Abstract
Objective
Laparoscopic donor nephrectomy (LDN) has been shown to be a safe approach with better morbidity results. Impact of multiple renal arteries (MRAs) and anatomical variations has been reviewed by many authors. In our study, the relationship between the donors with MRAs and risk of perioperative vascular complications related to donor nephrectomy was investigated.
Material and methods
Patients who underwent hand-assisted LDNs between January 2007 and February 2018 were reviewed retrospectively. Patient age, sex, body mass index (BMI), waist circumference, side of donor nephrectomies, donors with MRAs, intraoperative vascular complications, conversion rates, hospitalization durations, and operative times were extracted. Risk factors for perioperative vascular complications were defined.
Results
There were MRAs in 288 kidney donors (21.3%). The number of patients who underwent a right donor nephrectomy was 113 (8.4%). BMI, waist circumference, and postoperative hospital stay were not significantly different between donors with one artery and those with MRAs (p>0.05). The renovascular complication rate and overall conversion rate to open surgery were significantly higher in donors with MRAs (p<0.05).
Conclusion
Perioperative safety of the kidney donors is of crucial importance. Surgeons performing LDNs must be aware of the potential risks. Our analysis suggests that procurement of kidneys from donors with MRAs is a risk factor for renovascular complications.
Keywords: Hand-assisted laparoscopic donor nephrectomy, multiple renal arteries, vascular complications
Introduction
Donor nephrectomy is unique among major surgical procedures because it exposes an otherwise healthy patient to the risks of major surgery entirely for the benefit of another person. Laparoscopic donor nephrectomy (LDN) replaced open nephrectomy quickly after the initial report on the procedure from Ratner and colleagues.[1] Despite the benefits and worldwide acceptance of the LDN procedure, some questions remain unanswered. Issues related to perioperative morbidity and mortality are especially pertinent and, therefore, it is necessary to better define the limitations of the laparoscopic procedure and the potential pitfalls of the donor operation.[2,3] Right donor nephrectomy has been a significant challenge to LDN since its inception, with several groups avoiding or abandoning this procedure at the beginning.[4] The short right renal vein, the presence of friable venous branches draining into the inferior vena cava (IVC) in proximity to the right renal vein, and the need to mobilize the duodenum and mobilize and retract the liver have been challenging factors for many surgeons performing LDN. The right renal vein is considerably shorter than the left renal vein, and renal vein length can be another limiting factor during the recipient operation. Thus, a left kidney with multiple renal arteries (MRAs) and a longer renal vein compared to a right kidney with a single renal artery (SRA) is preferred by many surgeons, unless there is an obvious reason to choose the right kidney.[5] Many authors have reviewed the impact of MRAs and anatomical variations.[6,7] The purpose of this study was threefold: we reviewed our donors who underwent hand-assisted laparoscopic donor nephrectomy (HALDN); we investigated the risks of perioperative vascular complications related to donor nephrectomy for donors with MRAs; and we determined the incidence of ureteral strictures among the recipients.
Material and Methods
After obtaining ethical committee approval from Nigde Omer Halis Demir University and receiving patients’ informed consent, the data of all HALDNs performed by our group between January 2007 and February 2018 were reviewed retrospectively. Patients’ age, sex, body mass index (BMI), waist circumference, side of donor nephrectomies, donors with MRAs, intraoperative vascular complications, conversion rates, hospitalization durations, and operative times were extracted from their chart reviews. The number of renal arteries and intraoperative complications were determined from operative notes. Similarly, recipient data of the corresponding donors were taken from the discharge summaries. Delayed graft function (DGF) was defined as the need for at least one dialysis session within the first week after the kidney transplantation. We reviewed recipients’ operative notes to determine whether the accessory arteries were anastomosed separately or reconstructed. Cold ischemic time (CIT) was accepted as the time between the start of cooling the kidney with a cold perfusion solution infusion through the renal artery and unclamping of the renal vein and arteries after vascular anastomoses. All patients were operated with the standard HALDN technique. The operative technique for LDN (3–4 laparoscopic ports, left/right lower quadrant, or periumbilical extraction incision) has been described previously.[8] We usually placed the hand port through a 6 or 7 cm lower quadrant incision. One 5 mm camera port was placed at a point on the midclavicular line close to the costal arch and another 12 mm instrumental port was inserted lateral to the rectus sheath and 10 cm cranial to the 5 mm port (Figure 1).
