Abstract
Background
In the phase 3 RADIANT-2 study, everolimus plus octreotide long-acting repeatable (LAR) showed improvement of 5.1 months in median progression-free survival versus placebo plus octreotide LAR among patients with advanced neuroendocrine tumors associated with carcinoid syndrome. The progression-free survival P-value was marginally above the prespecified threshold for statistical significance. Here, we report final overall survival (OS) and key safety update from RADIANT-2.
Patients and methods
The RADIANT-2 trial compared everolimus (10 mg/day, orally; n = 216) versus placebo (n = 213), both in conjunction with octreotide LAR (30 mg, intramuscularly, every 28 days). Patients, unblinded at the time of progression or after end of double-blind core phase following primary analysis, were offered open-label everolimus with octreotide LAR (open-label phase). In the open-label phase, patients had similar safety and efficacy assessments as those in the core phase. For OS, hazard ratios (HRs) with 95% CIs using unadjusted Cox model and a Cox model adjusted for prespecified baseline covariates were calculated.
Results
A total of 170 patients received open-label everolimus (143 crossed over from the placebo arm; 27 in the everolimus arm continued to receive the same treatment after unblinding). The median OS (95% CI) after 271 events was 29.2 months (23.8–35.9) for the everolimus arm and 35.2 months (30.0–44.7) for the placebo arm (HR, 1.17; 95% CI, 0.92–1.49). HR adjusted for baseline covariates was 1.08 (95% CI, 0.84–1.38). The most frequent drug-related grade 3 or 4 AEs reported during the open-label phase were diarrhea (5.3%), fatigue (4.7%), and stomatitis (4.1%). Deaths related to pulmonary or cardiac failure were observed more frequently in the everolimus arm.
Conclusion
No significant difference in OS was observed for the everolimus plus octreotide LAR and placebo plus octreotide LAR arms of the RADIANT-2 study, even after adjusting for imbalances in the baseline covariates.
Clinical Trial Number
Key words: everolimus, neuroendocrine tumors, carcinoid syndrome, overall survival
Novartis Pharmaceuticals Corporation
Introduction
Neuroendocrine tumors (NET) are heterogeneous neoplasms originating from the diffuse neuroendocrine system at diverse primary sites [1]. NET can produce and secrete bioactive amines, peptides, and polypeptides. Excessive release of serotonin and other bioactive substances leads to the classical symptoms of diarrhea and flushing associated with carcinoid syndrome [2]. NET are often diagnosed at an advanced stage with metastatic disease in most cases; 65% of patients with NET die within 5 years of diagnosis [1]. The survival rates in patients with NET vary greatly by tumor origin and grade [1].
In the large, randomized, double-blind, placebo-controlled, phase 3 RADIANT-2 study, statistically not significant improvement of 5.1 months in median progression-free survival (PFS) was reported with everolimus plus octreotide long-acting repeatable (LAR) compared with placebo plus octreotide LAR in patients with low- or intermediate-grade advanced NET and a history of carcinoid symptoms (16.4 months versus 11.3 months; HR, 0.77; 95% CI, 0.59–1.00; one-sided log-rank test, P = 0.026; prespecified boundary for significance, P ≤ 0.0246) [3].
Here, we report an update on the secondary end point of overall survival (OS) and key safety information as of the final data cutoff date (13 June 2013) for the RADIANT-2 study. We also explored the prognostic or predictive value of baseline chromogranin A (CgA) by assessing the relationship between OS and baseline CgA.
Methods
Study design and participants
RADIANT-2 (NCT00412061) was a randomized, double-blind, placebo-controlled, multicenter, phase 3 trial and has been described previously [3]. PFS assessed by central radiology review was the primary end point. Efficacy and safety analyses from the double-blind phase have been reported earlier [3].
Patients ≥18 years with advanced (unresectable or metastatic), well or moderately differentiated NET and a history of symptoms attributed to carcinoid syndrome (flushing, diarrhea, or both) and progression within 12 months before randomization who had a WHO performance status score ≤2 were randomly assigned 1 : 1 to receive oral everolimus (10 mg/day) or placebo, both in conjunction with intramuscular octreotide LAR (30 mg every 28 days).
