Abstract
Background
Microsatellite instable/deficient mismatch repair (MSI/dMMR) metastatic colorectal cancers have been reported to have a poor prognosis. Frequent co-occurrence of MSI/dMMR and BRAFV600E complicates the association.
Patients and methods
Patients with resected stage III colon cancer (CC) from seven adjuvant studies with available data for disease recurrence and MMR and BRAFV600E status were analyzed. The primary end point was survival after recurrence (SAR). Associations of markers with SAR were analyzed using Cox proportional hazards models adjusted for age, gender, performance status, T stage, N stage, primary tumor location, grade, KRAS status, and timing of recurrence.
Results
Among 2630 patients with cancer recurrence (1491 men [56.7%], mean age, 58.5 [19–85] years), multivariable analysis revealed that patients with MSI/dMMR tumors had significantly longer SAR than did patients with microsatellite stable/proficient MMR tumors (MSS/pMMR) (adjusted hazard ratio [aHR], 0.82; 95% CI [confidence interval], 0.69–0.98; P = 0.029). This finding remained when looking at patients treated with standard oxaliplatin-based adjuvant chemotherapy regimens only (aHR, 0.76; 95% CI, 0.58–1.00; P = 0.048). Same trends for SAR were observed when analyzing MSI/dMMR versus MSS/pMMR tumor subgroups lacking BRAFV600E (aHR, 0.84; P = 0.10) or those harboring BRAFV600E (aHR, 0.88; P = 0.43), without reaching statistical significance. Furthermore, SAR was significantly shorter in tumors with BRAFV600E versus those lacking this mutation (aHR, 2.06; 95% CI, 1.73–2.46; P < 0.0001), even in the subgroup of MSI/dMMR tumors (aHR, 2.65; 95% CI, 1.67–4.21; P < 0.0001). Other factors associated with a shorter SAR were as follows: older age, male gender, T4/N2, proximal primary tumor location, poorly differentiated adenocarcinoma, and early recurrence.
Conclusions
In stage III CC patients recurring after adjuvant chemotherapy, and before the era of immunotherapy, the MSI/dMMR phenotype was associated with a better SAR compared with MSS/pMMR. BRAFV600E mutation was a poor prognostic factor for both MSI/dMMR and MSS/pMMR patients.
Trial identification numbers
NCT00079274, NCT00265811, NCT00004931, NCT00004931, NCT00026273, NCT00096278, NCT00112918.
Key words: colon cancer, microsatellite instability, deficient mismatch repair, recurrence, prognosis
Key Message
In recurring stage III colon cancer patients, microsatellite instable (MSI) was associated with a better survival and BRAF mutation with a worse one. In the era of immunotherapies for MSI and combination of targeted therapies for BRAF mutant mCRCs, having a better picture of the respective prognostic value of each of these two molecular markers may help to better design future clinical trials and tailor patients individual treatment.
Alt-text: Unlabelled Box
Introduction
Surgery alone or combined with adjuvant chemotherapy remains the cornerstone of treatment of non-metastatic colorectal cancer (CRC) [1., 2., 3., 4., 5., 6., 7., 8.]. Chromosomal instability and microsatellite instability (MSI) are distinct, well-described pathways of colorectal carcinogenesis that confer a different prognosis [9]. MSI/deficient DNA mismatch repair (MSI/dMMR) is considered a favorable prognostic factor in non-metastatic patients (stage I to III) tumors [9]; however, its prognostic role in metastatic CRC (mCRC) patients remains controversial.
A recent meta-analysis suggested that MSI/dMMR is a poor prognostic factor in patients eligible for the first-line treatment of non-resectable mCRC [10]. In this analysis, however, number of patients in each subgroup were limited which precluded definitive conclusions. Moreover, MSI/dMMR CRCs are often mutated for BRAFV600E, an established poor prognostic factor in mCRC that complicates the picture.
Clinical trials testing immunotherapy for MSI/dMMR mCRC patients, and targeted treatment of BRAFV600E mCRC patients, have yielded promising results. Having a clearer picture of the prognosis of each of these molecular subgroups is important given that approaches to their treatment is rapidly changing.
Among patients treated in seven phase III trials of adjuvant chemotherapy, we analyzed overall survival after disease recurrence in relationship to MMR and BRAFV600E status.