Figure 1.
Port sites for hand-assisted laparoscopic left donor nephrectomy
Statistical analysis
A Mann-Whitey U Test was performed for the analysis of quantitative variables. Categorical data were compared using the chi-square test. A p value greater than 0.05 was accepted as statistically significant.
Results
There were 1,350 HALDN patients who participated in our study. Six hundred and eleven (45.3%) of these patients were men and 739 (54.7%) were women; 1,062 (78.7%) and 288 (21.3%) donors had SRA and MRAs, respectively (Figure 2). Of the donors with SRA, 458 (43.1%) were men and 604 (56.9%) were women. For donors with MRAs, 153 (53.1%) were men and 135 (46.9%) were women. The number of male patients was significantly higher in donors with MRAs (p=0.002). The number of patients who underwent a right donor nephrectomy was 113 (8.4%) for the whole data set. Ninety-one (80.5%) of these donors had SRA and 22 (19.5%) of them had MRAs. The side of the donor nephrectomy was not significantly different between donors with SRA and MRAs (p=0.61). The mean ages and standard deviations of donors with SRA and MRAs were 46.4±12.9 and 44.9±12.5 years, respectively. Mean ages were not significantly different between the two donor groups (p=0.12). Other variables, including BMI, waist circumference, and postoperative hospital stay, were not found to be significantly different between donors with SRA and MRAs (p>0.05) (Table 1). However, the mean operative time of donors with MRAs was significantly longer than that of donors with SRA (p=0.00001).
Figure 2.
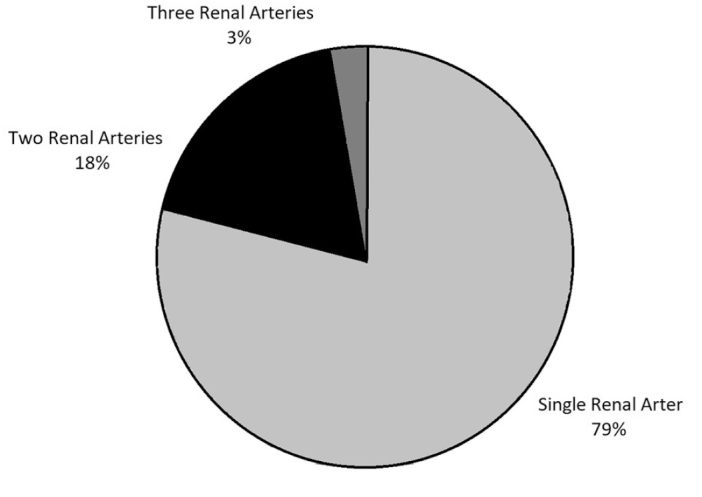
Percentages of donors according to the number of renal arteries
Table 1.
Demographic data of donors and rate of vascular complications for donors with and without multiple renal arteries
| Donors with SRA | Donors with MRAs | p | |
|---|---|---|---|
| No. of patients | 1,062 | 288 | N/A |
| Age (years) | 46.4±12.9 | 44.9±12.5 | 0.12 |
| Male/female | 458/604 | 153/135 | 0.002 |
| Waist circumference (cm) | 92.7±11.6 | 94.1±14.9 | 0.44 |
| Right/left kidney | 91/971 | 22/266 | 0.61 |
| No. of vascular complications (%) | 8 (0.7) | 12 (4) | 0.00002 |
| No. of conversion | 4 (0.375) | 5 (1.7%) | 0.01 |
| Mean operative time (min.) | 71.2±25.2 | 79.9±28.9 | 0.00001 |
| Hospital stay (day) | 2±1.2 | 1.9±1 | 0.44 |
| Ureteral stricture (recipient) | 7 | 3 | 0.5 |
SRA: single renal artery; MRAs: multiple renal arteries
When the risk of operative renovascular complications was considered and compared between donors with SRA and MRAs, we found that eight of 1,062 (0.7%) donors with SRA and 12 of 288 (4%) donors with MRAs experienced a renovascular complication. Overall, the renovascular injury rate was 1.5% in our cohort. A list of complications that occurred among participants is illustrated in Table 2. The renovascular complication rate was significantly higher in donors with MRAs (p=0.00002). The overall conversion to open surgery rate was 0.66% (only nine cases). Four (0.37%) patients had conversion to open surgery in donors with SRA, and five (1.7%) patients had conversion to open surgery in donors with MRAs. The conversion rate was found to be significantly higher in donors with MRAs (p=0.01).