Treatment in the double-blind phase (core phase) continued until disease progression, development of unacceptable adverse events (AEs), patient consent withdrawal, or primary analysis (cutoff date, 2 April 2010). Upon progression according to RECIST version 1.0 [4], crossover from the placebo arm to open-label everolimus plus octreotide LAR was allowed during the double-blind phase. At the end of the double-blind phase, all ongoing patients were unblinded. Patients initially assigned to placebo arm were offered to switch to open-label everolimus and those in the everolimus arm were transitioned to open-label everolimus (in both the cases patients received octreotide LAR). Patients who had already crossed over during the double-blind phase continued to receive open-label everolimus.
All patients who transitioned to or continued with open-label everolimus (denoted as open-label extension phase) had only local radiologic assessment. In the open-label extension phase, the same safety and efficacy assessments as those of the double-blind phase were conducted, except for pharmacokinetic and biomarker assessments [3]. The open-label extension phase continued until disease progression when patients discontinued everolimus and entered the follow-up phase, which involved monthly monitoring for survival information until the data cutoff date for final analysis (13 June 2013).
The study was conducted according to Good Clinical Practice guidelines and ethical principles of the Declaration of Helsinki. The institutional review board or independent ethics committee at each study site approved the protocol and all amendments. All patients provided written informed consent before randomization.
The full analysis set included all randomized patients. Patients were analyzed according to the intent-to-treat principle (i.e. the study treatment that they were assigned to at randomization), which was also applicable for the updated OS analysis when patients switched from placebo to open-label everolimus.
The safety set included all patients who received at least one dose of study drug during the double-blind phase and had at least one postbaseline safety assessment. Patients were included in the everolimus plus octreotide LAR arm if they received at least one dose of everolimus during the double-blind phase.
The open-label safety set included all patients who received at least one dose of open-label everolimus and had at least one safety assessment after the start of the treatment with open-label everolimus.
Statistical analyses
For the final OS results, no formal statistical testing was carried out because the primary end point of the study was not met (hierarchical testing for control of type I error). Only HRs with 95% CIs using unadjusted Cox model and a Cox model adjusted for prespecified baseline covariates [age (<65 years versus ≥65 years), sex (male versus female), race (Caucasian versus non-Caucasian), WHO PS (0 versus >0), and prior use of somatostatin analogs (SSAs; yes versus no)] were calculated for OS. A post hoc Cox model adjusted for baseline prognostic covariates that were unbalanced between the two arms [WHO PS (0 versus >0), baseline CgA (>2 × ULN versus ≤2 × ULN), lung as primary site (yes versus no), bone involvement (yes versus no)] was also developed. Annual survival rate up to 48 months was calculated using Kaplan–Meier estimates.
The relationship between OS and baseline CgA was explored. A comparison of OS within each treatment group by baseline CgA level using unstratified Cox model was carried out. HR and P value were obtained using a one-sided log-rank test.
All AEs recorded until the cutoff date were evaluated. The safety analyses included all on-treatment AEs that occurred within 28 days after discontinuation of the study treatment. All safety analyses were based on the safety and the open-label safety sets.
Results
Patients and treatment
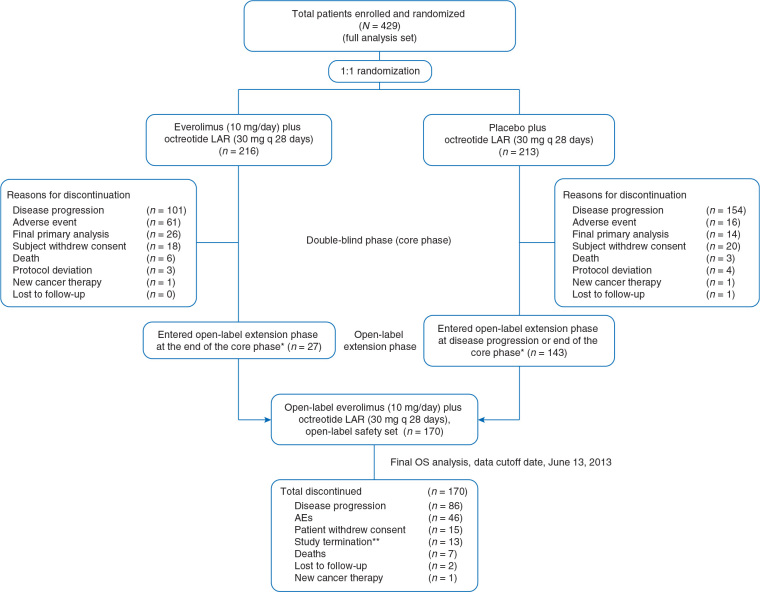
Between 10 January 2007 and 2 April 2010 a total of 429 patients were randomly assigned to receive everolimus plus octreotide LAR (n = 216) or placebo plus octreotide LAR (n = 213). Of these, 170 patients—27 initially randomized to everolimus plus octreotide LAR and 143 who crossed over from placebo plus octreotide LAR-received treatment with open-label everolimus (Figure 1).
