Abstract
Background
The survival advantage of induction chemotherapy (IC) followed by locoregional treatment is controversial in locally advanced head and neck squamous cell carcinoma (LAHNSCC). We previously showed feasibility and safety of cetuximab-based IC (paclitaxel/carboplatin/cetuximab—PCC, and docetaxel/cisplatin/5-fluorouracil/cetuximab—C-TPF) followed by local therapy in LAHNSCC. The primary end point of this phase II clinical trial with randomization to PCC and C-TPF followed by combined local therapy in patients with LAHNSCC stratified by human papillomavirus (HPV) status and T-stage was 2-year progression-free survival (PFS) compared with historical control.
Patients and methods
Eligible patients were ≥18 years with squamous cell carcinoma of the oropharynx, oral cavity, nasopharynx, hypopharynx, or larynx with measurable stage IV (T0–4N2b–2c/3M0) and known HPV by p16 status. Stratification was by HPV and T-stage into one of the two risk groups: (i) low-risk: HPV-positive and T0–3 or HPV-negative and T0–2; (ii) intermediate/high-risk: HPV-positive and T4 or HPV-negative and T3–4. Patient reported outcomes were carried out.
Results
A total of 136 patients were randomized in the study, 68 to each arm. With a median follow up of 3.2 years, the 2-year PFS in the PCC arm was 89% in the overall, 96% in the low-risk and 67% in the intermediate/high-risk groups; in the C-TPF arm 2-year PFS was 88% in the overall, 88% in the low-risk and 89% in the intermediate/high-risk groups.
Conclusion
The observed 2-year PFS of PCC in the low-risk group and of C-TPF in the intermediate/high-risk group showed a 20% improvement compared with the historical control derived from RTOG-0129, therefore reaching the primary end point of the trial.
Key words: LAHNSCC, C-TPF, PCC, cetuximab, induction chemotherapy
Key Message
This is a phase II trial with patients randomized to two cetuximab-based induction therapy arms followed by local therapy in locally advanced head and neck squamous cell carcinoma. The primary end point was improved 2-year progression-free survival compared with historical control, reached in the low-risk group of the PCC arm and the intermediate-/high-risk group of the C-TPF arm.
Alt-text: Unlabelled Box
Introduction
Despite recent progress in the treatment of head and neck squamous cell carcinoma (HNSCC), mortality is still 40% with an estimate of >250 000 deaths per year [1]. The majority of patients present with locoregionally advanced (LA) HNSCC (stage III–IVM0) and only 5% of patients present with distant metastases at diagnosis. Although there is much variability in primary site, associated symptomatology and prognosis, these cancers must be controlled locally with multimodality approach involving surgery, radiation therapy (RT) and chemotherapy to obtain a favorable therapeutic outcome. Distant disease recurrence is also an important problem and tends to correlate with site (i.e. nasopharynx) and the extent of nodal involvement at presentation (N2b-N3). Induction chemotherapy (IC) has proved to significantly reduce distant failure in the MACH-NC study and its update, however, its survival impact is controversial [2., 3., 4.]. Similarly, in the DECIDE trial, IC showed superior distant metastases-free survival in nonmetastatic N2/N3 HNSCC [5], and higher 2-year progression-free survival (PFS) rates for T4 or N3 patients when compared with historical control [6]. Taxane-based IC was superior to nontaxane-based combinations in randomized phase III trials [7, 8]. However, IC followed by locoregional treatment was not superior to locoregional treatment alone, possibly due to lack of sufficient statistical power [5, 9, 10], with the exception of a recent trial [4]. Our groups previously showed feasibility and safety of cetuximab-based IC (paclitaxel/carboplatin/cetuximab—PCC, and docetaxel/cisplatin/5-fluorouracil/cetuximab—C-TPF) followed by risk-based local therapy with a PFS of 87% in the overall population and 100% in human papillomavirus (HPV)-positive subgroup [11, 12].
There are several factors influencing the prognosis of HNSCC, in particular HPV status, smoking history and nodal stage [13]. The emerging body of knowledge regarding superior treatment outcomes for HPV-positive patients may render IC strategies particularly pertinent in this setting. It is evident that HPV-positive patients have significantly improved loco-regional control and a reduced rate of second cancers but not a statistically significant reduction in distant metastases rate with exception of the DECIDE trial [5].