Materials and methods
Study population and patients characteristics
Histologically proven stage III colon adenocarcinomas had been completely resected from all eligible patients in a pooled analysis of seven adjuvant trials: MOSAIC, NCCTG NO147, PETACC8, PETACC3, NSABP C07, NSABP C08, and AVANT [2., 3., 4., 5., 6., 7., 8.]. Patients were randomly assigned to receive 6 months of different regimens with fluoropyrimidines alone or with CPT11, or with oxaliplatin ± targeted therapy (bevacizumab, cetuximab), with regular monitoring, as described previously. Main results of all trials have been previously published. Among the 16,120 randomized patients, 4861 recurred and patients who had consented to translational research were tested for BRAFV600E and MMR status.
DNA extraction and BRAF mutation analysis
Tumor samples were prospectively banked in all trials except for MOSAIC for which tumor banking was done retrospectively.
Tumor DNA was extracted from FFPE tissues containing more than 50% of tumor cells using DNA extraction kits. Molecular analysis was carried out retrospectively. BRAFV600E (c.1799T>A/p.V600E) was detected by allele specific real-time PCR. All assays were alteration-specific and robustly detect ≥10% of mutated alleles for all mutations tested.
Microsatellite status determination
Mismatch repair (MMR) tumor status was determined by immunohistochemistry, or by MSI testing. MSI tumors were defined as showing loss of the expression of one or more MMR proteins or exhibiting high-level MSI by PCR testing. Microsatellite stable (MSS) tumors had normal MMR protein expression and/or MSS or low-level MSI status.
Statistical analyses
The primary outcome is survival after recurrence (SAR), defined as the time from recurrence to death due to all causes. Due to potential confounding and heterogeneities across studies, all analyses were based on multivariable models which adjusted for clinicopathologic variables, time to recurrence, and stratified per treatment groups within each study. The distribution of SAR between patient subgroups by biomarkers was estimated based on direct adjusted survival curves [11, 12]. Models were adjusted for age, sex, performance score, initial T/N stage, histologic grade, time from initial treatment to recurrence, primary tumor site and biomarkers when applicable. Two-sided P values are reported; P <.05 was considered statistically significant and was not adjusted for multiple comparisons. Analyses were carried out using SAS software (version 9.4; SAS Institute Inc).
Results
Study population
Among the 4861 patients randomized in the seven trials with disease recurrence, 2630 had consented to translational research with available material and thus, had complete data for BRAFV600Eand MMR status (see CONSORT diagram, supplementary Figure S1, available at Annals of Oncology online). In the CONSORT diagram (supplementary Figure S1, available at Annals of Oncology online) the 2230 patients excluded due to not having MSI or BRAF results are mixing patients for which no translational informed consent was available and patients for which BRAF or MSI status was not carried out due to inadequate materials or testings.
mdz208_Supplementary_Data.

Demographic and clinical characteristics of the patients in the molecular study (n = 2630) and the others (n = 2231) are summarized in supplementary Table S1, available at Annals of Oncology online.
Among the 2630 patients with MMR and BRAF data, 307 (11.7%) tumors harbored BRAFV600E and 271 (10.3%) were MSI/dMMR. Among MSI/dMMR tumors, 91 (33.6%) harbored BRAFV600E, and among BRAFV600E mutants, 91 (29.6%) were MSI/dMMR.
Clinical and pathological patients’ characteristics per study are summarized in supplementary Table S2, available at Annals of Oncology online.
Demographic and clinical characteristics according to MSI/dMMR and BRAF status
Patients with tumor recurrence and MSI/dMMR were more likely to be females and to have had a proximal primary tumor that was poorly differentiated, pT4/N2, BRAFV600E, had more lymph nodes examined, and were less frequently KRAS mutated (supplementary Table S3, available at Annals of Oncology online).
Patients with BRAFV600E tumors were more frequently females and to have had a proximal primary tumor that was poorly differentiated, pT4/N2, MSI/dMMR, and non KRAS mutated (supplementary Table S4, available at Annals of Oncology online).
Outcome in different molecular subgroups
In the overall population with recurrence (N = 4861), median follow-up was 77.3 months (95% confidence interval [CI] 75.4–80.5) and median SAR was 23.1 months (95% CI 22.3–23.9).
A single multivariable Cox model was fitted with variables listed in Tables 1 and 2 (clinic-pathologic variables plus MSI/MMR and BRAF variables). To better outline our results, the HRs associated with MSI/MMR and BRAF are reported in Table 2 (first two sections) and the median SAR and HRs are only reported for MMR and BRAF variables. In multivariable analysis adjusted for MSI/dMMR and BRAFV600E status, factors associated with a poor SAR were as follows: older age, male gender, T4/N2, proximal primary tumor location, poor differentiation, and early recurrence (by 1-year increase) (Table 1).
Table 1.