Figure 1.
Patient disposition. *All patients were unblinded at the time of progression or at the end of the double-blind phase, after primary analysis, and were allowed to cross over to open-label everolimus. Additional reasons for patient discontinuation in either arm of the double-blind phase included AEs, death, patient consent withdrawal, protocol violation, and loss to follow-up. **At the time of study termination, patients receiving everolimus were rolled over to study RAD001C2X01B (ClinicalTrials.gov identifier, NCT01789281) or to commercial everolimus. AE, adverse event; LAR, long-acting repeatable; OS, overall survival.
Several imbalances in baseline demographic and clinical characteristics were observed. Patients in the everolimus plus octreotide LAR arm had more frequently WHO PS score >0, primary tumor site in the lung, elevated baseline CgA, and previous use of chemotherapy than patients in the placebo plus octreotide LAR arm (supplementary Table S1, available at Annals of Oncology online) [3].
Supplementary Data.

Efficacy
The median duration of everolimus exposure, including both the double-blind and the open-label extension phases, was 37.0 weeks (range, 1–326) in patients randomized to everolimus plus octreotide LAR and 34.1 weeks (1–235) in patients randomized to placebo plus octreotide LAR who switched to open-label everolimus. Forty-three patients had ≥144 weeks of everolimus exposure, including 15 patients randomized to placebo plus octreotide LAR who switched to open-label everolimus.
The continued use of SSA therapy postprogression, i.e. octreotide or lanreotide treatment for ≥30 days after the end of the double-blind phase, was higher (+15.4%) in patients initially randomized to placebo arm [154 of 213 (72.3%)] than in those from everolimus arm [123 of 216 (56.9%)].
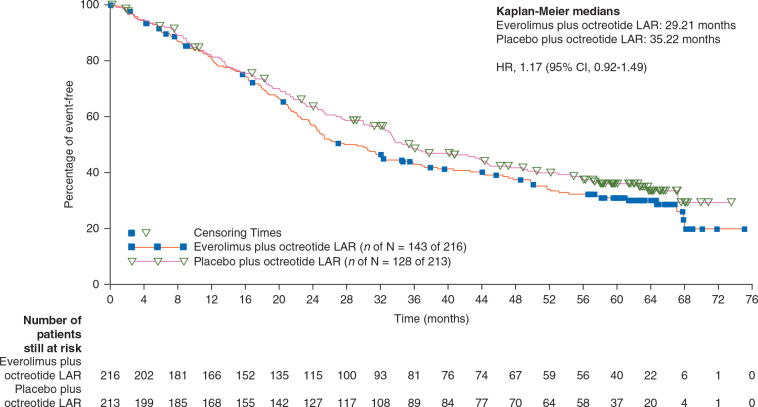
Of the total 429 patients, 271 had died (63%) by the data cutoff date for the final analysis. The incidence of deaths (double-blind and open-label phases combined) was higher by 6% in the everolimus arm [143 patients (66%) versus 128 patients (60%) in the placebo arm; Table 1]. Majority of the deaths (78%; 212 of 271) were attributed to the underlying malignancy. Median OS (95% CI) was 29.2 months (23.8–35.9) for everolimus plus octreotide LAR and 35.2 months (30.0–44.7) for placebo plus octreotide LAR (HR, 1.17; 95% CI, 0.92–1.49) (Figure 2). The survival rates at specific follow-up dates are displayed in Table 1.
Table 1.