We designed a phase II clinical trial with randomization to two cetuximab-based IC regimens (PCC and C-TPF) to compare the efficacy of each arm by PFS to historical control, followed by combined local therapy in patients with LAHNSCC stratified in different risk categories by HPV status and T-stage.
Patients and methods
Eligibility
Eligible patients were at least 18 years old with histologically confirmed SCC of the oropharynx, oral cavity, nasopharynx, hypopharynx, or larynx and measurable stage IV by AJCC seventh edition (T0–4N2b–2c/3M0). HPV by p16 IHC status was mandatory.
Metastatic disease, symptomatic peripheral neuropathy, prior chemotherapy or RT, or pregnancy excluded participation. The University of Texas MD Anderson Cancer Center and the Dana Farber Cancer Institute institutional review boards approved the study. All patients provided written informed consent.
Treatment
A multidisciplinary tumor board reviewed each patient’s plan of care. Patients were stratified by HPV status and T-stage to one of the two risk groups: (i) low-risk group (LR): HPV-positive and T0–3 or HPV-negative and T0–2; (ii) intermediate-/high-risk group (I/HR): HPV-positive and T4 or HPV-negative and T3–4. To further ensure the comparability in patient characteristics between the two treatments, Pocock–Simon dynamic allocation method was applied to achieve balanced randomization with respect to potentially important factors including smoking status (≤10 pack years versus >10 pack years) [14].
Patients in the PCC arm received carboplatin AUC 2 i.v. weekly weeks 1–6, paclitaxel 135 mg/m2 i.v. weekly weeks 1–6, cetuximab loading dose 400 mg/m2 i.v. week 1 then 250 mg/m2 i.v. weekly weeks 2–6. Patients in the C-TPF arm received cisplatin 100 mg/m2 day 1 i.v., docetaxel 75 mg/m2 i.v. day 1, 5-fluorouracil 700 mg/m2 24-h infusion by pump days 1–4; cycles were repeated every 3 weeks for 3 cycles and cetuximab loading dose 400 mg/m2 i.v. day 1 week 1 then 250 mg/m2 i.v. weekly weeks 2, 4–5, and 7–8.
Regardless of which induction treatment received, LR patients were to receive RT alone and I/HR patients received chemoradiation therapy (CRT) as definitive treatment following IC. However, loco-regional therapy decision was at the discretion of the treating physician. RT was delivered with standard IMRT fractionation.
End points and statistical considerations
This was a phase II trial with stratified randomization by risk group and study center with Pocock–Simon dynamic allocation with respect to smoking status [14]. Patients were randomized at 1 : 1 ratio to PCC or C-TPF. The primary objective was to compare the PFS of IC with PCC or C-TPF followed by definitive local therapy to historical control data from RTOG-0129 [13]. Secondary end points included overall survival (OS), objective response rate (ORR), duration of loco-regional control (DLC), patterns of tumor recurrence, and toxicity.
We proposed to enroll a total of 128 patients (64 to each arm), estimating an equal distribution between LR and I/HR. We hypothesized an improvement of the 2-year PFS rates from 0.75 to 0.95 in the LR and from 0.6 to 0.75 in the I/HR. For each arm comparison to standard therapy, we would have 82% power with a one-sided 10% significance level in the LR, 77% power in the I/HR. Using Bonferroni correction for multiple comparisons at an overall 10% type I error rate, we would have greater than 80% power for the treatment efficacy comparison in the combined group.
Chi-square test/Fisher’s exact test was used to test differences of category variables and Wilcoxon rank-sum test/Kruskal–Wallis test to detect differences for continuous variables between groups. The distribution of OS and PFS were estimated by the Kaplan-Meier method and log-rank test to test the difference in survival between groups. OS was defined as the time of randomization to the time of death and PFS as the time of randomization to the time of progression or death, whichever occurred first. SAS version 9.4 and S-Plus version 8.04 were used.
