al. reported the results of a phase II trial of 72 patients with metastatic melanoma treated with ipilimumab including 12 patients (24%) without neurologic symptoms achieved control of CNS disease, further highlighting that host antitumor immunity can be utilized against malignant cells in the CNS [149].
The potential importance of immune checkpoint guided therapy in breast cancer is underscored by recent reports of PD-1 inhibitor activity in TNBC. The monoclonal PD-1 antibody, pembrolizumab, was recently tested in a phase Ib trial in 32 female patients with PD-L1+ and heavily pretreated metastatic recurrent TNBC [150]. The disease control rate (partial response + disease stabilization rates) for ≥ 24 weeks was 25.9%. Three patients with brain metastasis were enrolled into this trial but efficacy endpoints were not reported for these patients. Avelumab, a PD-L1 monoclonal antibody, also showed signs of activity in a phase Ib trial showing stable disease in 40 patients (23.8%) with an overall disease control rate of 29.2% [151]. In patients with TNBC who had PD-L1+ immune cells within the tumor, 44.4% (4 of 9) had PRs, compared with 2.6% (1 of 39) for TNBC with PD-L1− immune cells. Notably, none of these trials reported results on patients with MBC and CNS involvement and only one of them allowed for accrual patients with CNS metastasis. Xiu et al. reported increased PD-L1 expression in 40% of brain tumor tissue of patients with primary TNBC, which was increased when compared to other metastatic sites such as liver (8%) or bone (17%) [152]. Analysis of 84 excised CNS breast cancer metastases derived from the four primary phenotypes including HR+/HER2− (n = 23), HR+/HER2+ (n = 21), HR−/HER2− (n = 21) and HR−/HER2+ (n = 19) has revealed CD4+ and CD8+ tumor-infiltrating lymphocytes (TIL) and CD68+ macrophage/microglial cells, as well as the expression of PD-1, PD-L1, and PD-L2 in brain tumor tissue. PD-L1 and PD-L2 expression was quantified using H-score, which incorporates staining intensity and frequency of positive cells [153]. PD-L1 (present in 53% of breast cancer brain metastases) did not correlate with CD4+ TIL (P = 0.31). Likewise PD-L2 (present in 38% of brain metastases) also did not with CD4+ TIL (P= 0.08). TILs PD-1 expression correlated positively (n = 17) with CD4+ (P= 0.03) and CD8+ (P= 0.005) TIL. In multivariate analysis, overall survival after resection of MBC in the brain was positively impacted by PD-1 expression on TILs (HR = 0.3; P = 0.003). PD-L1 and PD-L2 expression is a common occurrence in brain metastasis, irrespective of primary tumor and brain metastasis phenotypes. These data provide rationale for further investigation of checkpoint blockade in subsequent studies.
Discussion
Breast cancer complicated by CNS metastasis is a growing challenge as more effective therapy against the primary malignancy facilitates a more resilient systemic disease that leads to CNS metastatic infiltration. Many breast cancer patients presenting with multiple metastatic foci within the CNS are treated with radiation therapy as a first line option. Palliative chemotherapy after disease progression provides modest clinical benefit(s) with 1-year overall survival rates consistently <50%. Therefore, a better understanding of the biology governing the development and progression of breast cancer brain metastasis is essential to develop rational treatment strategies.
Individuals with HER2+ MBC possess greater benefit from sequential HER2-targeted therapies with prolonged survival [154, 155]. Moreover, hormone receptor-positive breast cancer treatments including newer targeted therapies against CDK and mTOR pathways prolong survival in the metastatic setting [104, 115]. A low accessibility to brain metastases has hampered biomarker based drug development in patients with MBC and CNS involvement. There are also limited data on biomarker statuses between CNS metastases and the primary breast tumor (concordance rates), although smaller studies indicate a discord between different sites of metastatic breast cancer including the brain [152, 156].Tumor-derived DNA isolated from the cerebral spinal fluid may allow for increased knowledge of genomic aberrations of MBC to the CNS and propel further drug development [157]. This is particularly relevant as cancer therapy development continues to utilize molecular biology as a guidance for combined drug approaches to different targets [158]. Incorporation of patients with CNS metastasis into early phase clinical trials for patients with progressive systemic disease is also a key component of future drug development [16].
Going forward, novel targeted therapies are required that cross the BBB to provide antitumor activity. This goal may be further enhanced by administering agents in combination with BBB disrupting agents through intra-arterial or nanoparticle-mediated transport [159., 160., 161.]. Furthermore, an important question remains with respect to whether CSF drug levels are a valid surrogate for estimating metastatic tumor drug exposure [162]. Therefore, future pre-clinical models will be needed to take into account the numerous unanswered questions for prioritizing future clinical drug development.
