Abstract
Background
Several studies have demonstrated the antitumor activity of first-generation somatostatin analogs (SSAs), primarily targeting somatostatin receptor (sstr) subtypes 2 and 5, in neuroendocrine tumors (NET). Pasireotide, a second-generation SSA, targets multiple sstr subtypes. We compared the efficacy and safety of pasireotide plus everolimus to everolimus alone in patients with advanced, well-differentiated, progressive pancreatic NET.
Patients and methods
Patients were randomized 1 : 1 to receive a combination of everolimus (10 mg/day, orally) and pasireotide long-acting release (60 mg/28 days, intramuscularly) or everolimus alone (10 mg/day, orally); stratified by prior SSA use, and baseline serum chromogranin A and neuron-specific enolase. The primary end point was progression-free survival (PFS). Secondary end points included overall survival, objective response rate, disease control rate, and safety. Biomarker response was evaluated in an exploratory analysis.
Results
Of 160 patients enrolled, 79 were randomized to the combination arm and 81 to the everolimus arm. Baseline demographics and disease characteristics were similar between the treatment arms. No significant difference was observed in PFS: 16.8 months in combination arm versus 16.6 months in everolimus arm (hazard ratio, 0.99; 95% confidence interval, 0.64–1.54). Partial responses were observed in 20.3% versus 6.2% of patients in combination arm versus everolimus arm; however, overall disease control rate was similar (77.2% versus 82.7%, respectively). No significant improvement was observed in median overall survival. Adverse events were consistent with the known safety profile of both the drugs; grade 3 or 4 fasting hyperglycemia was seen in 37% versus 11% of patients, respectively.
Conclusions
The addition of pasireotide to everolimus was not associated with the improvement in PFS compared with everolimus alone in this study. Further studies to delineate mechanisms by which SSAs slow tumor growth in NET are warranted.
Key words: everolimus, pasireotide LAR, pancreatic neuroendocrine tumors, mTOR signaling, somatostatin analog, insulin-like growth factor-1
Introduction
Pancreatic neuroendocrine tumors (pNET) comprise ∼5% of all pancreatic malignancies [1], and are characterized by the ability to secrete hormones that cause diverse clinical syndromes [2]. The majority of patients with pNET present at an advanced stage for which median survival durations are reported between 2 and 3 years [2,3]. Two molecular targeted agents, everolimus [4] and sunitinib [5] have been shown to prolong progression-free survival (PFS) in this setting [4,5]. First-generation somatostatin analogs (SSAs), including octreotide and lanreotide, target primarily somatostatin receptor subtype 2 (sstr2) and have been shown to be effective for both symptom and tumor growth control in gastroenteropancreatic NETs [6., 7., 8.].
Pasireotide (SOM230) is an investigational multiligand SSA that, compared with octreotide, has greater binding affinity for sstr1,3,5 and slightly lower affinity for sstr2 [9]. It has been reported to be active as a single agent in patients with metastatic NET [10]. Evidence from preclinical studies, albeit not in NET models, suggests that the combination of pasireotide and everolimus may be synergistic via dual inhibition of insulin-like growth factor-1 (IGF-1) and mammalian target of rapamycin (mTOR) signaling [11].
A combination of everolimus (10 mg/day) with pasireotide long-acting release (LAR) (60 mg/28 days) was found to be safe and associated with preliminary evidence of activity in patients with advanced NET [12]. We conducted a randomized phase 2 study to evaluate the efficacy and safety of everolimus in combination with pasireotide LAR compared with everolimus alone in patients with advanced, well-differentiated, progressive pNET.
Methods
Patients
Eligible patients included adults (age ≥18 years) with histologically confirmed, well-differentiated [World Health Organization (WHO) grade 1 or 2] [13], advanced pNET, with radiological documentation of disease progression within 12 months before randomization and measurable disease per Response Evaluation Criteria In Solid Tumors (RECIST v1.0) [14]. Additional inclusion criteria were WHO performance status (PS) ≤2, and adequate bone marrow, renal and hepatic function. Patients who had received prior mTOR inhibitors or clinically required SSA treatment were not eligible.