Summary of overall survival (full analysis set)
| Parameters | Everolimus plus octreotide LAR |
Placebo plus octreotide LAR |
|---|---|---|
| (n = 216) | (n = 213) | |
| No. of events, n (%) | 143 (66.2) | 128 (60.1) |
| No. censored, n (%) | 73 (33.8) | 85 (39.9) |
| Survival rate at | ||
| Year 1, % (95% CI) | 80.5 (74.5–85.3) | 81.8 (75.8–86.4) |
| Year 2, % (95% CI) | 57.0 (49.9–63.4) | 63.6 (56.6–69.8) |
| Year 3, % (95% CI) | 42.9 (36.0–49.6) | 48.5 (41.4–55.3) |
| Year 4, % (95% CI) | 37.4 (30.7–44.1) | 41.7 (34.7–48.6) |
| Median OS (95% CI), months | 29.2 (23.8–35.9) | 35.2 (30.0–44.7) |
| HR (95% CI), 1.17 (0.92–1.49) | ||
CI, confidence interval; HR, hazard ratio; LAR, long-acting repeatable; OS, overall survival.
Figure 2.
Kaplan–Meier plot of overall survival (full analysis set).
When adjusted for prespecified baseline covariates (age, sex, race, WHO PS, and prior SSA use), the HR was 1.08 (95% CI, 0.84–1.38). In a post hoc model when adjusted for the imbalanced baseline prognostic factors (WHO PS, baseline CgA, lung as primary site, bone involvement), the HR was 0.99 (95% CI, 0.77–1.27).
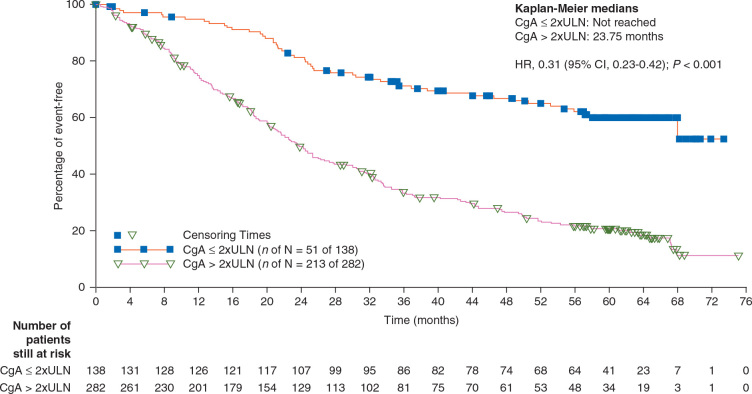
Baseline CgA level (low, <2xULN or high, ≥2xULN) was not predictive of an everolimus treatment effect on OS. The HRs for the everolimus arm compared with the placebo arm for low and high baseline CgA levels were consistent with the results obtained from overall population and were 1.08 (95% CI, 0.62–1.87) and 1.10 (95% CI, 0.84–1.45), respectively. Low CgA was a good prognostic factor for OS irrespective of treatment (HR, 0.31; 95% CI, 0.23–0.42) (Figure 3).
Figure 3.
Kaplan–Meier plot of overall survival by baseline CgA level, irrespective of treatment arm (full analysis set). HR was obtained from unadjusted Cox's model stratified by treatment group. P value was obtained from the 2-sided log-rank test stratified by treatment group.
Safety
AEs reported in patients who received open-label everolimus plus octreotide LAR (n = 170) were consistent with those observed in patients who received everolimus plus octreotide LAR during the double-blind phase (Table 2).
Table 2.