Multivariable associations between patient demographics and disease characteristics with survival after recurrence (SAR), adjusting for biomarkers (MSI/MMR and BRAF)a
| Events/total | Hazard ratio (95% CI) | P-valueb | |
|---|---|---|---|
| Age, 5-year increase | 1428/1987 | 1.04 (1.01–1.07) | 0.0057 |
| Gender | |||
| Female | 614/860 | 0.88 (0.79–0.98) | 0.0218 |
| Male | 814/1127 | Reference | |
| Primary tumor location | |||
| Distal | 657/1026 | 0.64 (0.57–0.72) | <0.0001 |
| Proximal | 771/961 | Reference | |
| T-stage | |||
| T1/2 | 60/104 | Reference | |
| T3 | 1048/1469 | 1.20 (0.92–1.57) | 0.1746 |
| T4 | 320/414 | 1.45 (1.09–1.92) | 0.0102 |
| N-Stage | |||
| N1 | 583/890 | Reference | |
| N2 | 845/1097 | 1.36 (1.22–1.51) | <0.0001 |
| Histologic grade | |||
| Low grade (grade 1–2) | 1022/1482 | Reference | |
| High grade (grade 3/4) | 406/505 | 1.36 (1.20–1.53) | <0.0001 |
| KRAS status | |||
| MT | 597/791 | 1.21 (1.07–1.36) | 0.0023 |
| WT | 831/1196 | Reference | |
| Performance score | |||
| 0 | 1077/1519 | Reference | |
| 1 | 341/457 | 1.18 (1.04–1.34) | 0.0089 |
| 2 | 10/11 | 3.54 (1.87–6.70) | 0.0001 |
| Time-to-recurrence, 1-year increase | 1428/1987 | 0.87 (0.82–0.91) | <0.0001 |
MSI, microsatellite instable; MMR, mismatch repair; BRAF; CI, confidence interval; KRAS; MT, mutated; WT, wild type.
A single multivariable Cox model was fitted with variables listed in the table, plus MSI/MMR and BRAF variables. The HRs associated with MSI/MMR and BRAF are reported in Table 2 (first two sections).
Stratified covariate Wald P-value.
Table 2.
Multivariable associations between patient biomarkers (MMR and BRAF status) with survival after recurrence (SAR), adjusting for demographics and disease characteristicsa
| SAR | Adjusted survival |
|||
|---|---|---|---|---|
| Event/total | Median (95% CI)KM | Hazard ratio (95% CI)Cox | P-value | |
| MMR status | 0.0290b | |||
| dMMR | 162/220 | 2.2 (1.9–2.7) | 0.82 (0.69–0.98) | |
| pMMR | 1266/1767 | 2.0 (1.9–2.2) | Reference | |
| BRAF status | <0.0001b | |||
| MT | 219/244 | 1.2 (0.9–1.4) | 2.06 (1.73–2.46) | |
| WT | 1209/1743 | 2.2 (2.1–2.4) | Reference | |
| dMMR patients only | ||||
| BRAF status | <0.0001b | |||
| MT | 64/77 | 0.8 (0.5–1.1) | 2.65 (1.67–4.21) | |
| WT | 98/143 | 1.9 (1.7–2.5) | Reference | |
| pMMR patients only | ||||
| BRAF status | <0.0001b | |||
| MT | 155/167 | 1.3 (0.9–1.5) | 2.12 (1.74–2.58) | |
| WT | 1111/1600 | 2.3 (2.2–2.4) | Reference | |
| BRAF WT patients only | ||||
| MMR status | 0.1030b | |||
| dMMR | 98/143 | 2.4 (1.9–3.5) | 0.84 (0.67–1.04) | |
| pMMR | 1111/1600 | 2.3 (2.2–2.4) | Reference | |
| BRAF MT patients only | ||||
| MMR status | 0.4299b | |||
| dMMR | 64/77 | 0.8 (0.7–1.1) | 0.88 (0.63–1.22) | |
| pMMR | 155/167 | 0.9 (0.8–1.2) | Reference | |
| Adjuvant FP + oxaliplatin patients only | ||||
| MMR status | 0.0476b | |||
| dMMR | 66/92 | 2.5 (1.8–4.6) | 0.76 (0.58–1.00) | |
| pMMR | 537/771 | 2.0 (1.9–2.2) | Reference | |
KMKaplan–Meier method; CoxCox model.
CI, confidence interval; dMMR, deficient mismatch repair; pMMR, proficient mismatch repair; FP, fluoropyrimidine; MT, mutated; WT, wild type.
Multivariable Cox models were fitted on all patients, and subgroups of patients as indicated. All models were adjusted by age, gender, PS, T-stage, N-stage, tumor location, histological grade, KRAS and years to progression. The median survival after recurrence and HRs were only reported for MMR and BRAF variables.