Study design
COOPERATE-2 (COmbination Of Pasireotide and evERolimus in Advanced Tumors of neuroEndocrine origin, second trial;www.clinicaltrials.gov, NCT01374451) is a randomized, global, multicenter, open-label, phase 2 trial. Patients were randomly assigned (1 : 1) to receive everolimus [10 mg/day, per oral (p.o.)] with pasireotide LAR (60 mg/28 days, i.m.) or everolimus alone. Patients were stratified by prior SSA treatment (yes or no) and the presence of elevated biomarkers at baseline [yes or no; defined as chromogranin A (CgA) >2 × upper limit of normal (ULN) and/or neuron-specific enolase (NSE) >1 × ULN; both measured centrally and chosen based on data from previous randomized studies demonstrating that elevation of these biomarkers was strongly associated with prognosis] [15., 16., 17., 18.]. Patients continued treatment until radiologically documented disease progression, start of a new anticancer therapy, intolerable toxicity, or withdrawal of consent. Crossover was not allowed. The study was approved by an independent ethics committee or institutional review board for each center and was monitored by an independent data monitoring committee. The study was conducted in accordance with Good Clinical Practice and ethical principles of the Declaration of Helsinki. All patients provided written informed consent before enrollment.
Assessments
The primary objective was to estimate the treatment effect on PFS assessed per RECIST v1.0 by local review. Objective response rate (ORR), disease control rate (DCR), overall survival (OS), pharmacokinetics (PKs), and safety were secondary objectives. Biomarker response was evaluated as an exploratory analysis. Please seesupplementary material, available at Annals of Oncology online for further details on study assessments.
Supplementary Data.

Statistical analysis
Sample size computation was based on the predictive probability of success of a subsequent phase 3 study [19]. With an observed hazard ratio (HR) of 0.783 (equivalent to clinically significant improvement of median PFS by 3 months, set as a threshold for success) or lower in this phase 2 study, the predictive probability of success in a following phase 3 study is 50% or greater. The probability of meeting the primary objective of this phase 2 study (i.e. observing HR of 0.783) was targeted to be ≥75% (power) assuming a true HR of 0.667, and at most 15% (alpha) assuming a true HR of 1. Eighty PFS events, and 150 patients enrolled and randomized in a ratio of 1 : 1 were required to meet these criteria.
Efficacy analysis was carried out on the full analysis set, which comprised all randomized patients (intention-to-treat population). PFS and OS were assessed by Kaplan–Meier method; treatment arms were compared using one-sided stratified log-rank test. Hazard ratios (HR) were estimated using a stratified Cox proportional hazards model. All patients, who received study medication and had ≥1 postbaseline safety assessment, were evaluated for safety. Please seesupplementary material, available at Annals of Oncology online for details on sample size calculation.
Results
Patient characteristics
Between July 2011 and December 2012, 160 patients were randomized. The two treatment arms were well balanced with regard to baseline demographics and disease characteristics (Table 1). More patients in the combination arm had received chemotherapy as their last treatment (27.8% versus 17.3%). Approximately one-third of patients in both the arms had received prior SSA therapy.
Table 1.
Patient characteristics
| Demographic variable |
Everolimus + pasireotide LAR |
Everolimus |
|---|---|---|
| n = 79 | n = 81 | |
| Median age, years (range) | 57 (22–79) | 59 (26–82) |
| Male,n (%) | 39 (49.4) | 47 (58.0) |
| Median time since initial diagnosis, months (range) | 28.5 (1–328) | 28.7 (1–335) |
| WHO performance status,n (%) | ||
| 0–1 | 79 (100) | 78 (96.3) |
| 2 | 0 | 3 (3.7) |
| Tumor grade,a,bn (%) | ||
| Well-differentiated (grade 1 or 2) | 77 (97.5) | 79 (97.5) |
| Poorly differentiated (grade 3) | 0 | 1 (1.2) |
| Unknown | 2 (2.5) | 1 (1.2) |
| Prior antineoplastic treatment,n (%) | ||
| No | 28 (38.4) | 31 (38.3) |
| Yes | 51 (64.6) | 50 (61.7) |
| Chemotherapy as last treatment,n (%) | 22 (27.8) | 14 (17.3) |
| Prior SSA treatment,n (%) | 26 (32.9) | 27 (33.3) |
| Elevated biomarkers,cn (%) | 45 (57.0) | 44 (54.3) |
CgA, chromogranin A; LAR, long-acting release; NSE, neuron-specific enolase; SSAs, somatostatin analogs; ULN, upper limit of normal; WHO, World Health Organization.