Results
Patient characteristics
From September 2010 to February 2014, 136 patients were randomized in this study, 68 to each arm. A total of 116 patients were treated at MD Anderson and 20 at Dana Farber. The data cut-off for analysis was July 2016.
Patient characteristics were similar between the two arms (Table 1).
Table 1.
Patient clinicopathological characteristics
| C-TPF N = 68 | PCC N = 68 | P-value | ||
|---|---|---|---|---|
| Age median | 57.01 | 58.44 | 0.38 | |
| Gender | Female | 13 (19.1) | 9 (13.2) | 0.35 |
| Male | 55 (80.9) | 59 (86.8) | ||
| Race | Asian | 2 (2.9) | 3 (4.4) | 0.24 |
| African American | 3 (4.4) | 0 (0) | ||
| Hispanic | 5 (7.4) | 2 (2.9) | ||
| Caucasian | 58 (85.3) | 63 (92.6) | ||
| ECOG performance status | 0 | 41 (60.3) | 51 (75) | 0.067 |
| 1 | 27 (39.7) | 17 (25) | ||
| Smoking status | Never | 23 (33.8) | 32 (47.1) | 0.29 |
| Former | 30 (44.1) | 24 (35.3) | ||
| Current | 15 (22.1) | 12 (17.6) | ||
| T stage | T0–2 | 38 (55.9) | 43 (63.2) | 0.38 |
| T3–4 | 30 (44.1) | 25 (36.8) | ||
| N stage | N0–2a | 1 (1.5) | 1 (1.5) | 1.0 |
| N2b–N3 | 67 (98.5) | 67 (98.5) | ||
| Cancer site | Oropharynx | 58 (85.3) | 52 (76.6) | 0.38 |
| Larynx | 3 (4.4) | 2 (2.9) | ||
| Hypopharynx | 1 (1.5) | 2 (2.9) | ||
| Oral cavity | 2 (2.9) | 4 (5.9) | ||
| Nasopharynx | 1 (1.5)a | 6 (8.8)b | ||
| Unknown Primary | 3 (4.4) | 2 (2.9) | ||
| HPV status | Negative | 14 (20.6) | 17 (25) | 0.54 |
| Positive | 54 (79.4) | 51 (75) | ||
| Risk group | Low | 50 (73.5) | 53 (77.9) | 0.55 |
| Intermediate/High | 18 (26.5) | 15 (22.1) | ||
| Risk group+smoking | Low risk <10 pack | 25 (36.8) | 27 (39.7) | 0.96 |
| Low risk >10 pack | 25 (36.8) | 26 (38.2) | ||
| Intermediate risk <10 pack | 5 (7.4) | 3 (4.4) | ||
| Intermediate risk >10 pack | 4 (5.9) | 4 (5.9) | ||
| High risk <10 pack | 3 (4.4) | 4 (5.9) | ||
| High risk >10 pack | 6 (8.8) | 4 (5.9) |
EBV (Epstein–Barr virus)-associated nasopharynx patient, WHO type 3.
Of the six nasopharynx patients, four were EBV-associated and WHO type 2/3, while two were WHO type 1.
Treatment delivery
Treatment delivery is shown in supplementary Figure S1, Tables S1 and S2, available at Annals of Oncology online.
Supplementary Data.

Efficacy
All patients were assessable for response to IC. The post-IC ORR was 79.1% for PCC and 91.8% for C-TPF. The post-CRT ORR was 97% for PCC and 98% for C-TPF. In the LR, postinduction ORR was 80.8% to PCC and 97.7% to C-TPF; post-CRT ORR was 100% in both arms. In the I/HR, ORR was 73.3% to PCC and 76.5% to C-TPF; post-CRT ORR was 86.7% to PCC and 93.8% to C-TPF (supplementary Table S2, available at Annals of Oncology online). After adjusting for risk group in a multivariate logistic regression model, low-risk category (P = 0.036) and C-TPF treatment (P = 0.046) were associated with a significantly higher post-IC response.
With a median follow-up of 3.2 years, PCC-treated patients’ PFS was 92.6% in the LR and 60% in the I/HR. In C-TPF-treated patients, PFS was 84% in the LR and 83.3% in the I/HR.