Table 2.
Vascular complications
| Single renal artery | Multiple renal arteries | |
|---|---|---|
| Lumbar vein injury | 1 | None |
| Renal vein injury | 2 | None |
| Renal artery injury | 3 | 10 |
| Adrenal vein injury | 1 | None |
| Stapler failure | 1 | 2 |
We also evaluated other risk factors for renovascular complications. However, there was no significant difference between donors with and without renovascular complications in terms of age, sex, BMI, waist circumference, or side of the nephrectomy (p<0.05) (Table 3). Ureteral stricture incidence in recipients after renal transplantation was also considered. Overall, ureteral stricture rate was 0.7% (10 patients). Of these 10 patients, 7 received kidneys from donors with SRA and 3 received kidneys from donors with MRAs. There was no correlation between the number of renal arteries and the incidence of postoperative ureteral stricture (p=0.5). Overall, the DGF rate was 2.3%. The incidence of DGF for kidneys with MRAs was similar to that for kidneys with SRA (p=0.22). Mean CITs were 119.21±20.4 and 121.1±26.1 minutes for kidneys with SRA and MRAs, respectively. There was no significant difference between the two donor groups regarding the CITs (p=0.76). All accessory arteries were anastomosed separately, except for five cases in which arterial reconstructions were performed either to correct vascular injuries or to reduce the number of MRAs.
Table 3.
Evaluation of risk factors for vascular complications during donor nephrectomy
| Vascular complication + | Vascular complication − | p | |
|---|---|---|---|
| No. of patients | 20 | 1330 | N/A |
| Age (years) | 48.5±13 | 46±12.8 | 0.36 |
| Male/female | 9/11 | 602/728 | 0.9 |
| Body mass index | 27.7±4.6 | 28.4±4 | 0.63 |
| Waist circumference (cm) | 97.5±13.8 | 93±12.4 | 0.52 |
| Right/left kidney | 1/19 | 112/1218 | 0.6 |
Discussion
LDN has been shown to be a safe and advantageous approach for procuring kidneys from living donors, not only because of better cosmesis, but also because of reduced morbidity and a shorter recovery time compared to open surgery.[9,10] The short right renal vein, the presence of friable venous branches draining into the IVC in proximity to the right renal vein, the need to mobilize the duodenum, and the need to mobilize and retract the liver have been a challenge to many surgeons performing a right LDN. The reluctance to perform right LDN also seems to be a consequence of technical difficulties during the recipient operation. The anatomical complexity of a short and thin renal vein on the right side has generally led surgeons to choose the left kidney for LDN. The main concerns are the high rates of venous complications associated with right donor nephrectomy in the recipient. This issue is evident from the results of earlier series. Higher rates of DGF and graft thrombosis have been shown to be associated with right LDN in these series.[11,12] Mandal et al.[13] reported that there were a total of three graft losses due to vascular thrombosis among the LDN procedures that were observed in their study; all three occurred in their initial experience and in right kidneys. The authors believed that thrombosis in all three cases was due to a short donor renal vein. Thus, a left kidney with MRAs and a longer renal vein compared to a right kidney with SRA is preferred by many donor surgeons.[14,15]
The incidence of SRA on both sides was reported to be between 70% and 75%, with the remainder being expected to have MRAs on either one side or both sides.[16,17] The risk of vascular and ureteral complications for the kidney recipients associated with MRAs has also been reported in prior research.[13,18–20] Theoretically, the transplantation of a kidney from a donor with MRAs has the risks of prolonged warm ischemia, DGF, and associated prolonged hospitalization or ureteral complications due to the deterioration of vascular supply to the ureter.[21–23] Currently, many other authors report that with the help of surgical advancements in laparoscopic technique and the accumulation of experience, the procurement and transplantation of the kidneys with MRAs can be performed safely.[21,24] However, we would argue that safety of donors with MRAs is still contentious, since most of the contemporary studies included small cohort sizes, only reviewed the literature for metanalysis, and provided insufficient details about the renovascular complications among kidney donors.[5,7,16,17,21,24]
In our study, we scrutinized donor nephrectomy-related renovascular complications, which could be a reason for the conversion to open surgery and, in some instances, a life-threatening condition for the donor. Unlike some of the previous studies, our study found MRAs to be a significant risk factor for renovascular complications and conversion to open surgery, although our vascular complication and conversion rates were comparatively low.[7,21] Moreover, it seems that the number of arterial complications was higher in donors with MRAs than in donors with SRA (Table 2). These data emphasize the effect of multiplicity of renal arteries on arterial injuries.