Patients with histological grade or WHO grade.
As per inclusion/exclusion criteria, patients with histological grade 1 or 2 were allowed; one patient with tumor grade 3 and three patients with unknown tumor grade were enrolled (protocol deviations). Two of the patients with unknown tumor grade in the combination arm were reported to have the Ki67 index of 5% and 7%).
Elevated biomarkers were defined as CgA >2 × ULN and/or NSE >1 × ULN. (CgA: ULN is 15 μg/l; NSE: ULN is 8.6 μg/l; both CgA and NSE were measured centrally).
Treatment exposure
All but one patient in the combination arm and all patients in the everolimus arm received study treatment (the one patient was randomized but had an abnormal postrandomization electrocardiogram, and was not treated). At the time of data cutoff (2 April 2014), treatment was ongoing in 19 patients (24%) in the combination arm versus 18 patients (22%) in the everolimus arm. The main reasons for discontinuation of treatment were disease progression, adverse events (AEs), or withdrawal of consent (CONSORT diagram,supplementary Figure S1, available at Annals of Oncology online). The median duration of exposure to everolimus was 49.4 weeks in the combination arm and 48.3 weeks in the everolimus arm. The median duration of pasireotide LAR exposure was 52.1 weeks. Dose interruption or reduction was more frequent in the combination arm (seesupplementary Table S4, available at Annals of Oncology online). The median (range) relative dose intensity of everolimus in the combination arm was 0.78 (0.26–1.00) and in the monotherapy arm was 0.98 (0.19–1.00).
Efficacy
The median PFS [95% confidence interval (CI)] was similar between the two arms: 16.8 (12.1–19.6) months versus 16.6 (11.1–19.5) months in the combination arm versus everolimus arm, respectively (HR, 0.991; 95% CI, 0.636–1.543) (Figure 1A). No differences in OS were observed, with a median follow-up of 22.6 months (Figure 1B). While sixteen patients (20%) in the combination arm and five patients (6%) in the everolimus arm experienced PR, the total number of patients who experienced tumor shrinkage was similar in both the arms (Figure 2) as was the overall DCR (CR + PR + SD; 77% in the combination arm versus 83% in the monotherapy arm).
Figure 1.

Kaplan–Meier curves for (A) progression-free survival (B) overall survival, assessed by local radiology review (full analyses set).aHazard ratio was obtained from the stratified unadjusted Cox’s model.bP value was obtained from the stratified one-sided log-rank test. CI, confidence interval; LAR, long-acting release.
Figure 2.
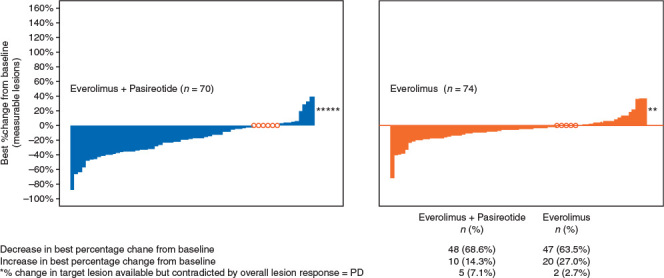
Best percentage change from baseline in sum of longest diameters of measurable lesions based on local radiology review (full analysis set). Patients, for whom percentage change in target lesions was not available and for whom investigator study assessment of PD did not correlate with tumor measurements, were excluded from the analysis (denoted as *). Patients with no change as their best response are denoted aso. Patients with no postbaseline tumor scan or only a scan with the overall lesion response ‘unknown’ were excluded from this analysis. PD, progressive disease.
Soluble biomarkers
Patients on combination therapy had a rapid and sustained decrease from baseline in IGF-1, IGF-2, and IGF binding protein 3 (IGFBP3) levels by 72%, 21%, and 37%, respectively, after 12 weeks. No consistent decrease in these markers was observed in patients treated with everolimus monotherapy (Figure 3). IGFBP2 levels increased in patients on everolimus and pasireotide combination therapy (by 85% at the beginning of cycle 4), but remained unchanged in the everolimus monotherapy arm. Among the biomarkers evaluated at day 1 of cycle 4, the decrease from baseline for CgA, NSE, IGF-1, IGF-2, IGFBP-3 was larger in the combination arm compared with the everolimus arm (supplementary Table S5, available at Annals of Oncology online).