Likelihood-ratio test.
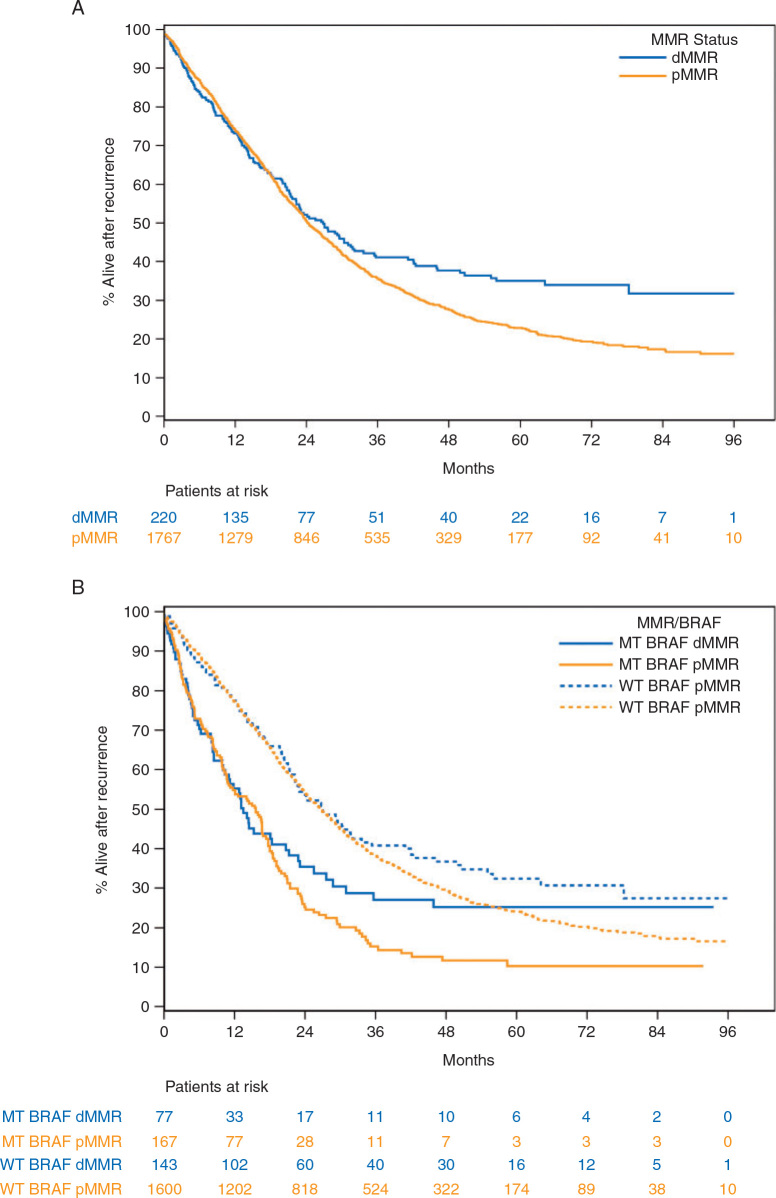
Multivariable analysis revealed that patients with MSI/dMMR tumors had significantly better SAR than did patients with MSS/proficient (p)MMR tumors (Table 2, Figure 1). This was also observed among patients treated with standard adjuvant fluoropyrimidine+oxaliplatin only. The same trends were observed when analyzing patients with MSI/dMMR tumors lacking BRAFV600E and mutant subgroups separately, without achieving statistical significance (Table 2).
Figure 1.
Survival after recurrence in patients with resected stage III colon cancer according to the MMR and BRAFV600Emutational status, adjusted for age, gender, PS, T-stage, N-stage, primary, grade, KRAS, recurrence years.
As previously described, poor SAR was observed in BRAFV600E versus nonmutated patients and this was also found in the subgroup of MSI/dMMR patients (Table 2).