Finally, efforts will be required to circumvent the challenge of accruing patients with MBC and CNS metastases who progress with systemic disease after multiple lines of therapy. Related efforts have already been implemented, including the multi-institutional ‘Brain metastases in breast cancer network Germany’ (BMBC, GBG 79) in which women with breast cancer brain metastases were analyzed in Germany [163]. Furthermore, efforts are also ongoing in Asia where a multinational cohort aims to retrospectively analyze cases of HER2+ MBC with CNS involvement and define patterns of disease occurrence and progression [164].
Funding
None declared. Grant support: not applicable.
Disclosure
The authors have declared no conflicts of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Barnholtz-Sloan J.S., Sloan A.E., Davis F.G. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 3.Frisk G., Svensson T., Backlund L.M. Incidence and time trends of brain metastasesadmissions among breast cancer patients in Sweden. Br J Cancer. 2012;106:1850–1853. doi: 10.1038/bjc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelletier E.M., Shim B., Goodman S., Amonkar MM. Epidemiology and economic burden of brain metastases among patients with primary breast cancer: results from a US claims data analysis. Breast Cancer Res Treat. 2008;108:297–305. doi: 10.1007/s10549-007-9601-0. [DOI] [PubMed] [Google Scholar]
- 5.Tham Y.L., Sexton K., Kramer R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107:696–704. doi: 10.1002/cncr.22041. [DOI] [PubMed] [Google Scholar]
- 6.DiStefano A., Yong Yap Y., Hortobagyi G.N., Blumenschein GR. The natural history of breast cancer patients with brain metastases. Cancer. 1979;44:1913–1918. doi: 10.1002/1097-0142(197911)44:5<1913::aid-cncr2820440554>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Olson E.M., Najita J.S., Sohl J. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013;22:525–531. doi: 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brufsky A.M., Mayer M., Rugo H.S. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17:4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. [DOI] [PubMed] [Google Scholar]
- 9.Gori S., Rimondini S., De Angelis V. Central nervous system metastases in HER-2 positive metastatic breast cancer patients treated with trastuzumab: incidence, survival, and risk factors. Oncologist. 2007;12:766–773. doi: 10.1634/theoncologist.12-7-766. [DOI] [PubMed] [Google Scholar]
- 10.Olson E.M., Abdel-Rasoul M., Maly J. Incidence and risk of central nervous system metastases as site of first recurrence in patients with HER2-positive breast cancer treated with adjuvant trastuzumab. Ann Oncol. 2013;24:1526–1533. doi: 10.1093/annonc/mdt036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin N.U., Claus E., Sohl J. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pestalozzi B.C., Zahrieh D., Price K.N. Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 13.Hicks D.G., Short S.M., Prescott N.L. Breast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30:1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Angulo A.M., Cristofanilli M., Strom E.A. Central nervous system metastases in patients with high-risk breast carcinoma after multimodality treatment. Cancer. 2004;101:1760–1766. doi: 10.1002/cncr.20530. [DOI] [PubMed] [Google Scholar]
- 15.Sezgin C., Gokmen E., Esassolak M. Risk factors for central nervous systemmetastasis in patients with metastatic breast cancer. Med Oncol. 2007;24:155–161. doi: 10.1007/BF02698034. [DOI] [PubMed] [Google Scholar]
- 16.Gounder M.M., Spriggs DR. Inclusion of patients with brain metastases in phase I trials: an unmet need. Clin Cancer Res. 2011;17:3855–3857. doi: 10.1158/1078-0432.CCR-11-0759. [DOI] [PubMed] [Google Scholar]
- 17.Cordon-Cardo C., O’Brien J.P., Casals D. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toth K., Vaughan M.M., Peress N.S. MDR1 P-glycoprotein is expressed by endothelial cells of newly formed capillaries in human gliomas but is not expressed in the neovasculature of other primary tumors. Am J Pathol. 1996;149:853–858. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R.D., Price J.E., Fujimaki T. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol. 1992;141:1115–1124. [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano A., Zimmerman HM. Fenestrated blood vessels in a metastatic renal carcinoma in the brain. Lab Invest. 1972;26:465–468. [PubMed] [Google Scholar]
- 21.Sun H., Dai H., Shaik N., Elmquist WF. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 2003;55:83–105. doi: 10.1016/s0169-409x(02)00172-2. [DOI] [PubMed] [Google Scholar]