Figure 3.
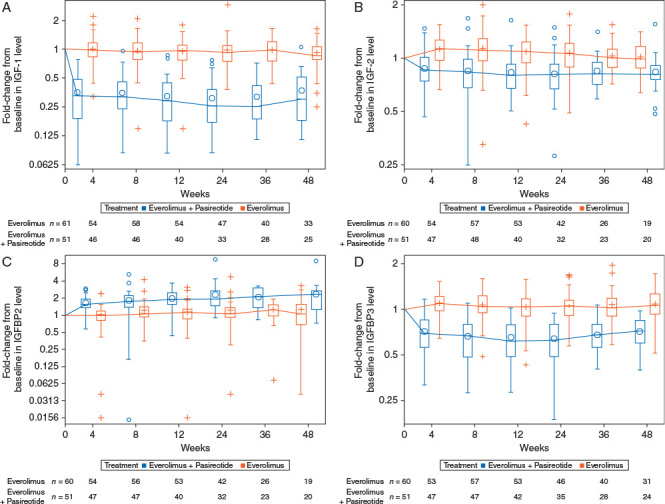
Fold-change from baseline in levels of (A) IGF-1, (B) IGF-2, (C) IGFBP2 and (D) IGFBP3. IGF-1, insulin-like growth factor 1; IGF-2, insulin-like growth factor 2; IGFBP2, insulin-like growth factor binding protein 2; IGFBP3, insulin-like growth factor binding protein 3.
Safety
AEs observed in the study were consistent with the known safety profiles of both the drugs (Table 2). AEs of any grade were reported in 78% versus 81% of patients, and grade 3 or 4 AEs were reported in 77% versus 69% of patients in the combination and monotherapy arms, respectively. Grade 4 drug-related AEs were infrequent and were reported in one patient, in each arm.
Table 2.
Selected adverse events of clinical relevance (≥5% incidence), irrespective of study drug relationship (safety set)
| AE, n (%) | Everolimus + pasireotide LAR |
Everolimus |
||||
|---|---|---|---|---|---|---|
|
n = 78 |
n = 81 |
|||||
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | |
| Hyperglycemia | 59 (75.6) | 28 (35.9) | 1 (1.3) | 22 (27.2) | 9 (11.1) | 0 |
| Diarrhea | 49 (62.8) | 4 (5.1) | 0 | 43 (53.1) | 3 (3.7) | 0 |
| Stomatitis | 46 (59.0) | 7 (9.0) | 0 | 51 (63.0) | 7 (8.6) | 0 |
| Abdominal pain | 21 (26.9) | 1 (1.3) | 0 | 29 (35.8) | 5 (6.2) | 0 |
| Diabetes mellitus | 20 (25.6) | 9 (11.5) | 0 | 6 (7.4) | 1 (1.2) | 0 |
| Hypoglycemia | 18 (23.1) | 4 (5.1) | 1 (1.3) | 4 (4.9) | 1 (1.2) | 0 |
| Pneumonitis | 6 (7.7) | 1 (1.3) | 0 | 10 (12.3) | 1 (1.2) | 0 |
AE, adverse event; LAR, long-acting release.
A higher incidence of hyperglycemia, diabetes, and elevated hemoglobin A1c (HbA1c) was observed in the combination arm. Postbaseline shift in HbA1c to ≥9% (from <7% of HbA1c at baseline) was observed in 23% of patients receiving combination therapy versus 5% of patients on monotherapy. Rates of diarrhea, stomatitis, abdominal discomfort, and pneumonitis were similar. Cardiac events, including syncope, QT-prolongation, loss of consciousness, ventricular fibrillation, were observed in four patients (5%) in combination arm and five patients (6%) in monotherapy arm. The proportion of patients requiring ≥1 dose reduction or dose interruption was higher in combination arm versus monotherapy arm (53% versus 36% and 65% versus 56%, respectively).