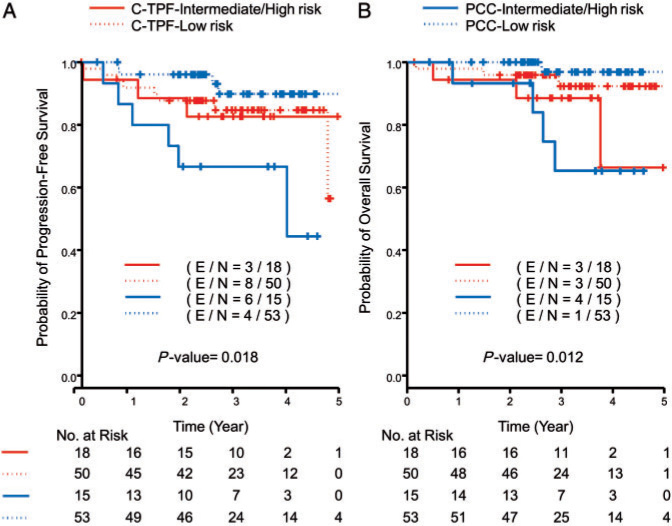
The 2-year PFS, the primary end point of the study, was 89% in the PCC arm and 88% in the C-TPF arm. When analyzed by risk categories, the 2-year PFS in the PCC arm was 96% in the LR and 67% in the I/HR; the C-TPF arm was 88% in the LR and 89% in the I/HR (Figure 1 and Table 2). Compared with historical control of 2-year PFS rates [13], 75% in the LR and 60% in the I/HR, the observed 2-year PFS of PCC and C-TPF in both risk groups were significantly higher (all P < 0.1), therefore reaching the primary end point (Table 2). Excluding nasopharyngeal carcinoma—considering it was not included in historical control—yielded an overall 2-year PFS that was slightly different in the PCC arm (92%) and in the I/HR for both arms (73% in PCC, 88% in C-TPF).
Figure 1.
Kaplan–Meier curves for all patients for (A) progression-free survival and (B) overall survival.
Table 2.
Two-year PFS and 3-, 5-year OS by risk groups
| PCC (%) | CTPF (%) | |
|---|---|---|
| Two-year PFS | ||
| All patients | 89 | 88 |
| Low risk | 96 | 88 |
| Intermediate/high risk | 67 | 89 |
| N2c-N3 | 78 | 88 |
| HPV-positive/never smokers | 100 | 90 |
| HPV-positive/≤10 pack years | 100 | 89 |
| HPV-positive/>10 pack years | 84 | 80 |
| HPV-negative | 82 | 100 |
| HPV-positive | 92 | 85 |
| Three-year OS | ||
| All patients | 89 | 92 |
| Low risk | 97 | 92 |
| Intermediate/high risk | 65 | 89 |
| Five-years OS | ||
| All patients | 89 | 86 |
| Low risk | 97 | 92 |
| Intermediate/high risk | NA*a | 66 |
PFS, progression-free survival; OS, overall survival.
All patients either died or were censored before 5 years.
The 3- and 5-year OS rates are reported in Table 2. The 5-year OS was 89% for PCC- and 86% for C-TPF-treated patients. Nine patients in the C-TPF and eight in the PCC arm recurred. Seven patients recurred locally, eight had distant metastases and two had both local and distant recurrence (supplementary Table S3, available at Annals of Oncology online). Median DLC was 18.2 months (range 6.6–57.5 months).
Local definitive treatment allocation
Per study design, patients in the LR after IC were expected to receive RT alone and patients in the I/HR CRT. Since the final choice of postinduction local therapy was left to the treating physician, the observed local therapy differed from the expected local therapy per protocol design.
Eighteen (34%) LR patients in the PCC arm and 20 (41%) in the C-TPF arm received the expected post-IC with RT alone (supplementary Table S4, available at Annals of Oncology online). Thirteen (87%) I/HR patients in the PCC arm and 16 (89%) in the C-TPF arm were treated with the expected post-IC with CRT (supplementary Table S4, available at Annals of Oncology online).