Drug-related adverse events reported in at least 10% of patients (safety set)
| Adverse event, n (%) | Everolimus plus octreotide LAR (double-blind phase) |
Everolimus plus octreotide LAR (open-label phase) |
||
|---|---|---|---|---|
| (n=215) |
(n=170) |
|||
| All grades | Grade 3 or 4 | All grades | Grade 3 or 4 | |
| Stomatitis | 102 (47.4) | 8 (3.7) | 60 (35.3) | 7 (4.1) |
| Rash | 80 (37.2) | 2 (0.9) | 49 (28.8) | 6 (3.5) |
| Fatigue | 68 (31.6) | 15 (7) | 40 (23.5) | 8 (4.7) |
| Diarrhea | 59 (27.4) | 13 (6) | 45 (26.5) | 9 (5.3) |
| Nausea | 44 (20.5) | 1 (0.5) | 31 (18.2) | 3 (1.8) |
| Dysgeusia | 36 (16.7) | 1 (0.5) | 27 (15.9) | 0 |
| Anemia | 35 (16.3) | 3 (1.4) | 23 (13.5) | 3 (1.8) |
| Thrombocytopenia | 32 (14.9) | 9 (4.2) | 16 (9.4) | 2 (1.2) |
| Weight reduction | 32 (14.9) | 1 (0.5) | 19 (11.2) | 2 (1.2) |
| Decreased appetite | 30 (14) | 0 | 21 (12.4) | 1 (0.6) |
| Aphthous stomatitis | 28 (13) | 2 (0.9) | 13 (7.6) | 1 (0.6) |
| Peripheral edema | 28 (13) | 0 | 23 (13.5) | 0 |
| Hyperglycemia | 26 (12.1) | 11 (5.1) | 12 (7.1) | 3 (1.8) |
| Dyspnea | 25 (11.6) | 3 (1.4) | 11 (6.5) | 1 (0.6) |
| Pruritus | 24 (11.2) | 0 | 13 (7.6) | 0 |
| Vomiting | 23 (10.7) | 1 (0.5) | 10 (5.9) | 2 (1.2) |
| Asthenia | 22 (10.2) | 2 (0.9) | 19 (11.2) | 6 (3.5) |
LAR, long-acting repeatable.
The most common AEs in the open-label safety set included stomatitis (35.3%), rash (28.8%), diarrhea (26.5%), and fatigue (23.5%).
Overall, 30 patients in the double-blind phase [19 (9%) in the everolimus arm and 11 (5%) in the placebo arm] and 22 patients in the open-label phase died on-treatment (i.e. during receipt of study medication or within the initial 28 days of discontinuing therapy; Table 3). Of these, 15 deaths (50%) during the double-blind phase and 13 (59%) in the open-label phase were attributed to the underlying malignancy or a disease progression. The causes for remaining deaths included cardiac disorders, respiratory and thoracic disorders, gastrointestinal disorders, or hepatobiliary disorders. Except one case (due to sepsis), all other deaths were deemed not to be related to the study treatment according to the investigators. A higher number of deaths related to respiratory or cardiac diseases were reported in the everolimus arm than in the placebo arm.
Table 3.
On-treatment deaths (safety set)
| Everolimus plus octreotide LAR (double-blind phase) |
Placebo plus depot octreotide (double-blind phase) |
Everolimus plus octreotide LAR (open-label phase) |
|
|---|---|---|---|
| n=215 (%) | n=211 (%) | n=170 (%) | |
| On-treatment deaths | 19 (9) | 11 (5) | 22 (13)a |
| Due to underlying malignancy or disease progression | 7 (3) | 8 (4) | 13 (8) |
| Due to other cause | 12 (6) | 3 (1) | 9 (5) |
| Pulmonary embolism | 2 (1) | 0 | 0 |
| Sudden death | 2 (1) | 0 | 2 (1) |
| Cardiac arrest | 1 (<1) | 0 | 1 (1) |
| Congestive cardiac failure | 1 (<1) | 0 | 0 |
| Cardiopulmonary failure | 1 (<1) | 0 | 0 |
| Respiratory failure | 1 (<1) | 0 | 1 (1) |
| Pneumonia | 1 (<1) | 0 | 1 (1) |
| Pulmonary sepsis | 1 (<1) | 0 | 0 |
| Small intestinal obstruction | 1 (<1) | 0 | 0 |
| Abnormal hepatic function | 1 (<1) | 0 | 0 |
| Arrhythmia | 0 | 1 (<1) | 0 |
| Cardiorespiratory arrest | 0 | 1 (<1) | 0 |
| Hepatic failure | 0 | 1 (<1) | 0 |
| Intestinal perforation | 0 | 0 | 1 (1) |
| Gastrointestinal hemorrhage | 0 | 0 | 1 (1) |
| Renal failure | 0 | 0 | 1 (1) |
| Sepsis | 0 | 0 | 1 (1) |
aAll but one patient were randomized to placebo during the double-blind phase of the study.