Because of the aforementioned anatomical complexity of the right renal vein, many surgeons prefer to procure the right kidney only if there is a clear advantage to the donor to retain the left kidney.[13] Hsu et al.[20] reported a high risk of early graft loss associated with right LDN. This finding, however, was not verified by many other studies that assessed the impact of right LDN on graft survival.[14] None of our grafts from the right LDN group were lost in the early period due to a vascular complication. There was also no significant difference between the right and left LDNs regarding vascular complications (p=0.6). We think that right LDNs can be performed safely, although more surgical expertise and experience is needed to handle the challenges faced during a right LDN and the benching of a right kidney.
The safety of kidney donors is paramount. It is, therefore, imperative that surgeons who prefer a longer left renal vein with MRAs to a short right renal vein with an SRA consider the perioperative risks while performing an LDN with MRAs. We would argue that procuring a kidney with MRAs is not always an acceptable tradeoff for the benefit of the donor, considering that our data showed that MRAs caused more morbidity with higher conversion to open surgery rates.
Contrary to the results reported by some of the previous studies, our study results from 1,350 HALDN cases showed that a donor kidney with MRAs was not a risk factor for ureteral stricture in recipients.[17,22] We believe that meticulous arterial dissection and preservation of the lower polar arteries during bench surgery are crucial for the prevention of ureteral strictures in recipients. CITs and DGF rates were also similar in both donor groups. We think this finding reflects a low number of arterial reconstructions during the bench surgery, which otherwise could have been a reason for longer CITs. Similar DGF rates were likely a result of comparable CITs of the kidneys with SRA and MRAs. Another explanation for similar DGF rates might be our sequential-clamping approach when performing anastomoses of a kidney transplant with MRAs. We usually perform arterial anastomoses separately, starting from the main renal artery and the vein and, if possible, by unclamping the vessels. We then reclamp the iliac artery distally and perform the other arterial anastomoses, especially the lower polar arteries.
Preoperative imaging modalities can now accurately detect the vascular anatomy of donor kidneys.[23,25] The sensitivity of radiologic tests to show abnormal renovascular structures or anatomical variations may vary between 70% and 99%, depending on the type of radiologic diagnostic tests.[23,25,26] An unexpected renovascular variation, including an extra vascular structure, is a challenging condition for every surgeon. In our series, there was discordance in 24 patients (1.7%) regarding the number of renal arteries. In all these cases, more renal arteries were found intraoperatively than the number of arteries reported preoperatively by radiologic diagnostic tests. Therefore, every donor surgeon should be alert and careful during renal hilar dissection in terms of uncovering an unexpected vascular structure.
Our study was a retrospective analysis of the data collected over 9 years and, therefore, it has some inherent limitations. Unfortunately, we could not analyze and compare the long-term graft survival due to the lack of significant follow-up data. However, our cohort was one of the largest series of HALDN patients from a single group, as it included nearly 1,500 patients worldwide.
In conclusion, perioperative safety of kidney donors is of crucial importance. Surgeons performing LDNs must be aware of the potential risks. Our analysis suggests that the procurement of kidneys from donors with MRAs is a risk factor for renovascular complications and possible conversion to open surgery.
Main Points.
MRAs were found to be a statistically significant risk factor for renovascular complications during LDN.
Right LDN was not a risk factor for renovascular complications and renal vein thrombosis in the recipient.