Toxicity and treatment delivery
Both treatments were of manageable toxicity (Table 3). There was a significant statistical difference in grade 3/4 side-effects between PCC and C-TPF for the following: skin rash (35% versus 3%), nausea (9% versus 25%), hypomagnesemia (1.5% versus 7.4%), and neutropenia (22% versus 30%) (P < 0.05, Table 3).
Table 3.
Maximum grade of most common adverse events by treatment arm
| Adverse event | Grade | PCC N (%) | C-TPF N (%) | P-value |
|---|---|---|---|---|
| Fatigue | 1 | 22 (32.4) | 15 (22.1) | 0.55 |
| 2 | 30 (44.1) | 37 (54.4) | ||
| 3 | 10 (14.7) | 11 (16.2) | ||
| Skin rash | 1 | 12 (17.6) | 33 (48.5) | <0.0001 |
| 2 | 28 (41.2) | 22 (32.4) | ||
| 3 | 24 (35.3) | 2 (2.9) | ||
| Nausea | 1 | 28 (41.2) | 20 (29.4) | 0.038 |
| 2 | 20 (29.4) | 23 (33.8) | ||
| 3 | 6 (8.8) | 17 (25) | ||
| Vomiting | 1 | 18 (26.5) | 18 (26.5) | 0.2 |
| 2 | 6 (8.8) | 13 (19.1) | ||
| 3 | 6 (8.8) | 9 (13.2) | ||
| Mucositis | 1 | 8 (11.8) | 10 (14.7) | 0.86 |
| 2 | 18 (26.5) | 14 (20.6) | ||
| 3 | 28 (41.2) | 29 (42.6) | ||
| Dysphagia | 1 | 6 (8.8) | 3 (4.4) | 0.24 |
| 2 | 25 (36.8) | 18 (26.5) | ||
| 3 | 25 (36.8) | 27 (39.7) | ||
| Dysgeusia | 1 | 33 (48.5) | 34 (50) | 0.41 |
| 2 | 20 (29.4) | 14 (20.6) | ||
| Xerostomia | 1 | 21 (30.9) | 17 (25) | 0.84 |
| 2 | 11 (16.2) | 11 (16.2) | ||
| 3 | 1 (1.5) | 2 (2.9) | ||
| Diarrhea | 1 | 23 (33.8) | 29 (42.6) | 0.36 |
| 2 | 17 (25) | 15 (22.1) | ||
| 3 | 4 (5.9) | 7 (10.3) | ||
| 4 | 0 (0) | 1 (1.5) | ||
| Constipation | 1 | 34 (50) | 26 (38.2) | 0.21 |
| 2 | 15 (22.1) | 14 (20.6) | ||
| 3 | 1 (1.5) | 0 (0) | ||
| Weight loss | 1 | 13 (19.1) | 20 (29.4) | 0.34 |
| 2 | 25 (36.8) | 17 (25) | ||
| 3 | 4 (5.9) | 6 (8.8) | ||
| Hypomagnesemia | 1 | 39 (57.4) | 43 (63.2) | <0.0001 |
| 2 | 2 (2.9) | 12 (17.6) | ||
| 3 | 1 (1.5) | 5 (7.4) | ||
| Alopecia | 1 | 35 (51.5) | 38 (55.9) | 0.92 |
| 2 | 4 (5.9) | 4 (5.9) | ||
| Neutropenia | 1 | 4 (5.9) | 1 (1.5) | 0.0039 |
| 2 | 15 (22.1) | 6 (8.8) | ||
| 3 | 11 (16.2) | 5 (7.4) | ||
| 4 | 4 (5.9) | 15 (22.1) | ||
| Neuropathy | 1 | 20 (29.4) | 21 (30.9) | 0.0082 |
| 2 | 10 (14.7) | 1 (1.5) | ||
| 3 | 2 (2.9) | 0 (0) |
Mean cumulative dose for PCC and C-TPF is reported and the compliance rate (actual dose received/total dose) was similar in both groups (supplementary Table S5, available at Annals of Oncology online).