Discussion
We report the largest study examining the role of MMR and BRAFV600Estatus among patients (n = 2630) with colon cancer recurrence following surgery and adjuvant chemotherapy. Among recurrent colon cancers, MSI/dMMR and BRAFV600Eeach had a low prevalence (around 10%). Whereas BRAFV600Eindicates a poor prognosis, we found that MSI/dMMR status was associated with a better SAR. Moreover, the association of BRAFV600Ewith poor prognosis was found in both MSI/dMMR and MSS/pMMR patients. Most studies report a poor prognostic value of BRAFV600E in CRC, and in MSI/dMMR patients in the metastatic setting [10]. The general explanation for the poor prognosis of MSI/dMMR tumors in the metastatic setting, as compared with their favorable prognosis in the adjuvant setting, is based on possible immunoescape and immuno-editing processes with strong immune infiltration [13]. MSI/dMMR tumors that recur appear to have escaped immune surveillance by diverse cellular and molecular processes, and thus tend to be more aggressive. Marissa et al. demonstrated recently that immune checkpoint expression cancels the prognostic relevance of tumor-infiltrating T cells in highly immunogenic colon tumors and predicts a poor outcome in MSI CRC patients [14]. Another explanation is the overlap between MSI and the poor prognostic BRAFV600E mutations.
This work has however some limitations including the fact that only 54% of recurring patients were analyzed due to missing patients consents of inadequate BRAF and MMR testing. Moreover, in contrast to prior reports, all patients had subsequent recurrent disease and were treated with previous adjuvant therapy. This may explain why our results differ compared with studies of patients with non-resectable mCRC eligible for a first-line trial [10]. However, a recent study of mCRC patients enrolled in a first-line study (CALGB/SWOG 80405) found that MMR status was not a poor prognostic factor [15].
In summary, our data demonstrate in a large cohort of MSI/dMMR patients with recurrence and before the era of immuno-oncologic treatments, that MSI/dMMR (versus MSS/pMMR) status is associated with a longer survival. Furthermore, BRAFV600E was observed to be a poor prognostic factor for both MSI/dMMR and MSS/pMMR patients. As both MSI and BRAF status influence recurring colon cancer patients’ outcome, these factors should be used to stratify patients in future clinical trials dedicated to MSI or BRAF mutant mCRC.
Acknowledgement
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U10CA180882. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number U10CA180882).
National Cancer Institute at the National Institutes of HealthU10CA180882
Disclosure
JT has received honoraria for speaker or/and advisory role from Merck KGaA, Sanofi, Roche Genentech, MSD, Lilly, Celgene, Servier, Pierre Frabre and Amgen. TA has participated in consulting or/and advisory boards for Astra-Zeneca, Amgen, BMS, MSD Oncology, HalioDx, Roche/Ventana, Sanofi, Sevier. FS has participated in consulting and/or advisory boards for Roche/Ventana Medical Systems and HalioDx. TY reports research funding from Chugai Pharmaceutical Co., Ltd., Sanofi, 423 K.K., and Sumitomo Dainippon Pharma Co., Ltd. All remaining authors have declared no conflicts of interest.
References
- 1.Taieb J., André T., Auclin E. Refining adjuvant therapy for non-metastatic colon cancer, new standards and perspectives. Cancer Treat Rev. 2019;75:1–11. doi: 10.1016/j.ctrv.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 2.André T., Boni C., Mounedji-Boudiaf L. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 3.Yothers G., O'Connell M.J., Allegra C.J. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29(28):3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Cutsem E., Labianca R., Bodoky G. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: pETACC-3. J Clin Oncol. 2009;27(19):3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 5.de Gramont A., Van Cutsem E., Schmoll H.-J. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13(12):1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 6.Alberts S.R., Sargent D.J., Nair S. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307(13):1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taieb J., Tabernero J., Mini E. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(8):862–873. doi: 10.1016/S1470-2045(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 8.Allegra C.J., Yothers G., O'Connell M.J. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29(1):11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaanan A., Shi Q., Taieb J. Role of deficient DNA mismatch repair status in patients with stage III colon cancer treated with FOLFOX adjuvant chemotherapy: a pooled analysis from 2 randomized clinical trials. JAMA Oncol. 2018;4(3):379–383. doi: 10.1001/jamaoncol.2017.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venderbosch S., Nagtegaal I.D., Maughan T.S. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20(20):5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makuch R.W. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35(6):437–443. doi: 10.1016/0021-9681(82)90058-3. [DOI] [PubMed] [Google Scholar]
- 12.Gail M.H., Byar D.P. Variance calculations for direct adjusted survival curves, with applications to testing for no treatment effect. Biom J. 1986;28(5):587–599. [Google Scholar]
- 13.Dunn G.P.1, Bruce A.T., Ikeda H. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 14.Marisa L., Svrcek M., Collura A. The balance between cytotoxic T-cell lymphocytes and immune checkpoint expression in the prognosis of colon tumors. J Natl Cancer Inst. 2018;110(1):68–77. doi: 10.1093/jnci/djx136. [DOI] [PubMed] [Google Scholar]
- 15.Innocenti F., Ou F.S., Qu X. Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 2019;37(14):1217–1227. doi: 10.1200/JCO.18.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]