- 22.Hooijberg J.H., Broxterman H.J., Kool M. Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res. 1999;59:2532–2535. [PubMed] [Google Scholar]
- 23.Renes J., de Vries E.G., Nienhuis E.F. ATP- and glutathione-dependent transport of chemotherapeutic drugs by the multidrug resistance protein MRP1. Br J Pharmacol. 1999;126:681–688. doi: 10.1038/sj.bjp.0702360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine R.L., Chen J., Balmaceda C. Randomized study of paclitaxel and tamoxifen deposition into human brain tumors: implications for the treatment of metastatic brain tumors. Clin Cancer Res. 2006;12:5770–5776. doi: 10.1158/1078-0432.CCR-05-2356. [DOI] [PubMed] [Google Scholar]
- 25.Gerstner E.R., Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol. 2007;25:2306–2312. doi: 10.1200/JCO.2006.10.0677. [DOI] [PubMed] [Google Scholar]
- 26.Morikawa A., Peereboom D.M., Thorsheim H.R. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol. 2015;17:289–295. doi: 10.1093/neuonc/nou141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regina A., Demeule M., Che C. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas F.C., Taskar K., Rudraraju V. Uptake of ANG1005, a novel paclitaxelderivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertrand Y., Currie J.C., Poirier J. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer. 2011;105:1697–1707. doi: 10.1038/bjc.2011.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumthekar P., Tang S.-C., Brenner A.J. ANG1005, a novel brain-penetrant taxane derivative, for the treatment of recurrent brain metastases and leptomeningeal carcinomatosis from breast cancer. J Clin Oncol. 2016;34 (suppl abstr 2004) [Google Scholar]
- 31.Gaillard P.J., Appeldoorn C.C., Dorland R. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101) PLoS One. 2014;9:e82331. doi: 10.1371/journal.pone.0082331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aftimos P.G., Milojkovic-Kerklaan B., Diéras V. Phase 1/2a study of glutathione PEGylated liposomal doxorubicin (2B3-101) in breast cancer patients with brain metastases [abstract]. In: Proceedings of the Thirty-Seventh Annual CTRC-AACR San Antonio Breast Cancer Symposium: 2014 Dec 9–13; San Antonio, TX. AACR; Philadelphia (PA): 2015. Cancer Res 75(9 Suppl)Abstract nr P6-16-04 2015. [Google Scholar]
- 33.Winkler F., Osswald M., Blaes J. Impact of blood-brain barrier integrity on tumor growth and therapy response in brain metastases. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-1327. [DOI] [PubMed] [Google Scholar]
- 34.Salphati L., Pang J., Plise E.G. Preclinical assessment of the absorption and disposition of the phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor GDC-0980 and prediction of its pharmacokinetics and efficacy in human. Drug Metab Dispos. 2012;40:1785–1796. doi: 10.1124/dmd.112.046052. [DOI] [PubMed] [Google Scholar]
- 35.Gajria D., Chandarlapaty S. HER2-amplified breast cancer: mechanisms of trastuzumab resistance and novel targeted therapies. Expert Rev Anticancer Ther. 2011;11:263–275. doi: 10.1586/era.10.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yarden Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 37.Graus-Porta D., Beerli R.R., Daly J.M., Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guy P.M., Platko J.V., Cantley L.C. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci U S A. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holbro T., Beerli R.R., Maurer F. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park Y.H., Jung H.A., Choi M.K. Role of HER3 expression and PTEN loss in patients with HER2-overexpressing metastatic breast cancer (MBC) who received taxane plus trastuzumab treatment. Br J Cancer. 2014;110:384–391. doi: 10.1038/bjc.2013.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia W., Petricoin E.F., 3rd, Zhao S. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15:R85. doi: 10.1186/bcr3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nafi S., Generali D., Kramer-Marek G. Nuclear HER4 mediates acquired resistance to trastuzumab and is associated with poor outcome in HER2 positive breast cancer. Oncotarget. 2014;5(15):5934–5949. doi: 10.18632/oncotarget.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harbeck N., Beckmann M.W., Rody A. HER2 dimerization inhibitor pertuzumab – mode of action and clinical data in breast cancer. Breast Care (Basel) 2013;8:49–55. doi: 10.1159/000346837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabindran S.K., Discafani C.M., Rosfjord E.C. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004;64:3958–3965. doi: 10.1158/0008-5472.CAN-03-2868. [DOI] [PubMed] [Google Scholar]