The number of patients who received concurrent CRT with cisplatin as post-IC local therapy was significantly higher in the PCC versus C-TPF arm (52% versus 20%; P = 0.001; supplementary Table S1, available at Annals of Oncology online). The number of patients receiving carboplatin as post-IC local therapy was significantly higher in the C-TPF versus PCC arm (56% versus 29%; P = 0.01; supplementary Table S1, available at Annals of Oncology online).
Discussion
Our trial is the first to examine and compare the efficacy of cetuximab-based IC with two different regimens (PCC and C-TPF) followed by combined loco-regional therapy in LAHNSCC, compared with historical control. This trial builds on our prior reporting of risk-based therapy [11] and adapts therapy according to risk as defined by stage, HPV status and balanced allocation by smoking status.
The role of IC in the treatment of LAHNSCC is still highly debated [2, 3, 5]. Taxane-based IC showed superior efficacy over nontaxane-based combinations in randomized phase III trials [7, 8]. However, IC followed by loco-regional treatment compared with loco-regional treatment alone did not show superiority [5, 9, 10]. Recent IC data in nasopharyngeal cancer are encouraging and demonstrates a survival advantage in these patients [15]. In HPV-related oropharyngeal cancer, IC could play a role as ‘chemoselection’ for de-intensification strategies as reported by E1308 [16]. Long-term follow-up of RTOG 91-11 observed a trend for better OS with IC compared with concomitant chemotherapy in laryngeal SCC [17].
We hypothesized that a risk-based approach by HPV status and disease stage could identify two major risk categories (low: HPV-positive and T0–3 or HPV-negative and T0–2; intermediate/high: HPV-positive and T4, HPV-negative and T3–4) that could be treated with different postinduction combined strategies. Per study design, LR patients were treated with post-induction RT alone and I/HR patients with CRT. Our trial met its primary end point with a 20% improvement of the 2-year PFS of 96% in LR patients treated with PCC and 89% in I/HR patients treated with C-TPF compared with historical control (Figure 1 and Table 2). C-TPF promoted the same excellent 2-year PFS regardless of risk category, suggesting that treatment intensification with four agents during IC should be considered for I/HR patients. In contrast, PCC 2-year PFS in the I/HR was not as good as the LR or those undergoing C-TPF. Nasopharyngeal carcinoma—WHO classification type 1 (two patients) and types 2/3 (five patients)—was included in our trial and not in historical control [13] (Table 1). A sub-group analysis of the primary end point in our trial excluding nasopharynx site revealed no significant differences.
Encouraging 3- and 5-years survival outcomes were reported in both arms (97% in PCC and 88% in C-TPF) (Table 2). The fact that the choice of postinduction local therapy was left to the treating physician’s preference might have caused a disproportionate rate of patients treated with combined CRT modality in the PCC LR arm (64% in PCC and 58% in C-TPF) (supplementary Table S4, available at Annals of Oncology online). The C-TPF arm was found to have a higher rate of patients receiving carboplatin as post-IC therapy (56%; supplementary Table S1, available at Annals of Oncology online) and reflects the routine use of carboplatin post-TPF based on the TAX324 study [7] and the increased toxicity seen with using cisplatin with RT post-TPF as noted in the TREMPLIN study [18]. Other limitations of our trial include: overselection of patients, highly specialized tertiary cancer centers where patients underwent treatment, and newer RT IMRT techniques and machinery that could represent a bias when compared with historical control.
The 5-year OS was 89% for PCC- and 86% for C-TPF-treated patients. Patterns of tumor recurrence between the arms were comparable. Nine (13%) patients in C-TPF and eight (12%) in the PCC arm recurred: seven (5%) locally, eight (6%) with distant metastases and two (1.5%) with local and distant metastases. Median DLC was 18.2 months (range 6.6–57.5 months).