In the double-blind phase, study drug discontinuation attributable to AEs occurred more often in the everolimus plus octreotide LAR arm [61 patients (28%) versus 16 patients (8%) in the placebo arm]. The median duration of exposure to everolimus was 37 weeks (1–180) during this phase. The most commonly reported AEs leading to study drug discontinuation in the everolimus arm were dyspnea, fatigue (each reported in five patients, 2.3%), diarrhea, pneumonia, interstitial lung disease, pneumonitis, and general physical health deterioration (each reported in four patients, 1.9%).
In the open-label phase, the median duration of exposure of everolimus was 40.8 weeks (1–235). A total of 48 patients (28.2%) discontinued study treatment because of AEs in the open-label phase. The most commonly reported AEs leading to study drug discontinuation were abdominal pain and stomatitis (each reported in four patients, 2.4%) and diarrhea (three patients, 1.8%).
Discussion
RADIANT-2 was one of the first, largest, phase 3 trials involving patients with advanced NET. The results, as per the primary analysis, had shown that addition of everolimus to octreotide LAR resulted in 5.1-month delay in disease progression compared with placebo plus octreotide LAR (HR, 0.77; 95% CI, 0.59–1.00; P = 0.026), albeit the difference marginally missed the prespecified threshold for statistical significance (0.024) [3]. The present final OS analysis of the RADIANT-2 study showed no statistically significant difference in the OS between the everolimus and placebo arms (median OS was 29.2 months for the everolimus arm and 35.2 months for the placebo arm; HR, 1.17; 95% CI, 0.92–1.49). It is possible that addition of everolimus to octreotide LAR failed to demonstrate a survival advantage over octreotide LAR alone because many of the randomized patients did not need aggressive therapy. This is supported by the long PFS in the placebo plus octreotide LAR arm of the study. Future studies may need to select for patients with more aggressive biology.
The lack of significant differences in OS between everolimus and placebo arms in the RADIANT-2 study is also not unexpected. PFS was the primary end point and the study was not powered to detect differences in OS between the treatment arms. In addition, the primary end point of PFS was not met in this study, and thus was unlikely to affect OS. The use of OS as an end point in clinical trials for patients with prolonged survival post progression remains challenging. For such studies, the power to detect OS difference is generally low [5]. In general, a longer postprogression survival in patients with NET has been reported; for example, pivotal trials for everolimus as well as sunitinib have reported a median postprogression survival of >12 months in patients with progressive pancreatic NET [6, 7]. A hypothetical phase 3 study with 80% power to detect 6 months’ improvement in OS (type I error, 0.05) would require approximately 1350 OS events, 2500 patients, and 5 years of study duration of the double-blind phase.
Several other factors, including SSA exposure, crossover design, or unbalanced baseline prognostic parameters such as WHO performance status, primary site, and baseline CgA, may have contributed to the differences in OS between both arms. Adjustment for unbalanced baseline parameters led to comparable median OS. However, in the absence of precision for other major prognostic parameters, like tumor burden and/or grading, the final weight of these baseline parameters remains uncertain. Longer exposure to SSA in the placebo arm but also crossover from placebo to everolimus likely influenced the final OS results. A higher number of deaths related to pulmonary or cardiac functions were reported in the everolimus arm and may have contributed to the survival results.
On exploring the relationship between OS and baseline CgA, we observed that the low CgA level at baseline was a good prognostic factor for OS, irrespective of treatment (HR, 0.31; 95% CI, 0.23–0.42). Baseline CgA level was not predictive of treatment response.
The safety data from open-label phase were similar to that seen in the double-blind phase and was consistent with the primary report [3]. Patients with carcinoid syndrome have more disease-related comorbidities, such as diarrhea and carcinoid heart disease, and they might be more susceptible to experience serious events as cardiovascular complications or infections compared with those with nonfunctional NET after treatment with everolimus [8, 9]. Adequate education of both physicians and patients to everolimus-related AEs, including risk of infections and pulmonary complications, plays an important role in patient management.