Four of the six deaths in the combination arm and five of the nine deaths in the everolimus arm were attributable to underlying cancer or disease progression. Overall, three deaths were suspected to be treatment related (ketoacidosis and sepsis in the combination arm; pulmonary embolism in the everolimus arm).
Discussion
Our study represents the first randomized study to evaluate the antitumor effect of everolimus combined with an SSA specifically in pNET. We found no difference in PFS between the two arms, and the overall DCR was similar. The safety findings observed for the combination were consistent with the known safety profiles of the individual drugs. We observed decreases in biochemical markers associated with the addition of pasireotide to everolimus in the combination arm.
While pasireotide did not contribute to an improved PFS, an antisecretory effect associated with pasireotide was confirmed by the suppression of tumor growth factors IGF-1 and IGF-2, and corresponding changes of the IGF-binding proteins, IGFBP2 and IGFBP3. Pasireotide is thought to decrease IGF-1 levels primarily through its ability to decrease growth hormone (GH) secretion in the pituitary gland, thereby decreasing the synthesis of IGF-1 in the liver [20]. The suppression of IGF-2 observed in this trial suggests that pasireotide may also act directly on autocrine/paracrine growth signals in the tumor, since IGF-2 is known to be secreted by tumors and surrounding cells [21., 22., 23.]. Of the 6 IGFBPs, IGFBP3 is the major carrier of IGF ligands and is also responsive to GH. The decrease in IGFBP3 provides additional evidence that pasireotide was associated with an antisecretory effect.
The safety and tolerability of everolimus and pasireotide LAR were consistent with the previous clinical experience for each drug [4,24]. Hyperglycemia and diabetes were more common in the combination arm. PK sampling, albeit in a limited cohort of patients, suggested that exposure to everolimus was higher in the combination arm of the study, and further suggested no clear relationship between everolimus PK levels and PFS. Differences in drug exposure, therefore, seem to be an unlikely explanation for the lack of observation of a PFS benefit observed with pasireotide in this study.
Based on evidence of an antiproliferative effect associated with the first-generation SSAs in gastroenteropancreatic NET [7,8] we anticipated observing a PFS benefit associated with adding pasireotide to everolimus. Data from studies in which SSAs have been combined with everolimus also suggested a potential benefit to adding SSAs. In the single-arm RADIANT-1 study, in patients with advanced pNET, patients receiving everolimus experienced a median PFS of 9.7 months while those receiving everolimus plus octreotide LAR had a PFS of 16.7 months [25]. Encouraging PFS durations were also observed in a study of patients with advanced, well-differentiated NET of gastroenteropancreatic and lung origin receiving everolimus and octreotide LAR [26].
A close analysis of patient demographics in both the arms failed to reveal obvious differences that would account for the observation that pasireotide failed to prolong PFS in our study. Central review was not required in our study, as it was designed as a randomized phase II study to estimate the probability of success in a formal phase III study. However, lack of central review and potential investigator bias seems an unlikely explanation for a null study. Patients were well balanced with regard to key prognostic factors, including age, tumor grade, and PS. We did note a relatively high rate of grade 2 tumors in both the treatment arms. However, the PFS duration observed in both the arms of our study was relatively longer (16.6 and 16.8 months) than those observed in the RADIANT-3 trial which also evaluated patients with advanced pNET [4].
While patients in our study were required to have had disease progression within 12 months of study entry, RECIST-defined progression was not required and no central review to confirm progression before study entry was carried out, and it is possible that there could have been differences in baseline progression status between arms. Additionally, sstr scintigraphy was not required and somatostatin receptor status was not known before enrollment, and it is possible that there could have been an imbalance in this variable affecting outcome.
It is also conceivable that differences in the biological effects of pasireotide and octreotide or lanreotide account for the lack of PFS benefit observed with pasireotide compared with what was observed in PROMID [7] and CLARINET [8]. In addition to its ability to bind to multiple sstr subtypes, pasireotide differs from octreotide in its ability to phosphorylate sstr2. Octreotide induces persistent phosphorylation and partial internalization of sstr2 while pasireotide does not induce sstr2 phosphorylation and induces only transient internalization of the receptor [27]. Whether this difference translates into a difference in the ability of the two drugs to slow tumor growth, however, is unclear.