Seiwert et al. [6] used a carboplatin/paclitaxel backbone with incorporation of cetuximab into induction strategy showing a similar ORR (91%) and 5-year distant failure for the entire cohort (6.6%) and HPV-negative cohort (7.2%). RTOG-0234 showed favorable outcomes with the addition of cetuximab to docetaxel-based CRT through reduction in distant metastases [19]. Similarly to other reported studies, addition of cetuximab in both arms was well tolerated with manageable hematologic and skin toxicities without any treatment-related deaths (Table 3). Feeding tube utilization was significantly higher in C-TPF-treated patients (P = 0.041) and I/HR patients (P = 0.0037), however, 12-month feeding tube dependence did not differ by treatment arm (P = 0.312) as previously reported with different cetuximab-based IC regimens (supplementary data, available at Annals of Oncology online) [6]. Therefore, patient selection becomes important for choice of IC. Although we investigated biomarkers including immune profiling, DNA sequencing for commonly mutated genes and cytokine profiling, no predictive associations were found.
HNSCC is now widely viewed as composed of two distinct clinical entities: HPV-positive and HPV-negative. A therapeutically relevant molecular classification system showed that HPV-positive tumors could be divided in two distinct gene expression subtypes: the inflamed/mesenchymal and the classical supergroup [20]. These molecular differences suggest clinical heterogeneity that might translate into differential treatment approaches. In first-line metastatic HNSCC, the addition of antiprogrammed cell death protein 1 (PD-1) antibody alone or in combination with platinum/5-FU chemotherapy has been recently shown to improve OS versus standard first-line chemotherapy [21]. This evidence supports the potential role of checkpoint inhibitors alone or in combination with chemotherapy as new first-line standard treatment. Recent published data demonstrated encouraging results of neoadjuvant immunotherapy with anti PD-1 blockade in patients with surgically resectable non-small-cell lung cancer with a clear predictive role of tumor mutational burden that highlights the importance of patient selection [22]. Similarly, encouraging results were shown in patients with oral cavity cancer treated with single agent pembrolizumab before undergoing surgical resection, leading to larger planned studies [23].
In conclusion, our study suggests that patients with low risk disease could be safely and effectively treated with PCC and those with intermediate-/high-risk disease should be offered a C-TPF-based approach.
Acknowledgements
The authors thank the patients, their families and caregivers, and all investigators involved in this study.
Funding
This work was supported by philanthropic funds for head and neck cancer research to the Department of Thoracic and Head and Neck Medical Oncology at the University of Texas MD Anderson Cancer Center. No grant numbers are applicable.
Disclosure
RH has consulted with BMS, Merck, Pfizer, Genentech, Astra Zeneca, Celgene and received research support from BMS, Merck, Pfizer, and Genentech. EM has participated in advisory boards of Genentech and Nektar Therapeutics, received honoraria from Astra Zeneca Pharmaceuticals, Merck & Co, and received research support from Pfizer, Astra Zeneca, Merck, BMS, GSK, and Tessa Pharmaceuticals. GB has received research funding from Bayer, Merck, Celgene, Novartis, Xcovery, Astra Zeneca, Genentech, GlaxoSmithKline, Kite, Immatics, Macrogenetics, Adaptimmmune, BMS, and has received personal fees from Bayer, Clovis, Merck, Celgene, AbbVie, Novartis, Xcovery, Astra Zeneca, ARIAD, BMS, Genentech. WW has received research support from Eli Lilly, Astellas, Merck, BMS, Boehringer Ingelheim; and honoraria from AstraZeneca, Eli Lilly, Merck, BMS, and Pfizer. RT has participated in an advisory board for EMD Serono Inc. KA has participated in advisory boards of Takeda, Regeneron, Astra Zeneca Pharmaceuticals, and Boehringer Ingelheim, received honoraria from Takeda, and received research funding from Pharmacyclics, Pfizer, BerGenBio, AbbVie, and Astra Zeneca. GR has participated in advisory boards of EMD Serono, Pfizer, and Regeneron. VP has participated in advisory boards of Nektar Therapeutics, Astra Zeneca Pharmaceuticals, Arrys Therapeutics, Merck & Co, LOXO Oncology, Araxes Pharma, F. Hoffman-La Roche Ltd, Janssen Research Foundation, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly & Co, Novartis Pharmaceuticals Corp., Takeda Pharmaceuticals, Abbvie, TRM Oncology and received grants from Eli Lilly & Co, Novartis, Merck, Astra Zeneca Pharmaceuticals, F Hoffman-La Roche, Nektar Therapeutics, Janssen, Bristol-Myers Squibb, Checkmate, and Incyte. All remaining authors have declared no conflicts of interest.