Donor Surgeons should be aware of the risks while making decision between a left kidney with MRAs and a right kidney with a SRA.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Nigde Omer Halis Demir University (Date: 13.06.2019 No:2019/18).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.A., B.K., B.G.; Design - E.A., C.K., B.A., D.Y.; Supervision - E.A., B.K.; Resources - E.A., D.Y., B.G., B.A.; Materials - E.A., B.G., D.Y.; Data Collection and/or Processing - E.A., D.Y., B.G.; Analysis and/or Interpretation - E.A., B.K., B.A., C.K.; Literature Search - E.A., B.A., D.Y.; Writing Manuscript - E.A., B.K.; Critical Review - B.K., C.K.; Other - C.K., B.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Ratner LE, Ciseck LJ, Moore RG, Cigarroa FG, Kaufman HS, Kavoussi LR. Laparoscopic live donor nephrectomy. Transplantation. 1995;60:1047–9. [PubMed] [Google Scholar]
- 2.Wilson CH, Sanni A, Rix DA, Soomro NA. Laparoscopic versus open nephrectomy for live kidney donors. Cochrane Database Syst Rev. 2011:CD006124. doi: 10.1002/14651858.CD006124.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Nanidis TG, Antcliffe D, Kokkinos C, Borysiewicz CA, Darzi AW, Tekkis PP, et al. Laparoscopic versus open live donor nephrectomy in renal transplantation: a meta-analysis. Ann Surg. 2008;247:58–70. doi: 10.1097/SLA.0b013e318153fd13. [DOI] [PubMed] [Google Scholar]
- 4.Troppmann C, Wiesmann K, McVicar JP, Wolfe BM, Perez RV. Increased transplantation of kidneys with multiple renal arteries in the laparoscopic live donor nephrectomy era: surgical technique and surgical and nonsurgical donor and recipient outcomes. Arch Surg. 2001;136:897–907. doi: 10.1001/archsurg.136.8.897. [DOI] [PubMed] [Google Scholar]
- 5.Broudeur L, Karam G, Chelghaf I, De Vergie S, Rigaud J, Perrouin Verbe MA, et al. Feasibility and safety of laparoscopic living donor nephrectomy in case of right kidney and multiple-renal artery kidney: a systematic review of the literature. World J Urol. 2019 doi: 10.1007/s00345-019-02821-8. doi: 10.1007/s00345-019-02821-8. [DOI] [PubMed] [Google Scholar]
- 6.Kwapisz M, Kieszek R, Bieniasz M, Jedrzejko K, Nita M, Sulkowska K, et al. Do Anatomical Anomalies Affect the Results of Living Donor Kidney Transplantation? Transplant Proc. 2018;50:1669–73. doi: 10.1016/j.transproceed.2018.03.119. [DOI] [PubMed] [Google Scholar]
- 7.Hsu TH, Su L, Ratner LE, Trock BJ, Kavoussi LR. Impact of renal artery multiplicity on outcomes of renal donors and recipients in laparoscopic donor nephrectomy. Urology. 2003;61:323–7. doi: 10.1016/S0090-4295(02)02124-6. [DOI] [PubMed] [Google Scholar]
- 8.Leventhal JR, Deeik RK, Joehl RJ, Rege RV, Herman CH, Fryer JP, et al. Laparoscopic live donor nephrectomy--is it safe? Transplantation. 2000;70:602–6. doi: 10.1097/00007890-200008270-00012. [DOI] [PubMed] [Google Scholar]
- 9.Brown SL, Biehl TR, Rawlins MC, Hefty TR. Laparoscopic live donor nephrectomy: a comparison with the conventional open approach. J Urol. 2001;165:766–9. doi: 10.1016/S0022-5347(05)66521-4. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs SC, Cho E, Foster C, Liao P, Bartlett ST. Laparoscopic donor nephrectomy: the University of Maryland 6-year experience. J Urol. 2004;171:47–51. doi: 10.1097/01.ju.0000100221.20410.4a. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs SC, Cho E, Dunkin BJ, Flowers JL, Schweitzer E, Cangro C, et al. Laparoscopic live donor nephrectomy: the University of Maryland 3-year experience. J Urol. 2000;164:1494–9. doi: 10.1016/S0022-5347(05)67014-0. [DOI] [PubMed] [Google Scholar]
- 12.Ratner LE, Montgomery RA, Kavoussi LR. Laparoscopic live donor nephrectomy. A review of the first 5 years. Urol Clin North Am. 2001;28:709–19. doi: 10.1016/S0094-0143(01)80027-6. [DOI] [PubMed] [Google Scholar]