- 45.Burris H.A., 3rd, Hurwitz H.I., Dees E.C. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 46.Rusnak D.W., Lackey K., Affleck K. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 47.de Azambuja E., Zardavas D., Lemort M. Phase I trial combining temozolomide plus lapatinib for the treatment of brain metastases in patients with HER2-positive metastatic breast cancer: the LAPTEM trial. Ann Oncol. 2013;24:2985–2989. doi: 10.1093/annonc/mdt359. [DOI] [PubMed] [Google Scholar]
- 48.Lin N.U., Carey L.A., Liu M.C. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pivot X., Manikhas A., Zurawski B. CEREBEL (EGF111438): a phase III, randomized, open-label study of lapatinib plus capecitabine versus trastuzumab plus capecitabine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2015;33:1564–1573. doi: 10.1200/JCO.2014.57.1794. [DOI] [PubMed] [Google Scholar]
- 50.Lin N.U., Freedman R.A., Ramakrishna N. A phase I study of lapatinib with whole brain radiotherapy in patients with Human Epidermal Growth Factor Receptor 2 (HER2)-positive breast cancer brain metastases. Breast Cancer Res Treat. 2013;142:405–414. doi: 10.1007/s10549-013-2754-0. [DOI] [PubMed] [Google Scholar]
- 51.Awada A., Colomer R., Inoue K. Neratinib plus paclitaxel vs trastuzumab plus paclitaxel in previously untreated metastatic ERBB2-positive breast cancer: the NEfERT-T randomized clinical trial. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.0237. [DOI] [PubMed] [Google Scholar]
- 52.Freedman R.A., Gelman R.S., Wefel J.S. TBCRC 022: phase II trial of neratinib for patients (Pts) with human epidermal growth factor receptor 2 (HER2+) breast cancer and brain metastases (BCBM) J Clin Oncol. 2014;32 doi: 10.1200/JCO.2015.63.0343. (suppl abstr 528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurihara H., Hamada A., Yoshida M. (64)Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015;5:8. doi: 10.1186/s13550-015-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baselga J., Cortes J., Kim S.B. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swain S.M., Baselga J., Miles D. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann Oncol. 2014;25:1116–1121. doi: 10.1093/annonc/mdu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chargari C., Idrissi H.R., Pierga J.Y. Preliminary results of whole brain radiotherapy with concurrent trastuzumab for treatment of brain metastases in breast cancer patients. Int J Radiat Oncol Biol Phys. 2011;81:631–636. doi: 10.1016/j.ijrobp.2010.06.057. [DOI] [PubMed] [Google Scholar]
- 57.Clinicaltrials.gov. Pertuzumab with high-dose trastuzumab in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC) with central nervous system (CNS) progression post-radiotherapy. http://www.clinicaltrials.gov accessed on 02/23/2016.
- 58.Askoxylakis V., Ferraro G.B., Kodack D.P. Preclinical efficacy of ado-trastuzumab emtansine in the brain microenvironment. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jacot W., Pons E., Guiu S. Trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer and brain metastases. San Antonio Breast Conference. 2015. [P6-17-03] [DOI] [PubMed] [Google Scholar]
- 60.CFerrarioEHamiltonNAucoin. [P4-14-20] A phase 1b study of ONT 380, an oral HER2-specific inhibitor, combined with ado trastuzumab emtansine (T DM1), in HER2+ metastatic breast cancer (MBC). San Antonio Breast Conference 2015.
- 61.Gojis O., Kubecova M., Rosina J. Expression of selected proteins in breast cancer brain metastases. Folia Histochem Cytobiol. 2013;51:213–218. doi: 10.5603/FHC.2013.0030. [DOI] [PubMed] [Google Scholar]
- 62.Hohensee I., Lamszus K., Riethdorf S. Frequent genetic alterations in EGFR- and HER2-driven pathways in breast cancer brain metastases. Am J Pathol. 2013;183:83–95. doi: 10.1016/j.ajpath.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 63.Baselga J., Norton L., Masui H. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993;85:1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- 64.Baselga J., Gomez P., Greil R. Randomized phase II study of the anti-epidermal growth factor receptor monoclonal antibody cetuximab with cisplatin versus cisplatin alone in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2013;31:2586–2592. doi: 10.1200/JCO.2012.46.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.RDBairdH-TArkenauSDeva. [P4-14-26] Phase I expansion of S-222611, a reversible inhibitor of EGFR and HER2, in advanced solid tumors, including HER2-positive breast cancer patients with brain metastases. San Antonio Breast Conference 2015.