Department of Thoracic and Head and Neck Medical Oncology
University of Texas MD Anderson Cancer Center
References
- 1.Hayes D.N., Van Waes C., Seiwert TY. Genetic landscape of human papillomavirus-associated head and neck cancer and comparison to tobacco-related tumors. J Clin Oncol. 2015;33(29):3227–3234. doi: 10.1200/JCO.2015.62.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pignon J.P., Bourhis J., Domenge C., Designé L. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet. 2000;355(9208):949–955. [PubMed] [Google Scholar]
- 3.Pignon J.P., le Maitre A., Maillard E., Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17, 346 patients. Radiother Oncol. 2009;92(1):4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Ghi M.G., Paccagnella A., Ferrari D. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II–III trial. Ann Oncol. 2017;28:2206–2212. doi: 10.1093/annonc/mdx299. [DOI] [PubMed] [Google Scholar]
- 5.Cohen E.E.W., Karrison T.G., Kocherginsky M. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32(25):2735–2743. doi: 10.1200/JCO.2013.54.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiwert T.Y., Melotek J.M., Blair E.A. Final results of a randomized phase 2 trial investigating the addition of cetuximab to induction chemotherapy and accelerated or hyperfractionated chemoradiation for locoregionally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2016;96(1):21–29. doi: 10.1016/j.ijrobp.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Posner M.R., Hershock D.M., Blajman C.R. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 8.Vermorken J.B., Remenar E., van Herpen C. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357(17):1695–1704. doi: 10.1056/NEJMoa071028. [DOI] [PubMed] [Google Scholar]
- 9.Haddad R., O'Neill A., Rabinowits G. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14(3):257–264. doi: 10.1016/S1470-2045(13)70011-1. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard P., Bourhis J., Lacas B. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol. 2013;31(23):2854–2860. doi: 10.1200/JCO.2012.47.7802. [DOI] [PubMed] [Google Scholar]
- 11.Kies M.S., Holsinger F.C., Lee J.J. Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol. 2010;28(1):8–14. doi: 10.1200/JCO.2009.23.0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haddad R.I., Tishler R.B., Norris C. Phase I study of C-TPF in patients with locally advanced squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27(27):4448–4453. doi: 10.1200/JCO.2009.22.1333. [DOI] [PubMed] [Google Scholar]
- 13.Ang K.K., Harris J., Wheeler R. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pocock S.J., Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 15.Sun Y., Li W.-F., Chen N.-Y. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–1520. doi: 10.1016/S1470-2045(16)30410-7. [DOI] [PubMed] [Google Scholar]
- 16.Marur S., Li S., Cmelak A.J. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx—ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35(5):490–497. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forastiere A.A., Zhang Q., Weber R.S. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31(7):845–852. doi: 10.1200/JCO.2012.43.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefebvre J.L., Pointreau Y., Rolland F. Induction chemotherapy followed by either chemoradiotherapy or bioradiotherapy for larynx preservation: the TREMPLIN randomized phase II study. J Clin Oncol. 2013;31(7):853–859. doi: 10.1200/JCO.2012.42.3988. [DOI] [PubMed] [Google Scholar]
- 19.Harari P.M., Harris J., Kies M.S. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: radiation therapy oncology group RTOG-0234. J Clin Oncol. 2014;32(23):2486–2495. doi: 10.1200/JCO.2013.53.9163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keck M.K., Zuo Z., Khattri A. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21(4):870–881. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 21.Burtness B., Harrington K.J., Greil R. KEYNOTE-048: phase 3 study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) ESMO 2018 Congress. 2018 Munich Germany. [Google Scholar]
- 22.Forde P.M., Chaft J.E., Smith K.N. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med. 2018;378(21):1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uppaluri R., Zolkind P., Lin T. Neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV negative head and neck squamous cell carcinoma (HNSCC) J Clin Oncol. 2017;35(Suppl 15):6012. 6012. [Google Scholar]