In summary, the combination of pasireotide LAR and everolimus resulted in an antisecretory effect manifested by decreased levels of proteins in the IGF-axis, but did not improve PFS compared with everolimus alone in patients with advanced pNET. Further studies, defining the mechanisms by which SSAs inhibit tumor growth and investigating potential interference between signaling pathways when everolimus is combined with SSAs, are warranted.
Acknowledgements
The COOPERATE-2 study group is grateful to all physicians and patients who contributed to the present study, and also to the worldwide network of research nurses, trial coordinators, and operations staff for their contributions. We appreciate the support of the colleagues of the COOPERATE-2 study team who contributed to the presented data. Additionally, we thank Du Lam, MD for assistance with protocol development and study conduct, David Chen, PhD, Herbert Schmidt, PhD, Amanda Wang, PhD and Parul Patel for biomarker analyses, and Rajasree Solipuram, PhD, Novartis Healthcare Pvt. Ltd. for providing medical editorial assistance with this manuscript.
Funding
This study was funded by Novartis Pharmaceuticals Corporation and there is no grant number.
Disclosure
MHK received personal fees for consulting or advisory role (Ipsen, Novartis, Lexicon). PR received honoraria (Novartis, Ipsen, Advanced Accelerator Applications), personal fees for consulting or advisory role (Novartis, Ipsen, Advanced Accelerator Applications), research funding (Novartis, Ipsen), and travel, accommodation expenses (Ipsen). EVC received research funding (Novartis). CL-B received personal fees for consulting or advisory role (Novartis). JWV received honoraria (Novartis), personal fees for consulting or advisory role (Novartis), personal fees to participate in a speakers' bureau (Novartis), research funding (Novartis), and travel accommodation expenses (Novartis). WWDH received honoraria (Ipsen, Novartis), personal fees for consulting or advisory role (Ipsen, Novartis), and research funding (Ipsen, Novartis). MP received honoraria (Novartis, Ipsen, Pfizer, Lexicon), personal fees for consulting or advisory role (Novartis, Ipsen, Pfizer, Lexicon), and research funding (Novartis, Ipsen). ED is an employee at Novartis and has stock or other ownership (Novartis). JCB is an employee at Novartis. LBP is an employee at Novartis and has stock or other ownership (Novartis). VM is an employee at Novartis and has stock or other ownership (Novartis, Pfizer, Bristol-Myers Squibb). RS received personal fees for consulting or advisory role (Novartis), personal fees to participate in a speakers' bureau (Novartis), and travel accommodation expenses (Novartis). IB received honoraria (Novartis, Ipsen, Pfizer, Bayer), personal fees for consulting or advisory role (Novartis, Ipsen), and research funding (Ipsen, Bayer). NF received honoraria (Novartis, Ipsen), personal fees for consulting of advisory role (Novartis, Ipsen), and research funding (Novartis). RR received honoraria (Novartis), personal fees for consulting or advisory role (Novartis, Ipsen), and travel, accommodations, expenses (Novartis, Ipsen). JCY received personal fees for consulting or advisory role (Novartis, Ipsen, Advanced Accelerator Applications, Merck, Necktar), and research funding (Novartis). DS and JC have declared no conflicts of interest.
Footnotes
Note: This study was previously presented in part at: European Neuroendocrine Tumors Society (ENETS) 2015, Barcelona, Spain.