- 66.Da Silva L., Simpson P.T., Smart C.E. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010;12:R46. doi: 10.1186/bcr2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Momeny M., Saunus J.M., Marturana F. Heregulin-HER3-HER2 signaling promotes matrix metalloproteinase-dependent blood-brain-barrier transendothelial migration of human breast cancer cell lines. Oncotarget. 2015;6:3932–3946. doi: 10.18632/oncotarget.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baldassarre G., Battista S., Belletti B. Negative regulation of BRCA1 gene expression by HMGA1 proteins accounts for the reduced BRCA1 protein levels in sporadic breast carcinoma. Mol Cell Biol. 2003;23:2225–2238. doi: 10.1128/MCB.23.7.2225-2238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beger C., Pierce L.N., Kruger M. Identification of Id4 as a regulator of BRCA1 expression by using a ribozyme-library-based inverse genomics approach. Proc Natl Acad Sci U S A. 2001;98:130–135. doi: 10.1073/pnas.98.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Esteller M., Silva J.M., Dominguez G. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92:564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 71.Bartkova J., Tommiska J., Oplustilova L. Aberrations of the MRE11-RAD50-NBS1 DNA damage sensor complex in human breast cancer: MRE11 as a candidate familial cancer-predisposing gene. Mol Oncol. 2008;2:296–316. doi: 10.1016/j.molonc.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heikkinen T., Karkkainen H., Aaltonen K. The breast cancer susceptibility mutation PALB2 1592delT is associated with an aggressive tumor phenotype. Clin Cancer Res. 2009;15:3214–3222. doi: 10.1158/1078-0432.CCR-08-3128. [DOI] [PubMed] [Google Scholar]
- 73.Tommiska J., Bartkova J., Heinonen M. The DNA damage signalling kinase ATM is aberrantly reduced or lost in BRCA1/BRCA2-deficient and ER/PR/ERBB2-triple-negative breast cancer. Oncogene. 2008;27:2501–2506. doi: 10.1038/sj.onc.1210885. [DOI] [PubMed] [Google Scholar]
- 74.Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–3790. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 75.McMullin R.P., Wittner B.S., Yang C. A BRCA1 deficient-like signature is enriched in breast cancer brain metastases and predicts DNA damage-induced poly (ADP-ribose) polymerase inhibitor sensitivity. Breast Cancer Res. 2014;16:R25. doi: 10.1186/bcr3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ostermann S., Csajka C., Buclin T. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 77.Jang N.Y., Kim D.H., Cho B.J. Radiosensitization with combined use of olaparib and PI-103 in triple-negative breast cancer. BMC Cancer. 2015;15:89. doi: 10.1186/s12885-015-1090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clinicaltrials.gov. Olaparib and temozolomide in treating patients with relapsed glioblastoma. http://www.clinicaltrials.gov (30 March 2016, date last accessed).
- 79.Mehta M.P., Wang D., Wang F. Veliparib in combination with whole brain radiation therapy in patients with brain metastases: results of a phase 1 study. J Neurooncol. 2015;122:409–417. doi: 10.1007/s11060-015-1733-1. [DOI] [PubMed] [Google Scholar]
- 80.De Meyts P. Insulin and its receptor: structure, function and evolution. Bioessays. 2004;26:1351–1362. doi: 10.1002/bies.20151. [DOI] [PubMed] [Google Scholar]
- 81.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 82.Thomson A.H., McGrane J., Mathew J. Changing molecular profile of brain metastases compared with matched breast primary cancers and impact on clinical outcomes. Br J Cancer. 2016;114:793–800. doi: 10.1038/bjc.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Improta G., Zupa A., Deng J. Use of protein pathway mapping of brain metastasis from breast and lung cancer patients to identify new therapeutic targets: a seed/soil study. J Clin Oncol. 2010;28 (suppl abstr 10620) [Google Scholar]