- 13.Mandal AK, Cohen C, Montgomery RA, Kavoussi LR, Ratner LE. Should the indications for laparascopic live donor nephrectomy of the right kidney be the same as for the open procedure? Anomalous left renal vasculature is not a contraindiction to laparoscopic left donor nephrectomy. Transplantation. 2001;71:660–4. doi: 10.1097/00007890-200103150-00015. [DOI] [PubMed] [Google Scholar]
- 14.Keller JE, Dolce CJ, Griffin D, Heniford BT, Kercher KW. Maximizing the donor pool: use of right kidneys and kidneys with multiple arteries for live donor transplantation. Surg Endosc. 2009;23:2327–31. doi: 10.1007/s00464-009-0330-9. [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Zhang P, Xu X, Fan M. Right Versus Left Laparoscopic Living-Donor Nephrectomy: A Meta-Analysis. Exp Clin Transplant. 2015;13:214–26. [PubMed] [Google Scholar]
- 16.Nunes-Carneiro D, Marques-Pinto A, Veiga C, Braga I, Cabral JF, Almeida M, et al. Which One Is the Best for Living Donation: A Multiple-Artery Left Kidney Nephrectomy or a Right Kidney Nephrectomy? Transplant Proc. 2019;51:1559–62. doi: 10.1016/j.transproceed.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Zorgdrager M, Krikke C, Hofker SH, Leuvenink HG, Pol RA. Multiple Renal Arteries in Kidney Transplantation: A Systematic Review and Meta-Analysis. Ann Transplant. 2016;21:469–78. doi: 10.12659/AOT.898748. [DOI] [PubMed] [Google Scholar]
- 18.Benedetti E, Troppmann C, Gillingham K, Sutherland DE, Payne WD, Dunn DL, et al. Short- and long-term outcomes of kidney transplants with multiple renal arteries. Ann Surg. 1995;221:406–14. doi: 10.1097/00000658-199504000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roza AM, Perloff LJ, Naji A, Grossman RA, Barker CF. Living-related donors with bilateral multiple renal arteries. A twenty-year experience. Transplantation. 1989;47:397–9. doi: 10.1097/00007890-198902000-00045. [DOI] [PubMed] [Google Scholar]
- 20.Hsu JW, Reese PP, Naji A, Levine MH, Abt PL. Increased early graft failure in right-sided living donor nephrectomy. Transplantation. 2011;91:108–14. doi: 10.1097/TP.0b013e3181fd0179. [DOI] [PubMed] [Google Scholar]
- 21.Bandin Musa AR, Montes de Oca J. Hand-assisted Laparoscopic Nephrectomy in Living-donor Kidneys With Multiple Arteries: Experience of a Transplant Center. Exp Clin Transplant. 2016;14:153–6. [PubMed] [Google Scholar]
- 22.Fuller TF, Deger S, Buchler A, Roigas J, Schonberger B, Schnorr D, et al. Ureteral complications in the renal transplant recipient after laparoscopic living donor nephrectomy. Eur Urol. 2006;50:535–40. doi: 10.1016/j.eururo.2006.03.025. discussion 540-1. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto S, Montgomery RA, Lawler LP, Horton KM, Fishman EK. Multi-detector row CT evaluation of living renal donors prior to laparoscopic nephrectomy. Radiographics. 2004;24:453–66. doi: 10.1148/rg.242035104. [DOI] [PubMed] [Google Scholar]
- 24.Saidi R, Kawai T, Kennealey P, Tsouflas G, Elias N, Hertl M, et al. Living donor kidney transplantation with multiple arteries: recent increase in modern era of laparoscopic donor nephrectomy. Arch Surg. 2009;144:472–5. doi: 10.1001/archsurg.2009.49. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto S, Tanooka M, Ando K, Yamano T, Ishikura R, Nojima M, et al. Diagnostic accuracy of a volume-rendered computed tomography movie and other computed tomography-based imaging methods in assessment of renal vascular anatomy for laparoscopic donor nephrectomy. Int Urol Nephrol. 2009;41:785–90. doi: 10.1007/s11255-009-9535-z. [DOI] [PubMed] [Google Scholar]
- 26.El Fettouh HA, Herts BR, Nimeh T, Wirth SL, Caplin A, Sands M, et al. Prospective comparison of 3-dimensional volume rendered computerized tomography and conventional renal arteriography for surgical planning in patients undergoing laparoscopic donor nephrectomy. J Urol. 2003;170:57–60. doi: 10.1097/01.ju.0000068039.79654.d3. [DOI] [PubMed] [Google Scholar]