Over several years with the development of targeted therapies, we have advanced our knowledge in the field of NET and learnt several lessons to design and conduct phase 3 studies. Owing the nature of the disease, PFS has now been accepted as a preferred primary end point for studies in NET [6]. Learnings from the RADIANT-2 contributed in designing scientifically more robust studies. The RADIANT-4 study, for example, was designed after careful consideration of the following aspects from RADIANT-2 study: selection of a more homogeneous population, inclusion of patients with more aggressive tumor behavior, not allowing crossover upon disease progression during the double-blind phase, stratification based on known confounding factors, and performing an independent real-time assessment for disease progression besides investigator assessment [10]. Nevertheless, ‘where does the combination therapy stand in the treatment landscape for NET?’ and ‘what is an ideal sequencing with the existing therapies?’ are among few of the questions that need to be examined in future studies.
Conclusions
In patients with advanced NET and carcinoid syndrome, addition of everolimus to octreotide LAR did not extend OS compared with octreotide LAR. Multiple factors, including heterogeneous study population, unbalanced patient characteristics, uneven SSA exposure, crossover study design but also potentially AEs in patients with slowly growing tumors who might not have needed early initiation of everolimus therapy, likely influenced the survival outcome. Future studies in patients with more aggressive biology might help identify patients who will be benefitted with combination therapy. Nonetheless, the findings from the RADIANT-2 study offered important insights to design and conduct studies with targeted therapies in NET.
Acknowledgements
This study was sponsored by Novartis Pharmaceuticals Corporation. We thank Du Lam, MD for his support in the study conduct and interpretation of data. We thank Bhavik Shah, PhD of Novartis Healthcare Pvt. Ltd. for providing medical editorial assistance with this manuscript.
Funding
This study was sponsored by Novartis Pharmaceuticals Corporation (no grant numbers apply). The study was designed by academic investigators and by representatives of the sponsor. Data were collected using the sponsor’s data management systems and were analyzed by the sponsor’s statistical team complying with study protocol and statistical analysis plan. All authors vouch for the accuracy and completeness of the reported data and attest that they had final responsibility for the decision to submit for publication. Writing assistance funded by the sponsor was provided.
Disclosure
MEP has received fees from Novartis for presentation or advisory board, and research funding for her university. EB received fees from Novartis, during the conduct of the study. KEÖ serves on advisory boards of and receives honoraria from Novartis. JDH has received research funding for his institute from Novartis. MV and NR are employees of Novartis and own shares in Novartis. MP has received personal fees and is on the speaker’s bureau for Novartis. DJG has received fees from Novartis for presentation or advisory board, and research funding for his research laboratory. JCY has served as a consultant and has received research funding for his institute from Novartis.
Key Message
Overall survival was not significantly different between everolimus + octreotide LAR and placebo + octreotide LAR arms of the RADIANT-2 study. Inclusion of heterogeneous population, uneven SSA exposure, and crossover likely influenced survival estimates. Prospective studies in patients with more aggressive biology are warranted to identify patients who will be benefitted with combination therapy.
Alt-text: Unlabelled Box
Footnotes
Note: Findings from the present analysis were presented in part at the European Cancer Congress/European Society for Medical Oncology, 27 September–1 October 2013, Amsterdam, The Netherlands.
References
- 1.Yao J.C., Hassan M., Phan A. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Clinical practice guideline in oncology. Neuroendocrine tumors version 2. 2014; http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf (3 January 2017, last date accessed).
- 3.Pavel M.E., Hainsworth J.D., Baudin E. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 4.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment Of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 5.Broglio K.R., Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulke M.H., Siu L.L., Tepper J.E. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–943. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao J.C., Lagunes D.R., Kulke MH. Targeted therapies in neuroendocrine tumors (NET): clinical trial challenges and lessons learned. Oncologist. 2013;18:525–532. doi: 10.1634/theoncologist.2012-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grozinsky-Glasberg S., Grossman A.B., Gross DJ. Carcinoid heart disease: from pathophysiology to treatment-‘Something in the way it moves'. Neuroendocrinology. 2015;101:263–273. doi: 10.1159/000381930. [DOI] [PubMed] [Google Scholar]
- 9.Broder M.S., Chang E., Romanus D. Healthcare and economic impact of diarrhea in patients with carcinoid syndrome. World J Gastroenterol. 2016;22:2118–2125. doi: 10.3748/wjg.v22.i6.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao J.C., Fazio N., Singh S. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]