- 84.Nahta R., Yuan L.X., Zhang B. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 85.Saldana S.M., Lee H.H., Lowery F.J. Inhibition of type I insulin-like growth factor receptor signaling attenuates the development of breast cancer brain metastasis. PLoS One. 2013;8:e73406. doi: 10.1371/journal.pone.0073406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Relf M., LeJeune S., Scott P.A. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 87.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 88.Miller K., Wang M., Gralow J. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 89.Robert N.J., Dieras V., Glaspy J. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 90.Berghoff A.S., Ilhan-Mutlu A., Dinhof C. Differential role of angiogenesis and tumour cell proliferation in brain metastases according to primary tumour type: analysis of 639 cases. Neuropathol Appl Neurobiol. 2015;41:e41–55. doi: 10.1111/nan.12185. [DOI] [PubMed] [Google Scholar]
- 91.Monsky W.L., Mouta Carreira C., Tsuzuki Y. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin Cancer Res. 2002;8:1008–1013. [PubMed] [Google Scholar]
- 92.Kim L.S., Huang S., Lu W. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metastasis. 2004;21:107–118. doi: 10.1023/b:clin.0000024761.00373.55. [DOI] [PubMed] [Google Scholar]
- 93.Lin N.U., Gelmen R.S., Younger W.J. Phase II trial of carboplatin (C) and bevacizumab (BEV) in patients (pts) with breast cancer brain metastases (BCBM) J Clin Oncol. 2013;31 (suppl abstr 513) [Google Scholar]
- 94.Kodack D.P., Chung E., Yamashita H. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci U S A. 2012;109:E3119–E3127. doi: 10.1073/pnas.1216078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bachmann C., Grischke E.M., Staebler A. Receptor change-clinicopathologic analysis of matched pairs of primary and cerebral metastatic breast cancer. J Cancer Res Clin Oncol. 2013;139:1909–1916. doi: 10.1007/s00432-013-1511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lien E.A., Wester K., Lonning P.E. Distribution of tamoxifen and metabolites into brain tissue and brain metastases in breast cancer patients. Br J Cancer. 1991;63:641–645. doi: 10.1038/bjc.1991.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Madhup R., Kirti S., Bhatt M.L. Letrozole for brain and scalp metastases from breast cancer–a case report. Breast. 2006;15:440–442. doi: 10.1016/j.breast.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 98.Goyal S., Puri T., Julka P.K., Rath GK. Excellent response to letrozole in brain metastases from breast cancer. Acta Neurochir (Wien) 2008;150:613–614. doi: 10.1007/s00701-008-1576-z. discussion 614615. [DOI] [PubMed] [Google Scholar]
- 99.Ito K., Ito T., Okada T. A case of brain metastases from breast cancer that responded to anastrozole monotherapy. Breast J. 2009;15:435–437. doi: 10.1111/j.1524-4741.2009.00756.x. [DOI] [PubMed] [Google Scholar]
- 100.Navarro Martin L.M., Ocana Fernandez A., Rodriguez Sanchez C.A. Durable clinical benefit with exemestane in leptomeningeal metastasis of breast cancer. Clin Transl Oncol. 2005;7:358–360. doi: 10.1007/BF02716553. [DOI] [PubMed] [Google Scholar]
- 101.Miyajima M., Kusuhara H., Takahashi K. Investigation of the effect of active efflux at the blood-brain barrier on the distribution of nonsteroidal aromatase inhibitors in the central nervous system. J Pharm Sci. 2013;102:3309–3319. doi: 10.1002/jps.23600. [DOI] [PubMed] [Google Scholar]
- 102.Dave N., Gudelsky G.A., Desai PB. The pharmacokinetics of letrozole in brain and brain tumor in rats with orthotopically implanted C6 glioma, assessed using intracerebral microdialysis. Cancer Chemother Pharmacol. 2013;72:349–357. doi: 10.1007/s00280-013-2205-y. [DOI] [PubMed] [Google Scholar]
- 103.. Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baselga J., Campone M., Piccart M. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adamo B., Deal A.M., Burrows E. Phosphatidylinositol 3-kinase pathway activation in breast cancer brain metastases. Breast Cancer Res. 2011;13:R125. doi: 10.1186/bcr3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bollig-Fischer A., Michelhaugh S.K., Wijesinghe P. Cytogenomic profiling of breast cancer brain metastases reveals potential for repurposing targeted therapeutics. Oncotarget. 2015;6:14614–14624. doi: 10.18632/oncotarget.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.CF PSVolivaM.Burger Biological characterization of NVP-BKM120, a novel inhibitor of phosphoinositide-3-kinase in phase I/II trials. Presented at the American Association for Cancer Re-search Annual Meeting, Washington, DC, April 17–21, 2010 (abstr 4098) 2010.