References
- 1.Hackeng W.M., Hruban R.H., Offerhaus G.J., Brosens L.A. Surgical and molecular pathology of pancreatic neoplasms. Diagn Pathol. 2016;11:47.. doi: 10.1186/s13000-016-0497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao J.C., Hassan M., Phan A. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. JCO. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Yao J.C., Eisner M.P., Leary C. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–3500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao J.C., Shah M.H., Ito T. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raymond E., Dahan L., Raoul J.L. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 6.Khan M.S., Caplin M.E. Therapeutic management of patients with gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer. 2011;18(Suppl1):S53–S74. doi: 10.1530/ERC-10-0271. [DOI] [PubMed] [Google Scholar]
- 7.Rinke A., Muller H.H., Schade-Brittinger C. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. JCO. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 8.Caplin M.E., Pavel M., Cwikla J.B. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 9.Schmid H.A., Schoeffter P. Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology. 2004;80(Suppl1):47–50. doi: 10.1159/000080741. [DOI] [PubMed] [Google Scholar]
- 10.Wolin E.M., Jarzab B., Eriksson B. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des Devel Ther. 2015;9:5075–5086. doi: 10.2147/DDDT.S84177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weckbecker G., Briner U., Lewis I., Bruns C. SOM230: a new somatostatin peptidomimetic with potent inhibitory effects on the growth hormone/insulin-like growth factor-I axis in rats, primates, and dogs. Endocrinology. 2002;143:4123–4130. doi: 10.1210/en.2002-220219. [DOI] [PubMed] [Google Scholar]
- 12.Chan J.A., Ryan D.P., Zhu A.X. Phase I study of pasireotide (SOM 230) and everolimus (RAD001) in advanced neuroendocrine tumors. Endocr Relat Cancer. 2012;19:615–623. doi: 10.1530/ERC-11-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klimstra D.S., Modlin I.R., Coppola D. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712. doi: 10.1097/MPA.0b013e3181ec124e. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Yao J.C., Pavel M., Phan A.T. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96:3741–3749. doi: 10.1210/jc.2011-0666. [DOI] [PubMed] [Google Scholar]
- 16.Pavel M.E., Hainsworth J.D., Baudin E. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 17.Modlin I.M., Gustafsson B.I., Moss S.F. Chromogranin A–biological function and clinical utility in neuro endocrine tumor disease. Ann Surg Oncol. 2010;17:2427–2443. doi: 10.1245/s10434-010-1006-3. [DOI] [PubMed] [Google Scholar]
- 18.Nikou G.C., Marinou K., Thomakos P. Chromogranin a levels in diagnosis, treatment and follow-up of 42 patients with non-functioning pancreatic endocrine tumours. Pancreatology. 2008;8:510–519. doi: 10.1159/000152000. [DOI] [PubMed] [Google Scholar]
- 19.Gotte H., Schuler A., Kirchner M., Kieser M. Sample size planning for phase II trials based on success probabilities for phase III. Pharm Stat. 2015;14:515–524. doi: 10.1002/pst.1717. [DOI] [PubMed] [Google Scholar]
- 20.Schmid H.A. Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74. doi: 10.1016/j.mce.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen A.A., Cullen K.J. Paracrine/autocrine regulation of breast cancer by the insulin-like growth factors. Breast Cancer Res Treat. 1998;47:219–233. doi: 10.1023/a:1005903000777. [DOI] [PubMed] [Google Scholar]
- 22.Sciacca L., Costantino A., Pandini G. Insulin receptor activation by IGF-II in breast cancers: evidence for a new autocrine/paracrine mechanism. Oncogene. 1999;18:2471–2479. doi: 10.1038/sj.onc.1202600. [DOI] [PubMed] [Google Scholar]
- 23.Li B., Tsao S.W., Chan K.W. Id1-induced IGF-II and its autocrine/endocrine promotion of esophageal cancer progression and chemoresistance–implications for IGF-II and IGF-IR-targeted therapy. Clin Cancer Res. 2014;20:2651–2662. doi: 10.1158/1078-0432.CCR-13-2735. [DOI] [PubMed] [Google Scholar]
- 24.Cives M., Kunz P.L., Morse B. Phase II clinical trial of pasireotide long-acting repeatable in patients with metastatic neuroendocrine tumors. Endocr Relat Cancer. 2015;22:1–9. doi: 10.1530/ERC-14-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao J.C., Lombard-Bohas C., Baudin E. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. JCO. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajetta E., Catena L., Fazio N. Everolimus in combination with octreotide long-acting repeatable in a first-line setting for patients with neuroendocrine tumors: an ITMO group study. Cancer. 2014;120:2457–2463. doi: 10.1002/cncr.28726. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed A., Blanchard M.P., Albertelli M. Pasireotide and octreotide antiproliferative effects and sst2 trafficking in human pancreatic neuroendocrine tumor cultures. Endocr Relat Cancer. 2014;21:691–704. doi: 10.1530/ERC-14-0086. [DOI] [PubMed] [Google Scholar]