- 108.Nanni P., Nicoletti G., Palladini A. Multiorgan metastasis of human HER-2+ breast cancer in Rag2-/-;Il2rg-/- mice and treatment with PI3K inhibitor. PLoS One. 2012;7:e39626. doi: 10.1371/journal.pone.0039626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bendell J.C., Rodon J., Burris H.A. Phase I, dose-escalation study of BKM120, an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 110.Asghar U., Witkiewicz A.K., Turner N.C., Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Musgrove E.A., Caldon C.E., Barraclough J. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11:558–572. doi: 10.1038/nrc3090. [DOI] [PubMed] [Google Scholar]
- 112.Yu Q., Sicinska E., Geng Y. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. doi: 10.1016/j.ccr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 113.Miller T.W., Balko J.M., Fox E.M. ERalpha-dependent E2F transcription can mediate resistance to estrogen deprivation in human breast cancer. Cancer Discov. 2011;1:338–351. doi: 10.1158/2159-8290.CD-11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thangavel C., Dean J.L., Ertel A. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–345. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turner N.C., Ro J., Andre F. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 116.Finn R.S., Crown J.P., Lang I. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 117.Cristofanilli M., Turner N.C., Bondarenko I. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 118.Wingate H., Zhang N., McGarhen M.J. The tumor-specific hyperactive forms of cyclin E are resistant to inhibition by p21 and p27. J Biol Chem. 2005;280:15148–15157. doi: 10.1074/jbc.M409789200. [DOI] [PubMed] [Google Scholar]
- 119.Porter D.C., Zhang N., Danes C. Tumor-specific proteolytic processing of cyclin E generates hyperactive lower-molecular-weight forms. Mol. Cell Biol. 2001;21:6254–6269. doi: 10.1128/MCB.21.18.6254-6269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Corin I., Di Giacomo M.C., Lastella P. Tumor-specific hyperactive low-molecular-weight cyclin E isoforms detection and characterization in non-metastatic colorectal tumors. Cancer Biol Ther. 2006;5:198–203. doi: 10.4161/cbt.5.2.2356. [DOI] [PubMed] [Google Scholar]
- 121.Akli S., Van Pelt C.S., Bui T. Overexpression of the low molecular weight cyclin E in transgenic mice induces metastatic mammary carcinomas through the disruption of the ARF-p53 pathway. Cancer Res. 2007;67:7212–7222. doi: 10.1158/0008-5472.CAN-07-0599. [DOI] [PubMed] [Google Scholar]
- 122.Akli S., Zheng P.J., Multani A.S. Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer Res. 2004;64:3198–3208. doi: 10.1158/0008-5472.can-03-3672. [DOI] [PubMed] [Google Scholar]
- 123.Keyomarsi K., Tucker S.L., Buchholz T.A. Cyclin E and survival in patients with breast cancer. N Engl J Med. 2002;347:1566–1575. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 124.Nanos-Webb A., Jabbour N.A., Multani A.S. Targeting low molecular weight cyclin E (LMW-E) in breast cancer. Breast Cancer Res Treat. 2012;132:575–588. doi: 10.1007/s10549-011-1638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Horiuchi D., Kusdra L., Huskey N.E. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J Exp Med. 2012;209:679–696. doi: 10.1084/jem.20111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Parry D., Guzi T., Shanahan F. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2010;9:2344–2353. doi: 10.1158/1535-7163.MCT-10-0324. [DOI] [PubMed] [Google Scholar]
- 127.Mitri Z., Karakas C., Wei C. A phase 1 study with dose expansion of the CDK inhibitor dinaciclib (SCH 727965) in combination with epirubicin in patients with metastatic triple negative breast cancer. Invest New Drugs. 2015;33:890–894. doi: 10.1007/s10637-015-0244-4. [DOI] [PubMed] [Google Scholar]
- 128.Michaud K., Solomon D.A., Oermann E. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–3238. doi: 10.1158/0008-5472.CAN-09-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.de Gooijer M.C., Zhang P., Thota N. P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Invest New Drugs. 2015;33:1012. doi: 10.1007/s10637-015-0266-y. [DOI] [PubMed] [Google Scholar]
- 130.McClendon A.K., Dean J.L., Rivadeneira D.B. CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle. 2012;11:2747–2755. doi: 10.4161/cc.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vidula N., Rugo HS. Cyclin-dependent kinase 4/6 inhibitors for the treatment of breast cancer: a review of preclinical and clinical data. Clin Breast Cancer. 2015;16(1):8–17. doi: 10.1016/j.clbc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 132.An H.X., Beckmann M.W., Reifenberger G. Gene amplification and overexpression of CDK4 in sporadic breast carcinomas is associated with high tumor cell proliferation. Am J Pathol. 1999;154:113–118. doi: 10.1016/S0002-9440(10)65257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roberts P.J., Bisi J.E., Strum J.C. Multiple roles of cyclin-dependent kinase 4/6 inhibitors in cancer therapy. J Natl Cancer Inst. 2012;104:476–487. doi: 10.1093/jnci/djs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goel S., Wang Q., Watt A.C. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell. 2016;29:255–269. doi: 10.1016/j.ccell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clinicaltrials.gov. A study of abemaciclib (LY2835219) in women With HR+, HER2+ locally advanced or metastatic breast cancer (monarcHER). http://www.clinicaltrials.gov (28 March 2016, date last accessed).
- 136.Raub T.J., Wishart G.N., Kulanthaivel P. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab Dispos. 2015;43:1360–1371. doi: 10.1124/dmd.114.062745. [DOI] [PubMed] [Google Scholar]
- 137.Sahebjam S., Rhun E.L., Kulanthaivel P. Assessment of concentrations of abemaciclib and its major active metabolites in plasma, CSF, and brain tumor tissue in patients with brain metastases secondary to hormone receptor positive (HR+) breast cancer. J Clin Oncol. 2016;34 (suppl abstr 526) [Google Scholar]
- 138.Salhia B., Kiefer J., Ross J.T. Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS One. 2014;9:e85448. doi: 10.1371/journal.pone.0085448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nair J.S., Ho A.L., Tse A.N. Aurora B kinase regulates the postmitotic endoreduplication checkpoint via phosphorylation of the retinoblastoma protein at serine 780. Mol Biol Cell. 2009;20:2218–2228. doi: 10.1091/mbc.E08-08-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vader G., Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 141.Dar A.A., Goff L.W., Majid S. Aurora kinase inhibitors–rising stars in cancer therapeutics? Mol Cancer Ther. 2010;9:268–278. doi: 10.1158/1535-7163.MCT-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Goldenson B., Crispino JD. The aurora kinases in cell cycle and leukemia. Oncogene. 2015;34:537–545. doi: 10.1038/onc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hole S., Pedersen A.M., Lykkesfeldt A.E., Yde CW. Aurora kinase A and B as new treatment targets in aromatase inhibitor-resistant breast cancer cells. Breast Cancer Res Treat. 2015;149:715–726. doi: 10.1007/s10549-015-3284-8. [DOI] [PubMed] [Google Scholar]
- 144.Clinicaltrials.gov. Phase I study of MLN0128 and MLN8237 in patients with advanced solid tumors and metastatic triple-negative breast cancer. http://www.clinicaltrials.gov (28 March 2016, date last accessed).
- 145.Motzer R.J., Escudier B., McDermott D.F. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:9–15. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 148.Sampson J.H., Archer G.E., Ashley D.M. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the “immunologically privileged” central nervous system. Proc Natl Acad Sci U S A. 1996;93:10399–10404. doi: 10.1073/pnas.93.19.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Margolin K., Ernstoff M.S., Hamid O. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 150.Nanda R., Chow L.Q., Dees E.C. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib keynote-012 study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.LYDirixITakacsPNikolinakos. [S1-04] Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase Ib JAVELIN solid tumor trial. San Antonio Breast Conference 2015.
- 152.Xiu J., Gatalica Z., Reddy S. Distinct biomarker features in triple-negative breast cancer metastases to the brain, liver and bone. San Antonio Breast Conference. 2015. [P3-07-27] [Google Scholar]
- 153.Duchnowska R., Peksa R., Radecka B. Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res. 2016;18:43. doi: 10.1186/s13058-016-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Verma S., Miles D., Gianni L. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Swain S.M., Baselga J., Kim S.B. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Thomson A.H., Purvis G., McGrane J. Changing molecular profile of brain metastases compared with matched breast primaries and impact on clinical outcomes. San Antonio Breast Conference. 2015. [P6-17-07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang Y., Springer S., Zhang M. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A. 2015;112:9704–9709. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kurzrock R., Giles FJ. Precision oncology for patients with advanced cancer: the challenges of malignant snowflakes. Cell Cycle. 2015;14:2219–2221. doi: 10.1080/15384101.2015.1041695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fortin D., Gendron C., Boudrias M., Garant MP. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in the treatment ofcerebral metastasis. Cancer. 2007;109:751–760. doi: 10.1002/cncr.22450. [DOI] [PubMed] [Google Scholar]
- 160.Fortin D., Desjardins A., Benko A. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: the Sherbrooke experience. Cancer. 2005;103:2606–2615. doi: 10.1002/cncr.21112. [DOI] [PubMed] [Google Scholar]
- 161.Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81. doi: 10.1016/s0169-409x(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 162.Donelli M.G., Zucchetti M., D’Incalci M. Do anticancer agents reach the tumor target in the human brain? Cancer Chemother Pharmacol. 1992;30:251–260. doi: 10.1007/BF00686291. [DOI] [PubMed] [Google Scholar]
- 163.Witzel I., Loibl S., Laakmann E. Brain metastases in breast cancer network Germany (BMBC, GBG 79): first analysis of 548 patients from the multicenter registry. San Antonio Breast Conference. 2015. [Google Scholar]
- 164.Clinicaltrials.gov. Breast cancer with over-expression of erbB2-BRAINSTORM. http://www.clinicaltrials.gov (5 April 2016, date last accessed).


